Significance
Many proteins enter the amyloid state, which is associated with human diseases and is also involved in many biological events. Amyloid formed by various proteins has a uniform “cross-β” structure with protein units stacking repetitively into fibrils. This unique structure brings amyloid favorable mechanical and chemical properties, and inspires the exploration of amyloid as a novel class of bionanomaterials. On the other hand, the uniform fibrillar structure limits the application of amyloid materials for a diverse function. This paper illustrates our discovery, structure characterization, design, and application of an amyloid-based structure, termed “amyloid-like nanosheet.” This nanosheet enriches the architectures of amyloid materials and will aid researchers in designing and investigating amyloid with new functions.
Keywords: functional amyloid material, peptide self-assembly, nanosheet, retrovirus transduction, beta-amyloid
Abstract
Using and engineering amyloid as nanomaterials are blossoming trends in bionanotechnology. Here, we show our discovery of an amyloid structure, termed “amyloid-like nanosheet,” formed by a key amyloid-forming segment of Alzheimer’s Aβ. Combining multiple biophysical and computational approaches, we proposed a structural model for the nanosheet that is formed by stacking the amyloid fibril spines perpendicular to the fibril axis. We further used the nanosheet for laboratorial retroviral transduction enhancement and directly visualized the presence of virus on the nanosheet surface by electron microscopy. Furthermore, based on our structural model, we designed nanosheet-forming peptides with different functionalities, elucidating the potential of rational design for amyloid-based materials with novel architecture and function.
Numerous proteins and polypeptides have been found to self-assemble into amyloid fibrils under certain conditions (1). They are associated not only with dozens of devastating diseases including Alzheimer’s and Parkinson’s diseases (2) but are integral to many biological processes such as hormone storage, signal transduction, and cell surface adhesion (3–5). Separate from the context of their parent proteins, synthesized peptide segments can self-assemble into amyloid-like fibrils in vitro as well (6, 7). Fibrils formed by diverse proteins and peptides all share a common cross-β structure, composed of interdigitated β-sheets termed “the zipper-like fibril spine” (8, 9). The self-assembly process is a consequence of backbone hydrogen bonding for the single β-sheet layer formation and side-chain interaction (e.g., hydrophobic interaction, π-stacking, and van der Waals) for pairing β-sheet layers together (10). Their highly repetitive and ordered architecture, in particular for the short peptide fibrils, exhibits favorable properties including high thermal stability and stiffness, biocompatibility, controllable self-assembly, surface patterning and integration of functionality, and inexpensive production by chemical synthesis (11–13). These exceptional properties promote the exploitation of amyloid fibril as an emerging class of bionanomaterials (14).
Several studies have demonstrated that natural amyloidogenic and designed amphiphilic peptides are capable of self-assembling into nanostructures with topographies including fibril, film, nanotube, hydrogel, and liquid crystals (15–21), and these nanostructures have been used for nanowires, biosensors, 3D culturing, environmental carbon capture, retroviral gene transfer, light harvesting, and catalysis (22–26). Amyloid fibrils were also hybridized with other nanomaterials such as graphene and DNA origami in hopes of creating new properties and functions (27–29). In this study, we expand the amyloid material field’s scope by the finding, structure characterization, and functionalization of a previously unidentified architecture—the amyloid-like nanosheet. We showed that KLVFFAK, a key amyloid-forming heptapeptide of the Italian familial form of Alzheimer’s Aβ (30), self-assembles into a 2D nanosheet with a width of over 200 nm, far larger than typical fibrils (10–20 nm) (Fig. 1). We characterized the molecular structure of the nanosheet with both biophysical and computational approaches. The unique structural architecture of the nanosheet enables us to use it as a highly effective enhancer for retroviral transduction and to directly observe virus condensation on the nanosheet. Based on our structural model, we further designed two series of artificial peptides. One highlights the potential of the nanosheet as a robust platform for integrating different functionalities, and the other sheds light on the de novo design of amyloid-like nanosheets.
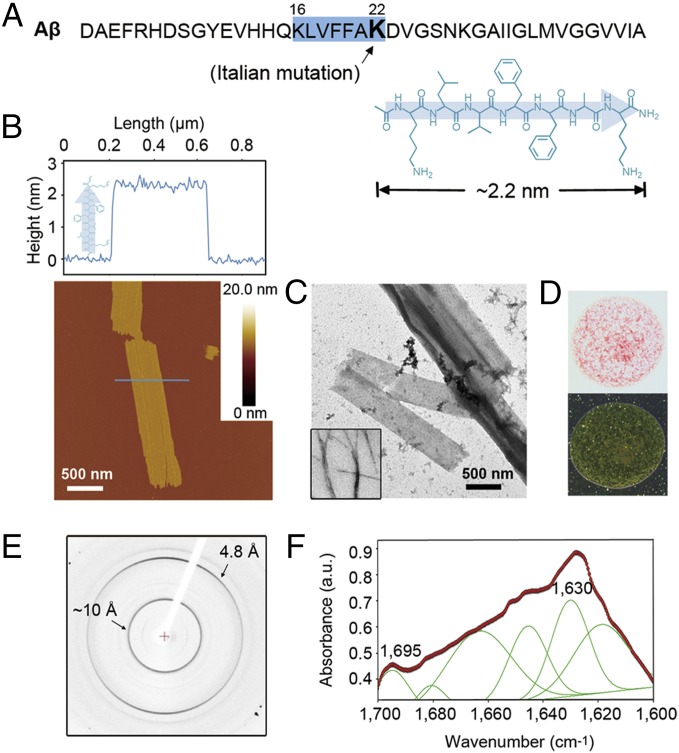
Fig. 1.
