Significance
Thiazolyl peptides are known antibiotics produced by diverse bacterial taxa. It has been believed that antibiotics are deployed by bacteria as weapons, providing them with an evolutionary advantage over other microbes. We show here that these weapons can also act as chemical tools that elicit biofilm production in the model bacterium Bacillus subtilis. Importantly, the biofilm-inducing (and therefore signaling) properties of these compounds are independent of their killing activity. We go on to use this biofilm-inducing activity to identify and confirm the presence of thiazolyl peptide gene clusters in other bacteria. These results indicate that thiazolyl peptides, and potentially other antibiotics, have the ability to alter bacterial behavior in ways important both to the environment and to human health.
Keywords: biofilm formation, Bacillus subtilis, Bacillus cereus, thiopeptides, thiazolyl antibiotics
Abstract
Bacteria have evolved the ability to produce a wide range of structurally complex natural products historically called “secondary” metabolites. Although some of these compounds have been identified as bacterial communication cues, more frequently natural products are scrutinized for antibiotic activities that are relevant to human health. However, there has been little regard for how these compounds might otherwise impact the physiology of neighboring microbes present in complex communities. Bacillus cereus secretes molecules that activate expression of biofilm genes in Bacillus subtilis. Here, we use imaging mass spectrometry to identify the thiocillins, a group of thiazolyl peptide antibiotics, as biofilm matrix-inducing compounds produced by B. cereus. We found that thiocillin increased the population of matrix-producing B. subtilis cells and that this activity could be abolished by multiple structural alterations. Importantly, a mutation that eliminated thiocillin’s antibiotic activity did not affect its ability to induce biofilm gene expression in B. subtilis. We go on to show that biofilm induction appears to be a general phenomenon of multiple structurally diverse thiazolyl peptides and use this activity to confirm the presence of thiazolyl peptide gene clusters in other bacterial species. Our results indicate that the roles of secondary metabolites initially identified as antibiotics may have more complex effects—acting not only as killing agents, but also as specific modulators of microbial cellular phenotypes.
Natural-product antibiotics have proven to be exquisite tools for combating infectious disease. Increasingly, a more elaborate role is being realized for these molecules formerly called “secondary” metabolites: as key signaling molecules for interspecies and intraspecies bacterial communication (1–7). Antibiotics at subinhibitory concentrations can trigger nonlethal physiological responses in human pathogens, including quorum sensing, virulence factor production, or biofilm formation (8–12). In some cases this activity has been tentatively linked to the mechanism of antibiosis of the small molecule. For example, quorum-sensing induction by the aminoglycoside tobramycin arises from sublethal inhibition of translation by RhlI/R system components (13).
More broadly, many natural-product antibiotics trigger biofilm formation, which has been interpreted as evidence that forming biofilms might be a general mechanism of defense against competitors (14). Biofilms are communities of bacterial cells living in a sticky, self-produced extracellular matrix on either a liquid or solid surface (15). The formation of bacterial biofilms can be both beneficial—such as on plant roots (16–18) or in wastewater treatment plants (19)—or detrimental—such as on in-dwelling medical devices or during infection (20, 21). Thus, understanding the chemical signals that induce and inhibit biofilm formation in bacteria has broad relevance.
The ability to form biofilms, like many bacterial phenotypes, is a result of bacteria differentiating into transcriptionally distinct cell types (22–24). Bacillus subtilis is a model organism whose cellular differentiation capabilities have been well characterized (23). Other members of the genus Bacillus can stimulate B. subtilis to differentiate into biofilm-matrix–producing cells (25). We monitored these interactions using a fluorescent transcriptional reporter for matrix gene expression. This reporter (PtapA–yfp) consists of PtapA [the promoter for the tapA operon (TasA anchoring/assembly protein A) that encodes TasA, the major protein component of B. subtilis biofilm matrix (26)], driving production of a yfp gene (encoding yellow fluorescent protein, YFP). Thus, when these B. subtilis cells are producing biofilm matrix, they also produce YFP. In particular, members of the Bacillus genus elicited B. subtilis biofilm formation via two distinct mechanisms (25). Some induced the matrix reporter via the sensor histidine kinase kinD (which activates the master transcriptional regulator for biofilm formation, Spo0A); others induced the matrix reporter through a separate Spo0A-dependent mechanism (25). This latter activity was linked to putative secreted metabolites present in conditioned medium that also had antibiotic activity (25).
Here, we pursue the identification of one of the biofilm-inducing metabolites secreted by Bacillus cereus ATCC 14579 that induced matrix formation in B. subtilis via both mechanisms (25). Using matrix-assisted laser desorption/ionization time-of-flight imaging mass spectrometry (MALDI-TOF IMS), we identified the thiocillins, members of the thiazolyl peptide class of natural products, as B. cereus-produced compounds that trigger biofilm formation in B. subtilis in a kinD-independent manner. The thiocillins are ribosomally encoded, posttranslationally modified peptide antibiotics that exert their activity by interfering with the interaction between the 23S rRNA and the protein L11 of the 50S ribosome (27, 28). The biosynthesis genes for the thiocillins have recently been elucidated (29), leading to the identification of related thiazolyl biosynthesis clusters in the genomes of diverse bacterial taxa. We show, using purified compounds and genetically modified variants of B. cereus, that the kinD-independent matrix-inducing activity of the thiocillins is independent of the antibiotic activity for which they are known. We further examine the structure–activity relationship of the thiocillins’ matrix-induction ability, and use this activity to identify putative thiazolyl peptide gene clusters in other Bacillus species.
