Significance
Humans are altering biodiversity globally and infectious diseases are on the rise; thus, there is considerable interest in understanding how changes to biodiversity affect disease risk. We show that the diversity of predators that consume parasites was the best negative predictor of infections in frogs, suggesting that predation on parasites can be an important mechanism of disease reduction. Follow-up experiments, field data, and mathematical models revealed that intraguild predators, predators that consume both hosts and parasites, decrease macroparasite infections per host less than predators that only consume parasites, representing a general trait of predators that predicts their ability to reduce disease. Consequently, managing assemblages of non-intraguild and intraguild predators is an underutilized tool to minimize human and wildlife diseases.
Keywords: biodiversity–ecosystem function, dilution effect, schistosomiasis, snail, trophic cascade
Abstract
Humans are altering biodiversity globally and infectious diseases are on the rise; thus, there is interest in understanding how changes to biodiversity affect disease. Here, we explore how predator diversity shapes parasite transmission. In a mesocosm experiment that manipulated predator (larval dragonflies and damselflies) density and diversity, non-intraguild (non-IG) predators that only consume free-living cercariae (parasitic trematodes) reduced metacercarial infections in tadpoles, whereas intraguild (IG) predators that consume both parasites and tadpole hosts did not. This likely occurred because IG predators reduced tadpole densities and anticercarial behaviors, increasing per capita exposure rates of the surviving tadpoles (i.e., via density- and trait-mediated effects) despite the consumption of parasites. A mathematical model demonstrated that non-IG predators reduce macroparasite infections, but IG predation weakens this “dilution effect” and can even amplify parasite burdens. Consistent with the experiment and model, a wetland survey revealed that the diversity of IG predators was unrelated to metacercarial burdens in amphibians, but the diversity of non-IG predators was negatively correlated with infections. These results are strikingly similar to generalities that have emerged from the predator diversity–pest biocontrol literature, suggesting that there may be general mechanisms for pest control and that biocontrol research might inform disease management and vice versa. In summary, we identified a general trait of predators—where they fall on an IG predation continuum—that predicts their ability to reduce infections and possibly pests in general. Consequently, managing assemblages of predators represents an underused tool for the management of human and wildlife diseases and pest populations.
In the last century there has been an unprecedented global increase in infectious diseases and decline and homogenization of biodiversity (1, 2). The controversial dilution effect hypothesis suggests that these two phenomena might be linked, specifically by proposing that biodiversity often decreases disease risk (3–11). Dilution effect research, for the most part, has focused on host diversity even though there is considerable evidence that selective predation on infected or uninfected hosts can strongly affect parasite transmission (7, 8) and that predation on parasites is widespread (9). As an example, in the well-studied Carpinteria Salt Marsh food web, 44% of trophic links are believed to involve predation on parasites (12). Despite the likely importance of predators to disease dynamics, we lack evidence supporting (i) the importance of predation to disease relative to more well-established factors known to affect parasite transmission, (ii) knowledge of environmental contexts that affect the impact of predators on disease, and (iii) the traits of predators that make them strong or weak “diluters” of disease risk [any species that reduces infections per focal host by removing parasites (equivalent to the solute] or serving as a less competent host than the focal host [equivalent to the solvent)]. This latter point is particularly important because it might facilitate identifying types of predators that can be managed to increase or decrease disease.
Many predators can consume both parasites and hosts (9), creating intraguild (IG) “predation” [IGP; predation can be substituted with natural enemy attack to capture both predators and parasites (13)] modules in food webs, defined as the killing and eating of potential competitors (14). These modules combine competition with predation and/or infection because the predator and parasite compete for a shared resource, the host, but at least one of the natural enemies can also benefit from consuming or infecting the other (13, 14). IGP is widespread, and it can structure and potentially stabilize communities (15, 16). However, it complicates predicting the impacts of predators on parasite transmission (9, 17, 18). For instance, by reducing the density of hosts, IG predators can increase the per capita exposure of the remaining hosts to parasites (10, 19), which could make IG predators weaker diluters of disease risk than predators that consume parasites but not the focal hosts (hereafter referred to as non-IG predators). In contrast, selective predation on infected hosts should reduce disease spread (7, 8). Additionally, IG predators often induce changes in traits of prey, such as behavior, growth, or morphology, which can also modulate parasite transmission (10, 17, 18). These effects can oppose or reinforce the reduction in parasite transmission associated with IG predators consuming parasites and it is the net effect of these potentially countervailing trait- and density-mediated indirect effects [TMIEs and DMIEs, respectively (20)] that will dictate the overall effect of predation on disease risk (18, 21).
