Significance
Conventional wisdom assumes that integrating different malaria intervention approaches will always act synergistically for malaria control. The complex and transient nature of malaria immunity significantly underlies this optimistic assumption. Here we use a fully parameterized mathematical model to investigate the interaction of treated bednets with vaccination in the population dynamics of malaria. We demonstrate that mixed interventions create nonlinear responses that modify the way in which humans acquire clinical protection from malaria infection. Our results indicate that vaccines will not necessarily provide a straightforward solution to malaria control, and that future programs need to consider both synergistic and antagonistic interactions between vaccines and other control measures.
Keywords: malaria control, malaria eradication, vaccines, treated bednets, combined intervention
Abstract
It is extremely likely that the malaria vaccines currently in development will be used in conjunction with treated bednets and other forms of malaria control. The interaction of different intervention methods is at present poorly understood in a disease such as malaria where immunity is more complex than for other pathogens that have been successfully controlled by vaccination. Here we develop a general mathematical model of malaria transmission to examine the interaction between vaccination and bednets. Counterintuitively, we find that the frailty of malaria immunity will potentially cause both synergistic and antagonistic interactions between vaccination and the use of bednets. We explore the conditions that create these tensions, and outline strategies that minimize their detrimental impact. Our analysis specifically considers the three leading vaccine classes currently in development: preerythrocytic (PEV), blood stage (BSV), and transmission blocking (TBV). We find that the combination of BSV with treated bednets can lead to increased morbidity with no added value in terms of elimination; the interaction is clearly antagonistic. In contrast, there is strong synergy between PEV and treated bednets that may facilitate elimination, although transient stages are likely to increase morbidity. The combination of TBV with treated bednets is synergistic, lowering both morbidity and elimination thresholds. Our results suggest that vaccines will not provide a straightforward solution to malaria control, and that future programs need to consider the synergistic and antagonistic interactions between vaccines and treated bednets.
Over 500 million annual cases of malaria worldwide kill roughly 1–3 million people and severely dampen economic growth and vitality in many African countries (1, 2). Although there are great challenges in developing a highly effective malaria vaccine, progress has accelerated considerably in last decade with ∼25 vaccine candidates at different stages of development (3). Farthest along is the preerythrocytic RTS,S vaccine, shown in recent phase III clinical tries to be between 25% and 50% effective against the disease (4, 5) and receiving a World Health Organization (WHO) recommendation to begin its use in 2015 provided it gains approval. Other candidates are also showing increased promise (6, 7). Malaria immunity is more complex, however, than that generated by other pathogens that have successfully been controlled by vaccination in the past (8–11), and is believed to provide protection only for 1–2 y (5). Therefore, for vaccination programs to be effective, a mixture of tactics will be needed, including levels of vaccine coverage similar or higher to those achieved for measles, but repeated annually throughout life. It follows that multiple approaches will be vital, and there will be a critical need to understand interactions between vaccines and treated bednets, and in particular, how these will modify and shape the immunological and susceptibility profile of the host population (8).
Here we develop a general model for the transmission dynamics of Plasmodium falciparum (Pf), the parasite responsible for the deadliest malaria in humans, and we examine the interaction of two different forms of intervention against malaria: treated bednets and each of the three major malaria vaccine families currently in development. In the past decade, insecticide-treated bednets (ITN) and long-lasting insecticide-treated nets have proven to be highly effective in reducing rates of malaria mortality in young children (12–14); differences between these are discussed in Methods. [Note that we use ITN to represent both insecticide-treated bednets and long-lasting insecticide-treated nets (LLINs)]. Mosquito nets act as physical barriers that provide personal protection against malaria to those using them, and insecticide treatment of the nets adds a chemical barrier, which either kills or repels, further reducing human–vector contact and increasing the protective efficacy for the individuals using the nets (12). High levels of treated bednet use are thought to reduce the overall vector population, hence also providing residual protection for the general community, including those who do not sleep under nets; an effect that is analogous to herd immunity (15–17). A recent study shows that relatively modest coverage (∼60%) may be sufficient for achieving equitable community-wide benefits (18). Nonetheless, according to WHO, neither treated bednets nor indoor residual spraying (IRS) will be sufficiently effective alone to achieve and maintain interruption of transmission to the point of elimination in endemic areas of Africa (1, 19–21). It is therefore recognized that there is priority in identifying new approaches for combining different strategies such as future vaccines (18, 22). Vaccines could also provide an alternative means of protecting people, particularly in areas where malaria is transmitted by crepuscular or diurnally active mosquitoes, and would also help protect people who need to work at night in areas where mosquitoes are active (23–26). Therefore, the quest to develop malaria vaccines continues apace (3, 6).
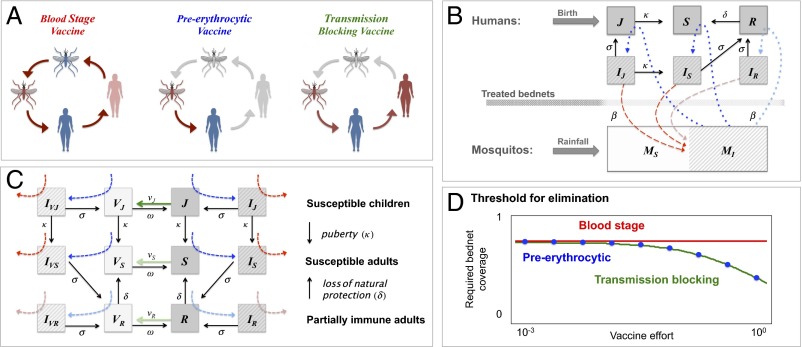
The complexity of malaria immunity has led vaccine development to pursue multiple approaches, focusing on different stages of the malaria life cycle (3, 7–9, 27). The leading vaccine candidates currently in development can be classified into three general families (Fig. 1A): (i) preerythrocytic vaccines (PEVs), such as the RTS,S (4, 5), which reduce the chances of a person becoming infected, (ii) blood-stage vaccines (BSVs), which reduce the level of disease severity and fatality, and (iii), transmission-blocking vaccines (TBVs), which target the sexual stages of the parasite and disrupt the malaria transmission cycle. In contrast to the first two, the TBV provides very limited benefit to the people vaccinated but potentially reduces the rate at which they infect others; for this reason it is commonly referred to as an altruistic vaccine (28–30). These three vaccine types are fundamentally different in their mode of action, and our modeling framework is constructed to capture how each of these integrates into the malaria transmission system. The model provides the means to both assess their interaction with other interventions such as treated bednets, as well as to compare between these three.