Characterization of the KLVFFAK nanosheet. (A) KLVFFAK is the key amyloid-forming segment of the Italian familial form of Aβ. Its chemical formation is shown in β-strand, and its length is ∼2.2 nm estimated from its structural model (SI Appendix, Materials and Methods). (B) AFM image (Lower) and section analysis (Upper) of the KLVFFAK nanosheet on mica. The thickness of the KLVFFAK nanosheet is about the length of the KLVFFAK β-strand, indicating an upright alignment of the peptide strands forming the nanosheet. (C) TEM images of the KLVFFAK nanosheet and a typical amyloid fibril of peptide VQIVYK (Inset). (D) Optical image (Upper) of the Congo red-stained nanosheet. It shows green birefringence when viewed between crossed polarizers (Lower). (E) X-ray diffraction image of nanosheet showing a cross-β diffraction pattern characteristically seen in fibrils (2). (F) FTIR profile indicating that the nanosheet is composed of antiparallel β-sheets.
Results
Characterization of the KLVFFAK Nanosheet.
We found that Italian familial Aβ16–22 (KLVFFAK) forms giant sheets in aqueous solution, distinct from typical long and unbranched fibrils formed by most amyloid-forming sequences (Fig. 1 B and C). The nanosheet exhibits a cross-β diffraction pattern from X-ray diffraction analysis (Fig. 1E), which is characteristic of amyloid fibrils (2), indicating the presence of the cross-β fibril spine (8). The thickness of the nanosheet was determined to be 2.3 ± 0.2 nm by atomic force microscopy (AFM) on mica and Si wafers under dry and hydrated conditions, respectively (Fig. 1B and SI Appendix, Figs. S1 and S2). Given the length of KLVFFAK is ∼2.2 nm, which was estimated from the length of an ideal β-strand (31, 32) and its structural model (SI Appendix, Materials and Methods), KLVFFAK stands upright, forming a monolayer nanosheet (Fig. 1 A and B). Circular dichroism (CD) spectroscopy showed a major content of antiparallel β-sheets in the nanosheet (SI Appendix, Fig. S3), and the Fourier transform infrared (FTIR) spectroscopic profile showed two peaks at around 1,630 cm−1 and 1,695 cm−1, also indicating the β-sheets are antiparallel (Fig. 1F). In addition, the nanosheet can bind Congo red and displays green birefringence when viewed between crossed polarizers (Fig. 1D). Taken together, despite its distinct morphology, the KLVFFAK nanosheet features most of the typical amyloid characteristics, and cross-β fibril spine is the building unit of the nanosheet.
Structural Modeling of the KLVFFAK Nanosheet.
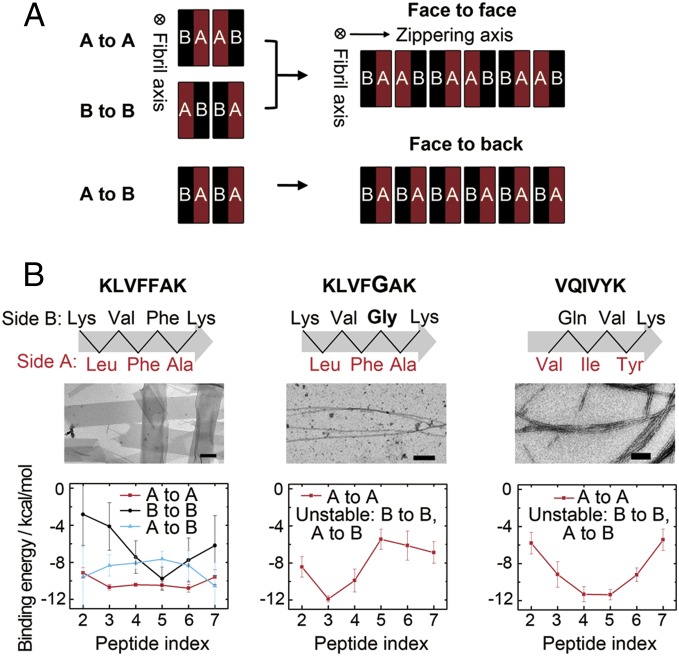
Based on the height of the KLVFFAK nanosheet measured by AFM (Fig. 1B) and the antiparallel β-sheet content characterized by CD and FTIR spectroscopy (Fig. 1F and SI Appendix, Fig. S3), we built a starting model for molecular dynamics (MD) simulations and performed implicit-solvent MD simulations followed by binding energy calculation using molecular mechanics/generalized Born surface area (MM/GBSA) with conformational entropy ignored. During a typical amyloid fibril assembly, the fibril elongates infinitely upon the peptide stacking into β-sheets via hydrogen bonding interaction. Perpendicular to the fibril axis, β-sheets are paired into “steric zippers” with hydrophobic complementary interfaces composed of side chains on one side of the β-sheets (2, 8). In a pair of β-sheets, there are three possible pairing conformations. The two sides of one β-sheet are referred to as “side A” and “side B,” and we describe pairing patterns as “A to A,” “B to B,” or “A to B” (Fig. 2A). In a typical amyloid-forming peptide assembly, one pairing interface always dominates with low binding energy, thus limiting packing along the zippering axis (“VQIVYK” in Fig. 2B and SI Appendix, Fig. S5). In contrast, in KLVFFAK self-assembly, the three interfaces have similar negative binding energies (“KLVFFAK” in Fig. 2B and SI Appendix, Figs. S4 and S5), indicating they are all energetically favorable. The structural stability of these interfaces enables β-sheets to stack along the zippering axis comparably in two possible patterns: “face to face” via A to A and B to B interfaces existing alternately, and “face to back” via A to B interface (8), thus spanning the amyloid architecture from a 1D fibril to a 2D sheet (Fig. 2A).
Fig. 2.
