Summary
The Rho GTPases—Rho, Rac and Cdc42—act as molecular switches, cycling between an active GTP-bound state and an inactive GDP-bound state, to regulate the actin cytoskeleton. It has recently become apparent that the Rho GTPases can be activated in subcellular zones that appear semi-stable, yet are dynamically maintained. These Rho GTPase activity zones are associated with a variety of fundamental biological processes including symmetric and asymmetric cytokinesis and cellular wound repair. Here we review the basic features of Rho GTPase activity zones, suggest that these zones represent a fundamental signaling mechanism, and discuss the implications of zone properties from the perspective of both their function and how they are likely to be controlled.
Introduction
Many of the most remarkable examples of cell shape change, including cytokinesis, wound healing and morphogenesis are powered by transient contractile arrays based on actin filaments (F-actin) and myosin-2. Such arrays assemble rapidly at a particular place and time, undergo constriction and then disassemble. Due to the fundamental importance of such structures in a variety of normal and pathological processes, one of the major goals of modern biomedical research is to understand signaling mechanisms regulating transient contractile arrays.
The Rho GTPases—Rho, Rac, and Cdc42—are essential regulators of the transient contractile arrays that underlie cytokinesis (e.g. Ref. 1), wound healing (e.g. Refs. 2,3), and morphogenesis (e.g. Ref. 4). The Rho GTPases are targeted to membrane compartments by lipid modification and act in a switch-like manner, such that, in their active (GTP-bound) state, they bind to specific effector proteins that modulate different aspects of actin cytoskeleton function.(5) For example, Rho-GTP binds to and activates ROCK, which, in turn, promotes myosin-2 assembly and activation by elevating phosphorylation of the myosin-2 regulatory light chain.(6) Rho-GTP also binds to and activates formins which, in turn, promote assembly of unbranched actin filaments.(7) Rac-GTP promotes actin assembly by activation of the WAVE complex and other targets,(8) while Cdc42-GTP binds and activates N-WASP, which activates the Arp2/3 complex, thereby promoting formation of branched actin filament networks.(9)
Because the Rho GTPases are intrinsically inefficient enzymes, hydrolyzing a single GTP every 5–50 minutes,(10) most of the regulation of the Rho GTPase cycle depends on several classes of regulatory proteins: (1) guanine nucleotide exchange factors (GEFs) promote the exchange of GDP for GTP, (2) GTPase activating proteins (GAPs) promote GTP hydrolysis, and (3) guanine nucleotide dissociation inhibitors (GDIs) stabilize the GDP-bound state and mask the lipid moeity, facilitating cytoplasmic solubility for GTPases.(11,12) The standard view of the role of Rho regulators is that a given actin-dependent process is initiated primarily by GEF-dependent Rho GTPase activation and eventually terminated by GAP-dependent Rho GTPase inactivation, with GDIs passively converting inactivated Rho GTPases to a soluble form (Fig. 1).
Figure 1.
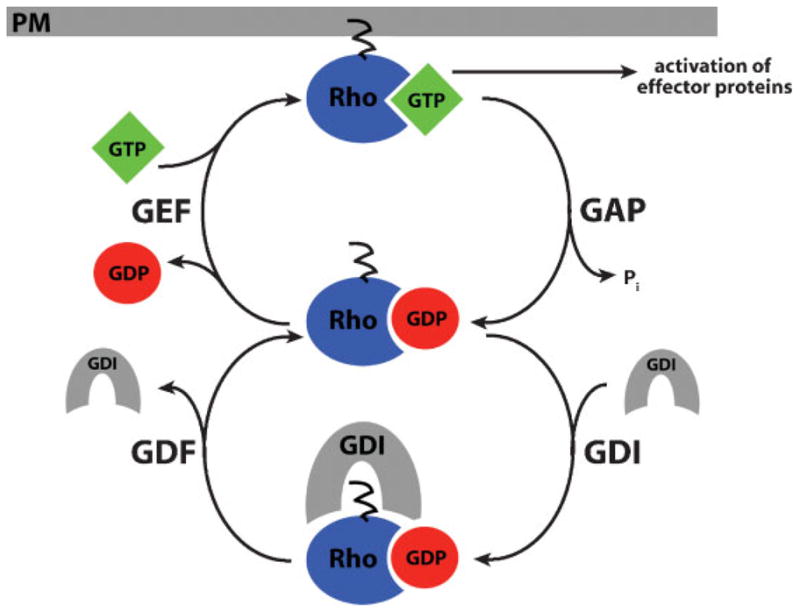
The Rho GTPase cycle. Rho GTPases cycle between an active, GTP-bound state and an inactive, GDP-bound state. Their activity is regulated by four factors: (1) Guanine nucleotide exchange factors (GEFs) activate Rho GTPases by promoting the exchange of GDP for GTP, (2) GTPase activating proteins (GAPs) promote GTP hydrolysis, (3) guanine nucleotide dissociation inhibitors (GDIs) stabilize the GDP-bound state and mask the lipid moeity, maintaining Rho GTPases in an inactive state in the cytoplasm, and (4) GDI displacement factors (GDFs) disrupt GDI-GTPase binding, facilitating GTPase activation. Only when Rho is in its active, GTP-bound conformation at the plasma membrane (PM) can it interact with downstream effector proteins to modulate the cytoskeleton.
In addition to the three steps of Rho GTPase regulation in the canonical pathway, a fourth step likely occurs. The generically termed “GDI displacement factors” (GDFs) partially or completely disrupt GTPase–GDI binding. GDFs include both proteins and phospholipids.(12) For example, ERM proteins, such as radixin, disrupt Rho–GDI interaction in vitro,(13) and the phospholipid inositol 4,5 bisphosphate (PIP2) can destabilize Rho–GDI interaction.(14) Thus, GDFs would facilitate GTPase activation by increasing the access of GEFs to GDP-bound GTPases (Fig. 1).