Here, we use field surveys, experiments, and mathematical models to identify the mechanisms driving predation-dependent patterns of infection in a trematode–amphibian system. In this system, free-living, parasitic trematode cercariae are transmitted from snails, the first intermediate host, to tadpoles, the second intermediate host (22) (see SI Background for a description of the life cycle) and several vertebrate and invertebrate taxa are known to consume cercariae (e.g., refs. 9 and 23–25; see SI Background for more details). To develop our hypotheses, we turned to the rich literature of predator–prey interactions because it has a longer history than the host–parasite literature (18). Consistent with both the dilution effect hypothesis (3–5) and a meta-analysis that revealed that predator diversity on average supresses prey (26), we hypothesized that, at ecologically relevant densities, increased diversity of parasite predators would decrease trematode infections in frogs. We postulated that that this effect of parasite predators would be of a strength comparable to that of more well-established factors known to affect trematode abundance (discussed below). Finally, there is considerable evidence that the efficacy of predator diversity in controlling pest species is dependent on IG predation and nonconsumptive or trait-mediated effects (26–29). Consequently, we hypothesized that the effects of predators on parasite transmission would depend on the sum of density- and trait-mediated effects and the relative abundance of IG versus non-IG predator species.
Results and Discussion
Wetland Survey.
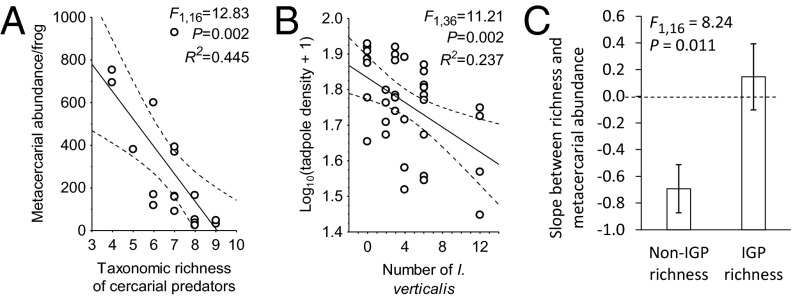
We surveyed 18 wetlands in Minnesota (see Fig. S1 for map) to evaluate whether the taxonomic richness of potential cercarial predators predicted the numbers of metacercariae (encysted cercariae) per frog per wetland and to evaluate its predictive ability relative to other plausible predictors of these infections, such as host (frog and snail) species richness; snail abundance; melanomacrophage densities in frogs (immune cells that fight trematodes); and concentrations of nitrate, phosphate, calcium, and the herbicide atrazine. The multimodel inference analyses revealed that taxonomic richness of potential cercarial predators was the best predictor of metacercarial infections per frog per wetland. The numbers of metacercariae per tadpole were lower in wetlands with more species of cercarial predators (sum of seven species of metacercariae; model averaged coefficient ± SE = −0.344 ± 0.121, F1,16 = 12.83, R2 = 0.45, P = 0.002; Fig. 1A and Table S1). Richness of cercarial predators appeared in >90% of the models with ΔAICc <4 and had a relative importance score of 0.96 (Table S1). A jackknife analysis revealed that the significance and direction of this effect was robust to the removal of individual taxa or even all dragonflies and damselflies (odonates) (Table S2), highlighting that an assemblage of cercarial predators was associated with the decline in metacercarial infection in frogs. The herbicide atrazine was the only other significant predictor of infections (Table S1), but it was a positive predictor supporting previous findings (30).
Fig. 1.
The relationship between the taxonomic richness of potential cercarial predators in a wetland and the number of metacercariae per frog per wetland (A), effects of the density of I. verticalis on the survival of R. clamitans tadpoles in a mesocosm experiment (B), and the standardized slope parameters (±1 SE) between the number of metacercariae per frog per wetland and either the taxonomic richness of non-IG (those that only eat cercariae) or IG predators (those that regularly eat cercariae and tadpoles) in a wetland (C) (see A for the relationship of the two groups combined). In the scatterplots, best-fit lines and 95% confidence bands are presented.
Given that the diversity of cercarial predators predicted infections in frogs, we next sought to elucidate mechanisms by which predators affect these infections. We focused on larval odonates as our predator guild because they are important predators of cercariae in ponds (23–25) and some species can also be predators of tadpole hosts (e.g., ref. 31).
Foraging Experiment Without Interspecific Interactions.
We first conducted laboratory experiments to quantify the cercarial foraging rates of several odonate species in the presence and absence of interspecific interactions (see below for results of interspecific interactions). All four larval odonate species significantly reduced cercariae through foraging (df = 4, χ2 = 63.45, P < 0.0001; all pairwise comparisons with control P < 0.0001; Fig. S2). The damselfly, Ischnura verticalis, had a higher cercarial foraging rate than that of the other three odonate species (all P < 0.0001; Fig. S2), which did not differ from one another (0.212 < P < 0.759; Fig. S2). Hence, based on cercarial foraging rates alone, I. verticalis might be expected to be the strongest diluter of disease risk.
Mesocosm Experiments.
We conducted an experiment to elucidate mechanisms by which density and diversity of larval odonates affect abundance of three species of parasitic trematodes in Rana clamitans (green frog) tadpoles. Each of these three trematode species was found commonly in our wetland survey (see ref. 27). This experiment used a 2 × 2 × 2 × 2 fully factorial design, which crossed the presence or absence of three species of odonates [late instar I. verticalis (damselfly), or early instar Pachydiplax longipennis or Sympetrum semicinctum (dragonfly)], with one of two odonate densities, 6 or 12 larvae. I. verticalis was the only IG predator. This design resulted in three odonate densities (0, 6, and 12 larvae) and four odonate diversity treatments (0–3). In our wetland survey, all wetlands had one to three odonate species (27.8%, 55.6%, and 16.7%, respectively) and the lower of the two densities in our experiment is more ecologically relevant (32); thus, we focus on the richness and density levels most commonly found in the field (see SI Results and Discussion for a discussion of the other richness and density levels).
