Abstract
In this study, a multifunctional poly(β-L-malic acid)-based nanoconjugate with a pH-dependent charge conversional characteristic was developed for tumor-specific drug delivery. The short branched polyethylenimine-modified poly(β-L-malic acid) (PEPM) was first synthesized. Then, the fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride were covalently attached to the PEPM to form the nanoconjugate, HDPEPM. In this nanoconjugate, the 2,3-dimethylmaleic anhydride, the shielding group, could shield the positive charge of the conjugate at pH 7.4, while it was selectively hydrolyzed in the tumor extracellular space (pH 6.8) to expose the previously-shielded positive charge. To study the anticancer activity, the anticancer drug, doxorubicin, was covalently attached to the nanoconjugate. The doxorubicin-loaded HDPEPM nanoconjugate was able to efficiently undergo a quick charge conversion from −11.62 mV to 9.04 mV in response to the tumor extracellular pH. The electrostatic interaction between the positively charged HDPEPM nanoconjugates and the negatively charged cell membrane significantly enhanced their cellular uptake, resulting in the enhanced anticancer activity. Also, the tumor targetability of the nanoconjugates could be further improved via the fragment HAb18 F(ab′)2 ligand–receptor-mediated tumor cell-specific endocytosis.
Keywords: nanoconjugate, charge-conversional, PMLA, pH-sensitive
Video abstract
Introduction
To improve the anticancer effects and minimize side effects of cancer chemotherapy, tumor-specific delivery of toxic anticancer drugs are one of the most effective strategies.1–3 Recently, drug delivery systems at the nanoscale, also known as nanocarriers, have shown great potential in the delivery of drugs to tumor,4–7 through either size-dependent “passive” tumor targeting due to the enhanced permeability and retention effect,8 or their surface-modified tumor-specific moieties, eg, small molecule-, aptamer-, peptide-, and antibody-mediated “active” tumor-targeting.9,10 Active tumor targeting requires these targeting “ligands” to get close enough to and interact with their corresponding “receptors” on the surface of the tumor cells. Its efficacy, therefore, is intrinsically limited by cellular heterogeneity, which mainly results from intrinsic genetic instability, epigenetic diversity, and stochastic noise in phenotype expression of cancer cells that exists both within and among tumors.11–13 Nanocarriers have been successfully used to decrease drawbacks of conventional chemotherapeutics such as nonspecific cytotoxicity, poor pharmacokinetics, and chemical instability to improve drug efficacy.14
Drug delivery nanocarriers with positive charge can easily implement endocytosis through the electrostatic interaction with the negatively charged cell membrane.15,16 Nevertheless, for in vivo drug delivery, the nanocarriers with strong surface charge are not always favorable. The positive charge may cause nonspecific interactions of the nanocarriers with various negatively charged biological components including plasma proteins, other anions, and the cell membrane of noncancer cells, resulting in short circulation half-life and nonspecific distribution.17,18
The tumor extracellular microenvironment is typically more acidic (pH 6.8) than normal tissues (pH 7.4) due to the hypoxia-induced production of excess lactate and protons.19,20 Therefore, various pH-sensitive nanocarriers have been developed for tumor-targeted drug delivery. Among them, the pH-dependent charge- conversional nanocarriers are our focus. Here, the nanocarriers with the positive charge were first shielded to avoid nonspecific interaction during blood circulation. Then, they were deshielded to expose the positive charge in response to the low pH once accumulated in the tumor extracellular microenvironment.21–25
To build a nanocarrier, the biocompatible polymers are of particular interest since they can be custom synthesized and engineered to fit various biomedical applications. Among them, both polyethylene glycol (PEG) and poly(lactic-co-glycolic acid) approved by the US Food and Drug Administration have been widely used as nanocarriers for cancer therapy.26,27
Poly(β-L-malic acid) (PMLA) is a natural aliphatic polyester obtained from the microorganism myxomycete Physarum polycephalum, which is degraded first to malic acid and then to carbon dioxide and water in the tricarboxylic acid cycle in vivo.28 As a nanocarrier building block, PMLA was proven to be biodegradable, nontoxic, and nonimmunogenic.29,30 Furthermore, compared to PEG and poly(lactic-co-glycolic acid), which contain a limited number of the conjugation sites, PMLA could provide numerous pendant carboxyl groups for easy conjugation with various types of molecules including chemotherapeutics, targeting ligands, and other functional groups.31
However, the difficulty involved in the preparation of PMLA limits its application. In a previous study, we have optimized the synthesis and purification and have been able to obtain the PMLA with definite molecular weight. The natural PMLA is a polymer with a strong negative charge, which would decrease the cellular uptake of the PMLA-based nanocarriers. Polyethylenimine (PEI) is a cationic polymer that has been widely used as a carrier for gene delivery.32,33 In this study, PMLA was first modified with PEI to allow the polymer to bear positive charge. Then, pH-sensitive 2,3-dimethylmaleic anhydride (DMA) was used to decorate the polymer backbone to shield its positive charge and impart the pH-dependent charge-conversional property to the nanoconjugate. Furthermore, to improve tumor-targeting, the fragment HAb18 F(ab′)2 (an antibody for human hepatocellular carcinoma cell)34,35 was conjugated to the PMLA. Additionally, doxorubicin (DOX) was chosen as the model anticancer drug and covalently attached to the nanoconjugates (Figure 1). In the tumor microenvironment, with the selective hydrolysis of the charge-shielding groups, DMA, the nanoconjugates underwent a quick charge-conversion from negative to positive. Meanwhile, the nanoconjugates could attach to tumor cells through the affinity between antibody and its antigen for receptor-mediated endocytosis. In the in vitro study, the nanoconjugates exhibited an enhanced cellular uptake via both the electrostatic interaction and receptor-mediated endocytosis, resulting in higher anticancer activity.