Fig. 1.
(A) Vaccines interfering with the Pf life cycle: BSV protects from clinical disease but does not break the life cycle of the parasite because it does not block infection. PEV blocks infection, implying that hosts are both protected from disease and that the life cycle of Pf is broken. TBV does not provide any protection from disease, but it reduces the likeliness of secondary infections, hence breaking the life cycle of Pf (SI Appendix, section IV). (B) A sketch of the basic compartmental model with coupled hosts and vector dynamics (SI Appendix, sections I and II): mosquitoes are either susceptible to infection (MS) or infected (MI), whereas the human population is structured by (i) age: young children under the age of 5 (J + IJ) vs. older children and adults (S + IS + R + IR); (ii) infection status: infected hosts (IJ + IS + IR) vs. noninfected hosts (J + S + R); and (iii) disease risk: hosts susceptible to clinical disease (IJ + IS) vs. hosts with partial protective immunity susceptible only to mild or asymptomatic infections (IR) (Methods). By differentiating these classes, we can express the general occurrence of morbidity relative to asymptomatic infection and distinguish between morbidity in adults vs. young children (47). Transmission of infection takes place from infected mosquitoes to susceptible humans (τMH, in blue), and from infected humans to susceptible mosquitoes (τHM, in red). Treated bednets can reduce the biting rate β by providing physical and chemical barriers between mosquitos and humans. Duration of infection is 1/σ (d), 1/δ is the duration of natural immunity (y), and 1/κ = 5 y, the time children spend in the juvenile age group. (C) A vaccinated state is defined for each host class (VJ, VS, and VR). We consider young children are vaccinated at a higher rate than the rest of the population (νJ >> νS and νR) and the possibility that naturally immune hosts may be less susceptible to infection (τMH,J, τMH,S ≥ τMH,R ) and/or less infectious to mosquitoes when infected (τHM,IJ, τMH,I,S ≥ τMH,I,R; shades of green, blue, and red, respectively). Vaccine protection lasts 1/ω (y), but depending on vaccine type hosts may still be susceptible to infection (IVJ, IVS, and VIVR). A generalized version of the model with multiple age groups is provided in SI Appendix, section II. (D) The level of bednet coverage needed to cross the elimination threshold (R0 ≤ 1; Eq. 1) as a function vaccine effort log (νJ). Parameters: NH = 5,000, NM = 5,000, β = 0.5, ε = 0.2, ξ = 0.4, 1/σ = 60 d, 1/δ = 1/δV = 2, 1/κ = 5 y, 1/αJ = 1/αS = 1/μH = 30 y, 1/μM = 20 d, λrainfall = NM × μM, and νJ = νS/100 = νR/100(y−1). For all classes, i = J,S,R: τMH,I = τHM,i = 0.5, for BSV, τMH,V = τHM,V = 0.5, for PEV, τMH,V = 0, and for TBV, τMH,V = 0.5 and τHM,V = 0.
A fundamental challenge is how to correctly assess benefits of intervention (31–33) and how to appropriately quantify malaria disease burden (34–36); these measures obviously require a clear definition of an end goal (37–39). In highly endemic areas, young children under the age of 5 are at the highest risk of contracting severe clinical malaria (40). Hence one of the principle goals of malaria control has been to reduce mortality in younger age groups; this reduction is often the sole measure for quantifying the effectiveness of different interventions (41–44). In contrast, in regions where malaria transmission is lower or unstable, significant mortality and morbidity occurs in people of all ages (35); thus success in reducing malaria mortality rates in early childhood does not necessarily reflect reduction in mortality and/or morbidity in the older age classes (45). Our model therefore considers the clinical risks of malaria for several age groups (Fig. 1B), which also allows us to track changes in age dependency ratios following different forms of intervention. Because we are interested in the changes in clinical risks of malaria, we do not explicitly track case fatality but rather use morbidity as a general description for all forms of clinical disease, including mortality. (Note that explicitly incorporating disease-induced mortality into our model produces qualitatively identical results.)
Results
Calculation of the basic reproductive rate of our model (R0) provides a clear analytical expectation of how vaccine and treated bednets reduce infection prevalence and potentially lead to elimination (16, 46), highlighting several well-known targets of control (Methods): decreasing biting rate (β); increasing infection clearance (σ); increasing bednet coverage (b); increasing insecticide treatment of bednets and its effectively of killing (ξ); decreasing vector mobility (ε); decreasing transmissibility from vectors to hosts (τMH); decreasing transmissibility from hosts to vectors (τHM), and from vectors to hosts (τMH; e.g., through vaccine; Fig. 1D); and decreasing the ratio of vectors to humans host (e.g., through bednet use).
