Significance
Nanotechnology-based drug delivery is expected to bring new hope for cancer treatment by enhancing anticancer drug efficacy, overcoming drug resistance, and reducing drug toxicity. In this respect, we developed an innovative drug delivery system based on a self-assembling amphiphilic dendrimer, which can generate supramolecular nanomicelles with large void space in their core to encapsulate anticancer drugs with high loading capacity. The resulting drug-encapsulated nanomicelles can effectively enhance drug potency and combat drug resistance by promoting cellular uptake and decreasing efflux of the anticancer drug. Moreover, this drug delivery system can significantly reduce the systemic toxicity of the free drug. The present study illustrates a successful example of how advances in dendrimer nanotechnology can be advantageously implemented to foster therapeutic perspectives.
Keywords: amphiphilic dendrimers, supramolecular nanomicelles, drug delivery, cancer treatment, nanodrugs
Abstract
Drug resistance and toxicity constitute challenging hurdles for cancer therapy. The application of nanotechnology for anticancer drug delivery is expected to address these issues and bring new hope for cancer treatment. In this context, we established an original nanomicellar drug delivery system based on an amphiphilic dendrimer (AmDM), which could generate supramolecular micelles to effectively encapsulate the anticancer drug doxorubicin (DOX) with high drug-loading capacity (>40%), thanks to the unique dendritic structure creating large void space for drug accommodation. The resulting AmDM/DOX nanomicelles were able to enhance drug potency and combat doxorubicin resistance in breast cancer models by significantly enhancing cellular uptake while considerably decreasing efflux of the drug. In addition, the AmDM/DOX nanoparticles abolished significantly the toxicity related to the free drug. Collectively, our studies demonstrate that the drug delivery system based on nanomicelles formed with the self-assembling amphiphilic dendrimer constitutes a promising and effective drug carrier in cancer therapy.
Although considerable progress has been made in cancer therapy, the complete cure and eradication of cancer remains one of the greatest challenges at present. A well-known hurdle is the drug resistance induced by chemotherapeutics, causing high recurrence rate and therapeutic failure (1). Moreover, high systemic toxicity of traditional anticancer drugs is another reason for eventual poor clinical outcome. To address these problems, the application of nanotechnology for drug delivery is widely expected to bring new hope for cancer treatment (2–6). Nanoparticle-based drug delivery systems can repress many drawbacks of traditional chemotherapeutics, such as high systematic toxicity and low therapeutic efficacy caused often by poor drug bioavailability, frequently related to the stability, solubility, and nonspecificity of drugs (2–8). In addition, nanodrugs with tailored properties can overcome drug resistance by increasing the drug accessibility and drug sensitivity via high local drug concentration achieved at tumor lesion through enhanced permeation and retention (EPR) effect (9–11). As a result, there is an increasing interest to develop effective nanodrugs for cancer treatment, and some of such systems have already made their way to clinical trials (2, 12, 13).
Among various nanotechnology-based drug delivery systems, such as liposomes, nanomicelles, and nanotubes (7, 8, 14), nanomicelles have gained particular interest in cancer therapy by virtue of their appealing advantages such as high drug loading for effective therapeutic potency and small size (<30 nm) for deep tumor penetration (15, 16). The most common nanomicelles are constructed with lipids and amphiphilic polymers (12, 13). However, lipid-based nanomicelles have the drawback of limited stability, whereas polymers are plagued with dispersed molecular weight distribution. An optimal nanomicelles-based delivery system is expected to combine the advantages of lipid and polymer vectors while overcoming their shortcomings.