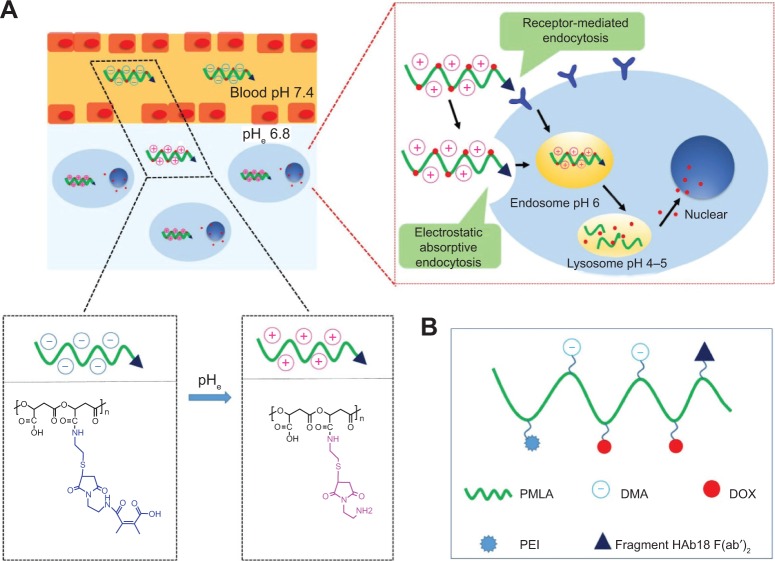
Figure 1.
Schematic illustration of the stealth property and promoted tumor cell uptake of nanoconjugates (A) and DOX-loaded nanoconjugates (DOX/HDPEPM) (B).
Notes: Depicted in (A), nanoconjugates minimize nonspecific interactions with serum components and change the surface charge of nanoconjugates in response to the tumor acidity (pHe), leading to promoted cell internalization by the combination of electrostatic absorptive endocytosis and receptor-mediated endocytosis.
Abbreviations: DMA, 2,3-dimethylmaleic anhydride; DOX, doxorubicin; HDPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid); PEI, polyethylenimine; PMLA, poly(β-L-malic acid).
Materials and methods
Materials
PEI with a molecular weight of 1.8 kDa, branched, and DOX hydrochloride were purchased from Sigma-Aldrich (St Louis, MO, USA). The bivalent fragment HAb18 F(ab′)2 was obtained from the Cell Engineering Research Center, Fourth Military Medical University (Xi’an, People’s Republic of China). DMA and succinic anhydride were purchased from Alfa Aesar (Ward Hill, MA, USA). N-(2-aminoethyl) maleimide trifluoroacetic acid was purchased from Energy Chemical (Shanghai, People’s Republic of China). 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) hydrochloride and N-hydroxysuccinimide (NHS) were purchased from TCI (Shanghai, People’s Republic of China). NHS-PEG-maleimide (Mal) and fluorescein isothiocyanate (FITC)-PEG-NH2 were purchased from Nanocs Inc. (New York, NY, USA). Other chemical reagents were purchased from Baotelai Chemicals (Xi’an, People’s Republic of China). All solvents were thoroughly dried and distilled before use. Dulbecco’s Modified Eagle’s Medium and fetal bovine serum (Hyclone Cell Culture and Bioprocessing brands) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The Eno-GeneCell™ counting kit-8 (CCK-8) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Beyotime Institute of Biotechnology (Jiangsu, People’s Republic of China).
Human hepatocellular carcinoma cell line Huh7 and human lung adenocarcinoma cell line A549 were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences.
Synthesis of PMLA
PMLA was synthesized by ring-opening polymerization and starting from the initial material L-aspartic acid.36 Briefly, L-aspartic acid and sodium bromide were dissolved in sulfuric acid solution, then sodium nitrite was added. After stirring for 2 hours, trifluoroacetic anhydride was added and stirred for another 2 hours. After dropwise addition of benzyl alcohol, the reaction mixture was stirred for 12 hours at 45°C. The concentrated product was dissolved in dichloromethane and stirred for 24 hours at 45°C. Then, the mixture was concentrated and purified by silica gel chromatography to afford pure lactone (benzyl-β-malolactonate [MLABz]). Using tetraethylammonium benzoate as the initiator, the polymerized MLABz (PMLABz) was synthesized by ring-opening polymerization of MLABz. Finally, PMLA was obtained by hydrogenation of PMLABz. The details of the synthesis process can be found in our previous work.37
Synthesis and characterization of nanoconjugates
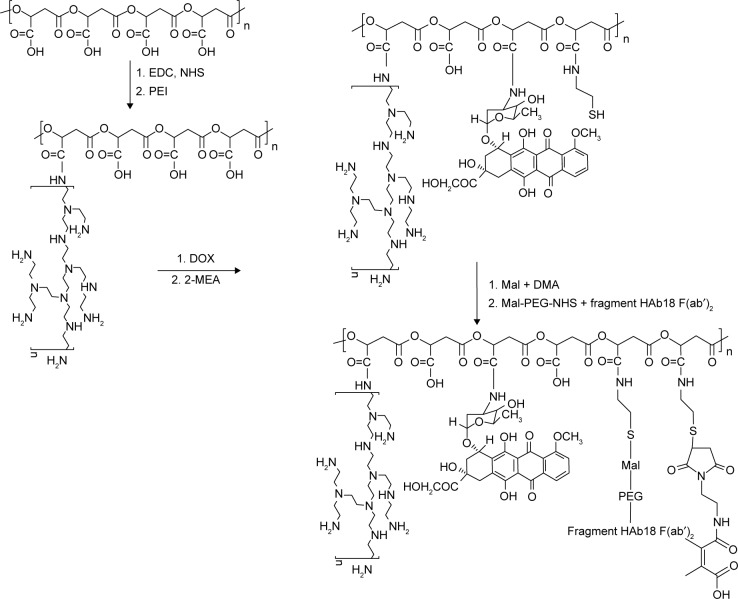
The DOX nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and DMA to PEI-modified PMLA(DOX/HDPEPM) was synthesized as shown in Figure 2. First, PMLA was modified by PEI to obtain PEI-PMLA (PEPM) nanoconjugate. DOX was covalently attached to the nanoconjugate to form the drug-loaded nanoconjugate (DOX/PEPM). Then, DMA was conjugated to the nanoconjugate using N-(2-aminoethyl)maleimide trifluoroacetic acid, and the DMA-protected nanoconjugate (DOX/DPEPM) was obtained. Finally, the bivalent fragment HAb18 F(ab′)2 was attached to DOX/DPEPM nanoconjugate by a linker NHS-PEG-Mal. Thus, the final nanoconjugate (DOX/HDPEPM) was obtained. To visualize the intracellular distribution of the nanoconjugate, the FITC-labeled nanoconjugate was synthesized by the similar methods, except that DOX monomer was replaced with NHS-PEG-FITC.
