Significance
Understanding the factors that cause mosquitoes to resist arbovirus infection could lead to new strategies to control disease transmission. One antiviral response that may play a role in mosquito immunity is apoptosis, a type of cell suicide that is often induced by virus infection. However, apoptosis is rarely seen in arbovirus-infected mosquitoes. To understand why, we infected mosquitoes with an arbovirus that expresses a proapoptotic gene called reaper and found that the Reaper-expressing virus had replication defects in mosquitoes. We also observed strong selective pressure against retention of reaper in the virus genome. These results indicate that apoptosis is a strong antiviral response in mosquitoes and may explain why it is usually not observed in coevolved arbovirus–vector relationships.
Keywords: apoptosis, arbovirus, mosquito, vector competence
Abstract
Millions of people are infected each year by arboviruses (arthropod-borne viruses) such as chikungunya, dengue, and West Nile viruses, yet for reasons that are largely unknown, only a relatively small number of mosquito species are able to transmit arboviruses. Understanding the complex factors that determine vector competence could facilitate strategies for controlling arbovirus infections. Apoptosis is a potential antiviral defense response that has been shown to be important in other virus–host systems. However, apoptosis is rarely seen in arbovirus-infected mosquito cells, raising questions about its importance as an antiviral defense in mosquitoes. We tested the effect of stimulating apoptosis during arbovirus infection by infecting Aedes aegypti mosquitoes with a Sindbis virus (SINV) clone called MRE/Rpr, in which the MRE-16 strain of SINV was engineered to express the proapoptotic gene reaper from Drosophila. MRE/Rpr exhibited an impaired infection phenotype that included delayed midgut infection, delayed virus replication, and reduced virus accumulation in saliva. Nucleotide sequencing of the reaper insert in virus populations isolated from individual mosquitoes revealed evidence of rapid and strong selection against maintenance of Reaper expression in MRE/Rpr-infected mosquitoes. The impaired phenotype of MRE/Rpr, coupled with the observed negative selection against Reaper expression, indicates that apoptosis is a powerful defense against arbovirus infection in mosquitoes and suggests that arboviruses have evolved mechanisms to avoid stimulating apoptosis in mosquitoes that serve as vectors.
The yellow fever mosquito, Aedes aegypti, is an important disease vector because of its ability to transmit a number of medically important arboviruses (arthropod-borne viruses). This species is the major vector for yellow fever and dengue viruses, which alone are responsible for more than 100 million infections and 50,000 deaths worldwide per year (1, 2). A. aegypti is also an important vector for chikungunya virus, an emerging pathogen in South Asia, Africa, Europe, and more recently the Caribbean region (3). The range of A. aegypti is broad, including tropic and subtropic regions around the world, and it primarily lives in close association with humans, making it an especially problematic disease vector (4–6).
Once a mosquito ingests an infectious blood meal, the virus must first infect the midgut epithelial cells and then escape the midgut and infect other tissues in the mosquito, including the salivary glands. To be transmitted to another host, the virus must replicate in the salivary glands and be expelled in the saliva during a subsequent blood meal (7, 8). In addition to overcoming physical barriers in the mosquito vector, the virus must also defeat innate immune defenses such as the Toll and JAK/STAT pathways and RNA interference (9–13). Although progress has been made in understanding antiviral immune mechanisms in mosquitoes in recent years, our knowledge is still far from complete.
One type of antiviral response that is known to be important in other virus–host systems is the ability of infected cells to commit suicide by apoptosis. There are many examples where apoptosis has been shown to be a defense against viruses in other insects and in mammals (14, 15). However, the role of apoptosis in arbovirus–vector interactions is unclear. Arbovirus infection often leads to apoptosis in vertebrate cells, but mosquito cells usually undergo nonlytic, persistent arbovirus infections (16–20), even though A. aegypti cells have a functional apoptosis pathway that largely resembles that of Drosophila melanogaster (21–23). Pathological effects resulting from arbovirus infection in mosquitoes have been reported in a few cases (24–27), but arbovirus infections are generally thought to have a minimal effect on mosquito vectors, although that assumption has been challenged (28). However, there have been a small handful of reports of apoptosis correlating with resistance of mosquitoes to infection by arboviruses. Apoptosis observed in salivary glands of Culex quinquefasciatus infected with West Nile virus has been proposed to be a defense against infection (24, 29, 30). In addition, West Nile virus infection in a refractory strain of C. quinquefasciatus correlated with extensive cell death in midgut tissue (31). Finally, infection of susceptible and refractory A. aegypti strains with dengue virus resulted in a rapid increase in expression of the proapoptotic gene michelob_x, a reaper homolog, in the refractory, but not the susceptible, strain (32). Nonetheless, in all of these studies, it is not clear whether apoptosis directly caused virus resistance or merely accompanied it. A recent study by our group tested the effect of inducing or inhibiting apoptosis during Sindbis virus (SINV) infection in A. aegypti by using RNA interference to silence expression of pro- and antiapoptotic factors in the mosquito. When the initiator caspase AeDronc was knocked down, decreased infection and dissemination were observed, whereas increased infection and spread were seen when apoptosis was increased by knockdown of AeIAP1 (33). These results suggested that apoptosis may actually facilitate arbovirus infection; however, secondary effects resulting from gene knockdown in the entire mosquito (as opposed to only affecting infected cells) could have affected the outcome of infection.
SINV is the type member of the genus Alphavirus in the family Togaviridae and can be transmitted by A. aegypti under laboratory conditions. SINV and A. aegypti have been used extensively as a model to study virus–vector interactions (34–38). SINV is well characterized molecularly and has been developed as an alphavirus transducing system in which a cDNA clone of the viral genome is engineered to allow expression of foreign genes during virus replication (36, 39, 40). SINV expression systems using the MRE-16 strain have high midgut infection and dissemination rates in A. aegypti mosquitoes when administered during a blood meal (41).
In this study, we used an MRE-16-based SINV clone that was engineered to express the proapoptotic protein Reaper from Drosophila to examine the effects of inducing apoptosis on the ability of SINV to infect A. aegypti mosquitoes. Our results demonstrate that induction of apoptosis by SINV is detrimental to its ability to replicate and cause disseminated infection in a mosquito vector, and we observed rapid selection against viral induction of apoptosis in infected mosquitoes. This may explain why apoptosis is rarely observed in successful coevolved arbovirus/mosquito relationships and suggests that apoptosis can be a factor in determining vector competence for arbovirus transmission.
Results
Using Recombinant SINV to Induce Apoptosis During Mosquito Infection.
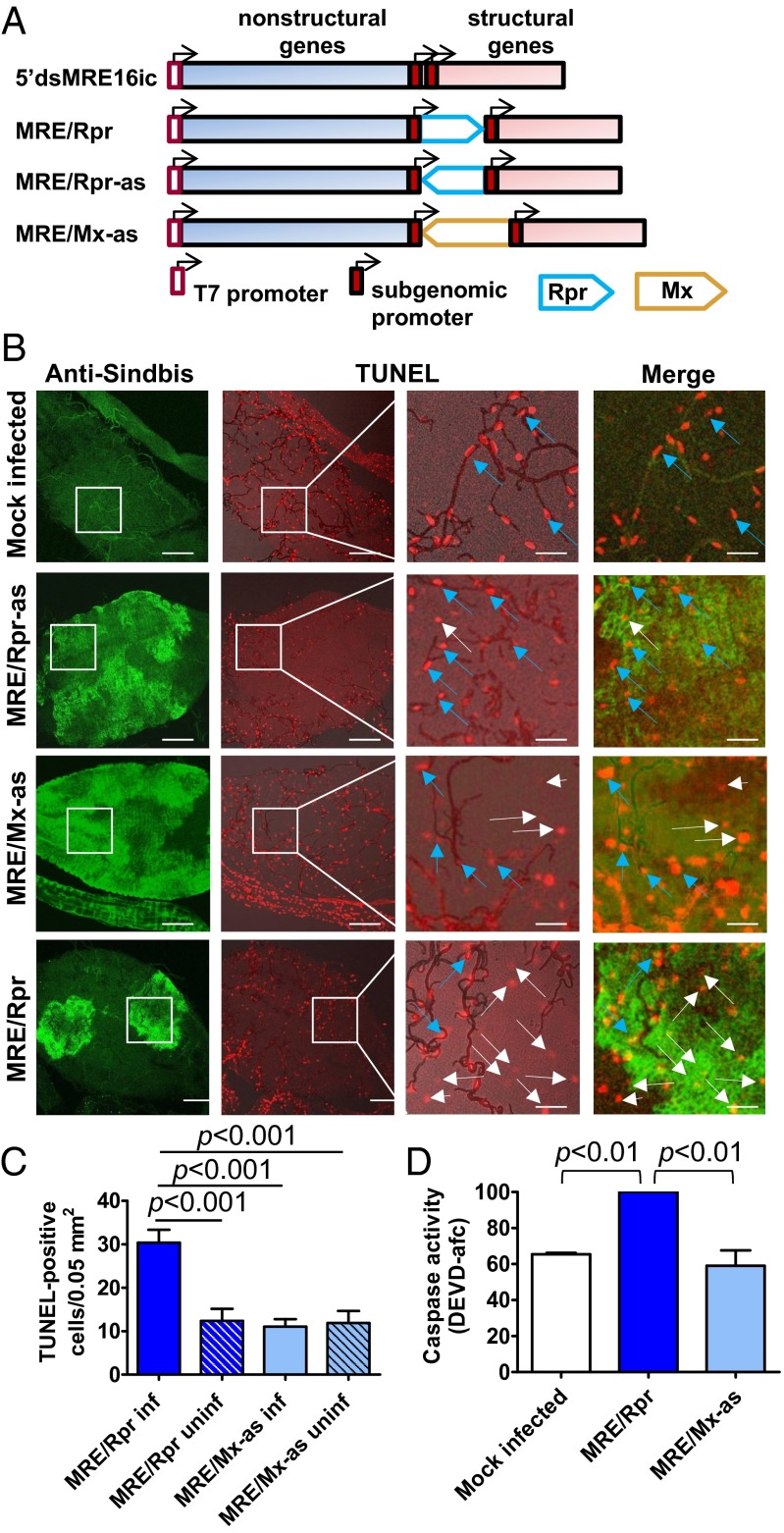
To examine the effects of inducing apoptosis on arbovirus–vector interactions, we used an infectious clone based on the SINV strain 5′dsMRE16ic (41) that was engineered to express the proapoptotic Drosophila gene reaper via a duplicated subgenomic promoter (42) (Fig. 1A). This recombinant SINV, called MRE/Rpr, has been previously shown to induce apoptosis in cultured mosquito cells (42). Two control constructs, MRE/Rpr-as and MRE/Mx-as, containing the sequence of the A. aegypti michelob_x gene, were used that contained noncoding (antisense orientation) sequences of similar size as reaper to control for the effects of genome size on virus replication.