Results
Using IMS to Connect Phenotype to Chemotype.
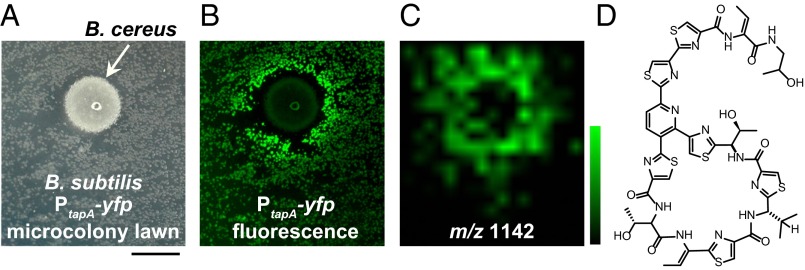
We indirectly monitored the response of B. subtilis to environmental signals that activated biofilm matrix production using the PtapA–yfp transcriptional fluorescent reporter strain. We used this strain to examine the consequences of coculturing B. subtilis with B. cereus under conditions in which B. subtilis does not normally produce biofilms (Fig. 1A). The B. subtilis colonies closest to B. cereus fluoresced, indicating that tapA gene expression is activated in these cells (Fig. 1B; ref. 25). We hypothesized that this biofilm activation was due to compounds secreted by B. cereus that diffused through the agar of the assay plate and altered the cellular physiology of B. subtilis.
Fig. 1.
IMS used to identify a matrix-inducing compound produced by B. cereus. (A) B. cereus grown as a colony on a microcolony lawn of B. subtilis. (Scale bar: 0.5 cm.) (B) This B. subtilis strain contains a fluorescent transcriptional reporter for biofilm matrix gene expression (PtapA–yfp); the B. subtilis colonies closest to B. cereus are fluorescing, indicating that matrix gene expression is activated. (C) IMS data showing the distribution of an ion with m/z = 1,142 (linear negative mode) that corresponds to the area of fluorescence observed in B; relative scale is from 0.7 to 1.8. (D) Structure of micrococcin P1, a thiazolyl peptide antibiotic produced by B. cereus with a molecular weight consistent with it being the ion observed in C.
The striking visual distribution of this coculture interaction inspired us to use MALDI-TOF IMS to connect this fluorescent signal with the molecular cue(s) responsible for inducing it. MALDI-TOF IMS involves collecting numerous mass spectra in a grid-like pattern across 2D samples, which can then be used to correlate chemical ion distributions with spatial biological phenotypes (30, 31). From the IMS data collected, we looked for ions whose spatial distributions corresponded to the region where the fluorescent signal for matrix was activated. One of these was an ion with m/z = 1,142, identified in linear negative mode (Fig. 1C and Fig. S1A). When we searched for known compounds produced by B. cereus with this molecular weight, we identified a variant of thiocillin, micrococcin P1 [M-H]− (Fig. 1D; ref. 29) as a potential match, suggesting that the thiocillins may be the cue produced by B. cereus that induces matrix production and PtapA–yfp fluorescence in B. subtilis. During the normal expression of the tcl biosynthesis cluster in B. cereus, a number of structural variants of thiocillin of different molecular weight are produced (29); we observed peaks corresponding to the masses of many of these structural variants, supporting our hypothesis that these ions observed in our IMS data may represent the thiocillins; we observed m/z = 1,141 micrococcin P2, [M-H]−), 1,155 (thiocillin 3, [M-H]−), 1,181 (thiocillin 1, [M-H+Na]−), 1,193 (YM-266184, [M-H+Na]−), and 1,195 (thiocillin 2, [M-H+Na]−) (Fig. S1B). We will refer to the thiocillins by their generic name here, unless using a specific purified variant.
Thiocillins Are Partially Responsible for B. cereus’ Ability to Induce B. subtilis Biofilm Matrix.
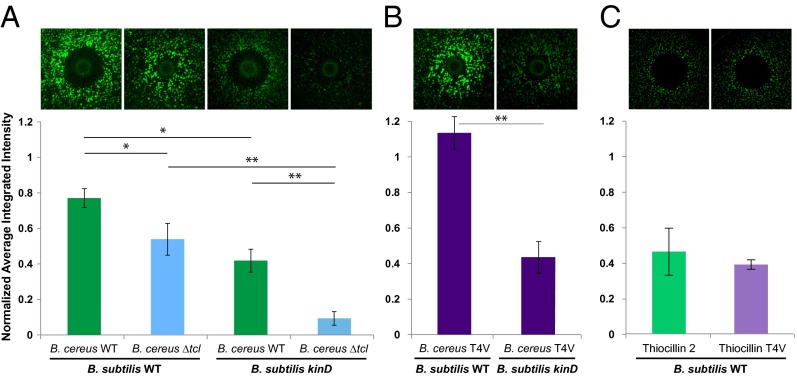
Using a mutant of B. cereus that does not contain the structural genes required to produce the thiocillins (ΔtclE-H) (32), we examined the ability of wild-type (WT) and ΔtclE-H B. cereus to induce PtapA-yfp expression in B. subtilis (Fig. 2A). Both the visual fluorescence of these interactions and their quantification indicate that the thiocillins are responsible for approximately half of the matrix-inducing ability of B. cereus (Fig. 2A, Left).