| [1] |
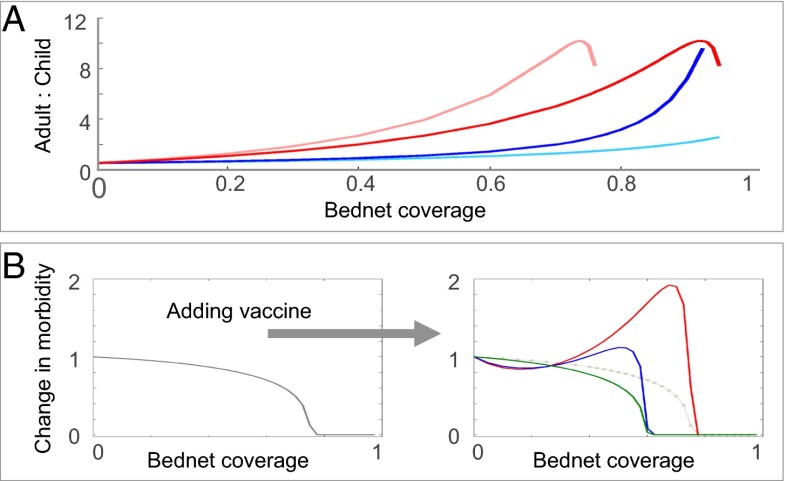
However, this measure does not give insight on the indirect effects that intervention has on age-dependent patterns of host morbidity because the relationship between transmission and morbidity is not as straightforward as it is for viruses, which create lifelong immunity; for malaria, protective immunity to clinical disease requires repeated exposure (11, 47) and quantifying the frailty of this immunity is central to the design of any malaria control scheme. In our model we distinguish between two types of infection: severe clinical disease occurring in young children under the age of 5 or in older children and adults (these compose the subgroups of humans suffering from morbidity in its different forms); and mild or asymptomatic infection in older children and adults (Fig. 1B). Our numerical simulations show that the relationship between the fraction of the host population using treated bednets and malaria prevalence and morbidity, display the “peak shift” phenomenon, suggesting that reductions in acquired immunity due to lower human–vector contact lead to increased levels of susceptibility and disease in older children and adults (Fig. 2A). This signature of immunity is a well-known epidemiological pattern characteristic to all immunizing infections (48). Specifically for Pf, peak prevalence of the parasite shifts to younger age groups as transmission intensity increases (10, 11, 40), whereas morbidity in the overall host population shifts to older age classes in areas of low-to-moderate transmission (10, 49). This outcome is relevant for control because it delineates the groups that are most at risk from clinical disease (35, 50); it also implies that reductions of transmission intensity through any form of control will lead to age-dependent shifts in malaria morbidity, from the young children to the older age classes (45, 51, 52). A major concern is that such age shifts caused by reductions in immunity at the population level may also reflect individual loss of immunity, implying that susceptibility of older hosts to severe disease is increasing (53). Thus, interventions aiming to reduce transmission will not necessarily reduce the burden of malaria (54, but see also refs. 55–57); ultimately, this raises concerns about using levels of malaria in young children as a simple index of regional malaria severity. Although these malaria in young children may decline when control is introduced, this will likely be matched by increased malaria in older age classes, a concomitant increase of malaria in older age classes is likely to follow.
Fig. 2.
(A) Changes in the ratio of adult to child morbidity with different levels of bednet use, calculated by dividing levels of morbidity with intervention to those without it. An increase in the ratio of relative morbidity demonstrates increased burden on the productive class. Different forms of insecticide treatment of repelling (ε) and killing (ξ) are considered (see Methods): pink: ε = 0, ξ = 0.2; red: ε = 0.2, ξ = 0.2; dark blue: ε = 0, ξ = 0; and light blue: ε = 0.2, ξ = 0. SI Appendix, Fig. III-1 shows that with higher bednet use child morbidity generally decreases while adult morbidity increases. Notably, when bednets are treated with relatively mild repellent (ε = 0.2) they are less effective than those that are not treated (ε = 0), indicating that the repulsion of mosquitoes from nets is likely to have a counter-productive effect that suppress their benefits if bednets do not also lead to enhanced mosquito mortality. This can occur as the nets age and the bednet declines in potency emphasizing the superiority of LLINs over ITNs. For further discussion on the implications of repelling vs. killing for control see SI Appendix, section III. (B) Relative morbidity as a function of bednet coverage calculated by dividing total morbidity with bednets to that without them. (Left) Without vaccine and (Right) with vaccine for BSV (red), PEV (blue), and TBV (green), where children are vaccinated on average once a year. Parameters are identical to those in Fig. 1.
The application of different vaccine types reveals several distinct patterns associated with host population immunity: PEV on its own has a direct effect on the general force of infection because vaccinated hosts are essentially removed from the pool of susceptibles. From the individual host level, this vaccine interferes with the acquisition of natural protective immunity and with its maintenance, but indirectly, it also leads to similar effects on the general host population (whether vaccinated or not). Thus, PEV leads to a shift in the peak of morbidity from younger ages to older ones, generating a similar epidemiological effect to that produced by bednets. In contrast, BSV does not interfere with the cycle of transmission and its effects on the vaccinated hosts are similar to those of naturally acquired immunity. Because exposed hosts can still be infected asymptomatically, these infections will build up and maintain protective immunity. Although vaccine protection wanes after roughly a year or two (5), we find that in circumstances of high transmission, BSV vaccinated hosts can both gain and maintain natural protective immunity for their whole lives, without ever having to experience episodes of clinical disease. In contrast, in areas of lower transmission, this protective immunity would eventually wane and hosts would have to depend on frequent revaccination for protection. At the other extreme, TBV does not provide any protection to the vaccinated host and the only way to gain immunity is through clinical infection. Unfortunately, the likeliness of maintaining this protection deteriorates as vaccine effort increases because of the decline in the general force of infection. We find that child morbidity is effectively reduced with both PEV and BSV, whereas TBV has very little benefit for children (SI Appendix, Fig. IV-2). However, both PEV and TBV can lead to increased levels of adult morbidity, whereas BSV is almost neutral, and even slightly positive, in its effects on the adult population. BSV is superior to the other two vaccines in this respect, because it can significantly reduce the burden of illness on children without generating costs for the rest of the host population. We see that an appropriate quantification of the benefits of intervention is not straightforward; a clear shortcoming of BSV is that it would not reduce the reservoir of the disease, and on its own would make a weak candidate for elimination. In contrast, both PEV and TBV reduce the elimination threshold of malaria.