Fig. 1.
A recombinant SINV clone expressing Drosophila Reaper induces apoptosis in A. aegypti midgut. (A) Recombinant SINV clones based on the infectious cDNA clone 5′dsMRE16ic. The clone MRE/Rpr produces infectious virus that expresses Reaper under control of the duplicated subgenomic promoter, whereas the control virus clones MRE/Rpr-as and MRE/Mx-as contain reaper or Mx sequences inserted in antisense orientation, and thus do not express any foreign protein. The T7 promoter is used to in vitro transcribe infectious RNA. (B) Midguts from A. aegypti given a virus-free blood meal (mock-infected) or a blood meal containing MRE/Rpr, MRE/Rpr-as, or MRE/Mx-as were harvested at 7 d after the blood meal. SINV infection was visualized using anti-SINV E1 antibody, and TUNEL staining was performed on the same midguts to label apoptotic cells. Panels in the third column from the left are higher-magnification views of the midgut surface outlined by white boxes in the first two columns. Merged images of the red and green channels are shown in the right-hand column. Tracheae overlying the midgut are visible as dark lines. Examples of TUNEL-positive, infected midgut epithelial cells are indicted by long white arrows, whereas TUNEL-positive, uninfected cells are indicated by short white arrows and examples of background staining of trachea-associated cells are indicted by blue arrows. (Scale bars, 200 μ in the left two panels and 50 μ in the right two panels.) Modified with permission from ref. 60. (C) TUNEL-positive cells were counted in infected (inf) and uninfected (uninf) areas of 10 midguts for each virus. Significance was determined by one-way ANOVA followed by Bonferroni’s posttest. Modified with permission from ref. 60. (D) Lysates from 7 dpi midguts fed a blood meal alone (mock infected) or a blood meal containing MRE/Rpr or control virus were assayed for ability to cleave the fluorogenic caspase substrate Ac-DEVD-AFC. MRE/Rpr values were set at 100. Three independent biological replicates were performed, and error bars indicate the SEM. Significance was determined by one-way ANOVA followed by Bonferroni’s posttest. Modified with permission from ref. 60.
We first tested whether infection with MRE/Rpr induced apoptosis in vivo. Adult female A. aegypti mosquitoes were given a blood meal containing either MRE/Rpr or control virus, and TUNEL assay was used to label apoptotic cells in midguts, the primary site of infection in the mosquito, at 7 d postinfection (dpi). SINV infection was simultaneously examined in midguts, using an antibody that recognizes the E1 structural protein of SINV. For unknown reasons, all midguts, regardless of infection status or whether the mosquitoes had been given a blood meal, displayed background staining of specific cells that were associated with trachea (Fig. 1B; blue arrows), which were ignored for the purposes of this experiment. In midguts that were infected with MRE/Rpr, regions that were positive for virus antigen contained significantly more TUNEL-positive cells (indicated by long white arrows) on the epithelial surface of the midgut than uninfected regions of the same midguts (short white arrows) (Fig. 1 B and C). Midguts from mosquitoes infected with control viruses exhibited low numbers of TUNEL-positive epithelial cells in both infected and uninfected regions (Fig. 1 B and C).
As an additional indicator of apoptosis, midgut lysate was analyzed for caspase activity at 7 dpi, using the fluorogenic caspase substrate N-Acetyl-Asp-Glu-Val-Asp-7-amido-4-trifluoromethylcoumarin (Ac-DEVD-AFC), a substrate of effector caspases. Lysate from midguts infected with MRE/Rpr displayed higher caspase activity than MRE/Mx-as-infected, MRE/Rpr-as-infected, or control blood-fed midgut lysate samples (Fig. 1D and Fig. S1). Together, these results indicate that expression of Reaper via the recombinant SINV clone MRE/Rpr induced effector caspase activation and apoptosis in infected A. aegypti midgut cells.
Effects of Apoptosis on Midgut Infection by SINV.
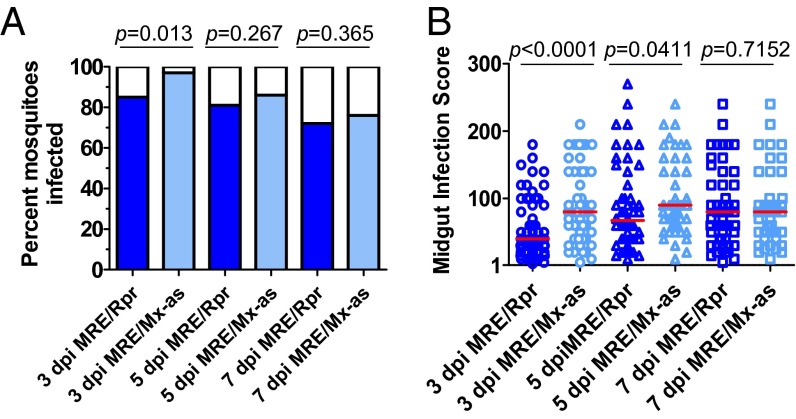
To assess whether increasing or decreasing apoptosis during virus replication affected the ability of SINV to infect the midgut, mosquitoes that had been given a blood meal containing MRE/Rpr or control virus were dissected and midguts were examined for levels of infection at 3, 5, and 7 dpi by immunofluorescence, using anti-E1 antibody. Each midgut was assigned an infection score based on the surface area that was infected and the intensity of the antibody staining, as previously described (42, 43). Prevalence of infection (the proportion of mosquitoes that were positive for SINV antigen in midgut) was significantly lower among mosquitoes infected with MRE/Rpr than control virus MRE/Mx-as at 3 dpi (Fig. 2A), although this difference was no longer significant at 5 and 7 dpi. Similarly, infection scores indicative of the extent of midgut infection were also significantly lower in mosquitoes infected with MRE/Rpr than in control-infected midguts at 3 and 5 dpi (Fig. 2B), but by 7 dpi, there was no longer a significant difference. These results suggest that stimulating apoptosis during virus replication in the midgut resulted in less infection in the midgut at earlier stages of infection, but that midgut infection by MRE/Rpr caught up to that of control virus by 7 dpi.
Fig. 2.
Expression of Reaper decreases midgut infection by SINV at early times after blood meal. (A) Prevalence of midgut infection in mosquitoes at 3, 5, and 7 dpi, based on presence or absence of staining in midgut using anti-SINV antibody. Fisher’s exact test was used to determine one-tailed P values. (B) Individual midgut infection scores based on staining with anti-SINV antibody. Infection scores were determined as described in Methods. Only infected midguts were included in the analysis of infection scores. Mann–Whitney U test was used to determine significance. Graphs represent combined results from at least 3 biologically independent experiments. Red lines indicate the median. Modified with permission from ref. 60.
Effects of Apoptosis on SINV Replication and Dissemination.