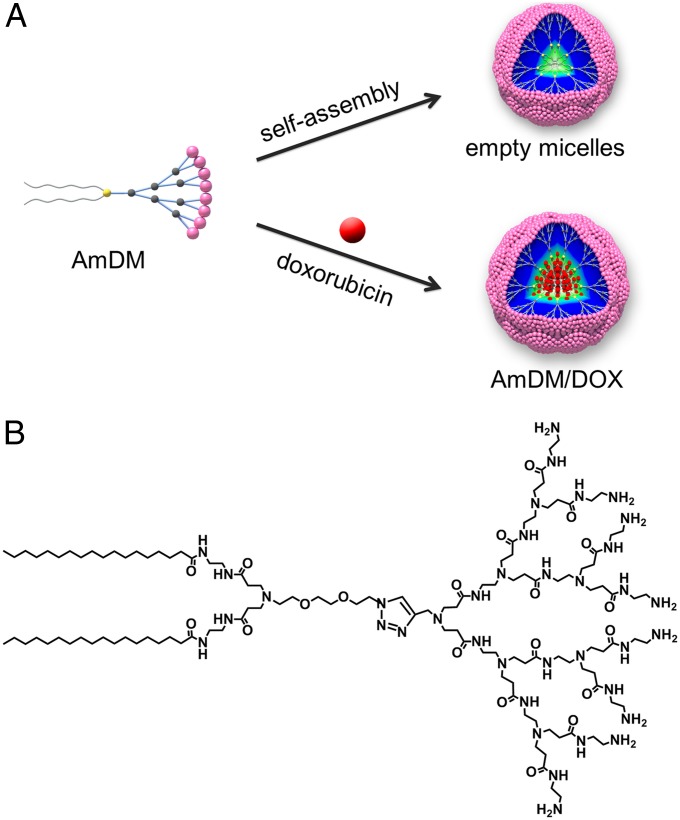
We have been particularly interested in establishing novel nanomicellar drug delivery platform based on amphiphilic dendrimers, i.e., hybrid molecules composed of a hydrophobic lipid entity entailing molecular self-assembly in a water environment, and a hydrophilic dendritic polymer component. Thus, nanoassemblies formed by amphiphilic dendrimers are able to combine the stability and mechanical strength of dendrimeric polymers and the micelle-forming feature of lipids (17). In addition, owing to the unique radiate dendritic structure, the nanomicelles composed by dendrimer amphiphiles harbor large void space within their inner cores, which can be harnessed for high drug-loading efficiency (Fig. 1A). Here, we report a nanomicelle-based drug delivery system generated by the dendritic amphiphile AmDM (Fig. 1B). Notwithstanding their small size (10 nm), these nanomicelles can carry the anticancer drug doxorubicin (DOX) at very high payload efficiency. The resulting AmDM/DOX system can effectively enhance anticancer drug efficiency and combat drug resistance through a combination of promoted cellular uptake and decreased drug efflux. Moreover, AmDM/DOX nanomicelles can significantly increase the therapeutic potency and reduce the systemic toxicity of doxorubicin in vivo by virtue of preferential tumor targeting via EPR effect alongside with deeper tumor penetration. This original nanomicelle delivery system based on the self-assembling amphiphilic dendrimer constitutes therefore a promising and effective drug carrier in cancer therapy.
Fig. 1.
Nanomicelles constructed with AmDM as a drug delivery platform. (A) Formation of empty AmDM nanomicelles and DOX-encapsulated AmDM/DOX nanomicelles. (B) Molecular structure of amphiphilic dendrimer AmDM.
Results and Discussion
Amphiphilic Dendrimer-Based Nanomicelles Encapsulate Doxorubicin with Small Size and High Drug Payload.
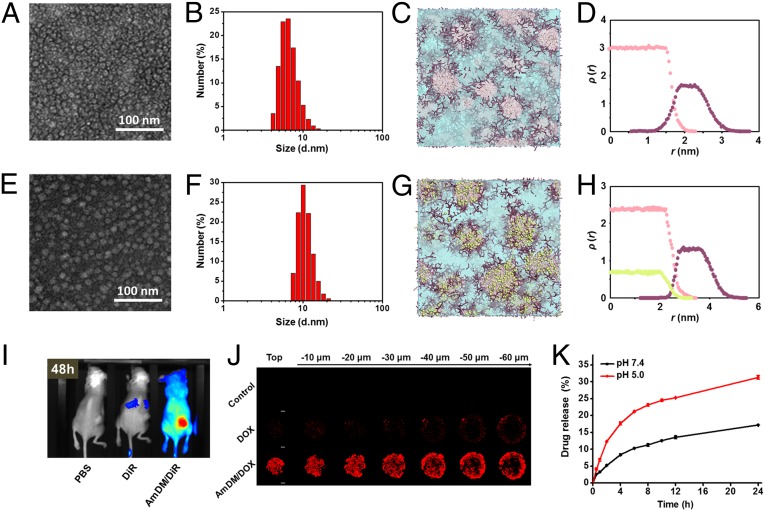
The amphiphilic dendrimer AmDM used in this work (Fig. 1B) bears a small hydrophilic poly(amidoamine) (PAMAM) (18, 19) dendron and two hydrophobic C18 alkyl chains bridged via click chemistry (20, 21). Using the film dispersion method, AmDM could easily form nanomicelles, as revealed by transmission electron microscopy (TEM) imaging (Fig. 2A) and dynamic light-scattering (DLS) analysis (Fig. 2B). The so-formed AmDM assemblies were spherical in shape (6.8 nm in diameter) and well-dispersed [polydispersity index (PDI): 0.21 ± 0.02] with a critical micelle concentration (CMC) of 3.2 μM (Fig. S1A). Computer simulations also predicted the aggregation of AmDM into small, spherical micelles, as shown in Fig. S2A. Insights into the structural properties of these micelles were further gained by performing mesoscale computational studies using dissipative particle dynamics (DPD) (22), a well-established technique in soft matter simulations to study amphiphilic systems at the nanoscale (23–25). These simulations highlighted the hydrophobic nature of the micellar core (Fig. 2C), whose average size Rcore was estimated to be 1.3 ± 0.2 nm. The hydrophilic portion of the micelles then forms a corona, shielding the core from the solvent environment, as proven by the corresponding density distribution profiles illustrated in Fig. 2D. The overall micellar radius Rm derived from DPD calculations was Rm 3.1± 0.3 nm, in line with the DLS and TEM results.
Fig. 2.