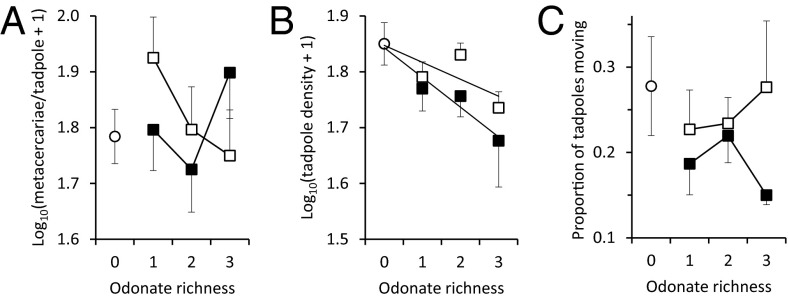
We first tested for main and interactive effects of odonate density and diversity on total metacercariae per tadpole [excluding the zero diversity treatment so it was not a missing cells design; multivariate ANOVA (MANOVA) on all three trematode species and univariate ANOVAs produced similar results, Table S3]. At the lowest odonate density, total metacercariae per tadpole decreased with increasing odonate richness from one to three species (Fig. 2A). At the highest odonate density, total metacercariae per tadpole decreased as odonate richness went from one to two species but increased from two to three species (density: F1,45 = 5.82, P = 0.020; diversity: F1,45 = 4.17, P = 0.047; interaction: F1,45 = 4.07, P = 0.050; Fig. 2A).
Fig. 2.
Results of the mesocosm experiment showing effects of larval odonate density (0, 6, or 12 individuals; ○, □, and ■, respectively) and diversity (zero to three species) on (A) metacercarial infections per tadpole, (B) tadpole survival (i.e., density), and (C) tadpole activity. Shown are means ±1 SE. See text for sample sizes and statistics.
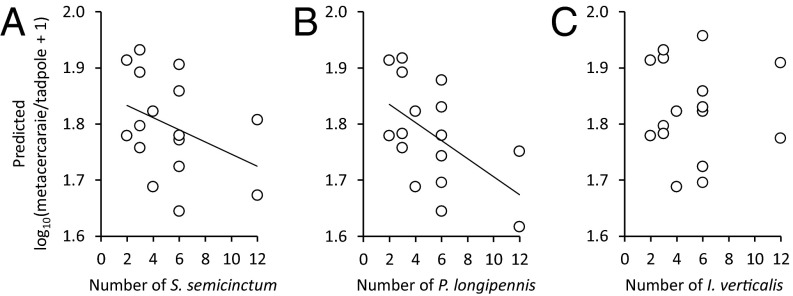
The previous statistical analysis ignored odonate species identity. To elucidate which odonate species were driving the density and diversity patterns in infections, we tested for the main effects of overall odonate density (a continuous predictor), each odonate species, and all two-way interactions between odonate species. There were no significant interactions between odonate species (P > 0.115), suggesting that interspecific interactions were not driving the infection patterns. Additionally, each odonate species had similar effects on each trematode species, providing little evidence that odonates specialized or had search images for particular cercarial species. Hence, in this experiment, we had little evidence of niche complementarity (see refs. 28 and 33) as a mechanism for the predator diversity effects on parasite transmission. The MANOVA revealed significant negative effects of densities of the non-IG predators, S. semicinctum (Wilk’s F4,42 = 3.28, P = 0.020; Fig. 3A) and P. longipennis (Wilk’s F4,42 = 2.83, P = 0.036; Fig. 3B), on total metacercariae per tadpole. However, despite being the most voracious cercarial predator in our foraging experiment (Fig. S2), I. verticalis densities surprisingly did not reduce metacercarial infections in tadpoles (Wilk’s F4,42 = 0.44, P = 0.777; Fig. 3C).
Fig. 3.
Relationships between metacercariae per tadpole and the densities of two non-IG predators (those that only eat cercariae), (A) S. semicinctum and (B) P. longipennis and (C) an IG-predator (eats cercariae and tadpoles), I. verticalis. Shown are the predicted values from a model with temporal block and the main effects of each species, the presence-only treatments for each species, and best-fit lines for significant relationships.
Next, we sought to elucidate the mechanisms for the observed patterns in metacercariae per tadpole (Fig. 2). We postulated that the observed patterns in metacercarial infections were driven by some combination of differences among treatments in (i) tadpole densities that affected per capita exposure to cercariae (10), (ii) tadpole growth rates and thus resources available for immunity (34), (iii) odonate interspecific interactions that affected their cercarial foraging rates, (iv) tadpole anticercarial behaviors (35–37), and (v) the relative abundance of diluting versus nondiluting odonates species. We conducted several additional analyses and experiments—taking a hypothetico-deductive approach—to gather support for or against each of these hypotheses.