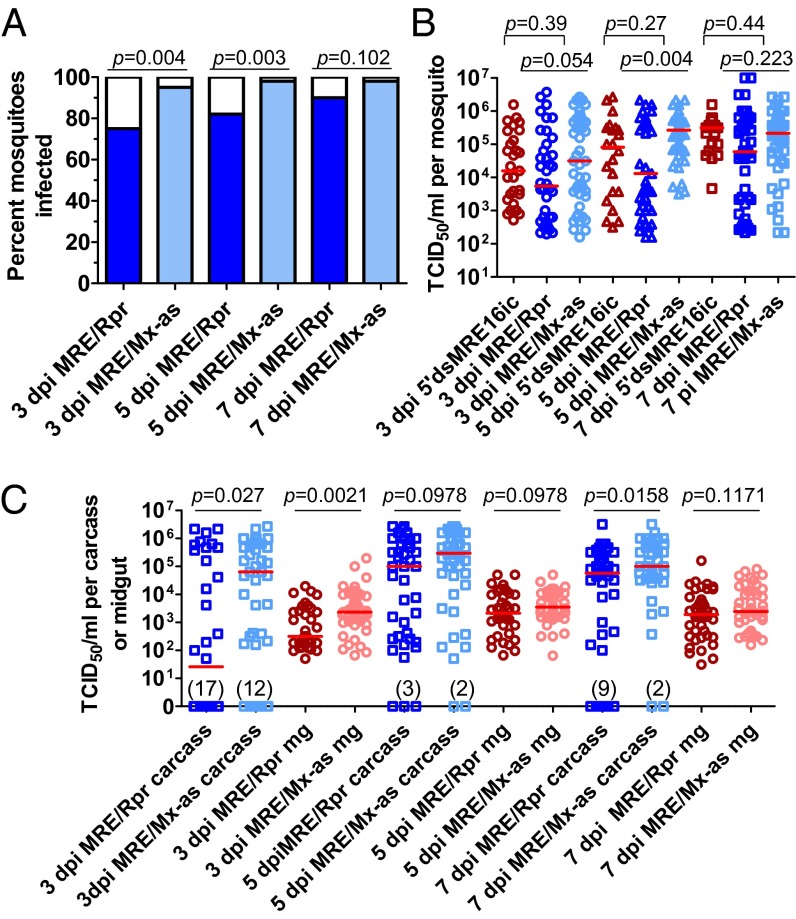
To more directly examine the levels of virus replication and dissemination, the amount of infectious virus per individual mosquito was determined by median tissue culture infectious dose (TCID50) assay. The parental 5′dsMRE16ic clone with no foreign insert was included as an additional control. Similar to what was observed by immunostaining of viral antigen, mosquitoes that were infected with MRE/Rpr had a significantly lower prevalence of infection at early times compared with infection with MRE/Mx-as, with the biggest difference being at 3 dpi (Fig. 3A). Among infected mosquitoes, virus titers were also lower in mosquitoes infected with MRE/Rpr than MRE/Mx-as- or 5′dsMRE16ic-infected mosquitoes, with the difference being significant at 5 dpi (Fig. 3B). No significant difference was observed between the titers of MRE/Mx-as and 5′dsMRE16-ic, and a single experiment performed with the control virus MRE/Rpr-as indicated similar trends (Fig. S2). This indicates that the presence of a ∼300-nt or smaller noncoding insertion did not significantly affect virus replication in the time frame examined. In addition, this also indicates that potential RNA interference-mediated knockdown of endogenous Mx expression, resulting from the presence of antisense Mx in the MRE/Mx-as genome, did not affect the outcome of infection. The decreased titers of MRE/Rpr further indicate a delay in the ability of SINV to establish infection in A. aegypti when Reaper is expressed during virus replication.
Fig. 3.
Reaper expression decreases SINV replication and dissemination in A. aegypti. (A) Prevalence of infection in mosquitoes, as determined by detection of infectious virus. Fisher’s exact test was used to determine one-tailed P values. (B) TCID50 assays were performed to measure the amount of infectious virus in individual mosquitoes. Only infected mosquitoes were included in the analysis, and Mann–Whitney U tests were used to determine P values. Red lines indicate the median. (C) Individual midguts were titered separately from the rest of the body (carcass), and results were analyzed as described earlier. Four to five individual biological replicates were performed for the above experiments, and the results were combined. Only infected samples were included in the analysis of body titers. Modified with permission from ref. 60.
A distinct group of mosquitoes infected with MRE/Rpr had lower titers ranging from 102 to 104 TCID50/mL; this group was most pronounced at 5 dpi (Fig. 3B). To determine whether these lower titers represented mosquitoes in which the virus had not escaped the midgut, we dissected the midgut from the rest of the mosquito and titered the individual midguts and bodies lacking midguts (referred to hereafter as carcasses) separately (Fig. 3C). At 3 dpi, 50% (17/34) of the mosquitoes fed MRE/Rpr did not contain detectable virus in the carcass versus 24.5% (12/49) of the mosquitoes fed MRE/Mx-as control virus. This indicated a significant difference in virus dissemination at 3 dpi (P = 0.01 by Fisher’s exact test). In addition, titer values of MRE/Rpr-infected midguts and carcasses were significantly lower than those that were control-infected at 3 dpi (Fig. 3C), correlating with lower midgut infection scores at this point. Carcass titers were not significantly different at 3 dpi if uninfected carcasses were omitted from the analysis, suggesting that if infection escaped the midgut, it reached normal levels. At 5 dpi, virus could be detected in most of the carcasses (37/40 MRE/Rpr and 44/46 MRE/Mx-as), and MRE/Rpr midgut and carcass titers had no significant difference compared with control titers (Fig. 3C). However, at 7 dpi, 9/45 mosquitoes that had been fed MRE/Rpr had no detectable carcass titer compared with only 2/47 mosquitoes fed with control virus, which was again a significant difference in viral dissemination, as determined by Fisher’s exact test (P = 0.02). Carcass titers were also significantly different at this time because of these uninfected samples. However, at 7 dpi, MRE/Rpr midgut titers were not significantly different from control (Fig. 3C), which is also consistent with the midgut infection scores at this point. The group of lower titer values seen in whole-body titer samples in Fig. 3B was consistent with the range of titers seen in midgut samples (Fig. 3C); however, some of the carcasses also had titers in this range. Thus, although the majority of MRE/Rpr-infected mosquitoes had high carcass titers by 5 dpi, indicating that the virus was able to escape the midgut in most cases, there was a significant difference in the rates of disseminated infection between MRE/Rpr and control virus at 3 and 7 dpi. It therefore appears that the lower group of whole-body titers seen in Fig. 3B was a result of lower initial infection in the midgut and either decreased or delayed midgut escape. This further reinforces the notion that Reaper expression decreases infection and dissemination of SINV.
Apoptosis Also Delays SINV Replication When the Midgut Is Bypassed.
These experiments involved oral infection, which is the natural route of arbovirus infection. To determine whether Reaper expression would also have an effect when the midgut barrier was bypassed, mosquitoes were infected by intrathoracic injection. Doses of 10, 100, or 1,000 plaque-forming units (PFU) were injected per mosquito, and samples were collected for TCID50 assays at 1, 3, and 5 dpi. Mosquitoes injected with MRE/Rpr had significantly lower titers than control virus at 1 dpi with each of the injected doses (Fig. S3). However, by 3 dpi, replication of MRE/Rpr had caught up with the control virus, and there was no significant difference in titer between MRE/Rpr and control at 3 or 5 dpi. These results indicate that even when the midgut barrier was bypassed, expression of Reaper during virus replication delayed SINV replication within the mosquito during the initial stages of infection.
Effect of Apoptosis on Accumulation of SINV in the Saliva of Infected Mosquitoes.
An important question was whether apoptosis affected the amount of virus in the saliva of infected mosquitoes, as this is what ultimately determines whether a mosquito will be able to transmit the virus. We were not able to find any published data that quantified SINV in A. aegypti saliva, and only a few transmission studies have been reported, which vary on how successfully SINV is transmitted by A. aegypti (43, 44). However, the presence of SINV in A. aegypti saliva has been reported by 9 d after oral infection (45). We collected saliva from orally infected mosquitoes at 10 and 14 dpi and determined virus titers. There was no significant difference in the proportion of mosquitoes having virus-positive saliva for MRE/Rpr versus control virus (Fig. 4A), with only 10–20% of saliva samples containing detectable virus at 10 dpi and less than 10% being virus-positive at 14 dpi. However, consistent with the delay seen in virus replication in midgut and whole body, MRE/Rpr saliva titers were significantly lower than control virus at 10 dpi (Fig. 4B). No significant difference was observed at 14 dpi; however, sample sizes at this time were small. These results again indicate that Reaper expression slowed the replication and dissemination of SINV during A. aegypti infection.
Fig. 4.
Reaper expression results in lower amounts of virus in the saliva early after the extrinsic incubation period. Saliva was collected from infected mosquitoes at 10 and 14 dpi. (A) Prevalence of virus in the saliva, as determined by TCID50 assay. Fisher’s exact test was used to determine one-tailed P values. (B) Virus titers in individual saliva samples. Mann–Whitney U tests were used to determine P values for TCID50 samples. Four independent biological replicates were performed for these experiments. Modified with permission from ref. 60.
Effects of Infection and Apoptosis on Mosquito Life Span.
Once a mosquito is infected with SINV, the virus will continue to replicate in its tissues for the remainder of the mosquito’s life (46). Therefore, we were interested to know whether infecting a mosquito with a virus expressing a proapoptotic factor would affect the life span of the mosquito. We monitored survival after giving mosquitoes a single, virus-containing blood meal. Infection with control viruses MRE/Mx-as or 5′dsMRE16ic had a small, but statistically significant negative effect on life span compared with mock-infected (blood-fed) mosquitoes, with increased mortality beginning at about 35 dpi (Fig. S4). However, mosquitoes infected with MRE/Rpr had an even lower survival rate than the other viruses tested, with increased mortality beginning at around 20 dpi; this difference was statistically significant compared with control viruses (Fig. S4). These results indicate that infection with a SINV that induces apoptosis reduced the life span of the mosquito more than just SINV infection alone.