AmDM self-assembles into nanomicelles with a large void space in their inner core available for DOX encapsulation. (A) TEM image and (B) DLS analysis of AmDM nanomicelles. (C) Detailed mesoscale morphology of AmDM micelles. Micellar hydrophilic shell and hydrophobic core are highlighted as purple and pink sticks, respectively. Solvent is depicted as light blue field. (D) Density distributions of AmDM hydrophobic core (pink dots) and hydrophilic shell (purple dots) segments as a function of distance from the center of the micelle. (E) TEM image and (F) DLS analysis of AmDM/DOX nanomicelles. (G) Detailed mesoscale morphology of AmDM/DOX micelles: AmDM micelles and solvent represented as in D; DOX shown as green spheres. (H) Density distributions of AmDM hydrophobic core (pink dots), hydrophilic shell (purple dots) segments, and DOX (green dots) as a function of distance from the center of the micelle. (I) Fluorescent imaging of biodistribution of AmDM nanomicelles loaded with a near-infrared fluorescent dye, DiR, 48 h postadministration via i.v. injection in NSG mice bearing MCF-7R tumors. (J) Drug penetration of AmDM/DOX nanomicelles into 3D-cultured MCF-7R tumor spheroids imaged with a two-photon microscope. (K) Time course of DOX released from AmDM/DOX micelles at pH 5.0 and pH 7.4 at 37 °C.
We next constructed the anticancer drug DOX encapsulated AmDM assemblies using the same film-dispersion method. Gratifyingly, the resulting AmDM/DOX systems also consisted in spherical nanoparticles with a size of ∼10 nm (PDI: 0.32 ± 0.01) (Fig. 2 E and F). It is important to mention that small-sized nanoparticles are particularly beneficial for drug delivery in cancer therapy because they can successfully avoid kidney excretion and spleen sequestration; concomitantly, they are able to accumulate at tumor site and penetrate deeper into the inner regions of tumor lesions (16, 26, 27). Indeed, biodistribution experiments using a near-infrared fluorescent dye, DiR, demonstrated that the AmDM nanosystem had a longer circulation time, and was effectively accumulated and retained in the tumor (Fig. 2I and Fig. S3)—evidence that could indeed be ascribed to the EPR effect. Further drug penetration experiments using 3D-cultured tumor spheroids unambiguously showed that, by virtue of their small size, the AmDM/DOX nanomicelles could effectively penetrate deeper into the interior of the tumor spheroids with respect to free DOX (Fig. 2J). These data were further supported by the tumor penetration results obtained in vivo (Fig. S4).
Also of note is that the AmDM/DOX nanoparticles were slightly larger in size than the empty AmDM micelles, as evidenced by both TEM image and DLS analysis (Fig. 2 A, B, E, and F). This size difference could be reasonably ascribed to a micellar expansion required to accommodate doxorubicin within the hydrophobic micellar core to reach high loading (Fig. 1A). In silico mesoscale simulations confirmed that DOX-loaded AmDM micelles still exhibited a spherical core/shell architecture (Fig. 2G), with an average micellar radius Rm of 4.6 ± 0.2 and hydrophobic core radius Rcore of 2.5 ± 0.2 nm, respectively. The distribution of DOX inside each micelle was also obtained by plotting the relative density profiles. As shown in Fig. 2H, the distribution of the drug is consistent with that of the core segments, confirming that DOX is predominantly entrapped in the hydrophobic core of the micelle. Accordingly, these results confirm the hypothesis outlined above that the slight increase in micellar dimensions upon drug loading could be substantially ascribed to the increase of its inner core to accommodate the high DOX payload.
We further investigated drug-loading content and encapsulation efficiency of the AmDM/DOX micelles by varying dendrimer/drug ratio. The maximal drug-loading efficiency could be attained up to 42% (Fig. S2B). This high drug-loading capacity can stem from the unique lipid/dendrimer hybrid molecular structure of AmDM, which, by virtue of its branched dendritic hydrophilic component, generates micelles with low-density hydrophobic cores large enough to accommodate considerable amounts of drug payloads.
Drug Release from AmDM Nanomicelles Is Enhanced in Acid Environment.
A key point of a performing drug delivery system in cancer therapy is the controlled release of the therapeutic agent at the tumor site, reducing the toxicity to normal tissues. Tumor lesions are usually more acidic than normal tissues because of hypoxia microenvironment and acidic organelles of cancer cells; therefore, acid-promoted drug release is a beneficial advantage in cancer therapy (28, 29). We hence assessed doxorubicin release from the AmDM/DOX micelles under acidic condition (pH 5.0) in contrast to the neutral physiological pH 7.4. As exhibited in Fig. 2K, doxorubicin release from AmDM/DOX nanomicelles at pH 5.0 was more rapid and efficient than that at pH 7.4. This evidence could be rationalized taking into account the well-known proton sponge effect of the PAMAM dendrimer component of AmDM (20, 21). Indeed, at physiological condition (pH 7.4), only the terminal primary amines of the PAMAM dendron are protonated, but under acidic environment (pH 5.0), the tertiary amines in the interior of the dendron also become protonated, making the entire dendrimer entity highly positively charged (Fig. S1B); this, in turn, generates intense electrostatic repulsion among the dendrimer branches, ultimately leading to enhanced drug release. In addition, the encapsulated amine-bearing DOX can be more favorably charged at pH 5.0 than at pH 7.4; this would contribute extra electrostatic repulsion and further facilitate drug release under acidic condition. This acid-promoted drug release feature will endow the AmDM/DOX system with a preferential drug release profile at acidic tumor lesions rather than in normal tissues at neutral pH.