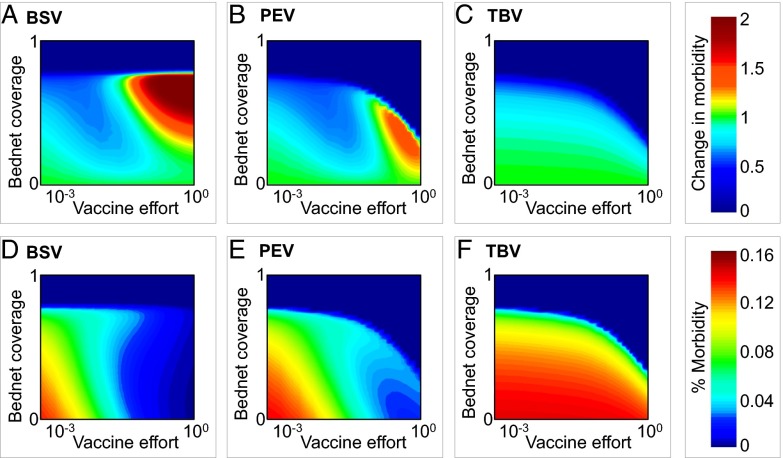
With our model we identify both synergistic and antagonistic interactions in the combined application of treated bednets with different vaccine types. The use of bednets in communities treated with BSV can lead to increased levels of morbidity, which can be even higher than those expected in the absence of nets (Fig. 3A). However, these changes in morbidity levels are generally lower than would be expected for populations vaccinated with PEV or TBV (Fig. 3 B and C). Nonetheless, because BSV can play no role in promoting elimination on its own, it would be necessary to use it in conjunction with treated bednets to achieve this goal; this implies that unless vaccination levels are kept low, the path to successful eradication with BSVs requires crossing over an intermediate peak of enhanced morbidity (Fig. 3 A and D).
Fig. 3.
Combined intervention of treated bednets with BSV, PEV, and TBV. (A–C) Contour plots show changes in total morbidity calculated by dividing the observed level of morbidity with bednets by its level in their absence. Cold colors represent synergistic interactions, and warm colors antagonistic ones. (D–F) Absolute fraction of hosts suffering of clinical illness (morbidity). Vaccine effort is defined as log(νJ). All other parameters are identical to those in Fig. 2. In SI Appendix, section V, we demonstrate robustness of our result to different epidemiological parameters and with multiple age classes.
The application of treated bednets to communities treated with PEV shows more complicated immunity patterns. We find regions of decreased morbidity when vaccination levels are low, but also increased morbidity when vaccination levels are high (Fig. 3 B and E). In contrast to BSV, PEV can play a significant role in promoting elimination (Fig. 3 A and D). To successfully achieve this goal, however, a conflict arises: higher levels of both PEV coverage and treated bednet use increase the likeliness of elimination, but this also involves crossing a peak of enhanced morbidity (Fig. 3B). Finally, in contrast to BSVs and PEVs, the application of treated nets to communities treated with TBV always leads to significant decreases in morbidity, while also increasing the probability of elimination. Although TBV on its own has minor benefits on population levels (in comparison with BSV and PEV), the addition of bednet protection provides significant benefits both on the population level and also directly to the individuals using nets. The absence of an intermediate morbidity peak highlights the uniqueness of TBV relative to the other two vaccines (Fig. 3 C and F).
Discussion
Our analyses illustrate that use of mixed interventions against malaria will create nonlinear responses that modify the way in which human hosts acquire protection against future disease. Though there are always short-term benefits to individuals that use a bednet or receive a viable protective vaccine (PEV/BSV), these may be accompanied by significant short-term costs to those who are not using a bednet, but are exposed to increased bites from the same mosquito population (Methods). On the longer term, both protected and nonprotected hosts could also suffer from loss of natural protective immunity as a consequence of insufficient boosting of immunity.
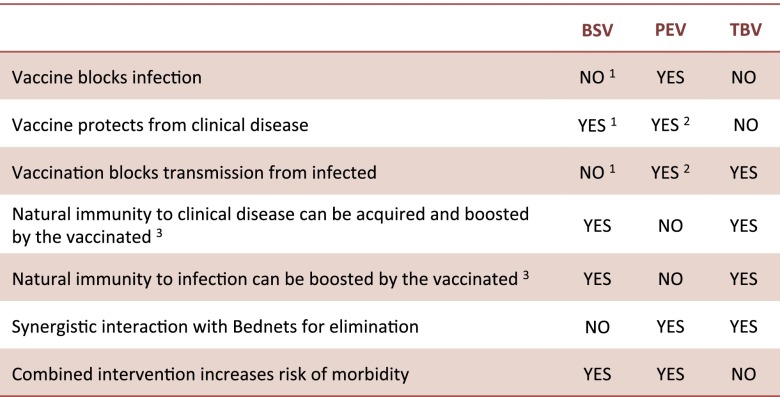
Our results suggest that the combination of treated bednets with transmission-blocking vaccines (also known as altruistic vaccines) (7, 28) achieves the most efficient control. In contrast, when treated bednets are combined with either blood or preerythrocytic vaccines, then synergistic interactions reduce levels of natural immunity in the population and increase levels of morbidity in older age classes (see Fig. 4 for a summary). These effects may be masked in malaria control programs that simply use reductions in levels of infant mortality as an index of success (see for example refs. 41–44).