Figure 2.
Synthesis of DOX-loaded, PEI, fragment HAb18 F(ab′)2 and DMA decorated PMLA (DOX/HDPEPM) nanoconjugates.
Abbreviations: DMA, 2,3-dimethylmaleic anhydride; DOX, doxorubicin; EDC, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide; HDPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid); Mal, maleimide; NHS, N-hydroxysuccinimide; PEG, polyethylene glycol; PEI, polyethylenimine; PMLA, poly(β-L-malic acid).
The nuclear magnetic resonance (NMR) spectra were obtained on a Varian 400 mHz NMR (Bruker, Rheinstetten, Germany). Chemical shifts were expressed as parts per million (ppm). The particle size of the nanoconjugate was measured by Delsa™ Nano C Particle Analyzer (Beckman Coulter, Brea, CA, USA). Ultraviolet-visible (UV-vis) spectroscopy (Shimadzu Corporation, Kyoto, Japan) was used to quantify the amount of DOX and FITC conjugated on the nanoconjugates. Briefly, DOX-loaded nanoconjugates were dissolved in deionized water, and the absorbance of the solutions at 481 nm was measured. Similarly, the content of FITC was also determined by UV-vis spectrometry using the absorbance at 492 nm in 0.1 mol/L borate buffer (pH 9.0).
Zeta potential analysis
The zeta potentials of nanoconjugates were measured by photon correlation spectroscopy using the Delsa™ Nano C Particle Analyzer, at 25°C. Briefly, 2 mg of nanoconjugates were dissolved in 2 mL phosphate-buffered saline (PBS) solution (pH 7.4 or pH 6.8). After incubating for 1.5 hours, the zeta potential of the nanoconjugates was measured.
In vitro drug release profile
The release rate of DOX from the nanoconjugates was investigated by a dialysis method. Typically, 1 mL of DOX/HDPEPM nanoconjugates solution (2 mg/mL) was dialyzed (molecular weight cut-off: 3.5 kDa) against 20 mL PBS solution (pH 7.4, 6.8, or 5.0) at 37°C. At given intervals, 100 μL of external buffer was collected and replaced by the same volume of PBS solution. The concentration of DOX released from DOX/HDPEPM was quantified by UV-vis spectroscopy at 481 nm.
Biocompatibility evaluation
Hemocompatibility assay
The animal experiment was approved by the Animal Research Committee of the Fourth Military Medical University and conducted in accordance with the international standards on animal welfare. Blood was collected from rabbit by vacuum blood collection, and erythrocytes were obtained by centrifugation (1,000× g for 5 minutes) and stored at 4°C. The erythrocytes were washed three times with isotonic saline buffer (0.15 mol/L sodium chloride [NaCl], pH 7.4) before diluting with buffer. Then, 4 mL erythrocyte was suspended in 5 mL 0.9% NaCl solution and incubated with nanoconjugates for 1 hour, followed by centrifugation at 1,000× g for 3 minutes. The supernatant was measured at 545 nm by UV-vis spectroscopy. The 0.9% NaCl solution and distilled water were used as negative and positive controls, respectively. The hemolysis rate (%) was calculated as follows:
| (1) |
where OD is optical density.
Protein absorption
Freshly prepared nanoconjugates solution (2 mg/mL) was added into an equal volume of standard bovine serum albumin (BSA) solution (100 μg/mL) and incubated under shaking at 37°C. After 6 hours of incubation, samples were centrifuged at 3,000× g for 5 minutes. Then, 1 mL supernatant was added into 5 mL coomassie brilliant blue G-250 solution and mixed for 5 minutes. Finally, the samples were measured at 595 nm by UV spectrometer. The amounts of protein (BSA) adsorption were calculated by the standard curve equation of BSA.
Flow cytometry
Huh7 cells were seeded into 6-well plates at a density of 3×105 cells/well and incubated for 24 hours. For cellular internalization study, cells were incubated with free DOX or DOX-loaded nanoconjugates at an equivalent DOX concentration of 5 μg/mL of fresh culture medium at pH 7.4 or 6.8, respectively. After incubation for 8 hours, the cells were washed three times with PBS solution. The cells were then harvested by trypsinization and centrifuged at 1,000 rpm for 5 minutes. The cell pellet was suspended with 500 μL PBS and analyzed by a FACScan instrument (Becton Dickinson, Franklin Lakes, NJ, USA).
Confocal microscopy studies
Confocal fluorescent microscopy was used to compare the cellular uptake of FITC-loaded nanoconjugates. Similar to flow cytometry, Huh7 cells were seeded into glass-bottom dishes at a density of 3×105 cells/well and incubated for 24 hours. Then, the cells were treated with various FITC-labeled nanoconjugates in fresh culture medium at pH 7.4 or 6.8. The concentration of FITC was 1 μg/mL. After 8 hours of incubation, the cells were washed three times with PBS solution to remove the remnant growth medium and fixed in 4% paraformaldehyde for 10 minutes, followed by cell nuclei staining with DAPI for 15 minutes. After the cells were washed with PBS solution, fluorescent images of cells were analyzed by using a FV1000 confocal microscope (Olympus Corporation, Tokyo, Japan).
In vitro cytotoxicity
The cytotoxicity of DOX-loaded nanoconjugates against Huh7 and A549 cells was investigated by using the CCK-8 assay. The cells were seeded into 96-well plates at a density of 1×104 cells/well and repeated in five wells. Then the medium was replaced by free DOX or DOX-loaded nanoconjugates in cell culture medium with different pH (6.8 or 7.4) and incubated for 48 hours. Briefly, 10 μL CCK-8 solution was added to each well of the plate. Then, the plate was incubated for 2 hours. Cell viability was determined by scanning with a microplate reader at 490 nm. The cell viability (%) was calculated according to the manufacturer’s instructions.
Statistical analysis
All data are presented as the mean ± standard deviation. The statistical significance of the differences between groups was evaluated by one-way ANOVA with a Bonferroni post hoc test. Statistical significance was established at P<0.05, and extreme significance was set at P<0.01.