AmDM/DOX Nanomicelles Show Enhanced Antiproliferation Efficiency via Rapid and Effective Cellular Uptake.
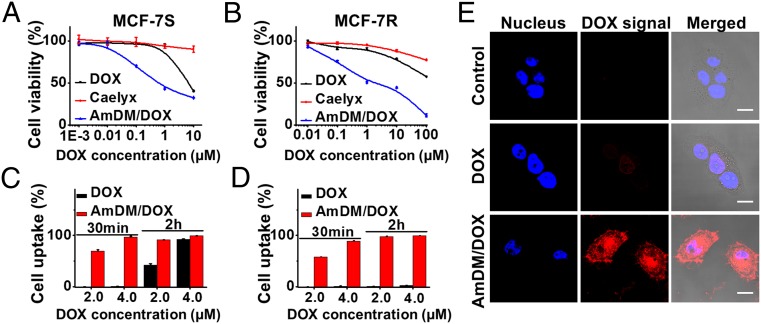
Impelled by the favorable drug release profile of AmDM/DOX nanomicelles, we went on to evaluate their antiproliferation efficiency in various cancer cells, including DOX-sensitive breast cancer MCF-7S cells, DOX-resistant breast cancer MCF-7R cells, castration-resistant prostate cancer PC-3 cells, hepatoma HepG2 cells, and cervical cancer HeLa cells (Fig. 3 and Fig. S5). In all cases, the antiproliferation effect of AmDM/DOX was much higher than that exerted by the clinical anticancer drug DOX and by the clinical DOX nanodrug Caelyx (Fig. 3 and Fig. S5). Specifically, in both DOX-sensitive MCF-7S cells and resistant MCF-7R cells, the IC50 values of AmDM/DOX (0.62 and 2.0 μM, respectively) were much lower than those of free DOX (6.1 and >100 μM) and of Caelyx (>100 μM). These results clearly demonstrated that AmDM/DOX micelles were considerably more effective than free drug DOX and nanodrug Caelyx in reducing cell proliferation in both drug-sensitive and drug-resistant cell lines, ultimately leading to apoptosis induced anticancer activity (Fig. S6).
Fig. 3.
AmDM/DOX nanomicelles show enhanced antiproliferation efficiency via rapid and effective cellular uptake. The antiproliferative activity of free DOX, DOX nanodrug Caelyx, and AmDM/DOX nanomicelles on (A) drug-sensitive breast cancer MCF-7S cells and (B) drug-resistant breast cancer MCF-7R cells was measured using an MTT assay. The cellular uptake in (C) MCF-7S and (D) MCF-7R cells was quantified using flow cytometry after treatment with DOX and AmDM/DOX, respectively. (E) The cellular uptake was imaged using confocal microscope following treatment with free DOX and AmDM/DOX in MCF-7R cells. (Scale bar: 10 μm.)
To understand the mechanisms underlying the enhanced antiproliferation activity of AmDM/DOX nanomicelles, we first inspected their uptake in both MCF-7S and MCF-7R cells using flow cytometry. Effectively, doxorubicin-encapsulated nanomicelles promoted the internalization and accumulation of doxorubicin in a dose- and time-dependent manner (Fig. 3 C and D); this was particularly significant in doxorubicin-resistant MCF-7R cells, where the cellular uptake was much faster and more effective with AmDM/DOX than with the free DOX. Indeed, the drug uptake in MCF-7R cells was practically null with free DOX after 2-h treatment (Fig. 3D); by contrast, the AmDM/DOX system could rapidly enter MCF-7R cells and led to substantial intracellular drug accumulation. This enhanced cellular uptake of AmDM/DOX in MCF-7R cells was further confirmed by confocal laser scanning microscopy (Fig. 3E). Collectively, these results suggest that AmDM/DOX nanomicelles successfully enter into both drug-resistant and drug-sensitive cancer cells via a rapid and efficient internalization.