Fig. 2.
Thiocillin contributes to the ability of B. cereus to induce PtapA–yfp gene expression in B. subtilis in a kinD- and antibiotic-independent manner. (A) Colonies of WT and ΔtclE-H B. cereus spotted onto a lawn of WT or kinD B. subtilis PtapA–yfp microcolonies and quantification of the fluorescence (n = 3). (B) Colonies of the thiocillin T4V mutant of B. cereus spotted onto the same B. subtilis lawns, as well as quantification of the fluorescence (n = 3). (C) A total of 450 ng of purified thiocillin or T4V thiocillin spotted onto a lawn of WT B. subtilis PtapA–yfp microcolonies and quantification of the fluorescence (n = 3). The halos visible in C are not due to B. subtilis cells dying, but by being physically moved during spotting of the compound. *P < 0.1; **P < 0.05.
B. cereus uses two distinct mechanisms to induce PtapA-yfp fluorescence in B. subtilis: One requires the sensor histidine kinase KinD, and the other correlates with an antibiotic activity. We previously proposed that this latter mechanism increases biofilm matrix production via a cell-type-specific killing of non-matrix-producing cells, thus increasing the subpopulation of matrix producers (25). To investigate which of these two B. cereus activities thiocillin might be responsible for, we examined the ability of these B. cereus strains to induce PtapA-yfp expression in a B. subtilis kinD strain. These data (Fig. 2A, Right) show that the B. cereus WT–B. subtilis kinD interaction has approximately half of the overall fluorescence observed in the B. cereus WT–B. subtilis WT interaction. This remaining signal is thus attributable to the activity produced by B. cereus that is acting via the kinD-independent pathway; it is virtually eliminated in the B. cereus ΔtclE-H–B. subtilis kinD interaction (Fig. 2A), indicating that the thiocillins are responsible for the kinD-independent activation of matrix induction in B. subtilis.
The Matrix-Inducing Activity of the Thiocillins Is Separable from Their Antibiotic Activity.
To test whether the thiocillins were increasing PtapA–yfp fluorescence in B. subtilis by specifically killing non-matrix-producing cells, we examined the activity of a B. cereus strain engineered to produce thiocillins containing a T4V mutation (Fig. 2B); T4V thiocillin has no antibiotic activity against B. subtilis (32) (Fig. S2). Remarkably, when we grew this B. cereus strain in our matrix-induction assay, it induced PtapA–yfp fluorescence in B. subtilis to the same extent as WT B. cereus (Fig. 2 A and B). B cereus producing the T4V thiocillin variant also induced changes in B. subtilis colony morphology, where wrinkling correlates with biofilm production (Fig. S2). This finding was true for both the WT and kinD B. subtilis interaction (Fig. 2B). These results indicate that T4V thiocillin induces both PtapA gene expression and changes in colony morphology associated with biofilm formation, and thus that the antibiotic activity of the thiocillins are separable from their matrix-inducing activity.
Purified Thiocillin Increases the Proportion of Matrix-Producing B. subtilis Cells.
To determine whether purified thiocillin has the ability to activate matrix gene expression in B. subtilis, we examined its effect on tapA gene expression. We first spotted 450 ng of purified YM-266183 or T4V thiocillin (Fig. S3) on microcolony lawns of B. subtilis PtapA–yfp and quantified the resulting fluorescence (Fig. 2C). Both the antibiotically active and antibiotically dead versions of the thiocillins induced PtapA–yfp expression to the same extent (Fig. 2C).
Our T4V data indicated that the antibiotic activity of thiocillin was not necessary for PtapA–yfp matrix induction in this structural mutant. To explore whether the antibiotic activity was also unnecessary for matrix induction by the native thiocillins, we determined thiocillin’s minimal inhibitory concentration (MIC). To do this assessment, we grew B. subtilis in liquid shaking culture in non-matrix-inducing growth medium [Lennox Luria broth (LB)] in the presence of purified YM-266183 or DMSO (as a control) and measured the OD600 absorbance over time. MICs are typically determined as an endpoint after overnight growth in varying concentrations of the antibiotic. We noted that this MIC concentration (50 nM) was significantly higher than the concentration at which B. subtilis showed no growth inhibition at any point during growth (3.125 nM) (Fig. S4).
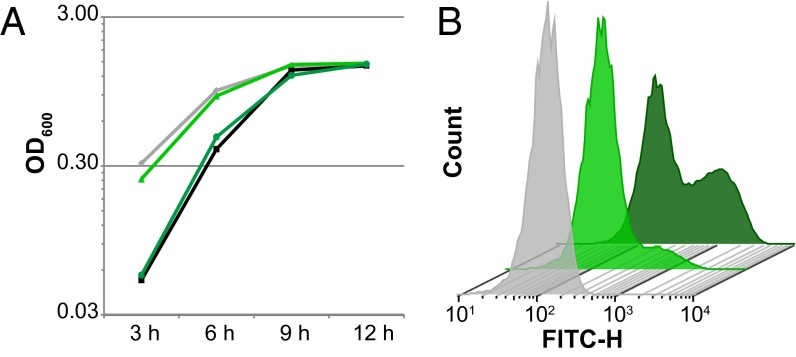
We selected a concentration intermediate between these two concentrations (12.5 nM), and selected a time point (9 h) at which there was no difference in the OD600 of the DMSO- or thiocillin-treated cultures (Fig. 3A). At this time point, we fixed the cells with paraformaldehyde and measured the fluorescence of individual cells using flow cytometry. When B. subtilis PtapA–yfp cells were grown in the presence of DMSO, only a small percentage of the population was fluorescent (11%), whereas a much larger percentage of the population (36–41%) expressed PtapA–yfp when the cells were grown with YM-266183 (Fig. 3B). Interestingly, even at earlier time points (4 h), when B. subtilis growth is being inhibited, the percentage of the population that was expressing matrix was still higher in the thiocillin-treated cells than in those treated with DMSO (56% vs. 34%) (Fig. S4C). Thus, purified thiocillin has the ability to increase the proportion of matrix-producing cells when added to B. subtilis liquid cultures, irrespective of its antibiotic activity.