Results and discussion
Synthesis of PMLA
Due to low toxicity, nonimmunogenicity, and biodegradability, PMLA has been a promising polymeric drug carrier. The PMLA-based nanoconjugates contained multiple active sites and were ready for further engineering and modification. However, the preparation of PMLA was difficult, which was one of the major challenges of the development of PMLA-based drug carriers. The PMLA could be produced by either chemical synthesis or biological fermentation from the slime mold Physarum polycephalum.
However, by the fermentation method, pure PMLA was difficult to obtain, and the separation and purification process were complicated. Also, the molecular weight of PMLA was hard to control. The low yield of chemical synthesis was the main obstacle of PMLA’s application. Of the whole synthesis process, the synthesis of MLABz was the crucial step. We optimized the reaction time and temperature of MLABz’s synthesis, and the yield was raised from 12%36 to 32%. Accordingly, the yield of PMLA has been improved. Furthermore, PMLA with different definite molecular weights could be synthesized by adjusting monomer/initiator ratio in the polymerization reaction.37 The proton NMR (1H NMR) spectrum of PMLA is shown in Figure 3A, and its principal peaks are at 2.95 and 5.38 ppm.
Figure 3.
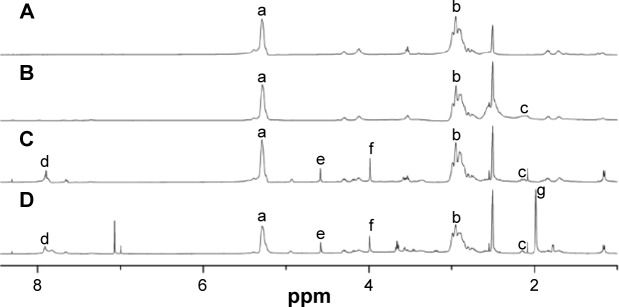
1H NMR spectra of synthesized nanoconjugates.
Notes: (A) PMLA, (B) PEPM, (C) DOX/PEPM, and (D) DOX/DPEPM; DMSO was used as the solvent.
Abbreviations: DMSO, dimethyl sulfoxide; DOX, doxorubicin; 1H NMR, proton nuclear magnetic resonance; DPEPM, nanoconjugate formed by covalent attachment of 2,3-dimethylmaleic anhydride to polyethylenimine-modifid poly(β-L-malic acid); PEPM, polyethylenimine-modified poly(β-L-malic acid); PMLA, poly(β-L-malic acid); ppm, parts per million.
Synthesis of nanoconjugates and characterization
As shown in Figure 2, DOX/HDPEPM nanoconjugate was synthesized through a series of reactions. First, in the presence of EDC, PEPM was synthesized by coupling of carboxyl groups of PMLA with the amino groups of PEI. The 1H NMR spectrum is shown in Figure 3B. The peak at 2.1 ppm was assigned to PEI. Next, The DOX/PEPM nanoconjugate was synthesized via the formation of amide bonds between free amino groups of DOX and the carboxylic groups of PEPM. Compared with the PEPM, DOX/PEPM showed the peaks of the DOX moieties (7.07, 4.58, and 3.64 ppm) in the 1H NMR spectrum (Figure 3C). Then, DOX/PEPM reacted with 2-aminoethanethiol to obtain sulfhydryl for conjugating DMA. DMA reacted with N-(2-aminoethyl) maleimide trifluoroacetic acid, thus forming a Mal terminal group. Therefore, the tumor pH-responsive DOX/DPEPM nanoconjugate was obtained by the reaction of sulfhydryl with the terminal group (Mal). Compared with DOX/PEPM, DOX/DPEPM exhibited a principal peak related to the DMA moieties (1.98 ppm) in its 1H NMR spectrum (Figure 3D). As a control, DOX/SPEPM nanoconjugate was synthesized by reacting DOX/PEPM with succinic anhydride. Finally, the DOX/HDPEPM was conjugated with the bivalent fragment HAb18 F(ab′)2 by the coupling reagent NHS-PEG-Mal.
The corresponding FITC-labeled nanoconjugate was obtained by the similar route. The FITC content of the nanoconjugate was in the range of 0.84%–0.91%. The mean diameter of HDPEPM nanoconjugates was 201.6 nm with polydispersity index of 0.268, determined by dynamic light scattering. According to UV-vis absorbance, the DOX-conjugating content of the nanoconjugate was 21.8% for DOX/PEPM, 22.6% for DOX/HPEPM, 20.4% for DOX/DPEPM, 20.9% for DOX/SPEPM (nanoconjugate formed by covalent attachment of 2,3-dimethylmaleic anhydride to polyethylenimine-modifid poly(β-L-malic acid), and 20.1% for DOX/HDPEPM. These results indicate that the synthetic nanoconjugates had good loading efficiency of the DOX.
Zeta potential analysis
As shown in Figure 4A and B, no charge-conversional behaviors were observed, and the zeta potentials remained negative in DOX/PEPM, DOX/HPEPM, and DOX/SPEPM when the pH was decreased from 7.4 to 6.8. In contrast, both DOX/DPEPM and DOX/HDPEPM nanoconjugates showed a significant charge conversion when the pH went down. The zeta potentials of all the nanoconjugates were about −11 mV at pH 7.4, while at pH 6.8, the zeta potentials of DOX/DPEPM and DOX/HDPEPM nanoconjugates were 9.08 mV and 9.04 mV, respectively. DMA was used to mask the positive charge at neutral pH, and the resultant β-carboxylic acid amides on the nanoconjugates have been reported to be acid-labile and could be quickly hydrolyzed under mild acidic environment.38,39 Therefore, the nanoconjugates with negative surfaces could avoid the electrostatic interaction with the negatively charged proteins or blood cells. When the DOX/DPEPM or DOX/HDPEPM nanoconjugates reached the tumor site, the weak acidic tumor microenvironment (pH 6.8) could trigger the pH-dependent hydrolysis of the DMA modified amide bonds, resulting in exposing the amines. As a result, the zeta potential of the nanoconjugates would change rapidly from negative to positive charge.
Figure 4.