Tadpole survival at the end of the experiment was negatively associated with both odonate density (F1,55 = 6.03, P = 0.017) and diversity (F1,55 = 6.03, P = 0.017; includes the zero diversity controls; Fig. 2B), but this entire effect seemed to be driven by I. verticalis, which was the only odonate species that was observed depredating tadpoles and was the only odonate for which its density was negatively associated with final tadpole density (β ± SE = −0.487 ± 0.146, F1,36 = 11.21, P = 0.002; Fig. 1B; other two species P > 0.805). Metacercariae per tadpole was a nonmonotonic function of diversity and density (Fig. 2A), whereas tadpole densities seemed to decline monotonically with odonate density and diversity, suggesting that tadpole density alone could not completely account for the pattern in metacercarial infections (Fig. 2B). Additionally, we found no evidence that available resources for immunological resistance or odonate interspecific interactions on cercarial foraging rates could account for the observed infection patterns across treatments (SI Results and Discussion and Fig. S3).
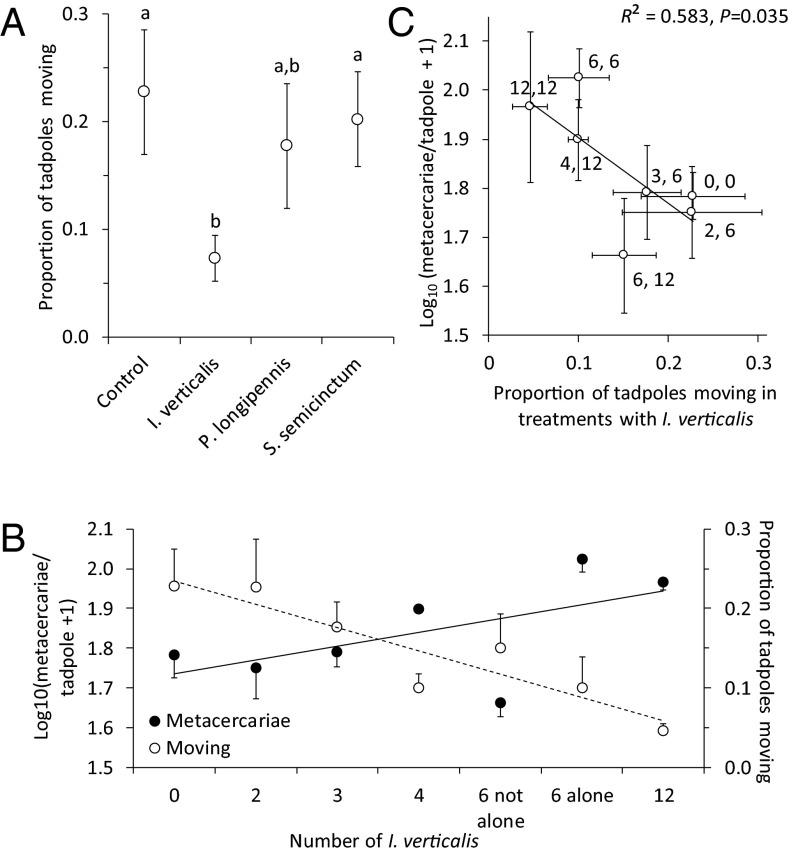
We also hypothesized that the pattern of infections across treatments was a function of tadpole antiparasite behaviors and the relative abundance of odonate species that did and did not reduce infections. Given that I. verticalis consumed tadpoles, that many amphibians possess alarm chemicals that can reduce their activity and affect their space use (38, 39), and that activity and cercarial avoidance are well-documented anticercarial behaviors (35–37), we hypothesized that I. verticalis density would decrease tadpole activity, increasing metacercarial infections. Indeed, I. verticalis was the only species that significantly reduced activity in monospecific tanks relative to controls (Fig. 4A). Importantly, the general pattern of metacercariae per tadpole as a function of diversity and density treatments (Fig. 2A) was the inverse pattern of tadpole activity (Fig. 2C), suggesting a cause–effect relationship. This is likely a product of the relative abundance of I. verticalis generally decreasing and the relative abundance of the “diluters,” S. semicinctum and P. longipennis, generally increasing as diversity increased (Figs. 2A and 4), a mechanism that also seems to drive the classic dilution effect observed in the Lyme disease system (albeit, in that case, via increased relative abundances of competent hosts) (4, 40, 41). Indeed, as I. verticalis density rose, tadpole activity generally decreased and metacercariae per tadpole generally increased (Fig. 4B). Furthermore, infections per tadpole declined with the proportion of tadpoles active in I. verticalis treatments (F1,5 = 5.23, P = 0.035; Fig. 4C). These findings are consistent with other studies that have shown that predator-induced reductions in host activity can increase metacercarial infections (10, 37, 42) and suggest that there are tradeoffs between defenses against parasites and predators that warrant further research (18, 34, 43).
Fig. 4.