Binding energy of the β-sheet pairs and nanosheet formation. (A) One β-sheet layer has two sides (A and B) composed of different side chains. When a pair of β-sheets forms the zipper spine, there are three possible pairing conformations: A to A, B to B, and A to B. When extending along the zippering axis to form nanosheets, there are two possible interaction patterns: face to face, which contains A to A and B to B interfaces, and face to back, which contains A to B interfaces. The fibril axis and zippering axis are perpendicular to each other, defining the two packing directions of amyloid-like nanosheet. (B) Binding energy of the three interfaces formed by peptides KLVFFAK, KLVFGAK, and VQIVYK. For KLVFFAK, the three interfaces have similar binding energy; thus, extensive packing along the zippering axis is possible via either face to face or face to back patterns. On the contrary, KLVFGAK and typical fibril-forming peptide VQIVYK have only one stable zippering interface of A to A, therefore only able to form fibrils rather than nanosheets. (Scale bars, 200 nm.)
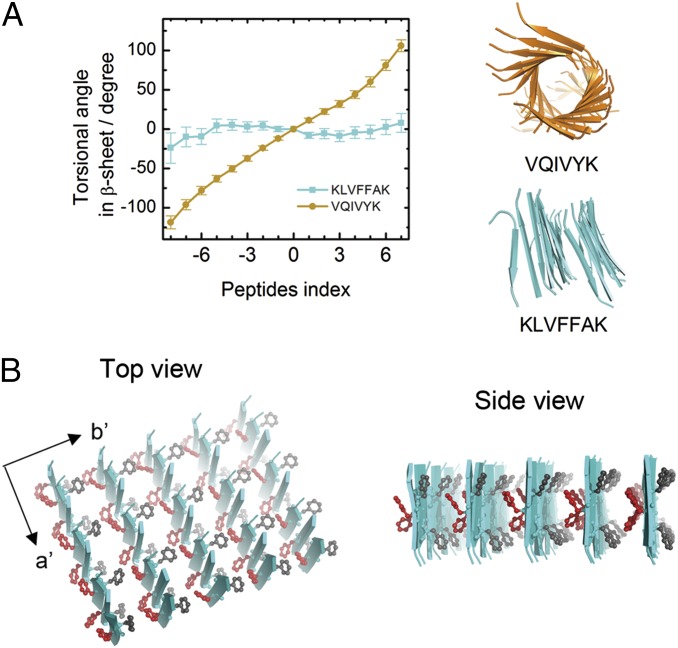
To determine whether the nanosheet is assembled via face to face or face to back pattern, we predicted the distortion of the three pairing interfaces of the KLVFFAK β-sheet pair (SI Appendix, Fig. S6). Amyloid fibril β-sheets are generally elongated with torsion due to amino acid chirality (Fig. 3A, VQIVYK). However, torsional β-sheet is deleterious and must be avoided in the flat KLVFFAK nanosheet. The MD simulations results showed that the β-sheet pair with the A to A interface is significantly twisted, whereas the B to B interface is flat (SI Appendix, Fig. S6). Because the face to face pattern incorporates both A to A and B to B interfaces, the twist of A to A interface is sufficient to break the β-sheet stacking in this pattern. The face to back pattern is stacked only via the A to B interface. The MD simulations showed that the A to B interface is rather flat, supporting the possibility of the β-sheet stacking along the zippering axis via the face to back pattern.
Fig. 3.
Structural model of the KLVFFAK nanosheet. (A) MD simulations of 2 × 16 β-sheets of VQIVYK with A to A interface and KLVFFAK with A to B interface. VQIVYK sheets showed a significant twist, whereas KLVFFAK sheets are rather flat. (B) Structural model of the KLVFFAK nanosheet. Along axis a′ (the fibril axis), the peptides are stacked via hydrogen bonds. Along axis b′ (the zippering axis), the packing is mediated by interactions of side chains between sides A and B of β-sheets.
Combining both the experimental and computational results, we proposed a structural model of the KLVFFAK nanosheet as shown in Fig. 3B. The nanosheet expands in two dimensions along the fibril axis (axis a′) and the zippering axis (axis b′). Along the fibril axis, KLVFFAK forms antiparallel β-sheets via main-chain hydrogen bonding interactions; along the zippering axis, the β-sheets stacks in face to back pattern via the side-chain steric hydrophobic interactions. To further prove the model, we mutated KLVFFAK to KLVFGAK. We performed binding energy analyses as a function of amino acid residue for the 2 × 8 KLVFFAK β-sheets with the three different packing interfaces (A to A, B to B, and A to B). The calculated binding energy in SI Appendix, Fig. S4 A–C showed that aromatic residue Phe in one layer of β-sheet exhibits the strongest binding affinity with the residues in the other layer. Meanwhile, hydrophobic residues Leu and Val also display strong interactions. The calculated binding energy and a snapshot of a 2 × 8 β-sheet with A to B interface in SI Appendix, Fig. S4 D and E showed that the side chains of the aromatic and hydrophobic residues form compact steric zippers and contribute dominantly to stabilizing the interface. Therefore, it is expected that the substitution of Phe5 in KLVFFAK for Gly (i.e., KLVFGAK) would greatly weaken the A to B interface, leading to the destabilization of the nanosheet assembly along the zippering axis. Consistent with our prediction, we observed that KLVFGAK only forms typical thin and long fibrils instead of nanosheets (Fig. 2B and SI Appendix, Fig. S5).
Controllable Growth of the Amyloid-Like Nanosheet.