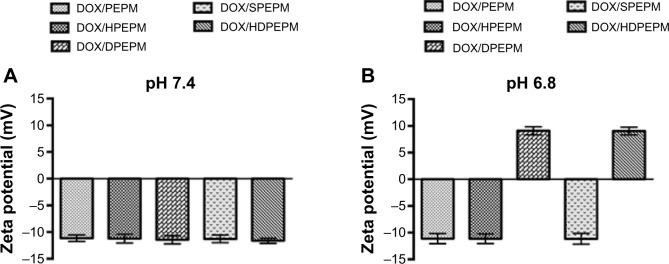
Zeta potentials of different DOX-loaded nanoconjugates at (A) pH 7.4 and (B) pH 6.8 (n=5).
Abbreviations: DOX, doxorubicin; HDPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid); PEPM, polyethylenimine-modified poly(β-L-malic acid); SPEPM, nanoconjugate formed by covalent attachment of succinic anhydride to polyethylenimine-modifid poly(β-L-malic acid).
In vitro drug release
Release of DOX from the nanoconjugates was measured in order to confirm the pH-sensitivity of the nanoconjugates. The in vitro DOX-release profile of the nanoconjugates was investigated at different pH. The results of DOX-release from DOX/HDPEPM nanoconjugates in PBS solution at pH of 5.0, 6.8, or 7.4 are shown in Figure 5. The nanoconjugates were relatively stable at pH 7.4 and pH 6.8, with less than 20% of DOX release after 12 hours, and there was no significant difference under these two pH conditions at most of the time points. As the pH declined to 5, a rapid drug release was observed. DOX was released in a biphasic manner, which exhibited an initial rapid release in 12 hours, followed by a slower release for up to 72 hours. This indicates that the nanoconjugates could hold the drugs under physiological condition but release the loaded drug in response to the endosomal (pH 5–6) or lysosomal pH (pH 4–5), to ensure most of drug release and function in the cell nucleus.
Figure 5.
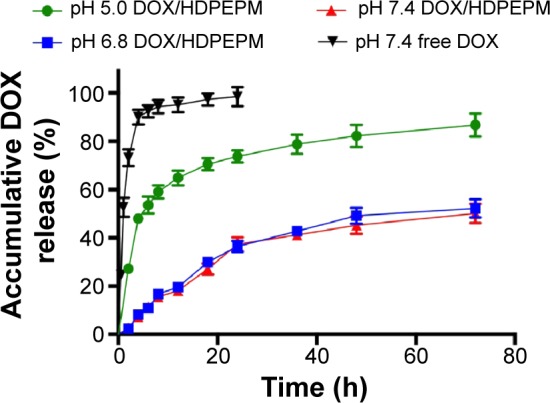
Release profile of DOX from DOX/HDPEPM or free DOX incubated at pH 5.0, pH 6.8, or pH 7.4 (n=3).
Abbreviations: DOX, doxorubicin; HDPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid).
Biocompatibility evaluation
In order to determine whether the nanoconjugates were safe for intravenous injection, the hemolytic potential and protein absorption were evaluated. The erythrocytes were incubated with DOX-loaded nanoconjugates at 37°C for 1 hour, and the absorbance was measured at 545 nm. As shown in Table 1, hemolysis rates of all the nanoconjugates were lower than 2%, which could be considered as nontoxic.40 The BSA adsorption rates were also determined, and the results showed that all the nanoconjugates’ protein adsorption rates were less than 5%. The results indicate that the nonspecific protein adsorption onto the nanoconjugates was negligible.
Table 1.
Hemolysis and protein absorption rates of DOX-loaded nanoconjugates
| Samples (1 mg/mL) | Hemolysis rate (%) | Protein absorption rate (%) |
|---|---|---|
| DOX/PEPM | 1.60±0.21 | 3.16±0.41 |
| DOX/HPEPM | 1.45±0.15 | 2.97±0.23 |
| DOX/DPEPM | 1.05±0.13 | 4.02±0.26 |
| DOX/SPEPM | 1.33±0.07 | 3.43±0.31 |
| DOX/HDPEPM | 1.31±0.18 | 3.77±0.34 |
Abbreviations: DOX, doxorubicin; HDPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid); PEPM, polyethylenimine-modified poly(β-L-malic acid); DPEPM, nanoconjugate formed by covalent attachment of 2,3-dimethylmaleic anhydride to polyethylenimine-modifid poly(β-L-malic acid); SPEPM, nanoconjugate formed by covalent attachment of succinic anhydride to polyethylenimine-modifid poly(β-L-malic acid); HPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 to polyethylenimine-modifid poly(β-L-malic acid).
Both the hemolysis and protein adsorption results suggest that these nanoconjugates possess good biocompatibility, which might be due to their anionic surface, and this property could benefit in vivo drug delivery in terms of the long blood circulation and passive tumor targeting.
Flow cytometry
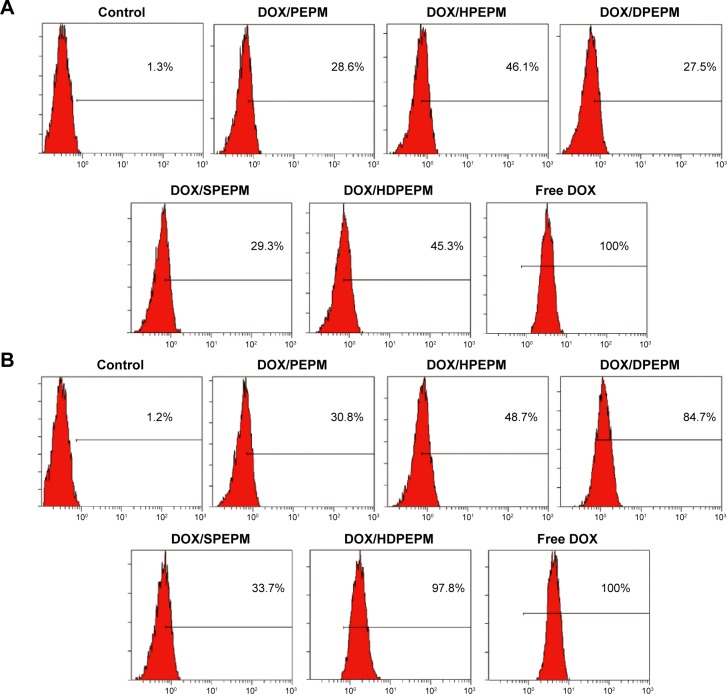
Flow cytometry was used to study the cellular uptake of the DOX-loaded nanoconjugates. As shown in Figure 6A, after incubation with Huh7 cells for 8 hours, all nanoconjugates showed relatively low cell internalization at pH 7.4. The cellular uptake of DOX/PEPM, DOX/HPEPM, DOX/DPEPM, DOX/SPEPM, DOX/HDPEPM, and free DOX in Huh7 cells were 28.6%, 46.1%, 27.5%, 29.3%, 45.3%, and 100%, respectively. Compared with DOX/PEPM nanoconjugates, DOX/SPEPM and DOX/DPEPM showed low cell internalization while the fragment HAb18 F(ab′)2-modified nanoconjugates, DOX/HPEPM and DOX/HDPEPM, had uptake improvement of approximately 1.6-fold. All five nanoconjugates were negatively charged at pH 7.4, indicating that the negative surface charges strongly affected the cellular uptake.