The widely repeated metaphor of Rho GTPases as “molecular switches” descends not only from the biochemical properties of small GTPases, but also from the early finding that experimental hyperactivation of one or another family member could prompt reorganization of the cell’s entire actomyosin network. If, however, the Rho-family GTPases are typically deployed in more localized regions and, if several different GTPases are at work in the same cell at the same time, then it makes more sense to consider the network of GTPase regulators and effectors as an intracellular pattern-formation system. Rather than switch the behavior of the entire cell from one regime to another, the Rho-family GTPases locally differentiate the dynamics of the cytoskeleton from one part of the cell to another, often very nearby. It becomes important to identify how the kinetics of GTPase regulation conspire with the mechanics of the cytoskeleton to make stable standing patterns of activation and inactivation.
Rho GTPase activity zones
It has recently become apparent that the Rho GTPases can direct formation of transient contractile arrays via activity “zones”. Rho GTPase activity zones are spatially constrained, plasma membrane (PM) regions characterized by high levels of Rho GTPase activity that persists for several minutes. These zones can be imaged by time-lapse multiple focal plane (4D) confocal microscopy using probes that specifically detect the GTP-bound, active state of Rho proteins (see Ref. 15 for a description of different probes used to detect Rho GTPases in live cells). Analysis of Rho and Cdc42 activity during wound healing in Xenopus oocytes provided the prime example of Rho GTPase zones associated with a transient contractile array.(3) In this model system, PM damage elicits actin and myosin-2 assembly in a precisely bounded zone around wound borders.(16) The actomyosin array segregates over time: while F-actin assembles throughout the wound array, myosin-2 becomes enriched on the interior circumference.(17) Subsequent studies showed that Rho GTPases direct the actomyosin response during wound healing and are likely responsible for the observed segregation of the actomyosin array. That is, wounding elicits simultaneous local activation of both Rho and Cdc42 about 20 seconds after wounding followed by segregation of active Rho and Cdc42 into complementary zones, with active Cdc42 circumscribing active Rho (Fig. 2). Disruption of either the Rho or Cdc42 activity zone interferes with wound healing.(3) In addition, there is some evidence that Rac activity zones may also participate in this process (H. Benink and W. Bement, unpublished data).
Figure 2.
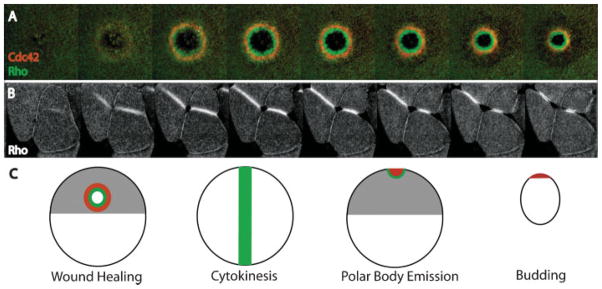
Rho activity zones regulate transient contractile arrays in diverse cellular contexts. A:Active Rho (green) and active Cdc42 (red) segregate into discrete zones during wound healing. Active Rho is concentrated in a ring that is circumscribed by a ring of active Cdc42. B: Rho activity zones predict the site of cleavage furrow formation during cytokinesis. The Rho activity zones form a stripe-like zone that remains tightly focused and moves inward in concert with the ingressing cleavage furrow. C: Rho GTPase activity zones are employed in diverse contexts including wound healing, cytokinesis, polar body emission and budding. Although the overall geometric organization may vary, the Rho activity zones share many common features (see text for details).
Similarly, a Rho activity zone develops at the site of incipient cytokinetic apparatus assembly in echinoderm and Xenopus embryos.(18) Moreover, Rho concentrates at the equator prior to cytokinesis in cultured mammalian cells.(19–23) Rho activity zones are typically spatially confined to regions with widths on the order of microns, while the linear dimension of the cells in which they form ranges from hundreds of microns (Xenopus oocytes, echinoderm zygotes) to tens of microns (echinoderm blastomeres, cultured mammalian cells). Rho itself was shown to be required for cytokinesis long ago,(1) but these findings, and the demonstration that active Rho dynamically senses microtubule perturbation,(18) imply that Rho zones are part of the long-sought link between microtubule geometry and stimulation of cytokinetic furrowing. It remains to be seen whether Cdc42 and/or Rac activity zones also participate in cytokinesis, but this is consistent with several findings. First, complementary zones of Rho and Cdc42 activity form during polar body emission, a highly asymmetric cytokinesis(24) (Fig. 2). Second, a study based on fluorescent resonance energy transfer (FRET) suggests that Cdc42 and Rac are activated in regions outside the cell equator during cytokinesis.(25) Third, genetic evidence indicates that Rac and Cdc42 maycooperate with Rho during cytokinesis in Drosophila.(26)
In addition to wound healing and cell division, we suspect that Rho GTPase zones are also employed in other contexts. Visualization of Cdc42 localization during bud formation in yeast indicates that the bud site represents a zone of tightly localized, semi-stable Cdc42 activity.(27–29) Other examples may be found during exocytosis, endocytosis, phagocytosis, and cell locomotion, all of which entail semi-stable, spatially constrained regions of Rho GTPase activity.(30–32)
These observations indicate that the Rho zones represent a widespread signaling mechanism harnessed in diverse cell types to assemble actomyosin-based arrays, differing in the overall geometric organization of the zones (Fig. 2). Further, while the zones associated with each of these processes are likely controlled by different sets of regulatory proteins, they share several important features. (1) The zones are spatially constrained; i.e., high levels of Rho or Cdc42 activity are maintained within a discrete region (Fig. 3). (2) In situations where both Cdc42 and Rho zones are present, the adjacent zones show remarkably little overlap (Fig. 3). (3) Zones are mobile and move in concert with the actomyosin array that they control. (4) Zones are semi-stable, such that they persist for as long as necessary to direct a particular actomyosin-dependent process and then disappear. The commonality of these dynamic features suggests that, while the details of zone generation and maintenance may differ, general regulatory principles are likely to operate in all cases.
Figure 3.