Fig. 3.
Purified thiocillin increases the proportion of matrix-producing B. subtilis cells in liquid culture, even when not inhibiting growth. (A) Growth curves of B. subtilis from growth in shaking liquid culture with 12.5 nM YM-266183 or an equivalent volume of DMSO (gray diamonds, WT with DMSO; black squares, WT with YM-266183; light green triangles, PtapA–yfp with DMSO; dark green circles, PtapA–yfp with YM-266183). (B) Flow cytometry of the fluorescence intensity of B. subtilis cells harvested from the 9-h time point from A (front, WT cells with DMSO; middle, PtapA–yfp cells with DMSO; back, PtapA–yfp cells with YM-266183). A total of 30,000 cells were quantified for each sample.
Matrix Induction Is a General Property of Thiazolyl Peptides.
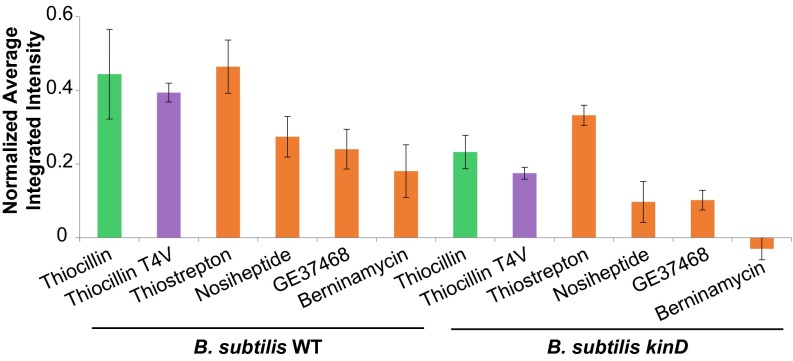
The thiocillins are ribosomally synthesized and posttranslationally modified peptides (RiPPs) in the class of bacteriocins known as thiazolyl peptides (33). We were therefore curious whether the biofilm-inducing activity of thiocillin was a generic property of this class of metabolites. We purified a range of structurally and functionally diverse thiazolyl peptides (thiostrepton, nosiheptide, berninamycin, and GE37468; Fig. S5), and tested whether they influenced PtapA–yfp expression in B. subtilis. We spotted 450 ng of these purified compounds (comparable to the amount of thiocillin used in Fig. 2C) onto B. subtilis WT and kinD lawns and quantified the resulting fluorescence (Fig. 4). All of these thiazolyl peptides induced PtapA–yfp expression. There was no significant difference between the fluorescence induction of any individual thiopeptide on the WT or kinD B. subtilis lawns (P > 0.1), indicating that, similar to thiocillin, all of these thiopeptides activate biofilm gene expression in a kinD-independent manner. Both thiostrepton and berninamycin demonstrated antibiotic activity at these concentrations. These data and those from Fig. 2 and 3 demonstrate that thiazolyl peptides possess structural or functional features that allow them to increase the number of matrix-gene–expressing cells in B. subtilis in a kinD-independent manner, regardless of their antibiotic activity.
Fig. 4.
Purified thiazolyl peptides induce PtapA–yfp gene expression in B. subtilis. Quantification of fluorescence intensity resulting from spotting 450 ng of the purified thiazolyl peptides onto a lawn of B. subtilis PtapA–yfp microcolonies (n = 3–13) is shown. There is no significant difference in PtapA–yfp fluorescence induction between the B. subtilis WT and kinD lawns.
Structure–Function Specificity of Thiocillin’s Activity.