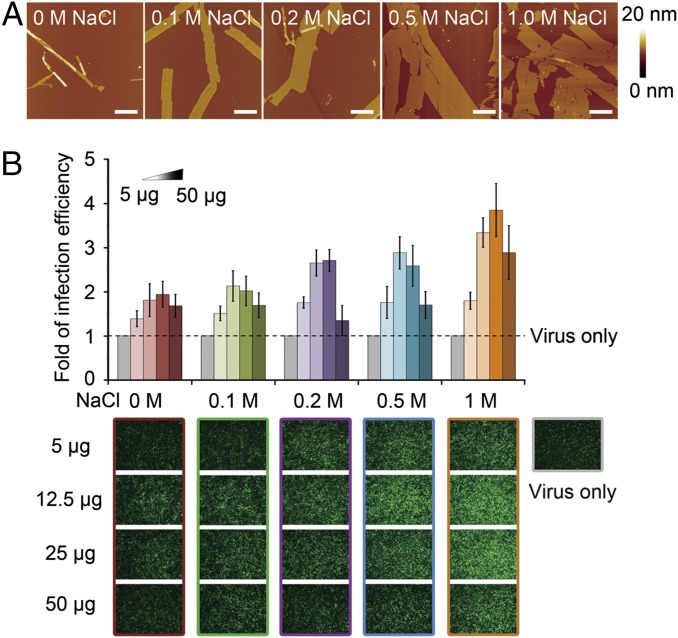
We found that the size and yield of an amyloid-like nanosheet can be fine tuned by altering ionic strength in aqueous solution. When increasing the NaCl concentration, the width of the nanosheet kept growing from 0.2–0.4 μm to 0.6–1.0 μm, plateauing at 0.5 M NaCl, and the yield increased accordingly (Fig. 4A and SI Appendix, Figs. S7 and S8). A similar trend was observed in the presence of MgCl2, but the plateau appeared at a lower MgCl2 concentration due to the stronger ionic contribution of the divalent magnesium cation (SI Appendix, Fig. S9). The possible reason behind this salt effect may be that salt improves the aggregation ability by screening out the repulsive interactions between the positively charged Lys–Lys contacts, which are concentrated on the nanosheet surfaces (33). In addition, we observed that the nanosheet morphology is stable for over 20 d at 37 °C with agitation, indicating it is thermodynamically favorable.
Fig. 4.
Salt effects on nanosheet growth and its efficiency of retroviral transduction enhancement. (A) AFM images of the KLVFFAK nanosheets under gradient NaCl concentrations. As the increase of NaCl concentration, the size and yield of nanosheets both increased. (Scale bars, 1 μm.) (B) Lentiviral transduction assay showed that the efficiency of enhancing transduction is positively related to the nanosheet size and yield. A gradient of nanosheet quantities, 5 μg, 12.5 μg, 25 μg, and 50 μg, were examined. GFP fluorescence is used for imaging and quantification of infected cells.
Application of the Nanosheet in Boosting Retroviral Transduction.
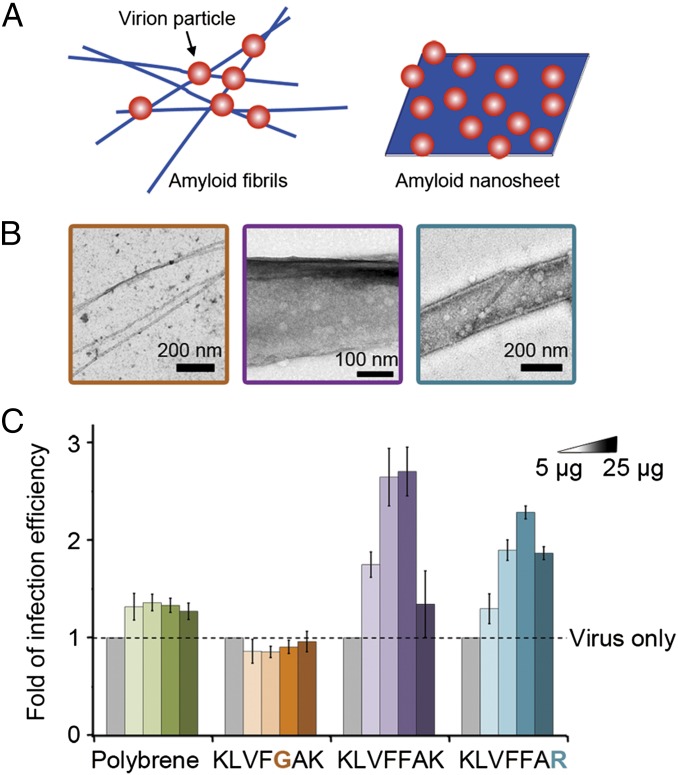
The structural model shows that lysine residues at the termini of the peptide KLVFFAK localize on the surface of the nanosheet, making both sides of the sheet highly charged. Previous studies have shown that positively charged amyloid fibrils with lysines exposed on the surface can mediate HIV infection and laboratorial retroviral gene transfer (24). Thus, we evaluated whether the KLVFFAK nanosheet can be used as an enhancer for viral infection. The retroviral transduction assay showed that it significantly enhanced lentiviral infection of HEK293T cells. This correlates positively with the size and yield of the nanosheet (Fig. 4B) and is significantly stronger than a commonly used enhancer—polybrene (Fig. 5C). More importantly, the nanosheet showed a significantly stronger enhancing effect than the KLVFGAK fibril even if KLVFGAK contains the same number of lysines as KLVFFAK (Fig. 5C), indicating that nanosheet architecture is superior for enhancing retroviral transduction.
Fig. 5.
Comparison of retroviral transduction enhancement by amyloid fibril and nanosheet. (A) Schematics of virus particles attaching on fibrils and nanosheet. Fibrils and nanosheet are shown in blue, representing the positive charges on their surfaces. Virus particles are in red, representing negatively charged surface. (B) Direct visualization of virus particles attaching on nanosheets formed by peptides KLVFFAK and KLVFFAR. As a comparison, the fibril of KLVFGAK is also shown. (C) Comparison of retroviral transduction enhancing efficiency of polybrene (a commonly used enhancer), KLVFGAK fibril, KLVFFAK, and KLVFFAR nanosheets. A single mutation of Phe to Gly transformed the nanosheet into a fibril and ameliorated the enhancing effect, indicating the nanosheet structure is favorable in this function.