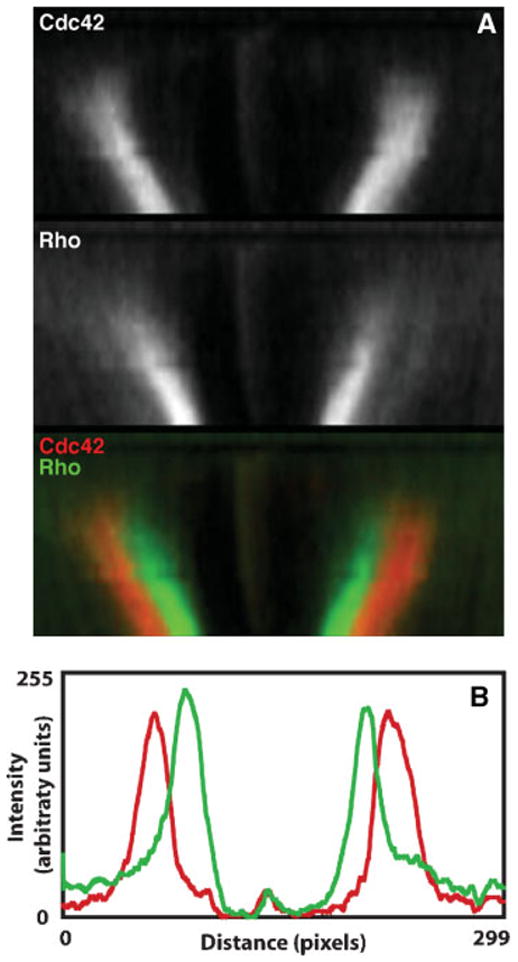
Rho activity zones are spatially segregated and tightly focused. Two common features of Rho activity zones are their sharp boundaries and spatial segregation. These features are illustrated here for the case of wound healing (see also Ref. 18 for an example from cytokinesis). A: Kymographs of active Cdc42 (red) and active Rho (green) during wound healing. Time is in the vertical direction with regions from successive time points aligned from top to bottom for each panel. The zones remain spatially segregated with active Rho localized on the inside, Cdc42 on the outside, and little overlap between the two. B: Line scans of the intensities of active Rho (green) and active Cdc42 (red). Notice that the peaks are tightly focused, with sharp boundaries between regions containing active or inactive GTPases.
How are zones generated?
Almost nothing is yet known about what triggers activation of Rho and Cdc42 during wound healing, except that calcium is required.(3) Conversely, Cdc42 activity during yeast budding has been studied intensely. Specifically, a Ras-related protein (Bud1p) and its corresponding GEF (Bud5p) and GAP (Bud2p) are recruited to the nascent bud site by proteins remaining from the previous round of cell division. Bud1p, Bud5p and Bud2p then recruit Cdc42p, its GEF (Cdc24p), and Bem1p, a putative scaffold protein that links the two.(33) Thus, Cdc24p can activate Cdc42p specifically at the bud site.
The currently favored model for generation of the Rho activity zone during cytokinesis posits that Ect2, a GEF with activity toward Rho, Rac and Cdc42, is responsible for Rho activation.(34–36) Ect2 associates with MgcRacGAP,(20–23,37) a component of the centralspindlin complex.(38) The other centralspindlin component, MKLP1, is a plus end-directed microtubule motor protein. Centralspindlin bundles microtubules in the spindle midzone,(38) and its components have also been localized to astral microtubules in the region near the equator prior to furrowing.(23,34) In addition, binding of Ect2 to MgcRacGAP may promote Ect2 GEF activity.(20) Disruption of MKLP, MgcRacGAP, or Ect2 prevents both cytokinesis and concentration of Rho at the cell equator.(20–23) Thus, it is thought that the centralspindlin complex translocates along microtubules to the cell equator, where it interacts with Ect2.(37) This interaction would concentrate Ect2 and stimulate its GEF activity, resulting in local Rho activation at the equator.
How are zones maintained?
Clearly, localized activation is part of the explanation for the phenomenon of GTPase activity zones. However, certain facts lead us to conclude that zones cannot simply represent sites where a given GTPase is activated. For example, GTPase zones exhibit a rapid rise to peak intensity, but maintain spatial confinement throughout their lifetime. Recall the low intrinsic rate of GTP hydrolysis by Rho GTPases and consider that, despite membrane anchoring, they would be expected to diffuse laterally along the membrane. A simple mathematical analysis shows that it would be impossible to maintain the spatially constrained activity zones without greatly altering these kinetic properties through regulators and physical constraints.
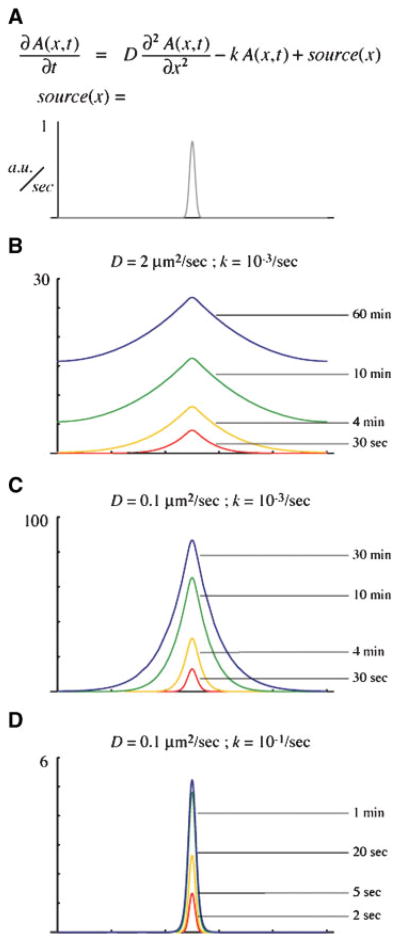
Imagine that active Rho is produced continuously by a narrow source, diffuses away from the source and experiences first-order decay. The reaction–diffusion equation relating the rate of change of concentration at a particular distance from the source to the existing spatial gradient and production rate can be solved numerically. Since the zones in question exist on two-dimensional membranes but have some axis of radial symmetry, we can use a one-dimensional version of the reaction–diffusion equation (see Fig. 4A). The typical Rho activity zone is confined to a scale on the order of microns, and the linear dimension of the cellular context ranges from hundreds of microns (Xenopus oocytes, echinoderm zygotes) to tens of microns (echinoderm blastomeres, cultured mammalian cells) or less (yeast). Consider a 100 micron domain joined circularly; this would be roughly appropriate for the cytokinetic zone on one of the ~30 micron-diameter cells in a 32-cell urchin embryo; such a cell would maintain a Rho activity zone about five microns wide.(18)
Figure 4.