Figure 6.
Cellular uptake of different DOX-loaded nanoconjugates in Huh7 cells at (A) pH 7.4 and (B) pH 6.8 by flow cytometry.
Abbreviations: DOX, doxorubicin; HDPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid); PEPM, polyethylenimine-modified poly(β-L-malic acid); HPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 to polyethylenimine-modifid poly(β-L-malic acid); DPEPM, nanoconjugate formed by covalent attachment of 2,3-dimethylmaleic anhydride to polyethylenimine-modifid poly(β-L-malic acid); SPEPM, nanoconjugate formed by covalent attachment of succinic anhydride to polyethylenimine-modifid poly(β-L-malic acid).
As shown in Figure 6B, when the pH was decreased to 6.8, DOX/PEPM, DOX/HPEPM, and DOX/SPEPM showed similar cellular uptake. However, the cellular uptake of DOX/DPEPM and DOX/HDPEPM was increased to about 84.7% and 97.8%, respectively. This might be due to the charge conversion. In brief, the charge conversion of DMA-modified nanoconjugates was confirmed by their zeta potential when the pH decreased from 7.4 to 6.8. Hence, for DOX/DPEPM and DOX/HDPEPM nanoconjugates, the negative charge reduced the cell internalization at pH 7.4, whereas the positive charge enhanced the cell internalization at pH 6.8.
Interestingly, when the two modification strategies (positive charge and fragment HAb18 F[ab′]2) were used alone, the cellular uptake of DOX/DPEPM nanoconjugates was about twice that of DOX/HPEPM nanoconjugates, suggesting that the electrostatic interaction with negatively charged cell membrane might have greater impact on the enhancement of cellular uptake than fragment HAb18 F(ab′)2-mediated endocytosis. When the two strategies were combined, the strongest cellular uptake, with about 3.18-fold increase over DOX/PEPM, was obtained by using DOX/HDPEPM nanoconjugates, indicating that the dual-targeting approach could have a synergic effect on cellular uptake.
Confocal microscopy studies
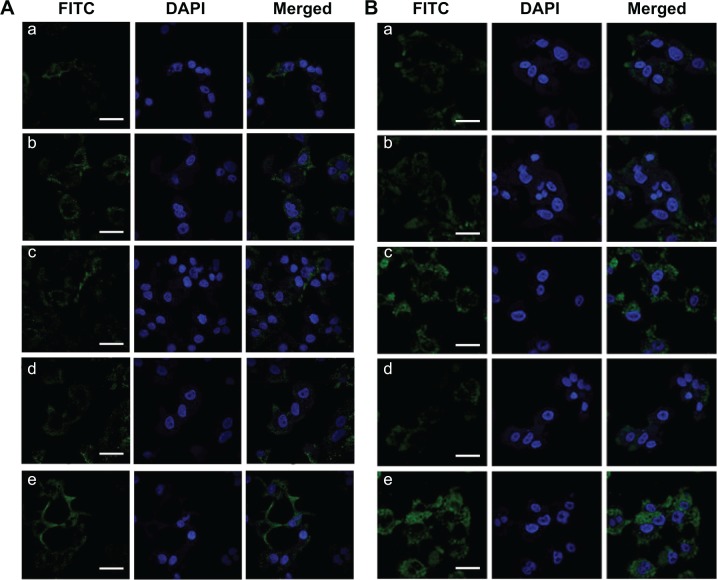
To confirm the influence of the charge conversion and fragment HAb18 F(ab′)2 on the cell internalization, the cellular uptake of nanoconjugates was investigated by confocal laser scanning microscopy. FITC-labeled nanoconjugates were used instead of DOX-loaded nanoconjugates. FITC-labeled nanoconjugates were incubated with Huh7 cells for 8 hours at pH 7.4 or 6.8, the cell nuclei were stained by DAPI, and the fluorescent images were analyzed with a confocal laser scanning microscope.
As shown in Figure 7A and B, the intracellular fluorescence was increased in FITC-HPEPM nanoconjugate-treated cells compared to that in FITC-PEPM nanoconjugate-treated cells at both pH 7.4 and 6.8. The results indicate that drug delivery efficiency could be increased by HPEPM nanoconjugates through receptor-mediated endocytosis. At pH 7.4, similar results were observed between FITC-HDPEPM and FITC-DPEPM nanoconjugates. Furthermore, at pH 7.4, the intensities of the intracellular fluorescence of all nanoconjugates were relatively low. Since cell membranes are negatively charged, these results indicate that the electrostatic repulsion at pH 7.4 between negatively charged nanoconjugates and cell membranes could decrease the cell uptake.
Figure 7.
Internalization of different FITC-labeled nanoconjugates in Huh7 cells at (A) pH 7.4 and (B) pH 6.8 imaged by confocal laser scanning microscopy.
Notes: (a) FITC-PEPM, (b) FITC-HPEPM, (c) FITC-DPEPM, (d) FITC-SPEPM, (e) FITC-HDPEPM; Scale bars =100 μm.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; HDPEPM, nanoconjugate formed by covalent attachment of HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid); PEPM, polyethylenimine-modified poly(β-L-malic acid); HPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 to polyethylenimine-modifid poly(β-L-malic acid); DPEPM, nanoconjugate formed by covalent attachment of 2,3-dimethylmaleic anhydride to polyethylenimine-modifid poly(β-L-malic acid); SPEPM, nanoconjugate formed by covalent attachment of succinic anhydride to polyethylenimine-modifid poly(β-L-malic acid).