Strong Negative Selection Against Maintenance of Reaper Expression Within Infected Mosquitoes.
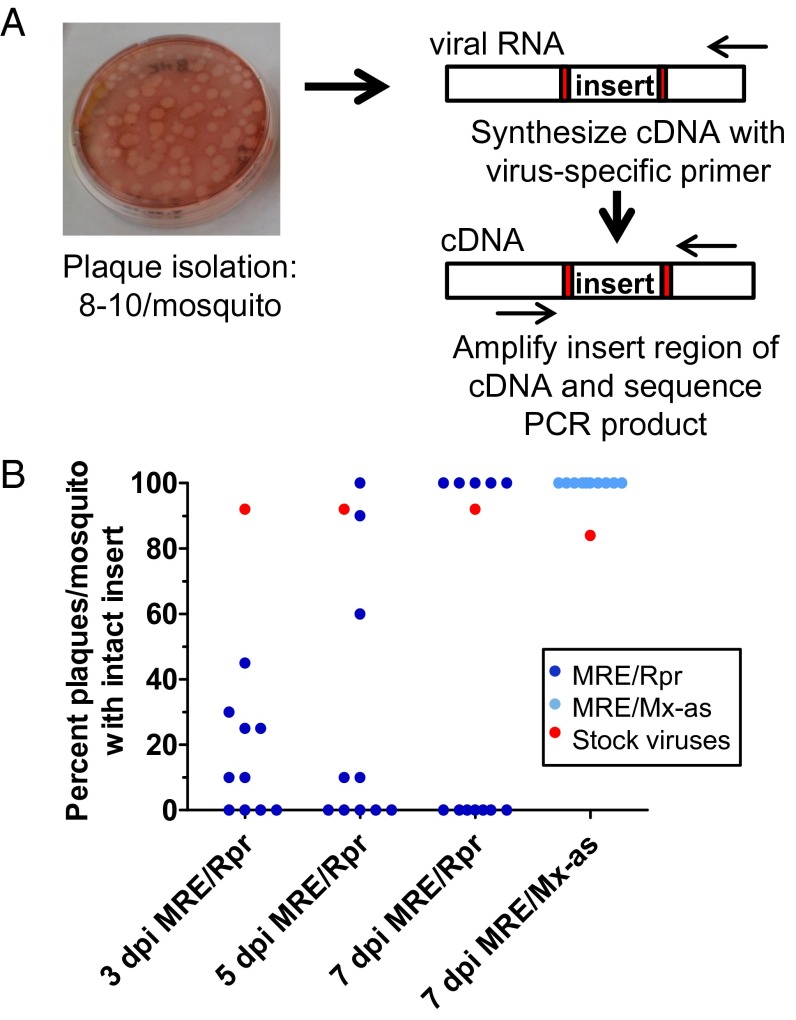
Given that the MRE/Rpr virus replicated less than control virus in the early stages of infection, but then caught up later, we asked whether the inserted reaper gene was remaining intact as SINV replicated in the mosquito during the experiments. To examine virus sequences in individual mosquitoes, we isolated plaques from titered whole-body samples. For each virus and time point, 8–10 plaques were isolated from each of 10–12 individual mosquitoes and amplified in BHK21 cells. Viral RNA was then isolated and the region of the genome containing the insert sequences amplified, followed by Sanger sequencing. A summary of the sequencing strategy and the results of the virus sequencing are shown in Fig. 5A and Table S1.
Fig. 5.
Negative selection against maintaining Reaper expression during mosquito infection by MRE/Rpr. (A) Strategy used for Sanger sequencing. Viruses collected from infected mosquitoes were isolated by plaque assay, and the region of the viral genome containing the duplicated subgenomic promoter and insert was amplified and sequenced from 8 to 10 plaque isolates from each of 10 mosquitoes. (B) Each data point indicates the percentage of sampled viruses (n = 8–10 plaque isolates) containing intact inserts from a single mosquito. The results are also presented in Table S1. Data obtained from sequencing individual plaque isolates obtained from stock viruses are indicated by red shapes (n = 25–40 plaque isolates). Modified with permission from ref. 60.
When individual plaque isolates were sequenced from the original MRE/Rpr stock virus used to infect mosquitoes, 92% (23/25) of the plaque isolates contained the complete reaper sequence, with no mutations or deletions. However, after only 3 d of replication in mosquitoes, the majority of sampled viruses isolated lacked the intact reaper cassette (Fig. 5B and Table S1). The most common type of mutations observed were deletions that removed all or a portion of the reaper ORF and/or the subgenomic promoter upstream of reaper; point mutations in the reaper ORF were rarely observed. At 5 dpi, 5 of 10 MRE/Rpr-infected mosquitoes had deletions in the reaper cassette in all sequenced plaque isolates, whereas four of the remaining five mosquitoes analyzed had mixtures of viruses with intact or deleted reaper inserts, and one mosquito had 10 of 10 plaque isolates with the intact reaper cassette (Fig. 5B and Table S1). At 7 dpi, the sampled plaque sequences in individual mosquitoes were homogenous; that is, either all of the sequenced isolates from a mosquito contained the intact insert (5 of 12 mosquitoes), or all of the sequenced isolates had deletions eliminating Reaper expression (7 of 12 mosquitoes) (Fig. 5B and Table S1). Linear regression analysis by ordinary least squares indicated there was no significant correlation between mosquito titer (Table S1) and presence or absence of the reaper insert (R2 = 0.01, 0.04, and 0.15 at 3, 5, and 7 dpi, respectively).
To further examine the MRE/Rpr viruses from the five mosquitoes that appeared to have 100% intact inserts at 7 dpi (mosquitoes 1, 3, 4, 6, and 7 in Table S1), one representative plaque isolate from each mosquito was used to infect C6/36 cells. Four of the five isolates did not induce apoptosis in C6/36 cells, suggesting Reaper was no longer being expressed from the majority of these viruses. When one of these plaque isolates was sequenced, a point mutation in the subgenomic promoter driving reaper was identified, which could possibly affect Reaper expression. Thus, it appears that in most cases, the viruses that had intact reaper inserts at 7 dpi had lost Reaper expression, either through point mutations in the subgenomic promoter or some other mechanism.
In contrast, when plaque isolates obtained at 7 dpi from mosquitoes infected with the control virus MRE/Mx-as were sequenced, all of them (88/88) contained the intact control insert and subgenomic promoter (Fig. 5B and Table S1). Similar results were obtained with MRE/Rpr-as (20 of 20 plaque isolates obtained from two mosquitoes contained the intact insert at 7 dpi). Thus, the accumulation of viruses with deletions in the insert strongly correlated with Reaper expression. These sequencing results indicate a strong negative selection against Reaper expression during replication of MRE/Rpr in mosquitoes.
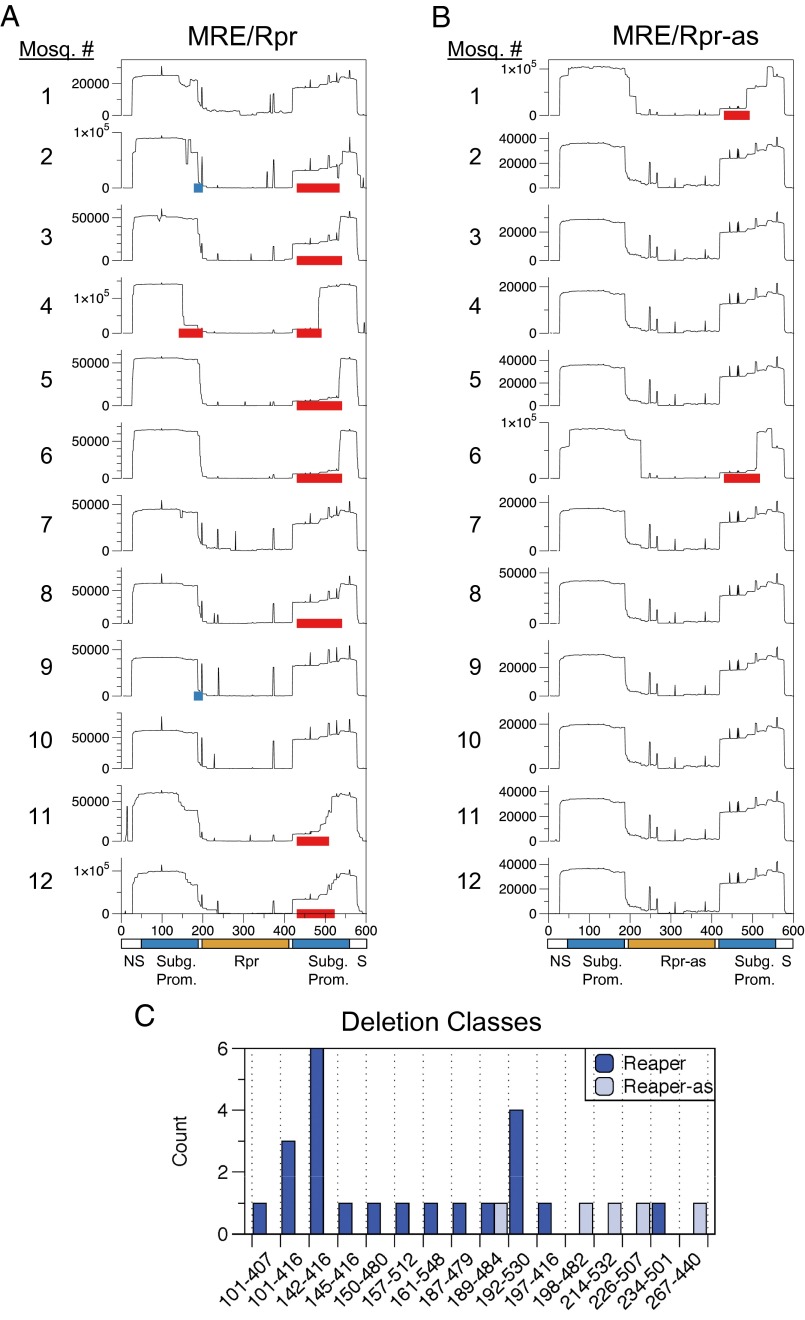
To obtain a more comprehensive picture of these virus populations, deep sequencing was performed on virus populations obtained from individual mosquitoes. Mosquitoes infected with MRE/Rpr or MRE/Rpr-as (12 mosquitoes per virus) were individually homogenized at 7 dpi, and RNA was isolated from the supernatant and converted to cDNA. The insert region was then PCR amplified and the products were separated by agarose gel electrophoresis (Fig. S5). PCR products ranging from 200 to 600 bp in length were excised from the gel and subjected to Illumina sequencing. For each mosquito, ∼30,000–70,000 reads were mapped to the reaper region, and their distribution was analyzed by counting the number of base pairs aligned at each position (Fig. 6). Because the reaper region was sequenced with paired-end Illumina sequencing for 250 cycles, the middle of the reaper insert was not sequenced.