Direct Visualization of Viral Attachment on an Amyloid-Like Nanosheet.
We next investigated the mechanism of how the nanosheet enhances viral infection. As proposed in previous studies, the positive charges on the amyloid fibril surface can concentrate viruses and facilitate the interaction between negatively charged cells and viruses (34). We examined the direct attachment of lentivirus on the surface of an amyloid-like nanosheet by electron microscope (EM) (Fig. 5B). We mixed the nanosheet with lentivirus and centrifuged to remove the solvent. Under EM, we observed that nearly all viruses reside on the nanosheet surface with few free ones, providing to our knowledge the first direct visualization of amyloid materials aggregating virus. This also explains why the nanosheet outperforms fibrils in enhancing viral transduction. Considering the sizes of a lentivirus (∼50–100 nm), fibril (10–20 nm), and nanosheet (0.2–1 μm), the interface between nanosheet and virus is significantly larger than that between fibril and virus (Fig. 5 A and B). Nanosheets provide an extensive surface large enough to support viral attachment, whereas fibrils are too thin to efficiently attach virus. In agreement with this hypothesis, a recent study has indicated that when fibrils cluster into plaques, they show stronger enhancement of retroviral gene transfer (35).
Interestingly, we also observed that HEK293T cells and amyloid-like nanosheets adhered to each other, most likely mediated by electrostatic interaction between the negatively charged cell membrane and the positively charged nanosheet (SI Appendix, Fig. S10). The adhesion facilitated the interaction between virus and cells for gene transfer, but was not observed to influence the cell proliferation.
Tunable Functionality of the Nanosheet.
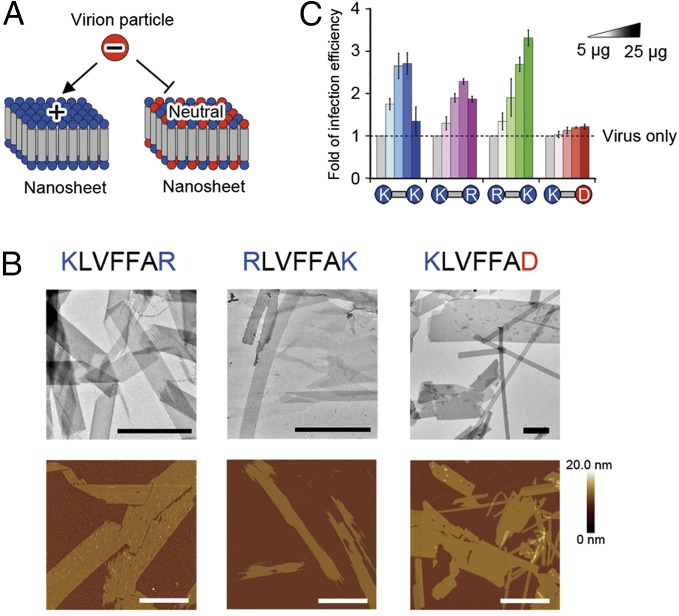
Unique architecture and useful application make amyloid-like nanosheets an attractive class of bionanomaterials. Next, we tested the tunability of their function by interchanging the amino acid side chains without disrupting the overall topology of the nanosheet. The structural model concludes “LVFFA” is the core sequence for nanosheet assembly. Therefore, we substituted the terminal lysine residues with arginine or aspartic acid. All designed sequences still form nanosheet structures (Fig. 6 and SI Appendix, Table S1), indicating that LVFFA is a robust nanosheet-forming sequence.
Fig. 6.
Effects of designed nanosheets on retroviral transduction. (A) Schematic illustration of nanosheets with positively charged and neutral surfaces. The positively charged nanosheet can attach a negatively charged retrovirus and mediate virus infecting cells; however, the nanosheet with a neutral surface cannot. (B) TEM and AFM images of nanosheets formed by three designed peptides. (Scale bars, 1 μm.) (C) Retroviral transduction assay proved that the enhancing efficiency depends on the surface charge of nanosheets.
More interestingly, the nanosheet functions are altered upon the integration of different amino acid residues (Fig. 6). When the C-terminal lysine is changed to aspartic acid, the surface of the KLVFFAD nanosheet becomes nearly neutral or slightly negative at pH 7, shown by the surface zeta potential (Fig. 6A and SI Appendix, Table S2). As a result, the KLVFFAD nanosheet showed no effect on retroviral transduction (Fig. 6C). On the contrary, the designed nanosheets of KLVFFAR and RLVFFAK showed similar effects on retroviral transduction as observed in KLVFFAK (Fig. 6C), because the surface electrostatic potential remains positive (Fig. 6A and SI Appendix, Table S2). This result further validates our working model and design strategy of the amyloid-like nanosheet.
Toward the Design of Artificial Nanosheet-Forming Peptides.
To enrich the class of amyloid-like nanosheets, we attempted to design the nanosheet from scratch. According to the structure of the KLVFFAK nanosheet, a nanosheet-forming peptide should contain the properties of fibril-forming peptides (36–38) and additionally be able to stack along the zippering axis, as shown in Fig. 2A, through face to face or face to back patterns. In the face to face pattern, interfaces A to A and B to B need to be energetically equivalent and preferred, whereas in the face to back pattern, interface A to B needs to be energetically preferred. We initiated our design with a simplified scenario where sides A and B are composed of the same residues to make the three interfaces equivalent (SI Appendix, Fig. S11A). In the five designed peptides, four formed nanosheet-like structures with much wider diameters than typical fibrils (SI Appendix, Fig. S11B). However, we noticed that the nanosheet formed by the designed peptides did not reach the size of that formed by KLVFFAK, indicating that factors important for nanosheet formation are not fully considered. Further design may incorporate computational calculations of binding energies and torsional angles in search of more complicated and diverse sequences for giant nanosheet formation.