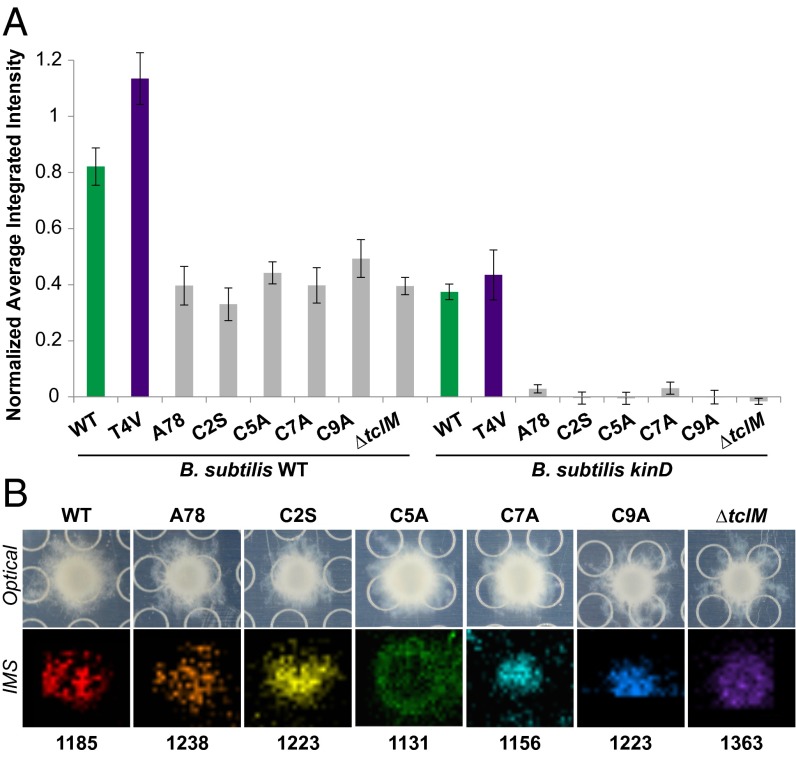
We next investigated the specificity of thiocillin’s activity. We began by examining the matrix-inducing activity of structural mutants of thiocillin, including those with disrupted thiazolyl rings (C2A, C5A, C7A, and C9A), those with a larger ring (A78), and those unable to form a ring (ΔtclM) (refs. 34 and 35 and Figs. S3 and S6). In all cases, when we spotted B. cereus strains engineered to produce these variants on a B. subtilis microcolony lawn, we observed that they induced B. subtilis PtapA–yfp expression to approximately half of the level that WT B. cereus did and that they had essentially no effect on PtapA–yfp expression in a lawn of kinD B. subtilis (Fig. 5A). These data are similar to those observed from the B. cereus ΔtclE-H mutant, which is unable to produce any of the thiocillins, suggesting that none of these structural variants are able to induce matrix expression in B. subtilis.
Fig. 5.
Structural modifications to thiocillin abolish its matrix-induction activity. (A) Quantification of fluorescence intensity of thiocillin mutant producers spotted onto a lawn of B. subtilis WT (Left) or kinD PtapA–yfp (Right) microcolonies (n = 3). None of the mutants (gray bars) were significantly different from one another on either the WT or kinD B. subtilis lawns, but each were significantly different from both WT and T4V B. cereus (by at least P < 0.05 on WT and at least P < 0.0001 on kinD). (B) Images of B. cereus colonies producing the mutant thiocillins indicated grown on agar on a MALDI plate with corresponding IMS data; colors represent ions of m/z indicated: WT [M+H+Na]+; A78 [M+H+Na]+; C2S [M+Na+K]+; C5A [M+H]+; C7A [M+H+Na]+; C9A [M+H+Na+K]+; ∆tclM [M+H]+.
To confirm that these strains were expressing these thiocillin variants, we used IMS to detect their expression in vivo. We grew colonies of the various B. cereus strains on 0.1× LB medium and assayed for the presence of the masses expected for the different thiocillin variants; each strain produced at least one compound of an expected mass (Fig. 5B). These data indicate that, although disrupting the antibiotic activity of thiocillin does not alter its ability to induce PtapA–yfp gene expression in B. subtilis, multiple other structural alterations appear to abolish its ability to induce biofilm gene expression in B. subtilis.
By quantifying the PtapA–yfp gene expression of B. subtilis in response to the naturally produced thiocillin variants YM-266183 and thiocillin 2, we observed that even minor differences in the thiocillins’ structure (Fig. S6) led to differences in their ability to induce biofilm in B. subtilis as purified compounds (Fig. S7A). We also observed that, although the matrix-induction ability of YM-266183 was strong at 450 ng, it showed a rapid, dose-dependent decrease in activity when diluted (Fig. S7A). Based on our IMS data, B. cereus appears to produce numerous thiocillin variants such as the two tested here (Fig. S1B); whether these compounds act in a synergistic or additive manner is unknown.
Bacteria with Cryptic Thiazolyl Biosynthesis Genes Induce Matrix in B. subtilis.
Other Bacillus species also have the ability to activate PtapA–yfp expression via an antibiotic-associated, kinD-independent activity (25). Here we quantified the PtapA–yfp fluorescence induced by these strains in kinD B. subtilis and compared it to the fluorescence they causes in WT B. subtilis (Fig. S7B). These data indicate that three of these strains induce PtapA–yfp fluorescence in B. subtilis via an entirely kinD-independent mechanism (Bacillus atrophaeus, Bacillus vallismortis, and Bacillus mojavensis); unfortunately, there are only partial genome sequences available for these strains, making it impossible to determine whether they possess cryptic thiazolyl biosynthesis genes that could be responsible for this activity. However, we used the sequences of the biosynthesis genes required for thiazolyl production [the lantibiotic dehydratase genes (shown in red in Fig. 6A)] (29), as well as the program antiSMASH (36) to search all available Bacillus bacterial genomes to identify strains containing cryptic thiazolyl peptides. We identified 10 Bacillus strains that contained these putative biosynthetic gene clusters, consistent with being capable of producing thiazolyl peptides (37). In addition to the lantibiotic dehydratase genes, these gene clusters included a YcaO homolog (a cyclodehydratase believed to catalyze production of thiopeptides’ thaizoles and oxazoles from cysteines and serines) and one to five copies of a putative structural gene, as well as other transporters and potential modification enzymes. Two of these bacterial strains were publicly available, and we tested them for their ability to induce PtapA–yfp gene expression in WT and kinD B. subtilis (Fig. 6B and Fig. S8). B. atrophaeus 1942 and Bacillus sp. 107 both induce matrix gene expression at levels comparable to B. cereus WT, but only B. atrophaeus 1942 does so in a kinD-independent manner (Fig. 6B). Interestingly, the biosynthetic gene cluster of Bacillus sp. 107 appears unique in that it does not contain a dehydrogenase; it is possible that it does not produce a typical thiazolyl peptide. Our data suggest that numerous Bacilli can induce expression of the PtapA–yfp biofilm reporter in B. subtilis via a kinD-independent manner and that some of these bacteria also contain putative thiazolyl-producing biosynthetic gene clusters.