Fig. 6.
Illumina sequencing of virus populations from individual mosquitoes. Twelve individual mosquitoes were infected with either MRE/Rpr (A) or MRE/Rpr-as (B), and total viral RNA was collected at 7 dpi from each mosquito. The insert region was amplified and subjected to Illumina sequencing. Shown are histograms for the 12 mosquitoes infected with MRE/Rpr or MRE/Rpr-as, with the read counts plotted at each position in the sequence. Regions containing large numbers of short indels (6 bp or less) are indicated by red boxes, and regions with numerous point mutations are indicated by blue boxes. NS, SINV nonstructural genes; S, structural genes. (C) Predominant deletion classes. The numbers of virus populations containing each deletion class are indicated. Three of the deletion classes (101–416, 142–416, and 192–530) were observed in more than one MRE/Rpr-infected mosquito.
Because both sequencing strategies suggested that most viruses obtained from mosquitoes infected with MRE/Rpr would contain large deletions, we first quantified the number and type of large deletions present, using the paired information for each read pair. All 12 of the virus populations derived from mosquitoes infected with MRE/Rpr had significant major deletions of the reaper gene and portions of the subgenomic promoters, whereas only 2 of the 12 mosquitoes infected with MRE/Rpr-as had virus populations with predominant deletions (Table S2). In all cases where deletion viruses predominated, one to three dominant deletion variants were present, suggesting that an early selective sweep resulted in these deletions becoming predominant during viral replication (Table S2). Deletions that removed the 5′ portion of the downstream promoter driving expression of the viral structural genes, the reaper insert, and the 3′ portion of the upstream promoter driving Reaper expression (e.g., the deletion 142–416) presumably resulted in a fused promoter still capable of driving structural gene expression; otherwise, these viruses would not be capable of replication.
Analyzing the Illumina reads for apparent small insertions and deletions (indels), as well as single nucleotide polymorphisms (SNPs), was complicated by the fact that the duplicated subgenomic promoters driving expression of Reaper and the downstream viral structural genes are identical 142-bp sequences, so not all apparent indels could be reliably called. Thus, this analysis likely underestimates the population of indels, especially close to the border of the 250 cycles of MiSeq sequence. However, the analysis revealed numerous SNPs and small deletions (6 bp or less), in addition to the major deletions listed in Table S2. Eight of the 12 mosquitoes infected with MRE/Rpr had populations of viruses with various small deletions in the promoter region (shown by red bars in Fig. 6). Although these small deletions are in the promoter driving structural gene expression, large deletions in these sequences removed the reaper insert; thus, the upstream promoter is presumably driving structural gene expression in these mutants. Two virus populations had large numbers of SNPs in the promoter region upstream of reaper (shown by blue bars in Fig. 6). In contrast, only two of the 12 virus populations resulting from infection with MRE/Rpr-as (the same two populations that had large deletions in Table S2) had virus populations with small deletions in the promoter region. In the regions well covered by sequence reads, SNPs were not observed in mosquitoes infected with MRE-Rpr-as (Fig. 6). In combination, these data indicate that expression of the reaper gene was under strong selective pressure to be eliminated from the viral genome during replication in mosquitoes.
Discussion
Evidence for Apoptosis Being a Defense Against Arbovirus Infection in Mosquitoes.
Most studies on the potential role of apoptosis in arbovirus–vector interactions have examined pathology in infected mosquitoes, in some cases correlating apoptosis with resistance to infection (22, 24–27, 30, 31, 37). A recent study from our group looked at the effects of silencing antiapoptotic or proapoptotic genes on SINV infection in A. aegypti mosquitoes and found that widespread induction of apoptosis actually exacerbated infection (33). However, there are significant differences in the approaches used in these two studies. In our previous experiments, apoptosis was stimulated by knockdown of Aeiap1 at 3 d before infection, and roughly 60% of the mosquitoes died within the first 2 d after gene knockdown (33). In addition, the midguts of the surviving mosquitoes showed obvious pathology at the time of infection, as a result of extensive cell death. Thus, induction of apoptosis in large numbers of cells, both infected and uninfected, likely weakened the physical midgut barrier. Immune defenses of the mosquitoes may have also been disrupted; for example, one of the main immune responses against arbovirus infection, RNA interference, was previously shown to be inhibited by apoptosis in Drosophila (47). This combination of factors likely was responsible for the increase in SINV infection observed when widespread apoptosis was stimulated by Aeiap1 knockdown before infection (33). In this study, we examined the effects of inducing apoptosis only in infected cells compared with widespread silencing of apoptotic factors. Our data indicate that inducing apoptosis during replication reduces or slows the ability of SINV to establish infection in the midgut, disseminate from the midgut, and infect salivary glands. In addition, we found evidence of strong selection against induction of apoptosis by viruses in infected mosquitoes.
We observed significantly less midgut infection by MRE/Rpr than control viruses at early times after infection, and a delay in virus dissemination from the midgut. Interestingly, however, by 5–7 dpi, MRE/Rpr had caught up to control viruses. This could be a result of at least two factors: first, Reaper expression from the viral subgenomic promoter occurs relatively late in the virus replication cycle, and thus may only cause a delay in cell-to-cell spread of virus. Some virus would likely be produced before MRE/Rpr-infected cells died by apoptosis, as was seen when cultured cells were infected (42). However, after the infected cells die, they would no longer be able to serve as virus factories, as they would in a normal persistent infection.
The second factor that likely allows MRE/Rpr to catch up over time is the potential replicative advantage of viruses containing mutations that eliminate the expression of Reaper. It is clear that there was strong selective pressure favoring viruses that no longer expressed Reaper, as the insert was found to be frequently deleted in mosquitoes infected with MRE/Rpr, but not with the control viruses. We can only speculate about what would have happened if the reaper insert was not able to be easily deleted from the MRE/Rpr genome, but we suspect that the effects on replication would have been even more drastic than what we observed. Reaper likely induces apoptosis by multiple mechanisms, including inhibition of IAP proteins, regulating mitochondrial fusion, and inhibiting cap-dependent protein translation (48–50). Because Reaper expression is up-regulated normally during Drosophila apoptosis (48), and many arbovirus mRNAs are capped, including SINV, inhibition of cap-dependent translation by Reaper homologs may play a role in decreasing arbovirus replication in apoptotic mosquito cells, regardless of how apoptosis is stimulated.
Two mosquitoes infected with MRE/Rpr-as had virus populations with the antisense insert deleted from the viral genome, suggesting that deletion events in the viral genome are quite common. These deletions most likely occur as a result of a mechanism of RNA recombination that is common among RNA viruses, called template switching, in which the viral RNA-dependent RNA polymerase detaches from one negative strand template and reattaches to another template (51, 52). If the polymerase reattaches at a different position, an insertion or deletion occurs. Most insertions and deletions would be deleterious to the virus, but if a deletion confers a selective advantage, it would be expected to rapidly become overrepresented in the viral population. In the case of the reaper gene, the strong selection to remove a gene that is cytotoxic could lead to rapid selection for virus clones lacking the gene or deficient in expressing the gene. In contrast, deletions removing the antisense reaper insert do not appear to confer a strong selective advantage, as the insert region is fairly small. During longer infection periods, deletion of the antisense insert may confer a replicative advantage because they remove a region without a fitness benefit that has some cost to be replicated.