Relationships between tadpole activity and metacercarial infections in R. clamitans tadpoles. (A) Effects of two non-IG predators (those that only eat cercariae), S. semicinctum and P. longipennis, and an IG-predator (eats cercariae and tadpoles), I. verticalis, in monospecific treatments on tadpole activity. Points with different letters are significantly different from one another (P < 0.05). (B) Tadpole activity and metacercarial loads as a function of the density of the IG predator I. verticalis, the only odonate species that significantly reduced tadpole activity. (C) Relationship between tadpole activity in treatments with I. verticalis and metacercarial infections per tadpole. The first number next to each point represents the number of I. verticalis in that treatment and the second number represents the total number of odonate larvae in that treatment. In each panel, means and 1 SE are displayed.
In summary, the reduction in trematode infections in tadpoles at higher densities of odonates (density: F1,45 = 5.82, P = 0.020) seems to be best explained by the abundance of S. semicinctum and P. longipennis, species that did not directly affect tadpole densities or behaviors (Fig. 4A) but that reduced tadpole exposure to parasites by consuming cercariae (Fig. 3 A and B). The diversity and diversity-by-density effects seem to be driven predominantly by the relative abundance of the non-IG predator species relative to I. verticalis, an IG predator that had opposing DMIEs and TMIEs on metacercarial infections, which likely explains the lack of a significant relationship between I. verticalis densities and numbers of metacerciae in tadpoles (Fig. 3C), despite its being the most voracious cercarial predator tested. Hence, the overall relationship between predator diversity and parasite transmission was a product of IG predation, nonconsumptive predator effects, and sampling (the increasing probability that species with traits that suppress pests increase as predator richness increases) rather than niche complementarity effects (see refs. 28 and 33). Although we did not find evidence for niche complementarity, it is possible that under natural conditions with more habitat complexity than in our mesocosms niche complementarity might be important.
Mathematical Model.
Given that the IG predator in our mesocosm experiment reduced infections less than the non-IG predators, we developed a mathematical model to evaluate whether IG predators generally caused weaker reductions in disease risk than non-IG predators. The model was derived from the classic macroparasite model by Anderson and May (44) and it is similar to a macroparasite–host–predator model examined by Packer et al. (8). The model includes differential equations for populations of intermediate hosts, focal hosts, infections in focal hosts, and free-living parasites (analogous to the snails, tadpoles, metacercariae in tadpoles, and cercariae in our experimental system, respectively), and incorporates top-down effects of a predator guild that can consume hosts and/or free-living parasites. Although in our experimental system antipredator behaviors (reduced activity) are opposite of antiparasite behaviors (increased activity), this might not be the case in other host–parasite systems and thus we conservatively did not include trait-mediated effects in our model.
Predation on the intermediate host had effects qualitatively similar to predation on free-living parasites that are released from these hosts (SI Results and Discussion), and thus we focus on contrasting the effects of predation on free-living parasites (non-IG predator) versus predation on both free-living parasites and focal hosts (IG predator). Intuitively, the model demonstrated that non-IG predators that only consumed free-living parasites increased focal host densities, reduced the population of parasites infecting focal hosts, and reduced mean burdens in focal hosts (Fig. 5 A and B; see also Fig. S4 and SI Results and Discussion). In contrast, the effect of IG predators was nonlinear. If IG predators preferred free-living parasites more than focal hosts or preferred infected focal hosts to uninfected focal hosts [i.e., healthy herd effect 7, 8)], they only weakly reduced infections on focal hosts, but if they strongly preferred focal hosts over free-living parasites, they could increase mean parasite abundance per focal host by increasing the per capita exposure rate of the surviving hosts (Fig. 5C; see also Fig. S4 and SI Results and Discussion). Mechanistically, this occurs because predation on either focal hosts or free-living parasites decreases the total population size of adult parasites (P*, Fig. 5B), but predation on hosts causes host density to decrease rapidly (H*, Fig. 5A). If host density decreases more rapidly than parasite density, then mean burdens can increase (P*/H*, Fig. 5C). This amplification effect is further supported by a partial solution for mean parasite burden at equilibrium (SI Results and Discussion). Hence, consistent with the experimental results, the model indicates that non-IG predators should generally reduce macroparasite infections per host more so than IG predators.
Fig. 5.
Epidemiological consequences of simultaneous predation on focal hosts and free-living parasites (correspond with tadpoles and cercariae in our experimental system) at equilibrial values. (A) Increasing predation on free-living parasites increases the equilibrium density of focal hosts. However, increasing predation on focal hosts strongly reduces their equilibrium density. (B) Both types of predation reduce the equilibrial density of parasitic infections within focal hosts. (C) Predation on free-living parasites causes a monotonic decrease in equilibrial mean burden of infection for individual focal hosts. In contrast, predation on focal hosts causes a unimodal response. Initially, predation on focal hosts slightly reduces mean burdens. However, as predation on focal hosts increases, equilibrial mean burden rises, eventually surpassing burdens in the absence of predation. Thus, predators of free-living parasites monotonically reduce mean burdens for definitive hosts. However, predators of focal hosts can only weakly reduce mean burden or even amplify infection risk for hosts. Simulation parameters are identical to those in Fig. S4.
Test of IG Predator Predictions in the Field.
Given that our experiment and mathematical model suggest that non-IG predators should reduce infections per host more so than IG predators, we returned to our wetland survey to test this hypothesis in the wild. As predicted, trematode infections in frogs decreased much more steeply with the richness of non-IG predators than with the richness of IG predators, which did not differ from zero (Fig. 1C).