Fig. 4.
A table comparing the three vaccine families in their direct and indirect effects on individual hosts, the overall host population, and their interaction with treated bednets. 1We consider BSVs that provide protection from clinical disease but without blocking infection or lowering transmission. Some evidence from animal models and clinical trials are showing that by reducing parasitemia, this vaccine may also reduce the production of gametocytes and therefore, transmission [see review on BSV (75)]. In such cases, if BSV fully blocks infection and/or transmission, the vaccine is effectively equivalent to PEV in the way it disrupts the Pf life cycle, and we therefore do not explicitly consider this option. Alternatively, we show in the sensitivity table of SI Appendix that for BSV partly blocking infection and/or transmission, our results do not change. 2As a by-product of blocking infection. 3BSV and TBV allow hosts to get infected, and we therefore assume natural immunity can be acquired and/or boosted while in a vaccinated state (as would be the case in the nonvaccinated state). In contrast, because PEV blocks infection, we do not consider natural immunity can be acquired and/or boosted during this period. It should be noted, however, that for a subgroup of PEV, antibodies against sporozoite proteins [such as circumsporozoite protein (CSP)] may potentially be boosted.
Several patterns supporting predictions of our model have been reported in African countries where signs of increasing malaria morbidity of adults coincided with mass treated bednet intervention over the past decade. Despite remarkable success in reducing malaria burdens between 2000 and 2010 through the use of treated bednets (12–14), the epidemiology of malaria in these settings has also become more complex (50, 58–60), which is likely reflective of transient dynamics of immunity (61). Notably, in many of the Sub-Saharan African countries, trajectories of adult and child mortality have been following seemingly unrelated trends over the past decade, with increasing ratios of adult to child morbidity, as illustrated in SI Appendix, section VI. One implication is the growing pressure on the productive population reflected by changes in the age dependency ratio. High morbidity and mortality clearly lead to major economic losses, particularly when these imply higher dependency ratios and excess childhood mortality. Nonetheless, the economic and social burdens of malaria are still not fully understood and are weakly defined. In financial terms, morbidity in our model can be directly reflected by the disability-adjusted life years (62); this is a measure of overall disease burden summarizing morbidity and mortality caused by clinical malaria, and expressing the number of years lost due to ill health, disability, or early death. Our findings highlight the importance of monitoring the effects of intervention across all age classes, and not only on young children (50).
Our results were shown to be robust to mode detailed age structure and to variation in epidemiological parameters (SI Appendix, section V), except for unrealistic extremes of durations of immunity and infectiousness comparable to host life span, which would make the population dynamics essentially those of a pathogen such as measles. Besides short-lived antibody responses to merozoites, strain diversity arising from variation in the major blood-stage antigen Pf erythrocyte membrane protein 1 (PfEMP1) is an additional process that effectively generates waning immunity (Methods). This diversity is still poorly understood in local parasite populations, particularly with respect to the enormous genetic diversity of the var multicopy gene family encoding PfEMP1 (63), and its mapping to phenotypic diversity and ultimately to cross-protection. Nonetheless, only if strain diversity were extremely low, we would expect the lifelong protection of a single exposure to last and to result in lifelong immunity. Given the large genetic diversity of the var system, lifelong protection is unlikely to apply in endemic regions. The questions of how strain diversity varies as intervention progresses and reduces transmission, (i.e., how rapidly and to what extent) are not well understood, although diversity is known to decrease with prevalence geographically. The consequences of this variation for vaccination should be investigated with more complex, agent-based models such as in Artzy-Randrup et al. (64), where exposure to different variants can explicitly be represented, as well as the dynamics of antigenic diversity per se, with the epidemiology emerging from within-host and between-host dynamics. Similarly, combining any type of vaccine with bednets is likely to initiate conditions under which selection may occur for malaria strains of increased virulence (65).
Considerable scientific and economic attention has rightly been focused on the development of malaria vaccines in the hopes that malaria can be successfully contained in the same way as smallpox, measles, and other childhood diseases. Our results confirm that each of the different types of vaccines has the potential to significantly control malaria. However, our results also strongly caution against the combined use of treated bednets and vaccines that directly protect those vaccinated, because this may lead to increased morbidity in older individuals in the population who are likely to also play a central role in the workforce. In contrast, the combination of treated bednets with transmission-blocking vaccines seems to provide highly efficacious means of malaria control. Different combinations of control may be suited for different locations and at different stages of local elimination (66). Simply assuming that vaccines, bednets, and drug treatment will act synergistically is likely to prove at best overoptimistic and potentially dangerous.
Methods
Transmission of Infection.
Mosquitoes bite human hosts at a constant attack rate (i.e., the total number of bites of a particular mosquito per unit time). Following a biting event, the probability that an infected host transmits to a susceptible mosquito depends on the rate of contact between susceptible mosquitos (MS) to infected hosts (IJ, IS, IR), and on the probability of successful transition from host to mosquito [τHM,I(J), τHM,I(S), τHM,I(R), respectively]. Similarly, the probability that an infected mosquito transmits to a susceptible host depends on the rate of contact between infected mosquitos (MI) to susceptible hosts (J, S, R), and on the probability of successful transition from host to mosquito (τMH,J, τMH,S, τMH,R, respectively). To simplify, we assume transmissibility is identical between all infectious classes and that mosquitos are equally attracted to all human hosts, regardless of their epidemiological state or immunological history (e.g., infected hosts with clinical symptoms are equally likely to attract mosquitos as any of the other hosts); both assumptions can easily be modified to reflect more complex possibilities. Our sensitivity analysis in SI Appendix, section V shows that these simplifications do not have a significant impact on our results, with the exclusion of two extreme scenarios: (i) only clinically ill hosts transmit, whereas asymptomatic ones do not, or, alternatively, (ii) mosquitos have full preference for biting the clinically ill.