Deep sequencing revealed that virus populations from individual mosquitoes tended to be composed of a small number of predominant virus clones (Table S2). This result may reflect the previous observation, made with Venezuelan equine encephalitis virus and Culex taeniopus, that only a small number of viruses escape the midgut and are responsible for establishing disseminated infection (53). Moreover, we found that three specific deletion genotypes were found more than once in virus populations from independent MRE/Rpr-infected mosquitoes. It is possible that these deletion genotypes were already present in the inoculum that was fed to the mosquitoes and emerged through natural selection. Alternatively, these sites may represent hot spots for copy choice recombination in this region.
Other studies have also suggested that early induction of apoptosis in mosquito midgut may result in nonpermissive virus infection. A laboratory strain of Culex mosquitoes that were refractory to West Nile virus exhibited increased apoptosis in midgut tissues at 3 dpi infection compared with uninfected individuals (31). In addition, early proapoptotic gene expression was observed in the midguts of A. aegypti adult mosquitoes that were refractory to dengue virus infection, but not in susceptible mosquitoes (32). Although these previous studies did not directly demonstrate that apoptosis was responsible for decreased infection, when taken together with this study, the evidence indicates that apoptosis can be an effective antiviral defense in mosquitoes that may be exploitable in controlling the transmission of arboviruses. Although most arboviruses do not appear to induce significant levels of apoptosis in their vector mosquitoes, we hypothesize that this is likely a result of natural selection favoring the evolution of arboviruses that do not induce apoptosis. A prediction arising from this hypothesis is that induction of apoptosis is more likely to be observed in noncompatible virus–vector combinations, which are not typically studied.
Can Apoptosis Be Exploited to Interrupt Arbovirus Transmission?
There are several factors to consider when looking at ways to decrease or block virus transmission by mosquitoes, including virus prevalence in the mosquito population, the amount and timing of virus salivated, and mosquito life span. Although SINV is naturally vectored by Culex mosquitoes, it can be transmitted by A. aegypti in the laboratory setting (34, 54). We found that prevalence of SINV in saliva was similar between MRE/Rpr and control viruses, but MRE/Rpr-infected mosquitoes did have significantly lower amounts of virus in saliva than control virus at 10 dpi, suggesting either it took longer for MRE/Rpr to successfully invade and replicate in salivary glands, or replication levels were decreased in this organ. Lowering the amount of virus in saliva may decrease virus transmission, although the magnitude of the decrease probably depends on the virus and its ability to infect the host. A recent report found increased apoptosis in SINV-infected salivary glands in A. aegypti compared with uninfected glands (37). This and other studies reporting apoptosis in arbovirus-infected salivary glands (29, 30) lend to the notion that apoptosis in salivary glands may naturally occur in some arbovirus–mosquito combinations and could explain why we observed decreased infection prevalence in saliva at 14 dpi compared with 10 dpi with all of the viruses tested. Thus, purposely stimulating apoptosis in salivary glands of infected mosquitoes, for example, by using transgenic approaches, may be a means of blocking virus transmission with fewer effects on mosquito health and survival than inducing apoptosis in midgut. The effectiveness of such an approach would require further testing with other more medically important arboviruses, as responses may vary in different virus–vector combinations.
In addition, we observed a significant reduction in the average life span of mosquitoes infected with MRE/Rpr compared with control viruses. If mosquitoes die before being able to transmit whatever pathogen they have acquired, transmission is blocked. In this case, the extrinsic incubation period of SINV in A. aegypti is around 9 d (45). However, MRE/Rpr-infected mosquitoes died well after 9 dpi, and approximately half of them lived past 40 dpi, although it is not known whether infection with an apoptosis-inducing virus would affect mosquito feeding behavior. Also, the length of time required for MRE/Rpr infection to cause lethality may have been affected by the loss of reaper expression over time in many of the virus genotypes. In contrast, more than 40% of mosquitoes in which AeIAP1 expression had been silenced died within 24–48 h after injection or topical application of dsRNA (33, 55). A balance between these two approaches may be able to achieve reduced life span, and thereby reduce the number of blood meals taken by a female mosquito without causing a severe evolutionary disadvantage, which would rapidly lead to loss of a genetic resistance trait in a transgenic mosquito. Such a system would also have to protect the overall health of the infected mosquitoes, as disruption of innate defenses or the midgut barrier could lead to increased virus infection and transmission if the mosquito survived past the extrinsic incubation period.
Together, these results provide the first direct evidence to our knowledge that apoptosis, if it is stimulated by an arbovirus, has detrimental effects on the ability of the virus to replicate in its vector. This probably explains why apoptosis is rarely observed in naturally coevolved arbovirus–vector relationships and suggests that apoptosis may be one of the defense responses that together decide the outcome of infection when a mosquito is exposed to an arbovirus.
Methods
Insect Rearing.
A. aegypti mosquitoes, Orlando strain, (obtained from James Becnel at the Agricultural Research Service US Department of Agriculture, Gainesville, FL) were reared at 27 °C, 80% humidity on a 12 h light/12 h dark cycle. They were allowed to feed on raisins and water before blood feeding, and sucrose, raisins, and water after blood meal. All experiments with SINV-infected mosquitoes were performed in an arthropod containment level 2 insectary at Kansas State University.
Propagation of Recombinant SINV Virus and Determination of Viral Titers.
Recombinant 5′dsMRE16ic-based SINV clones containing sense and antisense sequences for Drosophila reaper (MRE/Rpr) and an additional antisense control containing the antisense sequence of A. aegypti michelob_x (MRE/Rpr-as or MRE/Mx-as) were previously described (42). These viruses have insert sizes of 198 nt for MRE/Rpr and MRE/Rpr-as and 339 nt for MRE/Mx-as. Capped viral RNA was produced from linearized plasmids using AmpliScribe SP6 HighYield Transcription Kit (Epicentre Biotechnologies) and m7G(5′)ppp(5′)G Cap Analog (Ambion). Aliquots of each transcription reaction (10 μL) were used to transfect BHK21 cells in 1 mL Opti-MEM Reduced Serum Medium (Invitrogen) with 6 μL Lipofectamine 2000 (Invitrogen), as previously described (42). At 2–3 d posttransfection, medium was collected, aliquoted, and stored at −80 °C. Virus stocks were amplified once by using 100 µL virus to infect a T75 flask containing 90% confluent C6/36 cells, cultured in l-15 (Leibovitz) medium (Invitrogen) supplemented with 10% (vol/vol) FBS. At 2–4 dpi, virus was harvested, aliquoted, and stored at −80 °C. Viral titers were determined using TCID50 assays in BHK21 cells, as described here. Viruses used in this study were only passaged once and thawed once before use.
Oral Infection with SINV.
Two- to 3-d posteclosion mosquitoes were sorted for feeding while under cold-induced coma. Cages contained a 1:20 male-to-female ratio. Mosquitoes were given only water for 24 h before feeding. Defibrinated sheep blood (Colorado Serum Company) was mixed 1:1 with cell culture supernatant containing SINV for a final virus concentration of 1 × 107 TCID50/mL. Three- to 4-d-old mosquitoes were then administered an infectious blood meal using a Hemotek 5W1 feeding system (Discovery Workshops). Mosquitoes were allowed to probe and feed through parafilm for 30–60 min. Mosquitoes were chilled at 4 °C and sorted for fully engorged females. Blood-fed females were sorted into cages and given sucrose, raisins, and water until experiments were completed.
Intrathoracic Infection of SINV.
A Nanoinject II injector (Drummond Scientific) was used to inject 3–4 d posteclosion female mosquitoes with 69 nL DMEM containing 10–1,000 PFU of the indicated virus. PFU values were calculated by multiplying TCID50 values by 0.69, as previously described (56). Virus was injected intrathoracically while mosquitoes were knocked down with cold. After injection, mosquitoes were placed in cages and given sugar, raisins, and water until experiments were completed. Three independent biological replicates were performed.
Midgut Antibody and TUNEL Staining.
Dissected midguts were fixed in 4% (vol/vol) paraformaldehyde in PBS (137 mM NaCl, 7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). Fixed midguts were washed in PBS + 0.1% Triton X-100 and blocked with PBS + 10% (vol/vol) FBS + 1% BSA. Anti-E1 SINV monoclonal antibody 30.11a (57) (obtained from Carol Blair, Colorado State University) diluted 1:200 was used as a primary antibody, and goat anti-mouse Alexa Fluor 488 (Molecular Probes) diluted 1:500 was used as secondary antibody. Washed midguts were mounted on slides using Fluormount-G (Electron Microscopy Sciences). Each midgut was given an infection score as previously described (42, 43), determined by multiplying the estimated percentage of the midgut surface area that was infected by the brightness of the staining (scale 1–3, 1 = dull, 2 = moderate, 3 = bright). TUNEL was performed using the In Situ Cell Death Detection Kit, TMR red (Roche Applied Science) either after antibody staining was complete or with no antibody staining. TUNEL-stained and antibody-stained midguts were imaged using a laser scanning confocal microscope (Zeiss LSM 5 Pascal, Kansas State University Microscopy Facility). TUNEL-positive cells on the epithelial surface of the midgut were counted in one uninfected and two infected regions (230 µm × 230 µm), of ten midguts (7 dpi), per virus.
TCID50 Assay with Mosquito Samples.