Discussion
In this work, we discovered that KLVFFAK and its variants self-assemble into amyloid-like nanosheets and excel as nanomaterials in boosting retroviral gene transduction. Unlike fibrils, the nanosheet is a 2D expanding structure conferring features including a flat surface and increased density of functional groups. The discovery of 2D amyloid-like nanosheets adds depth to the field of amyloid materials and broadens their various applications in nanotechnology.
With unique chemical and physical properties, amyloid nanomaterials have shown beguiling potential as an emerging class of bionanomaterials (19, 39, 40). For versatile applications, it is highly desirable to have amyloid materials with diverse architectures, which feature different mechanical properties and provide scaffolds with distinct topographies for different functionalities. We discerned that amyloid morphology is mainly determined by sequence-specific side-chain interactions. Amyloidogenic peptides with various compositions and combinations of amino acids can potentially self-assemble into distinct, diverse structures. Therefore, peptide screening and computational design are likely to find more amyloid-related architectures.
For the same sequence, distinct morphologies of amyloid entities have also been observed under different conditions (41). This phenomenon is termed “amyloid polymorphism” (42). For instance, wild-type Aβ16–22 has been observed to form nanotubes, tape-like structures, and fibril bundles (15, 18, 43, 44). However, the mechanism underlying polymorphism is elusive, and the amyloid morphology upon conditions is barely predictable. Thus, further studies on the molecular mechanism of self-assembly will be essential to provide knowledge for accurate design and control of peptide self-assembly in a well-defined and ordered manner.
Materials and Methods
Nanosheet/Fibril Formation.
Peptides were synthesized by GL Peptide with purity over 98% (wt/wt). All peptides were synthesized with N-terminal acetylation and C-terminal amidation. Nanosheets/fibrils were grown with 4 mg⋅ml−1 peptide in 50 mM sodium phosphate buffer, pH 2. Various concentrations of NaCl and MgCl2 were used. The peptide solution was incubated at 37 °C with constant agitation for 3 d. Nanosheet and fibril formation was observed by transmission electron microscopy (TEM) and AFM.
TEM.
Each sample (4 μL) was applied to glow-discharged carbon film on 400 mesh copper grids and stained with 0.75% uranyl formate. Specimens were examined by using Tecnai G2 Spirit TEM operated at an accelerating voltage of 120 kV. Images were recorded using a 4K × 4K charge-coupled device camera (BM-Eagle, FEI Tecnai).
AFM.
AFM was operated in air on a Bruker MultimodeVIII SPM equipped with a J scanner. Experiments were performed in tapping mode with NSC11 tip (spring constant 48 N⋅m−1, MikroMasch) or in peakfore tapping mode with SNL10 tip (spring constant 0.35 N⋅m−1, Bruker). For AFM imaging under dry conditions, each sample (5 μL) was placed on a freshly cleaved mica or Si wafer substrate. Sample solution was allowed to adsorb for 5 min and then it was washed gently with 1 mL buffer followed by air drying. Muscovite mica was purchased from Ya’an Electrical Material Plant. Si wafer was purchased from Nanjing Zhongjingkeyi Technology. For imaging under hydrated conditions, 50 μL of buffer solution was placed on the above sample in a liquid cell.
Congo Red Staining.
Nanosheet/fibril solution (200 μL) was spun down at 20,000 × g for 10 min. Pellets were resuspended in 100 μL 0.1 mg⋅ml−1 Congo red dissolved in 10 mM Tris pH 8.0, 0.15 M NaCl, and incubated for 10 min at room temperature. The mixture was spun down again at 20,000 × g. Pellets were resuspended in 30 μL water. The washing process was repeated until the supernatant was no longer red. Pellets were finally resuspended with 5 μL water and then pipetted onto a glass coverslip. After being dried at room temperature, stains were observed by using an optical microscope equipped with crossed polarizers (Leica M205 FA).
FTIR Spectroscopy.
FTIR spectroscopy measurement was acquired using a Nicolet iZ10 Fourier transform infrared spectrometer (Thermo Scientific). Samples (400 μL) were freeze dried into powder. The powder and potassium bromide were compressed into a thin pellet. Data were Gaussian fitted using OMNIC software.
Nanosheet/Fibril X-Ray Diffraction.
Nanosheet/fibril pellets were washed with water twice by centrifugation and resuspension to remove salts. Pellets were finally suspended with 5 μL water. The suspension was pipetted and dried between two fire-polished glass rods for several hours. X-ray diffraction images were collected on a Rigaku Micromax-007 X-ray generator equipped with an R-Axis IV++ area detector.
MD Simulations.
All-atom MD simulations were performed using the Amber12 software package (45) with Amber99SB force field (46). All simulations were performed using the elastic boundary condition at a constant temperature of 300 K. The solvation effect was considered using the generalized Born (GB) model (47) and the cutoff for GB energy calculation was set to 3.0 nm. In the initial states, eight KLVFFAK peptide chains with β-strand conformations were in antiparallel alignment without β-sheet twisting and two layers of this β-sheet (2 × 8) were packed in three different side-chain interaction patterns (A to A: L-F-A to L-F-A, B to B: K-V-F-K to K-V-F-K, and A to B: L-F-A to K-V-F-K) with a sheet–sheet distance of 1.2 nm. For comparison, bilayer β-sheets for KLVFGAK and VQIVYK peptides were simulated in the same condition. MD simulations on a larger bilayer β-sheet (2 × 16) of KLVFFAK and VQIVYK were also performed to examine the β-sheet twisting in the three pairing patterns. Two 30-ns MD simulations were conducted for each system and the last 15-ns trajectories were used for analysis. The binding energy of each residue in one layer of β-sheet with the residues in the other layer was calculated using the MM/GBSA method (48). The binding energy was also estimated using the molecular mechanics/Poisson–Boltzmann surface area (MM/PBSA) method (49). The three systems with the (2 × 8) β-sheet were also simulated in explicit water solvent and the binding energy was calculated using both MM/GBSA and MM/PBSA methods. We performed the energy decomposition on a per-residue basis (50) using MMPBSA.py (a component of the AMBER package) (48) so that we could also estimate the contribution of each residue to the total binding energy. The details of this per-residue basis decomposition method can be found in ref. 50. More details about MD simulations and binding energy calculation are given in SI Appendix, Materials and Methods.