Fig. 6.
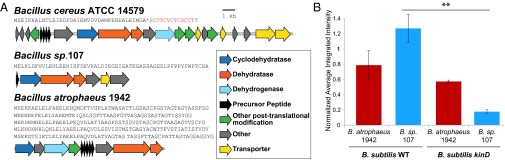
Bacterial strains containing cryptic thiazolyl peptide biosynthesis genes induce PtapA–yfp gene expression in B. subtilis. (A) Gene clusters comparing enzyme and peptide sequences for B. cereus, B. atrophaeus, and Bacillus sp. 107. The known precursor peptide sequence is shown in red, with the cleavage site marked with an asterisk. (B) Quantification of the fluorescence intensity induced by WT B. cereus, B. atrophaeus 1942, and Bacillus sp. 107 in WT and kinD B. subtilis microcolony lawns (n = 3). Bacillus sp. 107 induction of PtapA–yfp WT and kinD B. subtilis lawns are significantly different (P = 0.0094), but B. atrophaeus 1942 induction in these two strains is not significantly different, indicating a kinD-independent mechanism of induction. **P < 0.01.
Discussion
Antibiotics have frequently been considered merely weapons in a chemical arms race between soil microbes. Here we show that some of these killing agents also act as interspecies signals, altering bacterial gene expression and thus the development of their neighboring microbes. Specifically, we used a combination of coculture, fluorescent reporter assays, and MALDI-TOF IMS to identify and characterize the thiocillins—thiazolyl peptide natural products produced by B. cereus—as inducers of biofilm-matrix gene expression in B. subtilis. Antibiotic-mediated biofilm formation is not a new phenomenon, but in many instances, it has been thought to be a nonspecific response to antibiotic challenge (i.e., stress) that confers increased resistance. Indeed, we initially proposed cell-type–specific killing as a mechanism for the kinase-independent interaction between B. subtilis and B. cereus (25). However, using the T4V mutant of thiocillin, here we showed that thiocillin’s biofilm-activation activity can be divorced from its antibiotic activity.
This and previous work (25) indicate that B. cereus and the thiocillins activate matrix gene expression independently of the five sensor histidine kinases (KinA–KinE) known to control the activity of the master transcriptional regulator Spo0A in B. subtilis. Thus, thiocillins do not appear to fall into the class of small molecules that affect potassium ion concentration (like the lipopeptide surfactin), which activate matrix through a KinC-dependent pathway (38). We also found that thiocillin mutants with significant disruptions to the thiazolyl peptide backbone and major macrocycle were no longer biofilm-active, whereas structurally diverse thiazolyl peptides from several different bacterial species all induced matrix production in B. subtilis. These data suggest that biofilm induction may be generalizable to thiazolyl peptides as a class and that the potential sensor for these molecules may be capable of responding to general structural features of this diverse family of compounds. An example of a receptor for such largely varied structures is provided by the thiostrepton-inducible protein (Tip) promoter system, found in Streptomyces and Rhodococcus species, which is capable of recognizing and binding to thiazolyl peptides of diverse structures and highly up-regulating the expression of its own and other genes (39, 40). At present, no homolog to the Tip transcription factors could be identified in the genome of B. subtilis NCIB3610, but analogous receptors could exist in B. subtilis.
Thiazolyl peptides are a growing family of natural products, with new putative biosynthetic gene clusters being discovered in diverse bacterial taxa. Historically, many thiazolyl peptides have been isolated from common soil microbes such as Streptomyces or Bacillus species. Putative thiazolyl-like biosynthesis genes are present in ∼4.5% of the currently available Bacillus genome sequences (10 of 221 genomes), and ∼39% of the available Streptomyces genomes (35 of 89 genomes), as determined by antiSMASH (36). Putative thiazolyl-like biosynthetic gene clusters have also been identified in human skin- and gut-associated microbes like Propionibacterium acnes and Lactobacillus gasseri (29), as well as in other bacterial taxa isolated from a variety of human body sites (41). Thus, the biosynthetic capacity to generate thiazolyl peptides is not limited to a single bacterial genera or environmental niche. It is possible that, similar to other classes of microbial metabolites, thiazolyl peptides may act as signaling cues within and among these bacterial taxa. We explored this possibility by showing that two other Bacillus species (harboring related biosynthesis clusters for as-yet-uncharacterized thiazolyl peptides) were able to activate matrix gene expression when cocultured with B. subtilis, although only one did so in a kinD-independent manner. All of the purified thiazolyl peptides tested in our assay (thiostrepton, nosiheptide, berninamycin, and GE37468; all produced by Streptomyces) were able to activate the PtapA–yfp fluorescent reporter in B. subtilis to varying degrees, and they did so in a kinD-independent manner. Together, these data indicate that thiazolyl peptides can activate biofilm matrix gene expression and thus may mediate bacterial interspecies interactions.