Individual mosquitoes were placed in 500 μL DMEM (Invitrogen) supplemented with 15 μg/mL penicillin/streptomycin (Invitrogen) and 1 ng/mL gentamycin (Cellgro). Mosquitoes were triturated in 1.5-mL tubes with a disposable pestle, and then debris was spun down. Supernatant from each sample was used to perform a serial dilution. BHK21 cells in supplemented DMEM were used to seed 96-well plates. Each mosquito sample dilution (10 µL) was added to 8 wells of BHK21 cells (1 × 104 cells/well). Plates were scored for infection at 6 dpi by observing cytopathic effects. The number of infected wells per dilution was used to determine the TCID50 per mosquito (56).
Saliva Collection.
Saliva was collected from 10 or 14 dpi mosquitoes. Mosquitoes were starved of a sugar source for 24 h before saliva collection, then anesthetized by cold treatment, and their wings and legs were removed. The proboscis was placed in a pipette tip containing ∼20 μL FBS + 1 mM ATP. Mosquitoes were allowed to salivate for 60–90 min. After salivation the FBS was added to 100 μL DMEM containing penicillin/streptomycin and gentamicin. Samples were vortexed, spun down, and then used for TCID50 assays. Four independent biological replicates were performed per sample.
Longevity Assay.
Mosquitoes at 3–4 d posteclosion were allowed to feed on a noninfectious blood meal or a blood meal containing 1 × 107 PFU/mL of the indicated virus. Blood-fed mosquitoes were then placed in pint-sized containers and fed raisins and water throughout the experiment. Mortality was monitored daily for 42 d. Four independent biological replicates were performed.
Caspase Activity Assay.
Pools of 10 midguts were lysed in caspase buffer (20 mM Hepes-KOH, pH 7.5, 50 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT), using sonication. Debris was spun down, and supernatant was used for caspase activity assay. The concentration of protein in each lysate sample was quantified using BCA Protein Assay Kit (Pierce), and samples were diluted to equal protein concentrations. Fifty microliters of each sample were added to a 96-well white plate (Costar). After incubation at 37 °C for 10–15 min, Ac-DEVD-AFC substrate (MP Biomedicals) was added to each well at a concentration of 20 μM. Cleavage of fluorogenic substrate was then measured at 15-min intervals by fluorescence produced at excitation 405 nm and emission 535 nm, using a Victor3 1420 Multilabel Counter (Perkin-Elmer). The rate of substrate cleavage in the first 30 min was used to compare the amount of activity in each sample. Three independent biological replicates were performed for each experiment.
Plaque Isolation and Sequencing.
Plaques were isolated by diluting supernatant from titered individual mosquitoes in DMEM + 0.5% agarose, 10% FBS, 15 µg/mL penicillin/streptomycin, and 1 ng/mL gentamicin. BHK21 cells were overlaid with a mixture of DMEM, virus, and 0.5% agarose. At 3 dpi, 10 plaques from each mosquito or stock virus were collected and amplified once in BHK21 cells cultured in supplemented DMEM.
Plaque sizes varied but did not correlate with retention of the inserts. At 2–3 dpi, amplified virus was harvested from BHK21 cells. Viral RNA was isolated from each individual amplified plaque isolate using TRIzol LS reagent (Invitrogen). Reverse transcription was performed using ImProm-II Reverse Transcription Kit System (Promega) and the virus-specific primer 5′TACTGCGGAGGTCAATTGTT 3′. The region containing the insert was amplified by PCR with sense primer 5′CTGAGACACTGGCTACTGCG 3′, antisense primer 5′CGGCCGAGCATATTAAAGAA 3′, and GoTaq polymerase (Promega). Purified PCR products were sequenced by Genewiz, Inc. Sequences were analyzed using EMBOSS Kalign (www.ebi.ac.uk/Tools/msa/kalign/).
Deep Sequencing.
Purified PCR products from individual mosquitoes infected with the MRE or MRE antisense virus were individually barcoded during PCR amplification. Each PCR product from individual mosquitoes was gel extracted, followed by library preparation for Illumina sequencing with a NEBNext DNA Library Preparation kit for paired end samples. The libraries were quantified with an Agilent BioAnalyzer and equally loaded on a single lane of Illumina MiSeq, where they were sequenced for 250 cycles on each end. Because the samples from individual were barcoded during PCR, they were de-multiplexed into individual barcode libraries with a custom PERL script. To map the reads back to the MRE or MRE antisense insert region, we found that standard read mappers such as bwa REF and bowtie REF did not produce optimal alignments because of a tandem promoter repeat present in the sequenced region. Instead, a custom alignment pipeline was developed on the basis of the MUSCLE pairwise aligner REF (58). To align the reads, each read of a paired end set was analyzed to identify those which had the 5′ bar code inserted by PCR. The barcode sequence was then removed and the read aligned. Next, its mate pair corresponding to the 3′ end read was reverse complemented and aligned to the MRE or MRE antisense region, followed by combining both alignments into a single alignment. This process was then repeated for each of the read pairs from Illumina sequencing, and the entire result was combined into a single alignment file. To quantify the number of reads that mapped to each position in the MRE or MRE antisense region, the number of matching base pairs at each position was counted to produce a matrix of counts at each position. This matrix was used to produce a graph of the sequence count, using DataGraph 3.2 (Visual Data Tools, Inc.). SNPs and insertions and deletions (INDELS) were called with samtools 0.1.17 REF (59), allowing for a depth of up to 100,000 because of the read depth we obtained on each MRE PCR product. SNPs and INDELS were then filtered manually by z-score and depth and were filtered out of regions of low sequence quality. To quantify the presence of significant deletions that resulted in overlapping paired sequence reads, the alignments from above were scored with a custom PERL script that identifies the location where both mate-paired reads overlap and the corresponding location in the subgenomic promoter and reaper sequence. After initial identification and manual inspection, reads with less than 5 bp of overlap were filtered out because certain classes were a result of base call quality trimming near the end of the pair reads, not an actual deletion. Low-frequency deletions (less than 3%) had an ∼50% false discovery rate, and were filtered from the final analysis. Finalized sequences were submitted to the National Center for Biotechnology Information Sequence Read Archive (www.ncbi.nlm.nih.gov/sra) and were assigned accession no. PRJNA272945.
Supplementary Material
Acknowledgments
We thank Dan Boyle (Kansas State University Microscopy Facility) for microscopy assistance, Yueping Cao and Leigh Murry (Kansas State University Department of Statistics) for statistical consultation, James Becnel (US Department of Agriculture Agricultural Research Service) for providing the Orlando mosquito strain, Carol Blair (Colorado State University) for providing the α-Sindbis antibody, A. Lorena Passarelli (KSU Division of Biology) for helpful discussions, Hua Wang and Bart Bryant (Kansas State University Division of Biology) for construction of virus clones, and Alexander Franz (University of Missouri) for discussion and advice on experimental protocols. The work was supported by Grant R01AI091972 from the National Institutes of Health. This is contribution no. 13-299-J from the Kansas Agricultural Experiment Station.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424469112/-/DCSupplemental.
Data deposition: The sequences reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive, www.ncbi.nlm.nih.gov/sra (accession no. PRJNA272945).