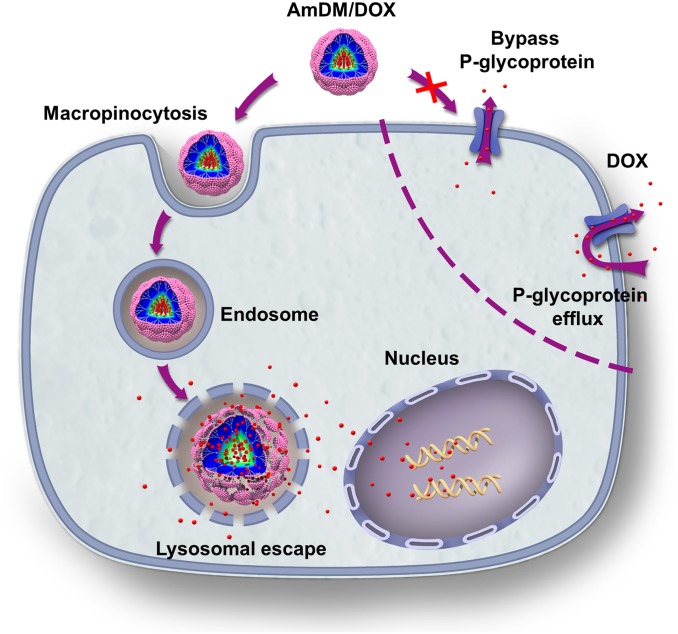
Further subcellular localization results showed that the doxorubicin fluorescence was partly colocalized with lysosomes, indicating that the AmDM/DOX nanomicelles were internalized and entered the lysosomes. Moreover, doxorubicin fluorescence was also observed in the cytoplasm, suggesting that the drug could successfully escape from the lysosomes into the cytoplasm (Fig. S7); this was mainly ascribed to the acidic environment of lysosomes (pH 4.0–5.0). As discussed previously, under these conditions, doxorubicin release from the AmDM micelles can indeed be facilitated by a combination of pH-dependent drug release and proton sponge effect of nanocarriers. Finally, the released doxorubicin from AmDM/DOX nanomicelles could effectively enter into nuclei (Fig. 3E), leading to the concrete anticancer activity. Together, these results qualify AmDM/DOX nanomicelles as promising drug delivery systems for cancer therapy, which significantly increase anticancer drug potency, even in drug-resistant cancer cells, through effectively enhanced drug uptake via nanoparticle formation.
AmDM/DOX Nanomicelles Combat Drug Resistance via Bypassing Drug Efflux Pumps and Inhibiting Drug Efflux.
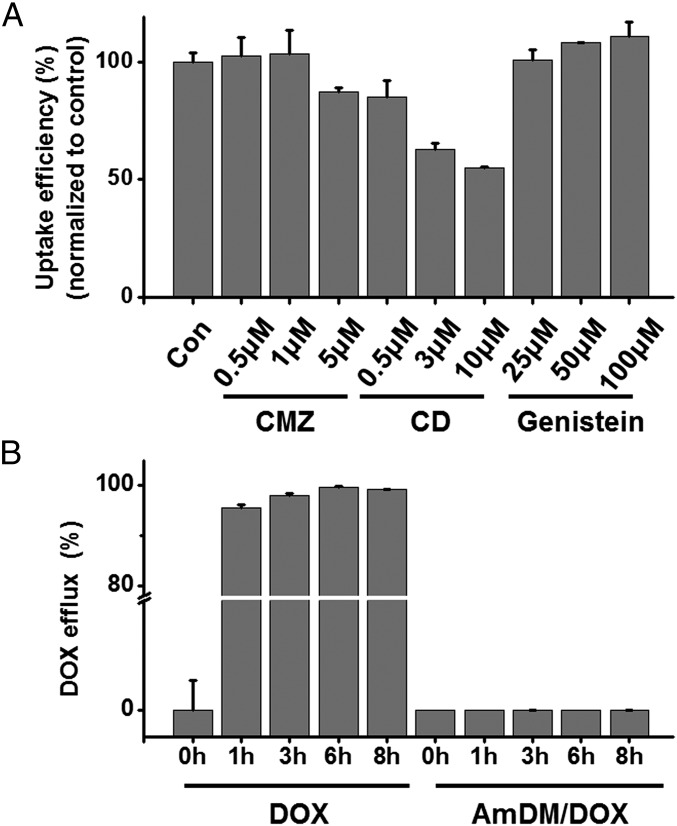
Drug resistance is often associated with the drug efflux pumps overexpressed on the cell membrane (30, 31). P-glycoprotein is reported to be the major efflux pump overexpressed on drug-resistant MCF-7R cells, opposing the cellular uptake of free DOX (32). The rapid and effective cell uptake of AmDM/DOX nanomicelles in MCF-7R is indicative of a nanoparticle-based cellular uptake pathway, which could bypass P-glycoprotein. We therefore investigated the uptake mechanism of AmDM/DOX nanomicelles using various endocytosis inhibitors (33) (Fig. 4A). Cytochalasin D (CD), an inhibitor of macropinocytosis-dependent endocytosis, significantly inhibited the internalization of AmDM/DOX in a dose-dependent manner, whereas neither genistein (an inhibitor of caveolae-mediated endocytosis) nor chlorpromazine (CMZ), an inhibitor of clathrin-mediated endocytosis, showed any notable inhibition on the cellular uptake (Fig. 4A). These results suggest that macropinocytosis was the main uptake mechanism by which AmDM/DOX could successfully bypass the recognition and capture of efflux proteins, contributing to the effective uptake of doxorubicin in drug-resistant cancer cells.
Fig. 4.