Conclusions
Our wetland survey uncovered a negative relationship between the diversity of potential cercarial predators and the total abundance of metacercariae in tadpoles. To our knowledge, this is the first field study comparing the predictive strength of predators of parasites to other factors known to affect parasite transmission. Our findings indicate that cercarial predators might have a larger influence on metacercarial infections in frogs than the richness and abundance of first and second intermediate hosts, frog immunity, and nutrients associated with elevated snail populations and trematode infections (30, 45) (Table S1). Moreover, the influence of cercarial predators on metacercarial infections in frogs was of comparable strength (but in the opposite direction) to the previously reported impacts of the herbicide atrazine on such infections (Table S1) (30).
Our experiment demonstrated that larval odonate density, diversity, and species composition, IGP, TMIEs, DMIEs, and a sampling effect (sensu ref. 33) all affected the abundance of metacercarial infections in anuran larvae. More specifically, under conditions that were most common in the field, increasing odonate richness reduced metacercarial infections in tadpoles, a pattern consistent with what was observed in our field survey. However, in the experiment and in the field, most of the decrease in infections per host was driven by the non-IG predators and our mathematical model suggests that non-IG predators should generally reduce macroparasite infections per host more so than IG predators.
Our findings have many similarities to the generalities that have emerged from the predator diversity–biocontrol literature. For example, similar to evidence that guilds of predators on average control pests better than single predator species (26–28), our findings suggest that entire guilds of predators can also regulate infections in hosts. Thus, managing predator assemblages might be more effective than managing single predator species to control disease (see SI Results and Discussion for examples). Again similar to our results, the predator diversity–biocontrol literature provides evidence that non-IG predators exhibit stronger biocontrol than IG predators and that biocontrol can be influenced by nonconsumptive effects of predators (26–29; see SI Results and Discussion for additional details on similarities). Overall, these similarities suggest that there might be general mechanisms for pest control regardless of whether the pest is a pathogen or consumer; thus, the general conclusions for disease control might match those for pest control. Specifically, releasing multiple non-IG predators will likely provide better pathogen suppression than releasing a single control agent (26), but the release of IG predators could decrease or increase pathogens, and thus the release of the single best control agent might provide better suppression of pathogen populations on average than the release of IG predators (29). Regardless of whether these conclusions hold, it seems clear that biocontrol research might inform disease management, and vice versa (18).
Finally, whereas we identified a general trait of predators—where they fall on an IG predation continuum—that predicts their ability to reduce disease and possibly pests in general, recent studies suggest that there might also be general traits of host species that predict their ability to dilute or amplify disease risk (46, 47) and herbivory (48). Consequently, to enhance infectious disease management and biocontrol we encourage further work that searches for traits of host and nonhost species that might be useful indicators of species that can increase or decrease parasite and pest populations.
Materials and Methods
Wetland Survey.
Methods of our wetland survey were reported in Rohr et al. (30) and Schotthoefer et al. (49) and thus we relegated a summary of these methods to SI Materials and Methods. The following taxa were considered potential predators of cercariae because they either filter-feed or actively feed on planktivorous prey of similar size to cercariae, or consume snails that can harbor cercariae: the insects Dytiscidae, Hydrophilidae, Chaoboridae, Belostomatidae, Corixidae, Nepidae, Notonectidae, Pleidae, Aeshnidae, Coenagrionidae, Libellulidae, other dragonfly and damselfly families, amphipods, and crayfish. Dytiscidae, Aeshnidae, damselflies, and crayfish were considered IG predators because they also regularly consume tadpoles.
Odonate Foraging Experiments.
To quantify the foraging rates of odonate larvae on cercariae, we exposed Echinostoma trivolvis cercariae to one of five odonate predator treatments: a nonpredator control to estimate background mortality of cercariae, one Anax junius larva, one E. simplicicolis larva, one S. semicinctum larva, or one I. verticalis larva (head widths of all odonates ranged from 2.11 to 3.21 mm). Fifty cercariae were transferred to a plastic predation arena (8.5 × 6.5 × 2.3 cm) containing 100 mL of water and an odonate larva. After 1 h we counted the number of cercariae that remained (see SI Materials and Methods for details).
We conducted a follow-up experiment to test whether interspecific interactions among the odonate species affected their cercarial foraging rates. The experiment had the same methods as the previous experiment except that there were eight treatments: a nonpredator control, one or three A. junius larvae, one or three P. longipennis larvae, or one or three I. verticalis larvae, and an interspecific interaction treatment where there was one of each of the three odonate species.
Mesocosm Experiment.
To examine whether larval odonate density and diversity influence trematode infections in amphibians, we conducted a 2 × 2 × 2 × 2 experiment in which the first three factors were the presence or absence of three species of odonates and the last factor was one of two odonate densities, 6 or 12 larvae per replicate. Each treatment was replicated two times in each of two temporal blocks (9-d duration each), with the exception of four replicates of the nonpredator control in each block. The experiment was conducted in clear rectangular plastic tubs (38 × 25 × 15 cm filled with 10 L of filtered pond water) each with 10 R. clamitans tadpoles (Gosner stage 25). To expose the tadpoles and odonates to cercariae, replicates received a single Planorbella trivolvis snail that was infected with one of three trematode species: E. trivolvis, Ribeiroia ondatrae, or a species from family Plagiorichiidae. The snails were rotated through the replicates so that tadpoles were exposed to each snail and each trematode species for the same amount of time, thus homogenizing tadpole exposure to the cercariae. There was no snail mortality during the experiment but some odonate larvae died and were replaced to maintain consistency in the predator treatments.