Acquisition and Boosting of Natural Immunity.
The acquisition and boosting of natural immunity to Pf infections is highly complex, involving multiple arms of the immune system, and still not fully understood (47). We distinguish between (i) immunity to clinical disease, gained during erythrocytic stages of infection, principally conferred by anti-PfEMP1 antibodies and merozoite antibodies, and (ii) immunity to infection, relevant to preerythrocytic stages of infection, likely to be provided by CD8+ T cells against the liver stages, as well as antibodies against sporozoite proteins, such as CSP. In cases where natural immunity reduces parasitemia, this may lead to a reduction in gametocytes production, hence lowering transmission.
In many endemic regions it is common to find that the reservoir of Pf infection is significantly larger than the number of clinical cases (47). Whereas natural immunity to clinical disease can be acquired after only a few exposure events, sterilizing immunity (i.e., natural immunity to infection) is likely to never be fully gained (47, 63). We capture this feature by assuming that natural immunity following infection provides protection from clinical disease, but never fully blocking infection itself. Hence, susceptible hosts can be in one of two states: (i) fully susceptible, where hosts suffer from clinical disease if infected, or (ii) partially immune, where hosts have immunity to clinical disease, suffering from only mild or asymptomatic disease if infected, and possibly less infectious to susceptible mosquitoes due to reduced production of gametocytes [i.e., τHM,I(i) ≥ τHM,A(i)]. In such cases, partial immunity may also make hosts less susceptible to becoming infected [i.e., τMH,S(i) ≥ τMH,R(i)].
Waning Immunity.
An important assumption is that natural immunity wanes, which is based on evidence showing that antibodies to merozoites antigens are relatively short-lived compared with antibodies to other pathogens, such as measles, that confer lifelong immunity (67) (report half-lives of 0.8–7.5 y). In addition, although immunity against PfEMP1 can be long-lasting [as has been shown for example for antibodies to Pfvar2 chondroitin sulfate A (Pfvar2-CSA)], an effect of waning immunity would still arise due to the diversity of these antigens in endemic regions [see, e.g., the simulations with the agent-based model in Artzy-Randrup et al. (64), where PfEMP1 diversity is explicitly incorporated]. That is, for this large and diverse multicopy gene family, at the epidemiological level of the host population, an effect of waning immunity arises when individuals are exposed to specific variable surface antigens like PfEMP1 that they have not encountered before. Hence, unless cross-protection is complete, we expect strain diversity to result in waning immunity; although this is a different mechanism than short-lived antibody responses to merozoites, both mechanisms lead to an effective loss of protection with time.
Bednets.
The rate at which a mosquito bites a nonprotected host is β(1 + εb/(1 − b))/NH, where NH is the human population size, b is the product of bednet efficiency and the proportion of humans using bednets (ranging from 0 to 1), and ε is the efficiency of mosquitoes to target nonprotected hosts in contrast to protected ones. To express mosquito foraging, i.e., the extent to which mosquitos can successfully locate viable blood meals, we use a type II functional response (68). Hence, when ε = 0, mosquitoes invest equal effort foraging between all hosts, but as ε increases, vectors preferentially target nonprotected hosts; this implies that for ε > 0, the rate at which nonprotected hosts are bitten increases with higher levels of bednet use, as would be the case when bednets are treated with a repellant. ε also captures the level of vector mobility, where physical restrictions on the movement of vectors imply lower values of ε. Studies are showing evidence of malaria vectors changing their biological behavior due to high levels of bednet coverage, including changes in the time of biting activity and changes in feeding preference (21, 24, 69–72); in our model, this would be interpreted as a selective advantage for higher levels of ε. In addition to repellants, bednets are commonly also treated with insecticides that increase vector mortality after contact. Hence, ξ is defined as the probability of mosquito mortality following an encounter with a treated net, and it follows that the rate of such events is ξβb(1 − ε). The value of ξ depends on the type of nets being used, including the rate at which the nets are retreated with insecticides (e.g., ITNs lose their efficiency after ∼6–12 mo in contrast to LLINs, which stay effective for several years) (12); the levels of mosquito resistance to insecticides; and the type of insecticide being used.
Calculating R0.
The basic reproductive rate (Eq. 1) is calculated as the number of secondary cases caused by a single infected mosquito in a fully susceptible human population × the number of cases caused by a single infected human in a fully susceptible mosquito population (73, 74), where N*H is the number of susceptible hosts in a disease-free equilibrium when the population is vaccinated (NH = N*H + N*V), N*M,b is the mosquito population size in the presence of bednets such that N*M,b = λrainfall/(μM + ξβb(1 − ε)), and τ*HM is the average probability of successful transmission from an infected host to a mosquito, such that for BSV, τ*HM = (N*H ×τHM + N*V × τHM,V)/(N*H + N*V); for PEV, τ*HM = (N*H × τHM)/(N*H); and for TBV, τ*HM = (N*H × τHM)/(N*H + N*V).
Supplementary Material
Acknowledgments
Y.A.-R. is grateful to the Department of Ecology and Evolutionary Biology at Princeton University for hosting her as an academic visitor during parts of this research. M.P. and Y.A.-R. thank the support of the Fogarty International Center at NIH (Program on the Ecology and Evolution of Infectious Diseases, NIH Grant R01TW009670). A.P.D.’s research on infectious disease dynamics is supported by a James McDonnell Foundation award and by Princeton University Grand Challenges. M.P. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 2925.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409467112/-/DCSupplemental.