AmDM/DOX nanomicelles overcome drug resistance via macropinocytosis-mediated endocytosis and inhibition of drug efflux. (A) Inhibition of the uptake of AmDM/DOX micelles on MCF-7R cells using specific endocytosis inhibitors. CD: inhibitor of macropinocytosis; genistein: inhibitor of caveolae-mediated endocytosis; CMZ: inhibitor of clathrin-mediated endocytosis. (B) Inhibition of doxorubicin efflux by AmDM/DOX nanomicelles was determined in MCF-7R cells.
It is also of note that P-glycoproteins are able to efflux a broad variety of structurally and functionally distinct anticancer drugs, preventing adequate drug accumulation in cancer cells and, thus, leading to drug resistance (34). We therefore assessed the drug efflux in drug-resistant MCF-7R cells using flow cytometry. The result showed that most of the free DOX was eliminated from MCF-7R cells within 1 h, whereas little doxorubicin carried by AmDM/DOX nanomicelles was expelled even after 8 h (Fig. 4B). These results highlighted that doxorubicin encapsulated into AmDM/DOX nanoparticles could effectively escape the drug efflux action of the P-glycoprotein. Overall, the enhanced anticancer activity of AmDM/DOX could be primarily attributed to the significantly promoted cellular uptake and considerably decreased efflux of doxorubicin (Fig. 5).
Fig. 5.
Proposed mechanism to elude drug resistance by AmDM/DOX micelles in doxorubicin-resistant breast cancer MCF-7R cells.
Potent Anticancer Effect and Significantly Reduced Systemic Toxicity of AmDM/DOX Nanomicelles.
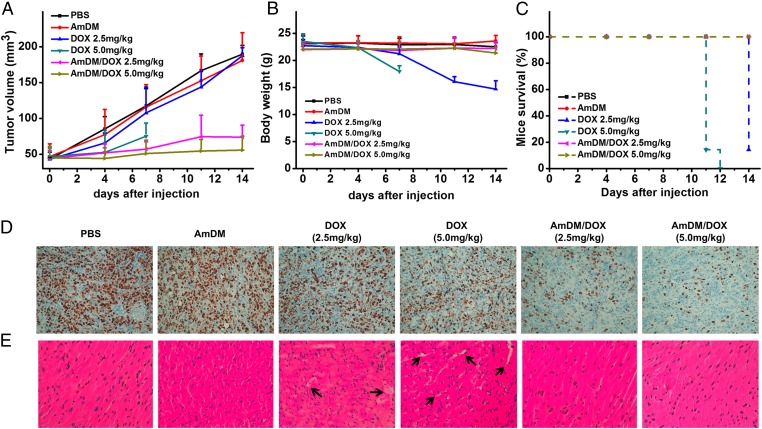
Encouraged by the promising in vitro results obtained with AmDM/DOX, alongside the good blood compatibility and nontoxic features of AmDM (Fig. S8), we performed in vivo experiments by assessing the tumor growth inhibition exerted by the AmDM/DOX nanomicelles in NOD scid gamma (NSG) mice bearing s.c. tumors derived from drug-resistant breast cancer MCF-7R cells. As shown in Fig. 6A, administration of free DOX only moderately retarded the tumor growth at high dosage (5.0 mg/kg), whereas little inhibition on tumor growth was observed at 2.5 mg/kg. However, treatment with AmDM/DOX nanomicelles significantly inhibited tumor growth at both dosages (2.5 and 5.0 mg/kg), which was further supported by the immunohistochemical (IHC) analysis to evaluate tumor cell proliferation. As shown in Fig. 6D, the Ki-67 level of AmDM/DOX treatment groups was much lower than those of other treatment groups, indicating lower cell proliferation in tumors and hence higher antitumor activity of AmDM/DOX. The markedly improved anticancer efficacy of the AmDM/DOX system can be ascribed to the beneficial nanomicellar formulation of DOX. Indeed, AmDM nanomicelles cannot only accumulate more efficiently at the tumor lesion via the EPR effect but also penetrate deeper within the tumor (Fig. 2 I and J and Figs. S3 and S4), generating higher local concentration of anticancer drug at the tumor site and, ultimately, exhibiting better antitumor activity compared with free drug.
Fig. 6.
AmDM/DOX nanomicelles significantly enhanced anticancer activity and reduced toxicity in tumor-xenograft mice. NSG mice were treated with free DOX and AmDM/DOX at doxorubicin dose of 2.5 and 5.0 mg/kg via i.v. administration (twice per week, n = 7). PBS, AmDM groups were used as control (n = 7). (A) Tumor volume and (B) mice body weight were measured twice per week. (C) Mice survival curves of different treatments. (D) Ki-67 IHC staining of tumor tissues to assess tumor proliferation. (E) Histological analysis of heart was determined to evaluate the toxicity.