For FITC-HDPEPM and FITC-DPEPM nanoconjugates, the fluorescence was significantly increased at pH 6.8 (Figure 7B) compared with that at pH 7.4 (Figure 7A). However, for the other three nanoconjugates, the fluorescence at pH 6.8 was similar to that at pH 7.4 in Huh7 cells. Thus, DMA-decorated nanoconjugates at pH 6.8 could greatly increase the cellular uptake since the DMA could be selectively hydrolyzed at weak acidic environment to regenerate the positive charge. Moreover, as shown in Figure 7B, FITC-HDPEPM nanoconjugates showed the highest fluorescence among the five groups. These results suggest that the charge shielding group (DMA) combined with antibody (fragment HAb18 F[ab′]2) had a synergistic effect on cellular uptake. Also, the intracellular fluorescence of FITC-DPEPM nanoconjugates was much higher than that of FITC-HPEPM nanoconjugates. This was consistent with the flow cytometry results, indicating that the positive charge played a more important role in the cell internalization than ligand–receptor-mediated interaction in this nanoconjugate.
In vitro cytotoxicity
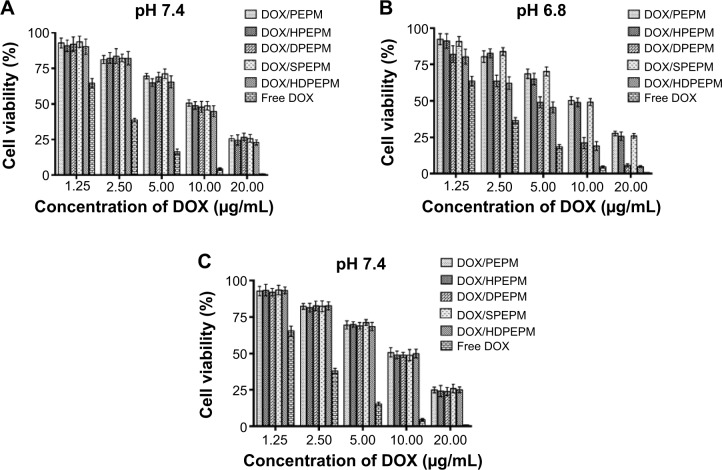
In vitro anticancer activities of drug-loaded nanoconjugates were evaluated by CCK-8 assay after incubation with Huh7 cells or A549 cells for 48 hours at pH 7.4 or 6.8, and 50% inhibitory concentration (IC50) was determined. As shown in Figure 8A, all nanoconjugates showed dose-dependent toxicity. The five nanoconjugates showed similar cell viabilities at pH 7.4. The IC50 of DOX/PEPM, DOX/HPEPM, DOX/DPEPM, DOX/SPEPM, DOX/HDPEPM nanoconjugates and free DOX were 9.43, 8.76, 9.23, 9.41, 8.21, and 2.49 μg/mL, respectively. Among them, the IC50 of DOX/HPEPM and DOX/HDPEPM nanoconjugates were slightly lower than those of the other nanoconjugates. This could be due to the enhanced cellular uptake via the antibody (fragment HAb18 F[ab′]2)-mediated endocytosis. The cytotoxicity of DOX-loaded nanoconjugates against the fragment HAb18 F(ab′)2 receptor-deficient cell line A549 was also evaluated. As shown in Figure 8C, the nanoconjugates containing fragment HAb18 F(ab′)2 could not increase the antitumor ability in A549 cells.
Figure 8.
Cell cytotoxicity of various DOX-loaded nanoconjugates and free DOX at (A) pH 7.4 and (B) pH 6.8 on Huh7 cells and at (C) pH 7.4 on fragment HAb18 F(ab′)2 receptor-negative A549 cells by CCK-8 assay (n=5).
Abbreviations: CCK-8, cell counting kit-8; DOX, doxorubicin; HDPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 and 2,3-dimethylmaleic anhydride to polyethylenimine-modified poly(β-L-malic acid); PEPM, polyethylenimine-modified poly(β-L-malic acid); HPEPM, nanoconjugate formed by covalent attachment of fragment HAb18 F(ab′)2 to polyethylenimine-modifid poly(β-L-malic acid); DPEPM, nanoconjugate formed by covalent attachment of 2,3-dimethylmaleic anhydride to polyethylenimine-modifid poly(β-L-malic acid); SPEPM, nanoconjugate formed by covalent attachment of succinic anhydride to polyethylenimine-modifid poly(β-L-malic acid).
As shown in Figure 8B, IC50 of DOX/PEPM, DOX/HPEPM, DOX/DPEPM, DOX/SPEPM, DOX/HDPEPM nanoconjugates and free DOX were 9.47, 8.87, 4.23, 9.41, 3.87, and 2.26 μg/mL at pH 6.8, respectively. DOX/HDPEPM nanoconjugates showed the highest toxicity, which was consistent with the results of the cellular uptake study. In addition, both DMA-conjugated nanoconjugates (DOX/DPEPM and DOX/HDPEPM) exhibited much higher cytotoxicity at pH 6.8 over pH 7.4 due to the charge conversion-induced cell internalization at acidic pH. Moreover, the IC50 value of DOX/HDPEPM nanoconjugates was about 1.10-fold and 2.30-fold lower than that of DOX/DPEPM and DOX/HPEPM nanoconjugates, respectively, indicating that the combined use of the positive charge and fragment HAb18 F(ab′)2 in the HDPEPM nanoconjugates might have a synergistic effect on cytotoxicity. Furthermore, DOX/DPEPM nanoconjugates displayed higher cytotoxicity than DOX/HPEPM nanoconjugates at pH 6.8, which confirmed that the charge conversion had a more important role than fragment HAb18 F(ab′)2 on the cellular uptake.
Conclusion
In this study, a PMLA-based multifunctional nanoconjugate (DOX/HDPEPM) was developed for effective and tumor-specific drug delivery. In the nanoconjugate, the backbone, PMLA, was engineered with PEI for efficient cell internalization, DMA as charge shielding groups, and fragment HAb18 F(ab′)2 for active tumor targeting. The nanoconjugates were negatively charged in a neutral pH environment (pH 7.4), while at weak acidic pH (pH 6.8), nanoconjugates became positively charged, which facilitates endocytosis. The results show that the DOX/HDPEPM nanoconjugates significantly enhanced cellular uptake of DOX in tumor cells via the combined effects of electrostatic interaction-mediated and receptor-mediated endocytosis, resulting in the enhanced antitumor activity in the in vitro studies. The study suggests the great potential to use DOX/HDPEPM nanoconjugates as a tumor-specific drug delivery platform for cancer treatment.