References
- 1.World Health Organization (WHO) World Malaria Report 2013. WHO; Geneva: 2013. [Google Scholar]
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) 2013. Tables of malaria vaccine projects globally: The rainbow tables. Available at www.who.int/immunization/research/development/Rainbow_tables/en/
- 4.Agnandji ST, et al. RTS,S Clinical Trials Partnership A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367(24):2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olotu A, et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368(12):1111–1120. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moorthy VS, Newman RD, Okwo-Bele JM. Malaria vaccine technology roadmap. Lancet. 2013;382(9906):1700–1701. doi: 10.1016/S0140-6736(13)62238-2. [DOI] [PubMed] [Google Scholar]
- 7.Hill AV. Vaccines against malaria. Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2806–2814. doi: 10.1098/rstb.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanisic DI, Barry AE, Good MF. Escaping the immune system: How the malaria parasite makes vaccine development a challenge. Trends Parasitol. 2013;29(12):612–622. doi: 10.1016/j.pt.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120(12):4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snow RW, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349(9066):1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Snow RW, Donnelly C, Newbold C. Acquired immunity and postnatal clinical protection in childhood cerebral malaria. Proc Biol Sci. 1999;266(1414):33–38. doi: 10.1098/rspb.1999.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) Insecticide Treated Mosquito Nets: A WHO Position Statement. WHO; Geneva: 2007. [Google Scholar]
- 13.Lim SS, et al. Net benefits: A multicountry analysis of observational data examining associations between insecticide-treated mosquito nets and health outcomes. PLoS Med. 2011;8(9):e1001091. doi: 10.1371/journal.pmed.1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisele TP, et al. Estimates of child deaths prevented from malaria prevention scale-up in Africa 2001–2010. Malar J. 2012;11:93. doi: 10.1186/1475-2875-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine PE. Herd immunity: History, theory, practice. Epidemiol Rev. 1993;15(2):265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature. 1985;318(6044):323–329. doi: 10.1038/318323a0. [DOI] [PubMed] [Google Scholar]
- 17.Dobson A. Climate variability, global change, immunity, and the dynamics of infectious diseases. Ecology. 2009;90(4):920–927. doi: 10.1890/08-0736.1. [DOI] [PubMed] [Google Scholar]
- 18.Killeen GF, et al. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med. 2007;4(7):e229. doi: 10.1371/journal.pmed.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaukat AM, Breman JG, McKenzie FE. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar J. 2010;9:122. doi: 10.1186/1475-2875-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin JT, et al. Reducing Plasmodium falciparum malaria transmission in Africa: A model-based evaluation of intervention strategies. PLoS medicine. 2010;7(8):e1000324. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karunamoorthi K. Vector control: A cornerstone in the malaria elimination campaign. Clin Microbiol Infect. 2011;17(11):1608–1616. doi: 10.1111/j.1469-0691.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 22.Chambers RG. UN Envoy’s response to estimates of global malaria mortality. Lancet. 2012;379(9817):707–708. doi: 10.1016/S0140-6736(12)60280-3. [DOI] [PubMed] [Google Scholar]
- 23.Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: Implications for vector control. Med Vet Entomol. 2006;20(4):425–437. doi: 10.1111/j.1365-2915.2006.652.x. [DOI] [PubMed] [Google Scholar]
- 24.Pates H, Curtis C. Mosquito behavior and vector control. Annu Rev Entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- 25.Trung HD, et al. Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: A challenge for vector control. Trop Med Int Health. 2005;10(3):251–262. doi: 10.1111/j.1365-3156.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- 26.Durnez L, et al. Outdoor malaria transmission in forested villages of Cambodia. Malar J. 2013;12:329. doi: 10.1186/1475-2875-12-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley EM, Stewart VA. Immune mechanisms in malaria: New insights in vaccine development. Nat Med. 2013;19(2):168–178. doi: 10.1038/nm.3083. [DOI] [PubMed] [Google Scholar]
- 28.DeWeerdt S. Vaccines: The take-home lesson. Nature. 2012;484(7395):S24–S25. doi: 10.1038/484S24a. [DOI] [PubMed] [Google Scholar]
- 29.Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. Malaria transmission-blocking vaccines—how can their development be supported? Nat Med. 2000;6(3):241–244. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- 30.Dinglasan RR, Jacobs-Lorena M. Flipping the paradigm on malaria transmission-blocking vaccines. Trends Parasitol. 2008;24(8):364–370. doi: 10.1016/j.pt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owusu-Agyei S, et al. Incidence of symptomatic and asymptomatic Plasmodium falciparum infection following curative therapy in adult residents of northern Ghana. Am J Trop Med Hyg. 2001;65(3):197–203. doi: 10.4269/ajtmh.2001.65.197. [DOI] [PubMed] [Google Scholar]
- 32.Shiff C. A call for integrated approaches to controlling malaria. Parasitol Today. 1997;13(3):125–, author reply 125–126. doi: 10.1016/s0169-4758(97)84872-7. [DOI] [PubMed] [Google Scholar]
- 33.Smith DL, Hay SI, Noor AM, Snow RW. Predicting changing malaria risk after expanded insecticide-treated net coverage in Africa. Trends Parasitol. 2009;25(11):511–516. doi: 10.1016/j.pt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hay SI, et al. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7(6):e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow RW, Craig M, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bull World Health Organ. 1999;77(8):624–640. [PMC free article] [PubMed] [Google Scholar]
- 36.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization (WHO) Roll Back Malaria Partnership (2008) The Global Malaria Action Plan for a Malaria-Free World. Available at www.rbm.who.int/gmap/index.html.