During the entire treatment period, the mice treated with PBS, AmDM, and AmDM/DOX did not show any abnormal behavior, and no significant alteration of mice body weight (Fig. 6B) was observed. In contrast, considerable weight loss was registered in mice treated with free DOX (Fig. 6B). Moreover, due to the high toxicity of free DOX (35–37), only one mouse treated with the lower DOX dose of 2.5 mg/kg survived the treatment period (Fig. 6C). To further assess the associated toxicity of different treatments, we performed histological studies using hematoxylin-eosin-safran (HES) staining method. For the mice administrated with free DOX, hyperemia and myocardial fiber breakage was observed, especially at the high dose of 5.0 mg/kg (Fig. 6E), suggesting possible cardiotoxicity associated with DOX (35–38). Liver damage was also detected in mice treated with 5.0 mg/kg free DOX (Fig. S9). In contrast, no visible tissue damage was observed in AmDM/DOX treatment groups (Fig. 6E and Fig. S9). The drastically reduced toxicity of AmDM/DOX can be reasonably explained by the nanomicellar formulation of DOX, which can preferentially accumulate in the tumor via EPR effect, thus reducing possible toxicity in normal tissues. Collectively, these results confirm that AmDM/DOX nanomicelles effectively improve DOX therapeutic effect while reducing its systemic toxicity via the EPR effect.
Conclusions
In this work, we disclosed an original supramolecular nanomicellar system based on the amphiphilic dendrimer AmDM, which is able to effectively deliver the clinical anticancer drug DOX, enhance anticancer activity, and combat drug resistance while obviating systemic toxicity. This AmDM/DOX nanomicelle features several favorable advantages as therapeutic options in cancer therapy, including (i) high drug loading. This property is primarily ascribable to the unique dendritic structure which, upon self-assembly, results in micelles with large, low-density hydrophobic core able to accommodate substantial amount of drugs; (ii) small micellar size (10 nm) with narrow size distribution; such small-sized nanoparticles favor deeper penetration into inner tumor regions and concurrently prolong overall circulation time for beneficial accumulation in tumor tissue via EPR effect; (iii) rapid and effective cellular uptake mainly via boosted macropinocytosis; (iv) enhanced drug release at acidic pH, leading to advantageously promoted drug release at tumor lesions, normally more acidic than healthy tissues; and (v) effective inhibition of drug efflux. Based on these multiple advantages, AmDM/DOX micelles can effectively enhance DOX therapeutic efficacy and combat drug resistance. Concomitantly, AmDM/DOX nanomicelles can drastically reduce the systemic toxicity of doxorubicin in vivo through preferential delivery at tumor site via combined EPR effect and acid-promoted drug release. Given these encouraging results, this drug delivery system based on amphiphilic dendrimer AmDM constitutes a concrete promise as a therapeutic entity in cancer treatment.
Materials and Methods
A full description of the materials and methods is provided in SI Materials and Methods.
AmDM/DOX Nanomicelles: Preparation, Characterization, in Vitro Anticancer Activity, Cell Uptake, and Drug Efflux Inhibition.
AmDM/DOX nanomicelles were prepared using film-dispersion method and characterized for size, morphology, drug-loading, and drug-release properties using DLS, TEM, and fluorescent spectroscopy. The antiproliferation activities of AmDM/DOX against different cancer cell lines were evaluated using an MTT assay. Cell uptake and drug efflux inhibitory effect of AmDM/DOX were studied using flow cytometry and confocal fluorescent microscope. For further details, please see SI Materials and Methods.
Deeper Tumor Penetration of AmDM/DOX Nanomicelles.
Multicellular tumor spheroids constructed with MCF-7R cells were incubated with AmDM/DOX, then analyzed using a two-photon microscope by scanning the tumor spheroids step-by-step with 10 μm thickness to obtain Z-stack images. For further details, see SI Materials and Methods.
In Vivo Anticancer Activity Assay, in Vivo Biodistribution, in Vivo Toxicity Assay, Immunohistochemistry, and HES Staining.
Animal experiments were performed in agreement with the Animal Ethics Committee of the Bouche du Rhône prefecture in France and the institutional Animal Care and Use Committee of Peking University in China. Mice bearing subcutaneous tumors derived from MCF-7R cells were treated with AmDM/DOX via tail vein injection, with PBS, empty AmDM, and free DOX as control groups. Mouse body weight was recorded and tumor volume was measured to assess anticancer activity and possible toxicity. In vivo biodistribution, in vivo toxicity assay, immunohistochemistry, and HES staining were performed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Remy Castellano for his assistance with in vivo optical imaging (TrGET preclinical assay platform facility, Centre de Recherche en Cancérologie de Marseille, INSERM, U1068; Institut Paoli-Calmettes). We acknowledge Mr. Patrick Gibier, Mr. Jean-Christophe Orsoni, and Mr. Olivier Cabaud, as well as other members in the animal facility of Centre de Recherche en Cancérologie de Marseille for their great help during the performance of in vivo experiments. Financial support was provided by the CAI YUAN PEI program, Association pour la Recherche sur les Tumeurs de la Prostate, Chinese Natural Science Foundation Key Project 31430031, National Distinguished Young Scholars Grant 31225009, Institte National du Cancer, Canceropôle Provence-Alpes Côte d'Azur, Agence Nationale de la Recherche (Project SANAM), State High-Tech Development Plan Grants 2012AA020804 and SS2014AA020708, Association Française Contre les Myopathies, CNRS, INSERM, Aix-Marseille Université, CNRS-CAS PhD Fellowship, China Scholarship Council, Strategic Priority Research Program XDA09030301 and the external cooperation program of BIC (121D11KYSB20130006) of the Chinese Academy of Sciences. Access to supercomputer Eurora (Consorzio interuniversitario per la gestione del centro di calcolo elettronico dell'Italia nord orientale) was granted via the Simulation of Biological Systems Project Iscra Supercomputing Grant (to S.P.). Two-photon imaging was performed using France-BioImaging infrastructure supported by the Agence Nationale de la Recherche (ANR-10-INSB-04-01, call “Investissements d'Avenir”).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418494112/-/DCSupplemental.