Acknowledgments
This study was supported by National Nature Science Foundation of China, grant number 81271687.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.den Hollander P, Savage MI, Brown PH. Targeted therapy for breast cancer prevention. Front Oncol. 2013;3:250. doi: 10.3389/fonc.2013.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Kearney AS. Prodrugs and targeted drug delivery. Adv Drug Deliver Rev. 1996;19(2):225–239. [Google Scholar]
- 4.Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42(3):1147–1235. doi: 10.1039/c2cs35265f. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreuter J. Nanoparticles – a historical perspective. Int J Pharm. 2007;331(1):1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 8.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target. 2007;15(7–8):457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 9.Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2014 Jul 9; doi: 10.1007/s00432-014-1767-3. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010;107(3):1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12(5):323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 12.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Adv Drug Deliv Rev. 2012;64(9):866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed Engl. 2003;42(27):3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 16.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum Gene Ther. 1996;7(16):1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Pardridge WM. Monoclonal antibody radiopharmaceuticals: cationization, pegylation, radiometal chelation, pharmacokinetics, and tumor imaging. Bioconjug Chem. 2003;14(3):546–553. doi: 10.1021/bc0256648. [DOI] [PubMed] [Google Scholar]
- 18.Ma SF, Nishikawa M, Katsumi H, Yamashita F, Hashida M. Cationic charge-dependent hepatic delivery of amidated serum albumin. J Control Release. 2005;102(3):583–594. doi: 10.1016/j.jconrel.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002;8(4):1284–1291. [PubMed] [Google Scholar]
- 20.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian H, Guo Z, Lin L, et al. pH-responsive zwitterionic copolypeptides as charge conversional shielding system for gene carriers. J Control Release. 2014;174:117–125. doi: 10.1016/j.jconrel.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Yuan YY, Mao CQ, Du XJ, Du JZ, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24(40):5476–5480. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- 23.Zhao BX, Zhao Y, Huang Y, et al. The efficiency of tumor-specific pH-responsive peptide-modified polymeric micelles containing paclitaxel. Biomaterials. 2012;33(8):2508–2520. doi: 10.1016/j.biomaterials.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Miura S, Na K, Bae YH. pH-responsive and charge shielded cationic micelle of poly(L-histidine)-block-short branched PEI for acidic cancer treatment. J Control Release. 2013;172(1):69–76. doi: 10.1016/j.jconrel.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90(3):363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 26.Chong CS, Cao M, Wong WW, et al. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J Control Release. 2005;102(1):85–99. doi: 10.1016/j.jconrel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG – a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81. doi: 10.1016/j.jconrel.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Portilla-Arias J, Patil R, Hu J, et al. Nanoconjugate platforms Development based in poly(β,L-malic acid) methyl esters for tumor drug delivery. J Nanotechnol. 2010;2010 doi: 10.1155/2010/825363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang ZW, Laurent V, Chetouani G, et al. New functional degradable and bio-compatible nanoparticles based on poly(malic acid) derivatives for site-specific anti-cancer drug delivery. Int J Pharm. 2012;423(1):84–92. doi: 10.1016/j.ijpharm.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljubimova JY, Fujita M, Khazenzon NM, et al. Nanoconjugate based on polymalic acid for tumor targeting. Chem Biol Interact. 2008;171(2):195–203. doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee BS, Fujita M, Khazenzon NM, et al. Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(beta-L-malic acid) for drug delivery. Bioconjug Chem. 2006;17(2):317–326. doi: 10.1021/bc0502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed M, Narain R. Cell line dependent uptake and transfection efficiencies of PEI-anionic glycopolymer systems. Biomaterials. 2013;34(17):4368–4376. doi: 10.1016/j.biomaterials.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Gaspar VM, Gonçalves C, de Melo-Diogo D, et al. Poly(2-ethyl-2-oxazoline)-PLA-g-PEI amphiphilic triblock micelles for co-delivery of minicircle DNA and chemotherapeutics. J Control Release. 2014;189:90–104. doi: 10.1016/j.jconrel.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Xu HY, Zhang Q, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res. 2007;5(6):605–614. doi: 10.1158/1541-7786.MCR-06-0286. [DOI] [PubMed] [Google Scholar]
- 35.Jin C, Qian N, Zhao W, et al. Improved therapeutic effect of DOX-PLGA-PEG micelles decorated with bivalent fragment HAb18 F(ab′)(2) for hepatocellular carcinoma. Biomacromolecules. 2010;11(9):2422–2431. doi: 10.1021/bm1005992. [DOI] [PubMed] [Google Scholar]
- 36.Cammas S, Renard I, Langlois V, Guéri P. Poly(β-malic acid): obtaining high molecular weights by improvement of the synthesis route. Polymer. 1996;37(18):4215–4220. [Google Scholar]
- 37.Qiao Y, Duan X, Fan L, Li W, Wu H, Wang Y. Synthesis of controlled molecular weight poly(β-malic acid) and conjugation with HCPT as a polymeric drug carrier. J Polym Res. 2014;21(4):397. [Google Scholar]
- 38.Oh NM, Kwag DS, Oh KT, Youn YS, Lee ES. Electrostatic charge conversion processes in engineered tumor-identifying polypeptides for targeted chemotherapy. Biomaterials. 2012;33(6):1884–1893. doi: 10.1016/j.biomaterials.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 39.Yoon SR, Yang HM, Park CW, Lim S, Chung BH, Kim JD. Charge-conversional poly(amino acid)s derivatives as a drug delivery carrier in response to the tumor environment. J Biomed Mater Res A. 2012;100(8):2027–2033. doi: 10.1002/jbm.a.34048. [DOI] [PubMed] [Google Scholar]
- 40.Jumaa M, Furkert FH, Müller BW. A new lipid emulsion formulation with high antimicrobial efficacy using chitosan. Eur J Pharm Biopharm. 2002;53(1):115–123. doi: 10.1016/s0939-6411(01)00191-6. [DOI] [PubMed] [Google Scholar]