- 38.Feachem RG, et al. Shrinking the malaria map: Progress and prospects. Lancet. 2010;376(9752):1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Modrek S, Gosling RD, Feachem RG. Malaria eradication: Is it possible? Is it worth it? Should we do it? Lancet Glob Health. 2013;1(1):e2–e3. doi: 10.1016/S2214-109X(13)70002-0. [DOI] [PubMed] [Google Scholar]
- 40.Snow RW, et al. Risk of severe malaria among African infants: Direct evidence of clinical protection during early infancy. J Infect Dis. 1998;177(3):819–822. doi: 10.1086/517818. [DOI] [PubMed] [Google Scholar]
- 41.Kleinschmidt I, et al. Marked increase in child survival after four years of intensive malaria control. Am J Trop Med Hyg. 2009;80(6):882–888. [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso PL, et al. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 5. Design and implementation of the trial. Trans R Soc Trop Med Hyg. 1993;87(Suppl 2):31–36. doi: 10.1016/0035-9203(93)90173-n. [DOI] [PubMed] [Google Scholar]
- 43.D’Alessandro U, et al. Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme. Lancet. 1995;345(8948):479–483. doi: 10.1016/s0140-6736(95)90582-0. [DOI] [PubMed] [Google Scholar]
- 44.Phillips-Howard PA, et al. Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68(4) Suppl:23–29. [PubMed] [Google Scholar]
- 45.O’Meara WP, et al. Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am J Trop Med Hyg. 2008;79(2):185–191. [PMC free article] [PubMed] [Google Scholar]
- 46.Chiyaka C, et al. Infectious disease. The stability of malaria elimination. Science. 2013;339(6122):909–910. doi: 10.1126/science.1229509. [DOI] [PubMed] [Google Scholar]
- 47.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1):13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolhouse ME. Patterns in parasite epidemiology: The peak shift. Parasitol Today. 1998;14(10):428–434. doi: 10.1016/s0169-4758(98)01318-0. [DOI] [PubMed] [Google Scholar]
- 49.Carneiro I, et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: A systematic review and pooled analysis. PLoS ONE. 2010;5(2):e8988. doi: 10.1371/journal.pone.0008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mharakurwa S, et al. Southern Africa ICEMR Team Changes in the burden of malaria following scale up of malaria control interventions in Mutasa District, Zimbabwe. Malar J. 2013;12:223. doi: 10.1186/1475-2875-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Meara WP, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372(9649):1555–1562. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–264. doi: 10.1016/s0065-308x(02)52013-3. [DOI] [PubMed] [Google Scholar]
- 53.Trape JF, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: A longitudinal study. Lancet Infect Dis. 2011;11(12):925–932. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 54.Trape JF, Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996;12(6):236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- 55.Lengeler C, Smith TA, Armstrong Schellenberg J. Focus on the effect of bednets on malaria morbidity and mortality. Parasitol Today. 1997;13(3):123–124, author reply 125–126. doi: 10.1016/s0169-4758(97)84870-3. [DOI] [PubMed] [Google Scholar]
- 56.D’Alessandro U, Coosemans M. Concerns on long-term efficacy of an insecticide-treated bednet programme on child mortality. Parasitol Today. 1997;13(3):124–125, author reply 125–126. doi: 10.1016/s0169-4758(97)84871-5. [DOI] [PubMed] [Google Scholar]
- 57.Keating J, Eisele TP. Epidemiology of malaria morbidity after control scale-up. Lancet Infect Dis. 2011;11(12):891–892. doi: 10.1016/S1473-3099(11)70212-2. [DOI] [PubMed] [Google Scholar]
- 58.Cotter C, et al. The changing epidemiology of malaria elimination: New strategies for new challenges. Lancet. 2013;382(9895):900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray CJ, et al. Global malaria mortality between 1980 and 2010: A systematic analysis. Lancet. 2012;379(9814):413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 60.Masquelier B, Reniers G, Pison G. Divergences in trends in child and adult mortality in sub-Saharan Africa: Survey evidence on the survival of children and siblings. Popul Stud. 2013;68(2):161–177. doi: 10.1080/00324728.2013.856458. [DOI] [PubMed] [Google Scholar]
- 61.Smith T, Schapira A. Reproduction numbers in malaria and their implications. Trends Parasitol. 2012;28(1):3–8. doi: 10.1016/j.pt.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Murray CJ. Quantifying the burden of disease: The technical basis for disability-adjusted life years. Bull World Health Org. 1994;72(3):429–445. [PMC free article] [PubMed] [Google Scholar]
- 63.Chen DS, et al. A molecular epidemiological study of var gene diversity to characterize the reservoir of Plasmodium falciparum in humans in Africa. PLoS ONE. 2011;6(2):e16629. doi: 10.1371/journal.pone.0016629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Artzy-Randrup Y, et al. Population structuring of multi-copy, antigen-encoding genes in Plasmodium falciparum. eLife. 2012;1:e00093. doi: 10.7554/eLife.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414(6865):751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- 66.Okiro EA, et al. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J. 2009;8:4. doi: 10.1186/1475-2875-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fowkes FJ, et al. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis. 2012;206(10):1612–1621. doi: 10.1093/infdis/jis566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holling CS. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol. 1959;91(5):293–320. [Google Scholar]
- 69.Russell TL, et al. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore SJ, Davies CR, Hill N, Cameron MM. Are mosquitoes diverted from repellent-using individuals to non-users? Results of a field study in Bolivia. Trop Med Int Health. 2007;12(4):532–539. doi: 10.1111/j.1365-3156.2006.01811.x. [DOI] [PubMed] [Google Scholar]
- 71.Ferguson HM, et al. Ecology: A prerequisite for malaria elimination and eradication. PLoS Med. 2010;7(8):e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56. doi: 10.1186/1475-2875-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford Univ Press; New York: 1991. [Google Scholar]
- 74.Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):1001–1012. doi: 10.1098/rstb.2001.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87(5):377–390. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.