Daily scan samples (9:00 AM and 4:00 PM) were used to quantify tadpole activity levels (number of tadpoles moving in 10 s). Tadpoles that died during the experiment were preserved in 70% ethanol and were not replaced. At the end of the experiment, the remaining tadpoles were counted, weighed, killed in 0.05% benzocaine, preserved in 70% ethanol, and cleared and stained to quantify the number of metacercarial infections of each trematode species, as described by Rohr et al. (21) (see SI Materials and Methods for additional details).
Mathematical Model.
To capture long-term dynamics and feedbacks that are important to IGP (50), our model used ordinary differential equations to track changes in the densities of focal (could be second intermediate or definitive) hosts, H, parasites that successfully infected hosts, P, intermediate hosts, I, and free-living parasites, Z. (Eqs. 1a–1d):
| [1a] |
| [1b] |
| [1c] |
| [1d] |
Focal hosts increase through density-dependent births, as determined by a maximum rate, bH, and a host carrying capacity, KH (Eq. 1a). Focal hosts are lost because of background deaths, at mortality rate, dH, predation by consumers, C, at per capita feeding rate, fH, and from parasitic infection, with virulence on survival, v. Parasitic infections of focal hosts, P, increase when hosts become infected by free-living parasites, Z. Following exposure (at per capita exposure rate, ε), hosts can become infected according to their per-parasite susceptibility, σ (0 ≤ σ ≤ 1; Eq. 1b). Parasitic infections decrease when hosts die (from background mortality, predation by consumers, or parasite virulence). The final term in Eq. 1b accounts for additional losses from parasite-induced mortality that occur because parasites are aggregated in focal hosts, indexed by θ, the aggregation parameter of the negative binomial distribution. Intermediate hosts also increase through density-dependent births, as determined by a maximum rate, bI, and their carrying capacity, KI (Eq. 1c). They are also lost from background deaths, at mortality rate, dI, and predation by consumers, C, at per capita feeding rate, fI. Finally, free-living parasites increase because of release (at per capita rate γ) by infected intermediate hosts (with infections assumed to be a saturating function of P, governed by the half-saturation constant, q; Eq. 1d). Free-living parasites are then lost following contact with focal hosts, ε, predation by consumers, fZ, or from background mortality, dZ.
The model assumes that the intermediate host population produces free-living parasites at a constant per capita rate. Additionally, we chose not to dynamically couple the predator guild to host or free-living parasite densities because most, if not all, of the predators in our focal system are broad generalists. A full analytical solution for this model is intractable, but we gained insight by examining a partial solution for mean parasite burden at equilibrium, P*/H*, by setting Eq. 1a equal to zero. Finally, we numerically simulated the model across a range of reasonable values for the predation rate on hosts, fH, and free-living parasites, fZ, with the lsoda function from the deSolve package in R statistical software and determined the equilibrium values of (i) focal host density, (ii) parasite density, and (iii) mean parasite burden in focal hosts for each simulation. See Fig. 5 and Fig. S4 for the state variables and parameters used in the epidemiological model.
Statistical Analyses.
For our field study, we used a multimodel inference approach in R statistical software (dredge and model.avg functions in the MuMIn package) to evaluate the importance of the taxonomic richness of potential cercarial predators to the number of metacercariae per frog per wetland (treating the wetland as the replicate) relative to other plausible predictors of this response variable (see Materials and Methods, Wetland Survey above). We limited the maximum number of variables in any model to three. To assess whether IG predators were generally weaker diluters than non-IG predators, we tested for a significant difference in the slope parameters between metacercariae per frog per wetland and the richness of IG and non-IG predators (nesting IG and non-IG predator richness within wetland).
For the two odonate foraging experiments, the response variable was the number of cercariae that were missing out of 50, the error distribution was binomial, each cercaria was nested within its test arena/replicate, and replicate was treated as a random variable to ensure proper error structure (lmer function in the lme4 package). For the mesocosm experiment, analyses were conducted with a general linear model with log trematode load as a response variable, temporal block as a random effect, and log diversity, log density, and their interaction as predictors for analyses focusing on richness and log density of each of the three odonate species as predictors for analyses focusing on species composition. We did not use a generalized linear model with a negative binomial error distribution because we were interested in conducting a multivariate analysis that incorporated all three, nonindependent trematode species in each tank as response variables and we are unaware of any multivariate analog for negative binomial error distributions. To test whether the richness, density, and odonate species had different effects on the abundance of the three metacercarial species, we conducted the same analyses as above, but nested trematode species within tank and tested for interactions between the predictors and this within-tank, trematode species factor.