Zeta Potential.
A total of 0.25 mL of freshly prepared KLVFFAK, RLVFFAK, KLVFFAR, and KLVFFAD nanosheet suspension was diluted to 1 mL in a solution containing 50 mM sodium phosphate, 0.1 M sodium chloride, pH 7, respectively. The zeta potential was measured using a Zeta Nanosizer (Nano-ZS 90, Malvern Instruments). Each sample was measured in triplicate.
Virus Package and Cell Transduction/Infection Assay.
Lentivirus was packaged with the standard protocol (51). Briefly, the HEK293T cells were transfected with lentiviral expression vector Lv-PGK-GFP together with the package vectors pVSVG, pMDL, and pREV (Invitrogen) using transfection reagents lipofectmin2000 according to the manufacturer’s protocol (Invitrogen). The medium containing virus particles was collected 48 h after transfection, filtered through a 0.45-μm filter (Millipore), and immediately used for infection assay.
For transduction/infection assay, HEK293T cells seeded in 24-well plates (2 × 106 cells/well) were infected with the solution comprising 150 μL virus particles containing medium, 350 μL DMEM/10% (vol/vol) FBS fresh medium, and different concentrations of fibril suspension. The fluorescent cell images were taken 48 h later, and the efficiency of transduction/infection was then quantified by FACs analysis of the percentage of the GFP positive cells.
Supplementary Material
Acknowledgments
This work was supported by the “1000 Talents” Program of China, the State High-Tech Development Plan (the “863 Program”) Award (Grant 2015AA020907), the National Natural Science Foundation (NSF) of China (Grant 31470748), and China Postdoctoral Science Foundation (Grants 2014M560366 and 2014T70446). Y.Z. acknowledges financial support from the National Basic Research Program of China (973 Program, Grant 2013CB932801) and the NSF of China (Grant 11274334). G.W. acknowledges financial support from the NSF of China (Grants 91227102 and 11274075). Simulations were performed at the National High Performance Computing Center of Fudan University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416690112/-/DCSupplemental.
References
- 1.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148(6):1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman MR, et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295(5556):851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maji SK, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325(5938):328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150(2):339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, et al. Out-of-register β-sheets suggest a pathway to toxic amyloid aggregates. Proc Natl Acad Sci USA. 2012;109(51):20913–20918. doi: 10.1073/pnas.1218792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleiholder C, Dupuis NF, Wyttenbach T, Bowers MT. Ion mobility-mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation. Nat Chem. 2011;3(2):172–177. doi: 10.1038/nchem.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawaya MR, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447(7143):453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 9.Jahn TR, et al. The common architecture of cross-beta amyloid. J Mol Biol. 2010;395(4):717–727. doi: 10.1016/j.jmb.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 10.Nelson R, et al. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435(7043):773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazit E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem Soc Rev. 2007;36(8):1263–1269. doi: 10.1039/b605536m. [DOI] [PubMed] [Google Scholar]
- 12.Knowles TP, Buehler MJ. Nanomechanics of functional and pathological amyloid materials. Nat Nanotechnol. 2011;6(8):469–479. doi: 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick AW, Park ST, Zewail AH. Exceptional rigidity and biomechanics of amyloid revealed by 4D electron microscopy. Proc Natl Acad Sci USA. 2013;110(27):10976–10981. doi: 10.1073/pnas.1309690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauser CA, Maurer-Stroh S, Martins IC. Amyloid-based nanosensors and nanodevices. Chem Soc Rev. 2014;43(15):5326–5345. doi: 10.1039/c4cs00082j. [DOI] [PubMed] [Google Scholar]
- 15.Lu K, Jacob J, Thiyagarajan P, Conticello VP, Lynn DG. Exploiting amyloid fibril lamination for nanotube self-assembly. J Am Chem Soc. 2003;125(21):6391–6393. doi: 10.1021/ja0341642. [DOI] [PubMed] [Google Scholar]
- 16.Reches M, Gazit E. Controlled patterning of aligned self-assembled peptide nanotubes. Nat Nanotechnol. 2006;1(3):195–200. doi: 10.1038/nnano.2006.139. [DOI] [PubMed] [Google Scholar]
- 17.Koutsopoulos S, Unsworth LD, Nagai Y, Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci USA. 2009;106(12):4623–4628. doi: 10.1073/pnas.0807506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Childers WS, Mehta AK, Ni R, Taylor JV, Lynn DG. Peptides organized as bilayer membranes. Angew Chem Int Ed Engl. 2010;49(24):4104–4107. doi: 10.1002/anie.201000212. [DOI] [PubMed] [Google Scholar]
- 19.Lakshmanan A, Zhang S, Hauser CA. Short self-assembling peptides as building blocks for modern nanodevices. Trends Biotechnol. 2012;30(3):155–165. doi: 10.1016/j.tibtech.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X, et al. Molecular self-assembly and applications of designer peptide amphiphiles. Chem Soc Rev. 2010;39(9):3480–3498. doi: 10.1039/b915923c. [DOI] [PubMed] [Google Scholar]
- 21.Guo C, Luo Y, Zhou R, Wei G. Probing the self-assembly mechanism of diphenylalanine-based peptide nanovesicles and nanotubes. ACS Nano. 2012;6(5):3907–3918. doi: 10.1021/nn300015g. [DOI] [PubMed] [Google Scholar]