Numerical solutions of a simple local source/global sink reaction–diffusion equation. A: The partial differential equation relates the rate of change of the concentration of some diffusible species “A”, at each point in space and time, to the spatial gradient (first term) and to the local concentration (second term). “D” is the diffusion coefficient, and “k” is the first-order decay rate. The source is a narrow Gaussian centered in the middle of the domain which creates “A” at a peak rate of ~1 arbitrary unit per second. The entire domain is 100 microns wide, and the edges are constrained to have the same level of “A” (periodic boundary conditions). B–D: Numerical solutions, computed using Mathematica, in which the concentration of “A” is plotted in arbitrary units across the entire domain. Four successive timepoints are shown for each of three conditions. B: With a diffusion coefficient similar to myristoylated yellow fluorescent protein and a decay rate similar to the intrinsic rate for Rho GTP hydrolysis, within seconds “A” has already appeared far from the source, and the domain slowly fills up with “A”. C: With a much smaller diffusion coefficient, the profile of “A” is still much wider than the source and also requires tens of minutes to reach steady state. D: The combination of slow diffusion and rapid turnover leads to a narrow zone, which reaches steady state quickly.
If Rho-GTP experiences a diffusion coefficient similar to myristoylated yellow fluorescent protein (~2 μm2/sec;)(39) and has an intrinsic hydrolysis rate on the order of one per 5–50 minutes, only a shallow, transient concentration gradient could be sustained, even with an infinitesimally narrow source. Furthermore, as the source continued to emit active Rho, the domain would simply fill up; the steady state, which would be reached after an hour or so, would have a high concentration of active Rho everywhere, but elevated by 20–30% near the source (Fig. 4B). This is clearly not what is observed in cells, nor does it seem likely to lead to efficient furrowing if it were.
Hypothesizing a 20-fold slower diffusion coefficient, as if Rho were trapped within cortical or membrane structure, improves matters: the steady state consists of a well-defined peak around the source, which falls to near zero far from the source (Fig. 4C). In such cases, we can extract a length scale as a convenient intuitive measure. If the source is very thin, the steady-state distribution converges on an exponential fall-off from the source with a length scale defined by the square root of the diffusion coefficient divided by the decay rate. This number corresponds to the distance over which concentration changes by 1/e, and is therefore a convenient measure for information-containing molecules. That is, if we conceive of Rho as a kind of morphogen for cortical organization, in order for effectors to respond differently at one position versus another, the concentration of active Rho must change significantly. For slow diffusion but slow hydrolysis, the length scale at steady state is about 10 microns. This is still too wide to match measured Rho zones. Since the structures that Rho organizes, such as the cytokinetic apparatus and the wound array, go from present to absent over a span of a few microns, tighter Rho-GTP localization is necessary. Furthermore, the slow diffusion plus slow hydrolysis takes tens of minutes to come to steady state, whereas Rho zones are observed to reach peak intensity within minutes.
In contrast, slow diffusion plus accelerated hydrolysis (i.e. by a GAP) yields length scales of the order of a micron if the source is itself confined sufficiently and, in addition, reaches steady state in less than a minute (Fig. 4D). Of course, since the length scale is a function of the ratio between the diffusion coefficient and the GTP turnover rate, a sufficiently small diffusion coefficient could yield a length scale around a micron even with slow turnover. For example, a diffusion coefficient of 0.001 square microns per second would be compatible with the intrinsic GTP hydrolysis rate of 5–50 minutes per GTP. However, this coefficient, which is about right for a pollen grain in motor oil, is physically unlikely. However, very rapid turnover would be compatible with fast diffusion, but would demand either that the cell make do with a proportionately lower peak concentration of Rho, or that the source activate Rho extremely quickly.
The simple construct of a reaction–diffusion model cannot account for the observed property that zone width scales linearly with cell dimension: the length scale for a simple local-source-distributed-sink model is independent of the spatial domain in which the process takes place. Nevertheless, the mathematical analysis underscores this conclusion: in order to achieve dynamic, spatially confined Rho GTPase zones, cells must restrict diffusion and, either globally or locally, accelerate turnover of active Rho. How could cells do this? In principle, four general hypotheses present themselves: (1) anchoring or corralling to restrict diffusion, (2) regulation of GTPase flux, (3) directed transport that counters diffusion and (4) feedback amplification of local dynamics or crosstalk between different pathways.
Anchors and corrals
Anchoring in order to restrict diffusion is simple to envision. The actin cytoskeleton likely contributes at least in part to the normal maintenance of zones, based on treatment of wounded and cytokinesing cells with cytochalasin or latrunculin, which disrupt F-actin. During wound healing, cytochalasin and latrunculin treatment reduce the intensity of the Rho zone and broaden both the Rho zone and the Cdc42 zone.(3) During urchin embryonic cytokinesis, Rho zones of approximately normal intensity and breadth appear in cells treated shortly before furrowing with either cytochalasin or latrunculin, implying that this zone is not strictly dependent on F-actin for formation. However, cytochalasin- or latrunculin-treated cells exhibit active Rho on dynamic, tubular PM extensions that are pulled into the cytoplasm, apparently on astral microtubules,(18) suggesting that at least some aspects of Rho-GTP’s normal distribution is perturbed.
Anchoring in an F-actin-independent manner could also contribute to zone maintenance. For example, a growing body of evidence suggests that Rho GTPases and their regulators can form complexes that are anchored to the PM. For example, AKAP proteins act as PM-anchored scaffolding proteins that associate with and regulate Rho GTPases(40) and could serve such a role. Likewise, effector binding itself could contribute to trapping active Rho, especially if active Rho must remain bound to its effectors to maintain their activity.