References
- 1.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12(11):958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow EK-H, Ho D. Cancer nanomedicine: From drug delivery to imaging. Sci Transl Med. 2013;5(216):216rv4. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- 4.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 5.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 7.Hubbell JA, Chilkoti A. Chemistry. Nanomaterials for drug delivery. Science. 2012;337(6092):303–305. doi: 10.1126/science.1219657. [DOI] [PubMed] [Google Scholar]
- 8.Bourzac K. Nanotechnology: Carrying drugs. Nature. 2012;491(7425):S58–S60. doi: 10.1038/491s58a. [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65(1-2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 11.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schütz CA, Juillerat-Jeanneret L, Mueller H, Lynch I, Riediker M. NanoImpactNet Consortium Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine (Lond) 2013;8(3):449–467. doi: 10.2217/nnm.13.8. [DOI] [PubMed] [Google Scholar]
- 13.Svenson S. Clinical translation of nanomedicines. Curr Opin Solid State Mater Sci. 2012;16(6):287–294. [Google Scholar]
- 14.Schroeder A, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12(1):39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 15.Cabral H, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan VP, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percec V, et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science. 2010;328(5981):1009–1014. doi: 10.1126/science.1185547. [DOI] [PubMed] [Google Scholar]
- 18.Tomalia DA, et al. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules. 1986;19(9):2466–2468. [Google Scholar]
- 19.Tomalia DA, Naylor AM, Goddard WA. Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl. 1990;29(2):138–175. [Google Scholar]
- 20.Liu X, et al. Adaptive amphiphilic dendrimer-based nanoassemblies as robust and versatile siRNA delivery systems. Angew Chem Int Ed Engl. 2014;53(44):11822–11827. doi: 10.1002/anie.201406764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu T, et al. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew Chem Int Ed Engl. 2012;51(34):8478–8484. doi: 10.1002/anie.201203920. [DOI] [PubMed] [Google Scholar]
- 22.Groot RD, Warren PB. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J Chem Phys. 1997;107(11):4423–4435. [Google Scholar]
- 23.Barnard A, et al. Double-degradable responsive self-assembled multivalent arrays—temporary nanoscale recognition between dendrons and DNA. Org Biomol Chem. 2014;12(3):446–455. doi: 10.1039/c3ob42202j. [DOI] [PubMed] [Google Scholar]
- 24.Chang H-Y, Sheng Y-J, Tsao H-K. Structural and mechanical characteristics of polymersomes. Soft Matter. 2014;10(34):6373–6381. doi: 10.1039/c4sm01092b. [DOI] [PubMed] [Google Scholar]
- 25.Posocco P, et al. Self-organization of mixtures of fluorocarbon and hydrocarbon amphiphilic thiolates on the surface of gold nanoparticles. ACS Nano. 2012;6(8):7243–7253. doi: 10.1021/nn302366q. [DOI] [PubMed] [Google Scholar]
- 26.Huang K, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6(5):4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong C, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci USA. 2011;108(6):2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 29.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sosnik A. Reversal of multidrug resistance by the inhibition of ATP-binding cassette pumps employing “generally recognized as safe” (GRAS) nanopharmaceuticals: A review. Adv Drug Deliv Rev. 2013;65(13-14):1828–1851. doi: 10.1016/j.addr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446(7137):749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Lu Q, Hu X. Down-regulation of P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by honokiol. Cancer Lett. 2006;243(2):274–280. doi: 10.1016/j.canlet.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 33.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 35.Raj S, Franco VI, Lipshultz SE. Anthracycline-induced cardiotoxicity: A review of pathophysiology, diagnosis, and treatment. Curr Treat Options Cardiovasc Med. 2014;16(6):315. doi: 10.1007/s11936-014-0315-4. [DOI] [PubMed] [Google Scholar]
- 36.Octavia Y, et al. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Herman EH, el-Hage AN, Ferrans VJ, Ardalan B. Comparison of the severity of the chronic cardiotoxicity produced by doxorubicin in normotensive and hypertensive rats. Toxicol Appl Pharmacol. 1985;78(2):202–214. doi: 10.1016/0041-008x(85)90284-4. [DOI] [PubMed] [Google Scholar]
- 38.Maksimenko A, et al. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc Natl Acad Sci USA. 2014;111(2):E217–E226. doi: 10.1073/pnas.1313459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.