How the two activities of the thiazolyl peptides (antibiosis and matrix induction) might interact to shape the distribution, growth, and development of bacteria in interspecies communities in native-like environments is still unresolved. Future experiments using reconstituted synthetic systems or natural environments seeded with specific microbes should allow us to evaluate the effects of the WT or antibiotic-null versions of the thiocillins. Our present data indicate that thiocillin has the ability to increase biofilm formation in B. subtilis and that the killing and bacterial differentiation activity of this antibiotic can be structurally distinguished. Whether other secondary metabolites produced by microbes (many of which are widely used as therapeutic antibiotics) possess bioactivities in addition to antibiosis is an intriguing question for future studies. However, the structural complexity of many secondary metabolites makes it conceivable that they may similarly exert more than one biological effect. Our work indicates that at least one class of secondary metabolites—the thiazolyl peptides—is able to induce biofilm differentiation in B. subtilis using a mechanism that is independent of antibiosis.
Materials and Methods
Strains.
Strains used in this work are listed in Table S1. The B. subtilis strain was NCBI3610 from our laboratory collection. The B. cereus strain 14579 was obtained from ATCC. Mutant B. cereus strains were constructed as in ref. 32.
Culture Conditions and Matrix Induction Assay.
Bacterial cultures were grown and microcolony lawn assays were performed as in ref. 25. Briefly, B. subtilis colonies were spread on dilute agar medium to form microcolony lawns, and B. cereus was spotted onto them for coculture growth. See SI Materials and Methods for details.
Fluorescence Quantification.
Typhoon data files were loaded into Metamorph (Version 7.1), and brightness and contrast were linearly adjusted. Quantification was performed as in ref. 25, except that thresholding was not used. See SI Materials and Methods for details.
Flow Cytometry.
B. subtilis liquid cultures with DMSO or Thiocillin (final = 12.5 nM) added were grown at 37 °C with shaking. Cells were fixed in 4% (wt/vol) paraformaldehyde, sonicated, and stored at 4 °C until analyzed by flow cytometry. See SI Materials and Methods for details.
MICs and Growth Curves.
B. subtilis cultures were initiated by diluting a midlog culture to OD600 = 0.004 in 1 mL of LB in 24-well plates (Falcon). Equivalent volumes of DMSO or thiopeptides were added to separate wells, and the plates were covered with Aeroseals and grown at 37 °C with shaking. OD600 measurements were taken by using a Tecan GENios plate reader.
IMS.
IMS was performed as described in ref. 31. Briefly, agar coculture samples were placed onto MALDI-TOF target plates and dried after being coated with Universal MALDI matrix. Mass spectra were collected from ∼600-μm pixels across the entire sample, and the distributions of masses were visualized by using false-color images. See SI Materials and Methods for details.
LC-MS.
LC-MS data were acquired on an Agilent 6520 Accurate-Mass Q-TOF mass spectrometer with an electrospray-ionization source in positive ion mode. The drying gas temperature was 350 °C, and the fragmentor voltage was 250 V. The thiazolyl peptides were separated by using a reverse-phase kinetex column; acetonitrile with 0.1% formic acid was run as a gradient from 2% to 100% over 15 min and held at 100% for 2 min against water with 0.1% formic acid.
Thiocillin Purification and Concentrations.
Thiocillin, its mutants, and other thiazolyl peptides were all purified as reported in ref. 32. Purified thiazolyl peptides were maintained as stocks at a concentration of 250 ng/µL in DMSO. For matrix induction assays, 1.8 µL (containing 450 ng) was spotted onto a dried plate freshly inoculated with a B. subtilis reporter microcolony lawn.
Statistics.
All P values were calculated with a Tukey’s honest significant difference pairwise analysis using JMP software.
Supplementary Material
Acknowledgments
We sincerely thank Roberto Kolter [Harvard Medical School (HMS)], in whose laboratory this work was initiated. We thank Hera Vlamakis (HMS) for insightful manuscript suggestions and Kirk Grubbs [University of North Carolina Chapel Hill (UNC-CH)] for assistance with the bioinformatic analysis. E.A.S. was supported by UNC-CH Start-Up Funds. R.B. was supported by a UNC-CH Royster Society Fellowship. Part of this work was supported by NIH Grants GM094802, AI095125, and S10RR029121 (to P.C.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414272112/-/DCSupplemental.