A mechanism related to scaffold-dependent anchoring of active GTPases is provided by PM-associated complexes that prevent lateral diffusion of membrane-anchored GTPases. For example, the septins act as barriers for lateral diffusion during bud formation in yeast.(41) However, while septins are associated with the cytokinetic apparatus in a variety of metazoan systems, and have been suggested to limit diffusion across the cleavage furrow,(42) a corral role would require that they border both sides of zones, but the available information suggests that, in metazoan systems, septins are localized to the middle of the cytokinetic apparatus.(42–44)
GTPase Flux
Anchoring and corralling are attractive hypotheses in that they could account for the ability of zones to move in concert with the actomyosin arrays that they control. However, anchoring cannot easily account for several observations. First, zones maintain a constant local intensity even as their overall size shrinks during contraction of the actomyosin arrays that they are associated with (Fig. 2). In other words, the total amount of active GTPase within a zone is decreasing once contraction begins. Second, the cytokinetic Rho zone disappears within 1–2 minutes when the spindle that it is supposed to bisect is physically moved to a new location.(18) Meanwhile, a new Rho zone appears just as rapidly as the old one disappears. Third, photobleaching experiments in budding yeast(45) and Candida albicans(46) indicate that Cdc42 at the bud site is turned over within ~5 seconds; photobleaching experiments in echinoderm embryos likewise indicate rapid turnover within cytokinetic Rho zones (10–30 seconds; unpublished results).
Thus, it seems inescapable that the zones are maintained by the balance of local activation and equally rapid inactivation. In this model, zones reflect regions where Rho GTPases move rapidly through the GTPase cycle. Effectors intercept a fraction of active GTPases, siphoning a trickle from the stream flowing from GEF to GAP; any active GTPases that fail to interact with an effector protein would be rapidly inactivated (Fig. 5). Implicit in this scenario is the hypothesis that effectors protect active GTPases, to some extent, from access by GAPs, which seems plausible since most effectors and regulators of Rho-family GTPases bind to a common surface around the switch region (e.g. Ref. 47). In an extreme case, the standing crop of free, active and diffusible GTPase might actually be quite low. This mechanism is superficially counterintuitive, in that locally elevating both activation and inactivation at the same time seems inherently wasteful. But as we argued above, spatial confinement of the zone practically requires elevated inactivation, and limiting the spread of the GTPase zones has the mechanistic virtue of ensuring that transient contractile arrays remain tightly focused. Moreover, precedent for such GTPase flux comes from yeast budding, where normal maintenance of Cdc42 at the incipient bud site is critically dependent on GTP hydrolysis.(29)
Figure 5.

The GTPase flux model. Rho activity zones are maintained by a balance of local GTPase activation (by GEFs) with local GTPase inactivation (by GAPs). This would result in the constant flux of Rho through the GTPase cycle, allowing cells to maintain tightly focused, dynamic Rho activity zones. In this way, any active Rho that does not immediately interact with an effector protein will be switched back off.
GDIs and GAPs provide the obvious means to direct GTPase flux in concert with elevated GEF activity. At least two different (but emphatically non-exclusive) scenarios can be envisioned: Rho GTPases are inactivated by local GAPactivity and then bound by GDIs, which drive them into a soluble pool, or active GTPases are directly bound by GDIs. The latter scheme is inconsistent with the idea that GDIs preferentially interact with inactive (GDP-bound) GTPases but, in fact, it has been reported that GDIs bind active and inactive Rho GTPases with similar affinity.(48) That GDIs and GTPase flux may be important in vivo is indicated by the finding that, while a constitutively active Cdc42 mutant can transform some cell types, this ability is suppressed by mutations that prevent interaction of the constitutively active Cdc42 with GDI.(49)
GDIs are abundant, soluble and active except when specifically inactivated by post-translational modifications such as phosphorylation.(12) Thus, GDIs can be viewed as acting as stable, soluble sinks for GTPase activity. While GAPs could also serve this role, the fact that they are frequently targeted and/or activated by interactions with other proteins implies that they could be more specifically involved in GTPase flux. During cytokinesis, MgcRacGAP is now well known to localize to the site of the Rho zone (see above). Thus, MgcRacGAP has the potential to promote GTPase flux. However, in vitro the Rho GAP activity of MgcRacGAP is relatively limited.(50,51) It has been reported that phosphorylation of MgcRacGAP by Aurora B kinase stimulates its Rho GAP activity,(52) and given that this kinase is activated prior to the onset of cytokinesis and localizes in the same general region as MgcRacGAP,(53) this could contribute to GTPase flux. However, based on structural considerations, the relevance of this phosphorylation event has been questioned.(54) Further, the available evidence on the importance of the GAP domain of MgcRacGAP is equivocal. Expression of GAP-dead MgcRacGAP has been reported to inhibit cytokinesis in some cases(55,56) but not others.(57) Whether these discrepancies reflect different experimental design or different requirements for GTPase flux in different systems remains to be seen, and will require in vivo studies where the GAP activity of MgcRacGAP is manipulated while the activity zones are directly monitored.