References
- 1.World Health Organization . 2014. Dengue and severe dengue. Factsheet No. 117. Available at www.who.int/mediacentre/factsheets/fs117/en/. Accessed January 12, 2015. [Google Scholar]
- 2.World Health Organization . 2014. Yellow fever. Factsheet No. 100. Available at www.who.int/mediacentre/factsheets/fs100/en/. Accessed January 12, 2015. [Google Scholar]
- 3.Vega-Rúa A, Zouache K, Girod R, Failloux AB, Lourenço-de-Oliveira R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J Virol. 2014;88(11):6294–6306. doi: 10.1128/JVI.00370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diallo M, et al. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999-2000: Entomologic findings and epidemiologic considerations. Emerg Infect Dis. 2003;9(3):362–367. doi: 10.3201/eid0903.020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott TW, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J Med Entomol. 2000;37(1):89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 6.Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42(5):844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- 7.Mellor PS. Replication of arboviruses in insect vectors. J Comp Pathol. 2000;123(4):231–247. doi: 10.1053/jcpa.2000.0434. [DOI] [PubMed] [Google Scholar]
- 8.Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 9.Sanders HR, et al. Sindbis virus induces transport processes and alters expression of innate immunity pathway genes in the midgut of the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2005;35(11):1293–1307. doi: 10.1016/j.ibmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4(7):e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell CL, et al. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 2008;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keene KM, et al. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101(49):17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Vargas I, et al. RNA interference, arthropod-borne viruses, and mosquitoes. Virus Res. 2004;102(1):65–74. doi: 10.1016/j.virusres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8(2):113–126. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 15.Clarke TE, Clem RJ. Insect defenses against virus infection: The role of apoptosis. Int Rev Immunol. 2003;22(5-6):401–424. doi: 10.1080/08830180305215. [DOI] [PubMed] [Google Scholar]
- 16.Schlesinger RW. Sindbis virus replication in vertebrate and mosquito cells: An interpretation. Med Biol. 1975;53(5):295–301. [PubMed] [Google Scholar]
- 17.Stollar V, Shenk TE, Koo R, Igarashi A, Schlesinger RW. Observations of Aedes albopictus cell cultures persistently infected with Sindbis virus. Ann N Y Acad Sci. 1975;266:214–231. doi: 10.1111/j.1749-6632.1975.tb35103.x. [DOI] [PubMed] [Google Scholar]
- 18.Karpf AR, Brown DT. Comparison of Sindbis virus-induced pathology in mosquito and vertebrate cell cultures. Virology. 1998;240(2):193–201. doi: 10.1006/viro.1997.8914. [DOI] [PubMed] [Google Scholar]
- 19.Levine B, et al. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361(6414):739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 20.Nava VE, et al. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72(1):452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant B, Blair CD, Olson KE, Clem RJ. Annotation and expression profiling of apoptosis-related genes in the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2008;38(3):331–345. doi: 10.1016/j.ibmb.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Clem RJ. Defining the core apoptosis pathway in the mosquito disease vector Aedes aegypti: The roles of iap1, ark, dronc, and effector caspases. Apoptosis. 2011;16(2):105–113. doi: 10.1007/s10495-010-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Clem RJ. The role of IAP antagonist proteins in the core apoptosis pathway of the mosquito disease vector Aedes aegypti. Apoptosis. 2011;16(3):235–248. doi: 10.1007/s10495-011-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard YA, Popov V, Wen J, Han V, Higgs S. Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2005;42(3):429–444. doi: 10.1093/jmedent/42.3.429. [DOI] [PubMed] [Google Scholar]
- 25.Mims CA, Day MF, Marshall ID. Cytopathic effect of Semliki Forest virus in the mosquito Aedes aegypti. Am J Trop Med Hyg. 1966;15(5):775–784. doi: 10.4269/ajtmh.1966.15.775. [DOI] [PubMed] [Google Scholar]
- 26.Bowers DF, Coleman CG, Brown DT. Sindbis virus-associated pathology in Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2003;40(5):698–705. doi: 10.1603/0022-2585-40.5.698. [DOI] [PubMed] [Google Scholar]
- 27.Weaver SC, Scott TW, Lorenz LH, Lerdthusnee K, Romoser WS. Togavirus-associated pathologic changes in the midgut of a natural mosquito vector. J Virol. 1988;62(6):2083–2090. doi: 10.1128/jvi.62.6.2083-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrechts L, Scott TW. Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc Biol Sci. 2009;276(1660):1369–1378. doi: 10.1098/rspb.2008.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girard YA, et al. Transcriptome changes in Culex quinquefasciatus (Diptera: Culicidae) salivary glands during West Nile virus infection. J Med Entomol. 2010;47(3):421–435. doi: 10.1603/me09249. [DOI] [PubMed] [Google Scholar]
- 30.Girard YA, et al. Salivary gland morphology and virus transmission during long-term cytopathologic West Nile virus infection in Culex mosquitoes. Am J Trop Med Hyg. 2007;76(1):118–128. [PubMed] [Google Scholar]
- 31.Vaidyanathan R, Scott TW. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis. 2006;11(9):1643–1651. doi: 10.1007/s10495-006-8783-y. [DOI] [PubMed] [Google Scholar]
- 32.Liu B, et al. P53-mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster. PLoS Pathog. 2013;9(2):e1003137. doi: 10.1371/journal.ppat.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Gort T, Boyle DL, Clem RJ. Effects of manipulating apoptosis on Sindbis virus infection of Aedes aegypti mosquitoes. J Virol. 2012;86(12):6546–6554. doi: 10.1128/JVI.00125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor RM, Hurlbut HS, Work TH, Kingston JR, Frothingham TE. Sindbis virus: A newly recognized arthropodtransmitted virus. Am J Trop Med Hyg. 1955;4(5):844–862. doi: 10.4269/ajtmh.1955.4.844. [DOI] [PubMed] [Google Scholar]
- 35.Olson KE, Beaty BJ, Higgs S. Sindbis virus expression systems for the manipulation of insect vectors. In: Miller LK, Ball LA, editors. The Insect Viruses. Plenum Press; New York: 1998. [Google Scholar]
- 36.Foy BD, Olson KE. Alphavirus transducing systems. Adv Exp Med Biol. 2008;627:19–34. doi: 10.1007/978-0-387-78225-6_2. [DOI] [PubMed] [Google Scholar]
- 37.Kelly EM, Moon DC, Bowers DF. Apoptosis in mosquito salivary glands: Sindbis virus-associated and tissue homeostasis. J Gen Virol. 2012;93(Pt 11):2419–2424. doi: 10.1099/vir.0.042846-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoo CC, Doty JB, Heersink MS, Olson KE, Franz AW. Transgene-mediated suppression of the RNA interference pathway in Aedes aegypti interferes with gene silencing and enhances Sindbis virus and dengue virus type 2 replication. Insect Mol Biol. 2013;22(1):104–114. doi: 10.1111/imb.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson KE, et al. The expression of chloramphenicol acetyltransferase in Aedes albopictus (C6/36) cells and Aedes triseriatus mosquitoes using a double subgenomic recombinant Sindbis virus. Insect Biochem Mol Biol. 1994;24(1):39–48. doi: 10.1016/0965-1748(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 40.Olson KE, et al. Development of a Sindbis virus expression system that efficiently expresses green fluorescent protein in midguts of Aedes aegypti following per os infection. Insect Mol Biol. 2000;9(1):57–65. doi: 10.1046/j.1365-2583.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- 41.Foy BD, et al. Development of a new Sindbis virus transducing system and its characterization in three Culicine mosquitoes and two Lepidopteran species. Insect Mol Biol. 2004;13(1):89–100. doi: 10.1111/j.1365-2583.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Blair CD, Olson KE, Clem RJ. Effects of inducing or inhibiting apoptosis on Sindbis virus replication in mosquito cells. J Gen Virol. 2008;89(Pt 11):2651–2661. doi: 10.1099/vir.0.2008/005314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myles KM, Pierro DJ, Olson KE. Comparison of the transmission potential of two genetically distinct Sindbis viruses after oral infection of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2004;41(1):95–106. doi: 10.1603/0022-2585-41.1.95. [DOI] [PubMed] [Google Scholar]
- 44.Dohm DJ, Logan TM, Barth JF, Turell MJ. Laboratory transmission of Sindbis virus by Aedes albopictus, Ae. aegypti, and Culex pipiens (Diptera: Culicidae) J Med Entomol. 1995;32(6):818–821. doi: 10.1093/jmedent/32.6.818. [DOI] [PubMed] [Google Scholar]
- 45.Phillips A, Mossel E, Sanchez-Vargas I, Foy B, Olson K. Alphavirus transducing system: Tools for visualizing infection in mosquito vectors. J Vis Exp. 2010;(45) doi: 10.3791/2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strauss JH, Strauss EG. The alphaviruses: Gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie W, Liang C, Birchler JA. Inhibition of RNA interference and modulation of transposable element expression by cell death in Drosophila. Genetics. 2011;188(4):823–834. doi: 10.1534/genetics.111.128470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steller H. Regulation of apoptosis in Drosophila. Cell Death Differ. 2008;15(7):1132–1138. doi: 10.1038/cdd.2008.50. [DOI] [PubMed] [Google Scholar]
- 49.Thomenius M, et al. Mitochondrial fusion is regulated by Reaper to modulate Drosophila programmed cell death. Cell Death Differ. 2011;18(10):1640–1650. doi: 10.1038/cdd.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colón-Ramos DA, et al. Direct ribosomal binding by a cellular inhibitor of translation. Nat Struct Mol Biol. 2006;13(2):103–111. doi: 10.1038/nsmb1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai MM. RNA recombination in animal and plant viruses. Microbiol Rev. 1992;56(1):61–79. doi: 10.1128/mr.56.1.61-79.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss BG, Schlesinger S. Recombination between Sindbis virus RNAs. J Virol. 1991;65(8):4017–4025. doi: 10.1128/jvi.65.8.4017-4025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forrester NL, Guerbois M, Seymour RL, Spratt H, Weaver SC. Vector-borne transmission imposes a severe bottleneck on an RNA virus population. PLoS Pathog. 2012;8(9):e1002897. doi: 10.1371/journal.ppat.1002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Echalier G. [Multiplication of an arbovirus, Sindbis, in the body of an experimental “vector”, the mosquito Aedes aegypti (L.)] C R Acad Sci Hebd Seances Acad Sci D. 1965;261(8):1920–1922. [PubMed] [Google Scholar]
- 55.Pridgeon JW, et al. Topically applied AaeIAP1 double-stranded RNA kills female adults of Aedes aegypti. J Med Entomol. 2008;45(3):414–420. doi: 10.1603/0022-2585(2008)45[414:taadrk]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 56.O'Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors: A laboratory manual. W. H. Freeman and Company; New York: 1992. [Google Scholar]
- 57.Myles KM, Pierro DJ, Olson KE. Deletions in the putative cell receptor-binding domain of Sindbis virus strain MRE16 E2 glycoprotein reduce midgut infectivity in Aedes aegypti. J Virol. 2003;77(16):8872–8881. doi: 10.1128/JVI.77.16.8872-8881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edgar RC. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, et al. 1000 Genome Project Data Processing Subgroup The sequence alignment/map (SAM) format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Neill K. PhD dissertation. Kansas State University; Manhattan, KS: 2013. The role of apoptotic factors in Sindbis virus infection and replication in the mosquito vector Aedes aegypti. Available at hdl.handle.net/2097/15378. Accessed January 12, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.