- 22.Chen AY, et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nat Mater. 2014;13(5):515–523. doi: 10.1038/nmat3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reches M, Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science. 2003;300(5619):625–627. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]
- 24.Yolamanova M, et al. Peptide nanofibrils boost retroviral gene transfer and provide a rapid means for concentrating viruses. Nat Nanotechnol. 2013;8(2):130–136. doi: 10.1038/nnano.2012.248. [DOI] [PubMed] [Google Scholar]
- 25.Li D, et al. Designed amyloid fibers as materials for selective carbon dioxide capture. Proc Natl Acad Sci USA. 2014;111(1):191–196. doi: 10.1073/pnas.1321797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rufo CM, et al. Short peptides self-assemble to produce catalytic amyloids. Nat Chem. 2014;6(4):303–309. doi: 10.1038/nchem.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Adamcik J, Mezzenga R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat Nanotechnol. 2012;7(7):421–427. doi: 10.1038/nnano.2012.62. [DOI] [PubMed] [Google Scholar]
- 28.Udomprasert A, et al. Amyloid fibrils nucleated and organized by DNA origami constructions. Nat Nanotechnol. 2014;9(7):537–541. doi: 10.1038/nnano.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Mezzenga R. The interplay between carbon nanomaterials and amyloid fibrils in bio-nanotechnology. Nanoscale. 2013;5(14):6207–6218. doi: 10.1039/c3nr01644g. [DOI] [PubMed] [Google Scholar]
- 30.Krone MG, et al. Effects of familial Alzheimer’s disease mutations on the folding nucleation of the amyloid beta-protein. J Mol Biol. 2008;381(1):221–228. doi: 10.1016/j.jmb.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moitra P, Kumar K, Kondaiah P, Bhattacharya S. Efficacious anticancer drug delivery mediated by a pH-sensitive self-assembly of a conserved tripeptide derived from tyrosine kinase NGF receptor. Angew Chem Int Ed Engl. 2014;53(4):1113–1117. doi: 10.1002/anie.201307247. [DOI] [PubMed] [Google Scholar]
- 32.Pauling L, Corey RB. Configurations of polypeptide chains with favored orientations around single bonds: Two new pleated sheets. Proc Natl Acad Sci USA. 1951;37(11):729–740. doi: 10.1073/pnas.37.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai B, et al. Salts drive controllable multilayered upright assembly of amyloid-like peptides at mica/water interface. Proc Natl Acad Sci USA. 2013;110(21):8543–8548. doi: 10.1073/pnas.1220711110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roan NR, et al. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol. 2009;83(1):73–80. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usmani SM, et al. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun. 2014;5:3508. doi: 10.1038/ncomms4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer-Stroh S, et al. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nat Methods. 2010;7(3):237–242. doi: 10.1038/nmeth.1432. [DOI] [PubMed] [Google Scholar]
- 37.Pawar AP, et al. Prediction of “aggregation-prone” and “aggregation-susceptible” regions in proteins associated with neurodegenerative diseases. J Mol Biol. 2005;350(2):379–392. doi: 10.1016/j.jmb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Li D, et al. Structure-based design of functional amyloid materials. J Am Chem Soc. 2014;136(52):18044–18051. doi: 10.1021/ja509648u. [DOI] [PubMed] [Google Scholar]
- 39.Cherny I, Gazit E. Amyloids: Not only pathological agents but also ordered nanomaterials. Angew Chem Int Ed Engl. 2008;47(22):4062–4069. doi: 10.1002/anie.200703133. [DOI] [PubMed] [Google Scholar]
- 40.Hauser CA, Zhang S. Designer self-assembling peptide nanofiber biological materials. Chem Soc Rev. 2010;39(8):2780–2790. doi: 10.1039/b921448h. [DOI] [PubMed] [Google Scholar]
- 41.Lu JX, et al. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154(6):1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kodali R, Wetzel R. Polymorphism in the intermediates and products of amyloid assembly. Curr Opin Struct Biol. 2007;17(1):48–57. doi: 10.1016/j.sbi.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Tao K, et al. Self-assembly of short aβ(16-22) peptides: Effect of terminal capping and the role of electrostatic interaction. Langmuir. 2011;27(6):2723–2730. doi: 10.1021/la1034273. [DOI] [PubMed] [Google Scholar]
- 44.Lin D, et al. Investigation of the aggregation process of amyloid-β-(16-22) peptides and the dissolution of intermediate aggregates. Langmuir. 2014;30(11):3170–3175. doi: 10.1021/la4048165. [DOI] [PubMed] [Google Scholar]
- 45.Case DA, et al. 2012. AMBER 12 (University of California, San Francisco)
- 46.Duan Y, et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 2003;24(16):1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
- 47.Onufriev A, Bashford D, Case DA. Modification of the generalized Born model suitable for macromolecules. J Phys Chem B. 2000;104(15):3712–3720. [Google Scholar]
- 48.Miller BR, et al. MMPBSA.py: An efficient program for end-state free energy calculations. J Chem Theory Comput. 2012;8(9):3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
- 49.Homeyer N, Gohlke H. Free energy calculations by the molecular mechanics Poisson-Boltzmann surface area method. Mol Inf. 2012;31(2):114–122. doi: 10.1002/minf.201100135. [DOI] [PubMed] [Google Scholar]
- 50.Gohlke H, Kiel C, Case DA. Insights into protein-protein binding by binding free energy calculation and free energy decomposition for the Ras-Raf and Ras-RalGDS complexes. J Mol Biol. 2003;330(4):891–913. doi: 10.1016/s0022-2836(03)00610-7. [DOI] [PubMed] [Google Scholar]
- 51.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1(1):241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.