Transport
Physical transport of active GTPases, either toward or away from the zones, is an easily overlooked but conceptually simple means to modulate their scale. Presently the only observation supporting this notion in animal cells is the observation that, following F-actin disruption, active Rho is translocated toward spindle poles, away from the Rho activity zone at the equator.(18) This occurs via tubular PM extensions and is sensitive to microtubule disruption. We do not yet know whether this is strictly an artifact of cortical F-actin disruption, or if F-actin disruption reveals a process that is not as obvious under normal circumstances. Regardless, it has the manifest consequence of physically removing active Rho from the cortex. For this mechanism to function to maintain zone boundaries, microtubules in regions flanking zones would have to preferentially remove active Rho. This would require either greater frequency of microtubule contact with regions flanking the zone, or a fundamental difference between the microtubules that contact the flanking regions and those that contact the zone itself. While the latter possibility might sound farfetched, not only is there precedent from yeast for differential protein loading onto different microtubules,(58) it is well known that dynamic microtubules associate differentially with proteins at their plus ends than do stable microtubules.(59) Further, there is good reason to believe that stable microtubules may somehow signal cytokinetic apparatus establishment.(60) Meanwhile, astral microtubules penetrate the polar cortex in echinoderm embryonic cells sooner and more densely than the equatorial cortex. Treatment of echinoderm blastomeres with low doses of nocodazole results in wider- and sloppier-than-normal Rho zones, which sometimes fail to furrow; likewise, physically restricting access to the cortex by astral microtubules leads to broad, poorly developed Rho activity zones. Thus, it seems plausible that something like microtubule-coupled endocytosis might sharpen the Rho zone during cytokinesis in large embryonic cells. This idea is given further plausibility by studies showing that disruption of actin-dependent endocytosis from the yeast bud site may impair Cdc42 removal.(61)
Feedback and crosstalk
Sharp zone boundaries dependent on GTPase flux could also be generated by self-amplification (aka positive feedback) as long as some other mechanism limits the spread of the self-amplified GTPase. Provided there exists an efficient positive feedback loop affecting GTPase activation, a relatively slight accumulation of Rho GTPase activity could be rapidly and locally amplified. Self-amplification has been described for Cdc42 activation during yeast bud site selection and may involve both direct modulation of Cdc42 by GEFs and GAPs or indirect modulation via actin-based transport.(28,29,42,61,62)
Another plausible positive feedback mechanism can be proposed for the cytokinetic Rho zones. Accumulation of active Rho might activate PIP kinase,(63) thereby promoting the observed accumulation of active PIP2 in the furrow.(64,65) The PIP2 could then act as a GDF to liberate Rho from the GDI–Rho–GDP complex (see above). Accumulated PIP2 could also promote recruitment of ERM proteins such as radixin, which localize to cytokinetic furrows(66) and act as GDFs (see above). Another potential mechanism for self-amplification could come from Rho-dependent stimulation of microtubule stabilization in the zone region,(67) assuming that such stable microtubules promote Rho activation.
Given the evidence that MgcRacGAP may stimulate the GEF activity of Ect2,(20) the Ect2–centralspindlin complex might even behave as an agent of Rho auto-activation. MgcRacGAP is a comparatively poor GAP toward Rho. If this slow turnover reflects not inefficient binding of GTP-bound Rho, but rather slow catalysis once bound, and if MgcRacGAP prefers GTP-bound to GDP-bound Rho, then the following scenario becomes appealing: the MgcRacGAP–Ect-2 complex binds, through the GAP domain, to a GTP-bound Rho. Whilst MgcRacGAP plods its way through that particular molecule of Rho, Ect-2 generates more GTP-bound Rho, which inserts in the membrane in the immediate vicinity of the one that initially attracted the attention of the complex. This positive feedback would be limited by the supply of GDP-bound Rho: once a significant fraction of Rho is activated, the supply rate would fall below the inactivation rate. During cytokinesis, it is not implausible that the furrow concentrates a significant fraction of the cell’s total complement of Rho, since the cytokinetic Rho zone is manifest using probes that recognize total Rho (including antibody staining and fluorescent protein derivatives of Rho). This might constitute a means to amplify an initially shallow asymmetry of Rho activation into a distinct Rho zone. In order for this speculative scheme to work as an amplifier, either most of the cell’s MgcRacGAP would have to be localized to begin with (which it may be), or MgcRacGAP binding or retention would have to be nonlinearly sensitive to active Rho concentration.
Another potential mechanism for boundary maintenance is crosstalk between the GTPases. The complementary Rho and Cdc42 zones observed during wound healing and polar body emission could mutually antagonize each other, thereby sharpening the boundaries between them. The precedent for negative crosstalk comes largely from biochemical and imaging analyses of whole cells, in which the activity of a given Rho GTPase seems to be inversely correlated with the activity of another Rho GTPase (e.g. Ref. 68). In addition, during wound healing, manipulations that eliminate the Rho zone result in a corresponding expansion of the Cdc42 zone,(3) suggesting that local Rho activity antagonizes local Cdc42 activity.
The molecular basis for such cross talk is poorly understood. The demonstration that positive crosstalk occurs via modulation of GDI activity(69) raises the possibility that GDIs could also mediate negative crosstalk. It is also possible that crosstalk could occur more indirectly, at the level of the cytoskeleton, for instance by controlling the local assembly and disassembly of a cytoskeletal scaffold. It has been reported that activation of Pak, a Rac effector protein, can antagonize the upregulation of Rho-dependent myosin light chain phosphorylation.(70) If actomyosin recruitment creates a structure that helps localize the Rho activity zone, high Cdc42 or Rac activity flanking the zone could maintain zone boundaries by directing local inactivation of contractility.
Contributions of the zones to contractile arrays
Sharp zones ensure the assembly of correspondingly sharp contractile arrays. Intuitively, a focused contractile array makes sense because it ensures that the maximum possible force is exerted where it is needed. Cytokinesis requires that one part of the cell surface is sufficiently different from another to overcome the inevitable point at which the curvature along the furrow matches the opposite curvature perpendicular to it. GTPase zones, by directing myosin recruitment/long filament assembly here, and not there, provide another means to develop a qualitative difference in the ability of the cortex to generate and bear tension. Clearly, focusing is not absolutely essential for cytokinesis in situations where either myosin-2 or Rho is inhibited.(71,72) However, when cytokinesis is monitored in living epithelial cells or epithelia in situ, until the terminal phase of the process, the cytokinetic apparatus is tightly focused, cutting through the cell like a knife. Moreover, broad cytokinetic arrays are associated with inefficient furrowing,(20) implying that focusing is important.