References
- 1.Romero D, Traxler MF, López D, Kolter R. Antibiotics as signal molecules. Chem Rev. 2011;111(9):5492–5505. doi: 10.1021/cr2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straight PD, Kolter R. Interspecies chemical communication in bacterial development. Annu Rev Microbiol. 2009;63:99–118. doi: 10.1146/annurev.micro.091208.073248. [DOI] [PubMed] [Google Scholar]
- 3.Ryan RP, Dow JM. Diffusible signals and interspecies communication in bacteria. Microbiology. 2008;154(Pt 7):1845–1858. doi: 10.1099/mic.0.2008/017871-0. [DOI] [PubMed] [Google Scholar]
- 4.Fajardo A, Martínez JL. Antibiotics as signals that trigger specific bacterial responses. Curr Opin Microbiol. 2008;11(2):161–167. doi: 10.1016/j.mib.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 2007;153(Pt 12):3923–3938. doi: 10.1099/mic.0.2007/012856-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, et al. Angucyclines as signals modulate the behaviors of Streptomyces coelicolor. Proc Natl Acad Sci USA. 2014;111(15):5688–5693. doi: 10.1073/pnas.1324253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311(5764):1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies J. Everything depends on everything else. Clin Microbiol Infect. 2009;15(Suppl 1):1–4. doi: 10.1111/j.1469-0691.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- 9.Davies J, Spiegelman GB, Yim G. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol. 2006;9(5):445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Seyedsayamdost MR. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc Natl Acad Sci USA. 2014;111(20):7266–7271. doi: 10.1073/pnas.1400019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Li L, Dong Z, Surette MG, Duan K. The YebC family protein PA0964 negatively regulates the Pseudomonas aeruginosa quinolone signal system and pyocyanin production. J Bacteriol. 2008;190(18):6217–6227. doi: 10.1128/JB.00428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh EB, et al. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Natl Acad Sci USA. 2002;99(26):17025–17030. doi: 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babić F, Venturi V, Maravić-Vlahovicek G. Tobramycin at subinhibitory concentration inhibits the RhlI/R quorum sensing system in a Pseudomonas aeruginosa environmental isolate. BMC Infect Dis. 2010;10:148. doi: 10.1186/1471-2334-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 15.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 16.Danhorn T, Fuqua C. Biofilm formation by plant-associated bacteria. Annu Rev Microbiol. 2007;61:401–422. doi: 10.1146/annurev.micro.61.080706.093316. [DOI] [PubMed] [Google Scholar]
- 17.Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci USA. 2013;110(17):E1621–E1630. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, et al. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol. 2013;15(3):848–864. doi: 10.1111/j.1462-2920.2012.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrout JD, Nerenberg R. Monitoring bacterial twitter: Does quorum sensing determine the behavior of water and wastewater treatment biofilms? Environ Sci Technol. 2012;46(4):1995–2005. doi: 10.1021/es203933h. [DOI] [PubMed] [Google Scholar]
- 20.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 21.Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008;100(1):1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6(3):199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 23.Lopez D, Vlamakis H, Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol Rev. 2009;33(1):152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 24.Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;67(2):254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shank EA, et al. Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proc Natl Acad Sci USA. 2011;108(48):E1236–E1243. doi: 10.1073/pnas.1103630108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59(4):1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 27.Cameron DM, Thompson J, March PE, Dahlberg AE. Initiation factor IF2, thiostrepton and micrococcin prevent the binding of elongation factor G to the Escherichia coli ribosome. J Mol Biol. 2002;319(1):27–35. doi: 10.1016/S0022-2836(02)00235-8. [DOI] [PubMed] [Google Scholar]
- 28.Harms JM, et al. Translational regulation via L11: Molecular switches on the ribosome turned on and off by thiostrepton and micrococcin. Mol Cell. 2008;30(1):26–38. doi: 10.1016/j.molcel.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Wieland Brown LC, Acker MG, Clardy J, Walsh CT, Fischbach MA. Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin. Proc Natl Acad Sci USA. 2009;106(8):2549–2553. doi: 10.1073/pnas.0900008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watrous JD, Dorrestein PC. Imaging mass spectrometry in microbiology. Nat Rev Microbiol. 2011;9(9):683–694. doi: 10.1038/nrmicro2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang JY, et al. Primer on agar-based microbial imaging mass spectrometry. J Bacteriol. 2012;194(22):6023–6028. doi: 10.1128/JB.00823-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acker MG, Bowers AA, Walsh CT. Generation of thiocillin variants by prepeptide gene replacement and in vivo processing by Bacillus cereus. J Am Chem Soc. 2009;131(48):17563–17565. doi: 10.1021/ja908777t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnison PG, et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30(1):108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowers AA, Acker MG, Koglin A, Walsh CT. Manipulation of thiocillin variants by prepeptide gene replacement: Structure, conformation, and activity of heterocycle substitution mutants. J Am Chem Soc. 2010;132(21):7519–7527. doi: 10.1021/ja102339q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowers AA, Walsh CT, Acker MG. Genetic interception and structural characterization of thiopeptide cyclization precursors from Bacillus cereus. J Am Chem Soc. 2010;132(35):12182–12184. doi: 10.1021/ja104524q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blin K, et al. antiSMASH 2.0—a versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res. 2013;41(Web Server issue):W204-12. doi: 10.1093/nar/gkt449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Liu W. Biosynthesis of thiopeptide antibiotics and their pathway engineering. Nat Prod Rep. 2013;30(2):218–226. doi: 10.1039/c2np20107k. [DOI] [PubMed] [Google Scholar]
- 38.López D, Fischbach MA, Chu F, Losick R, Kolter R. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci USA. 2009;106(1):280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami T, Holt TG, Thompson CJ. Thiostrepton-induced gene expression in Streptomyces lividans. J Bacteriol. 1989;171(3):1459–1466. doi: 10.1128/jb.171.3.1459-1466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong L, Nakashima N, Tamura N, Tamura T. Isolation and characterization of the Rhodococcus opacus thiostrepton-inducible genes tipAL and tipAS: Application for recombinant protein expression in Rhodococcus. FEMS Microbiol Lett. 2004;237(1):35–40. doi: 10.1016/j.femsle.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Donia MS, et al. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014;158(6):1402–1414. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.