Why a dynamic zone, in which Rho activation is locally balanced with inactivation and/or transport of active GTPases away from the zone? Using cytokinesis again as an example, a completely stable template would be less able to adjust to changes in spindle position. If the mitotic apparatus were simply to activate Rho at the equator, spindle displacement would be expected to result in the formation of a cytokinetic apparatus that spreads along and closes inward along the entire margin of the cell defined by the starting and ending position of the spindle midplane. However, a wealth of spindle displacement studies,(73) and direct imaging of the Rho activity zone,(18) indicate that both the cytokinetic apparatus and the Rho zone rapidly remodel in response to spindle movement. We have observed spindles to slip relative to the furrow, and the furrow to track the spindle, even in unmanipulated embryos. Thus, the forces exerted by cells upon each other are great enough to induce possible errors of this sort, and the dynamic Rho zone ensures that the cytokinetic apparatus closes between the separating chromosomes, even in situations where the spindle itself is unstable. Furthermore, even during unperturbed cytokinesis, the furrow must, from instant to instant, keep exquisite track of the spindle midplane: after all, the furrow is, as it constricts, moving along the cell surface! The cytokinetic apparatus is constantly shrinking and therefore changing its footprint on the cell surface; the phenomenon itself predicts that the Rho activity zone, which regulates the cytokinetic apparatus, must also be dynamic.
Why do cells form complementary Rho GTPase zones, as seen in both wound healing and polar body emission? Aside from the potential for crosstalk to sharpen zone boundaries, complementary zones may direct segregation of different Rho GTPase targets to different regions within the actomyosin array. During oocyte wound healing, for example, myosin-2 concentrates on the interior of the actomyosin array, while dynamic actin is concentrated around the outside of the array.(16,17) That this segregation results from the segregation of the Cdc42 and Rho activity zones is indicated both by the timing of segregation and the effects of experimentally broadening the Rho zone.(3)
This raises a further question, namely, why segregate different components of the contractile array? Is it not enough to simply locally activate contractility to drive local contraction? Perhaps, but a growing body of evidence indicates that transient contractile arrays as exemplified by the cytokinetic apparatus do not in fact behave in the textbook manner, in which the cell is simply pinched in half as a result of local actomyosin-based contraction at the cell equator.(74) During wound healing, the cell is trying to rebuild the missing cortex, not merely pinch shut the hole, and so it may make sense that a branched-actin-promoting, myosin-limiting zone follow on the heels of an unbranched-actin, myosin-recruiting zone. During cytokinesis, one might imagine that the cell has much the same challenge: to populate the stretched and newly inserted surface with structure, preferably without the interference of contractility. Sustained ingression of contractile arrays may be far more complex than generally appreciated, with different areas of the cortex making distinct contributions to cytokinesis.(74) Perhaps complementary zones create spatially distinct actin-dependent processes that cooperate to produce contractile array movement. For example, by directing rapid actin assembly–disassembly (via high Cdc42/Rac activity) in regions flanking high contractility (via high Rho activity), complementary zones could facilitate contraction by reducing resistance in adjacent regions of the cortex.(75)
In summary, we suggest that Rho activity zones represent a fundamental signaling mechanism for formation and maintenance of transient contractile arrays. The ideas presented in this review lead to a number of critically important questions that remain to be addressed. First, are the Rho activity zones in fact maintained by a balance of local activation and local inactivation (GTPase flux)? Second, how exactly do microtubules exert their control on the Rho zones? Third, what differences in molecular regulators allow different cell types to generate diverse Rho activity zone morphologies? Fourth, what are the contributions of different Rho family zones (Rho, Rac, Cdc42) to the contractile processes described? Finally, do Rho activity zones function in other processes such as developmental morphogenesis?
Abbreviations
- GTPase
guanine triphosphatase
- GTP
guanine triphosphate
- GDP
guanine diphosphate
- F-actin
filamentous actin
- GEF
guanine nucleotide exchange factor
- GAP
GTPase activating protein
- GDI
guanine nucleotide dissociation inhibitor
- GDF
GDI displacement factor
- PM
plasma membrane
References
- 1.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood W, Jacinto A, Grose R, Woolner S, Gale J, et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 5.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 6.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 7.Kovar DR. Molecular details of formin-mediated actin assembly. Curr Opin Cell Biol. 2006;18:11–17. doi: 10.1016/j.ceb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Soderling SH, Scott JD. WAVE signalling: from biochemistry to biology. Biochem Soc Trans. 2006;34:73–76. doi: 10.1042/BST0340073. [DOI] [PubMed] [Google Scholar]
- 9.Miki H, Takenawa T. Regulation of actin dynamics by WASP family proteins. J Biochem (Tokyo) 2003;134:309–313. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Zhang Y, Collins CC, Johnson DI, Zheng Y. A built-in arginine finger triggers the self-stimulatory GTPase-activating activity of rho family GTPases. J Biol Chem. 1999;274:2609–2612. doi: 10.1074/jbc.274.5.2609. [DOI] [PubMed] [Google Scholar]
- 11.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 12.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–364. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/ radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 14.Faure J, Vignais PV, Dagher MC. Phosphoinositide-dependent activation of Rho A involves partial opening of the RhoA/Rho-GDI complex. Eur J Biochem. 1999;262:879–889. doi: 10.1046/j.1432-1327.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- 15.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154:785–797. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9:579–587. doi: 10.1016/s0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- 18.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp Cell Res. 2004;295:300–314. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao WM, Fang G. MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis. Proc Natl Acad Sci USA. 2005;102:13158–13164. doi: 10.1073/pnas.0504145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, et al. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 24.Ma C, Benink HA, Cheng D, Montplaisir V, Wang L, et al. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr Biol. 2006;16:214–220. doi: 10.1016/j.cub.2005.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Avino PP, Savoian MS, Glover DM. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J Cell Biol. 2004;166:61–71. doi: 10.1083/jcb.200402157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richman TJ, Sawyer MM, Johnson DI. Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot Cell. 2002;1:458–468. doi: 10.1128/EC.1.3.458-468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 29.Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol. 2003;5:1062–1070. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- 30.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5:727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 31.Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell. 2004;15:3509–3519. doi: 10.1091/mbc.E03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 33.Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- 34.Hime G, Saint R. Zygotic expression of the pebble locus is required for cytokinesis during the postblastoderm mitoses of Drosophila. Development. 1992;114:165–171. doi: 10.1242/dev.114.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Prokopenko SN, Brumby A, O’Keefe L, Prior L, He Y, et al. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147:921–928. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 38.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 39.Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci USA. 2006;103:329–334. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baisamy L, Jurisch N, Diviani D. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J Biol Chem. 2005;280:15405–15412. doi: 10.1074/jbc.M414440200. [DOI] [PubMed] [Google Scholar]
- 41.Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt K, Nichols BJ. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr Biol. 2004;14:1002–1006. doi: 10.1016/j.cub.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 43.Fares H, Peifer M, Pringle JR. Localization and possible functions of Drosophila septins. Mol Biol Cell. 1995;6:1843–1859. doi: 10.1091/mbc.6.12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Field CM, Coughlin M, Doberstein S, Marty T, Sullivan W. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 2005;132:2849–2860. doi: 10.1242/dev.01843. [DOI] [PubMed] [Google Scholar]
- 45.Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bassilana M, Arkowitz RA. Rac1 and Cdc42 have different roles in Candida albicans development. Eukaryot Cell. 2006;5:321–329. doi: 10.1128/EC.5.2.321-329.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdul-Manan N, Aghazadeh B, Liu GA, Majumdar A, Ouerfelli O, et al. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich syndrome’ protein. Nature. 1999;399:379–383. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- 48.Nomanbhoy TK, Cerione R. Characterization of the interaction between RhoGDI and Cdc42Hs using fluorescence spectroscopy. J Biol Chem. 1996;271:10004–10009. doi: 10.1074/jbc.271.17.10004. [DOI] [PubMed] [Google Scholar]
- 49.Lin Q, Fuji RN, Yang W, Cerione RA. RhoGDI is required for Cdc42-mediated cellular transformation. Curr Biol. 2003;13:1469–1479. doi: 10.1016/s0960-9822(03)00613-4. [DOI] [PubMed] [Google Scholar]
- 50.Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, et al. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019–6023. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- 51.Jantsch-Plunger V, Gonczy P, Romano A, Schnabel H, Hamill D, et al. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minoshima Y, Kawashima T, Hirose K, Tonozuka Y, Kawajiri A, et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4:549–560. doi: 10.1016/s1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 53.Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the meta-phase to anaphase transition requires MKlp2. J Cell Biol. 2004;166:167–172. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishima M, Glotzer M. Cytokinesis: a logical GAP. Curr Biol. 2003;13:R589–R591. doi: 10.1016/s0960-9822(03)00521-9. [DOI] [PubMed] [Google Scholar]
- 55.Lee JS, Kamijo K, Ohara N, Kitamura T, Miki T. MgcRacGAP regulates cortical activity through RhoA during cytokinesis. Exp Cell Res. 2004;293:275–282. doi: 10.1016/j.yexcr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Zavortink M, Contreras N, Addy T, Bejsovec A, Saint R. Tum/ RacGAP50C provides a critical link between anaphase microtubules and the assembly of the contractile ring in Drosophila melanogaster. J Cell Sci. 2005;118:5381–5392. doi: 10.1242/jcs.02652. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein AY, Jan YN, Luo L. Function and regulation of Tumbleweed (RacGAP50C) in neuroblast proliferation and neuronal morphogenesis. Proc Natl Acad Sci USA. 2005;102:3834–3839. doi: 10.1073/pnas.0500748102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liakopoulos D, Kusch J, Grava S, Vogel J, Barral Y. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell. 2003;112:561–574. doi: 10.1016/s0092-8674(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 59.Vaughan KT. Microtubule plus ends, motors, and traffic of Golgi membranes. Biochim Biophys Acta. 2005;1744:316–3124. doi: 10.1016/j.bbamcr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Canman JC, Cameron LA, Maddox PS, Straight A, Tirnauer JS, et al. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- 61.Irazoqui JE, Howell AS, Theesfeld CL, Lew DJ. Opposing roles for actin in Cdc42p polarization. Mol Biol Cell. 2005;16:1296–1304. doi: 10.1091/mbc.E04-05-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozbudak EM, Becskei A, van Oudenaarden A. A system of counteracting feedback loops regulates Cdc42p activity during spontaneous cell polarization. Dev Cell. 2005;9:565–571. doi: 10.1016/j.devcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 63.Matsui T, Yonemura S, Tsukita S, Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidylinositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol. 1999;9:1259–1262. doi: 10.1016/s0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
- 64.Yonemura S, Nagafuchi A, Sato N, Tsukita S. Concentration of an integral membrane protein, CD43 (leukosialin, sialophorin), in the cleavage furrow through the interaction of its cytoplasmic domain with actin-based cytoskeletons. J Cell Biol. 1993;120:437–449. doi: 10.1083/jcb.120.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janetopoulos C, Borleis J, Vazquez F, Iijima M, Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev Cell. 2005;8:467–477. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Wong R, Hadjiyanni I, Wei HC, Polevoy G, McBride R, et al. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol. 2005;15:1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 67.Wadsworth P. Cytokinesis: Rho marks the spot. Curr Biol. 2005;15:R871–R874. doi: 10.1016/j.cub.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 68.Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 69.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15:117–127. doi: 10.1016/j.molcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 71.O’Connell CB, Wheatley SP, Ahmed S, Wang YL. The small GTP-binding protein rho regulates cortical activities in cultured cells during division. J Cell Biol. 1999;144:305–313. doi: 10.1083/jcb.144.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanada M, Nagasaki A, Uyeda TQ. Adhesion-dependent and contractile ring-independent equatorial furrowing during cytokinesis in mammalian cells. Mol Biol Cell. 2005;16:3865–3872. doi: 10.1091/mbc.E05-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rappaport R. Cytokinesis in Animal Cells. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 74.Wang YL. The mechanism of cortical ingression during early cytokinesis: thinking beyond the contractile ring hypothesis. Trends Cell Biol. 2005;15:581–588. doi: 10.1016/j.tcb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Mandato CA, Benink HA, Bement WM. Microtubule-actomyosin interactions in cortical flow and cytokinesis. Cell Motil Cytoskeleton. 2000;45:87–92. doi: 10.1002/(SICI)1097-0169(200002)45:2<87::AID-CM1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]