Supplementary Material
Acknowledgments
We thank M. Piwoni, J. Murphy, C. Johnson, A. Tremle, C. Lieske, N. Mateus-Pinilla, and the many highly skilled and dedicated technicians and undergraduate students who worked in our expansive field and/or laboratory studies, and B. Sears, T. McMahon, and anonymous reviewers for comments. This work was supported by grants to J.R.R. from the US Department of Agriculture (NRI 2008-00622 20 and 2008-01785), US Environmental Protection Agency (STAR R83-3835 and CAREER 83518801), National Institutes of Health (R01GM109499), and National Science Foundation (NSF) (DEB 0516227 and EF-1241889), and to V.R.B. from the US Environmental Protection Agency (Grant STAR R825867). P.W.C. was supported by a Research Opportunity Award Supplement to NSF Grant DEB 0516227.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415971112/-/DCSupplemental.
References
- 1.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olden JD, Leroy Poff N, Douglas MR, Douglas ME, Fausch KD. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol Evol. 2004;19(1):18–24. doi: 10.1016/j.tree.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Dobson A, et al. Sacred cows and sympathetic squirrels: The importance of biological diversity to human health. PLoS Med. 2006;3(6):e231. doi: 10.1371/journal.pmed.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keesing F, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9(4):485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PTJ, Thieltges DW. Diversity, decoys and the dilution effect: How ecological communities affect disease risk. J Exp Biol. 2010;213(6):961–970. doi: 10.1242/jeb.037721. [DOI] [PubMed] [Google Scholar]
- 7.Ostfeld RS, Holt RD. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front Ecol Environ. 2004;2(1):13–20. [Google Scholar]
- 8.Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP. Keeping the herds healthy and alert: Implications of predator control for infectious disease. Ecol Lett. 2003;6(9):797–802. [Google Scholar]
- 9.Johnson PTJ, et al. When parasites become prey: Ecological and epidemiological significance of eating parasites. Trends Ecol Evol. 2010;25(6):362–371. doi: 10.1016/j.tree.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Raffel TR, Hoverman JT, Halstead NT, Michel PJ, Rohr JR. Parasitism in a community context: Trait-mediated interactions with competition and predation. Ecology. 2010;91(7):1900–1907. doi: 10.1890/09-1697.1. [DOI] [PubMed] [Google Scholar]
- 11.Venesky MD, Liu X, Sauer EL, Rohr JR. Linking manipulative experiments to field data to test the dilution effect. J Anim Ecol. 2013;83(3):557–565. doi: 10.1111/1365-2656.12159. [DOI] [PubMed] [Google Scholar]
- 12.Lafferty KD, Dobson AP, Kuris AM. Parasites dominate food web links. Proc Natl Acad Sci USA. 2006;103(30):11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borer ET, Briggs CJ, Holt RD. Predators, parasitoids, and pathogens: A cross-cutting examination of intraguild predation theory. Ecology. 2007;88(11):2681–2688. doi: 10.1890/06-1707.1. [DOI] [PubMed] [Google Scholar]
- 14.Polis GA, Myers CA, Holt RD. The ecology and evolution of intraguild predation: Potential competitors that eat each other. Annu Rev Ecol Syst. 1989;20:297–330. [Google Scholar]
- 15.Bascompte J, Melian CJ. Simple trophic modules for complex food webs. Ecology. 2005;86(11):2868–2873. [Google Scholar]
- 16.Arim M, Marquet PA. Intraguild predation: A widespread interaction related to species biology. Ecol Lett. 2004;7(7):557–564. [Google Scholar]
- 17.Hatcher MJ, Dick JTA, Dunn AM. How parasites affect interactions between competitors and predators. Ecol Lett. 2006;9(11):1253–1271. doi: 10.1111/j.1461-0248.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 18.Raffel TR, Martin LB, Rohr JR. Parasites as predators: Unifying natural enemy ecology. Trends Ecol Evol. 2008;23(11):610–618. doi: 10.1016/j.tree.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Civitello DJ, Pearsall S, Duffy MA, Hall SR. Parasite consumption and host interference can inhibit disease spread in dense populations. Ecol Lett. 2013;16(5):626–634. doi: 10.1111/ele.12089. [DOI] [PubMed] [Google Scholar]
- 20.Werner EE, Peacor SD. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84(5):1083–1100. [Google Scholar]
- 21.Rohr JR, Raffel TR, Sessions SK, Hudson PJ. Understanding the net effects of pesticides on amphibian trematode infections. Ecol Appl. 2008;18(7):1743–1753. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- 22.Koprivnikar J, et al. Macroparasite infections of amphibians: What can they tell us? EcoHealth. 2012;9(3):342–360. doi: 10.1007/s10393-012-0785-3. [DOI] [PubMed] [Google Scholar]
- 23.Orlofske SA, Jadin RC, Hoverman JT, Johnson PTJ. Predation and disease: understanding the effects of predators at several trophic levels on pathogen transmission. Freshw Biol. 2014;59(5):1064–1075. [Google Scholar]
- 24.Orlofske SA, Jadin RC, Preston DL, Johnson PTJ. Parasite transmission in complex communities: Predators and alternative hosts alter pathogenic infections in amphibians. Ecology. 2012;93(6):1247–1253. doi: 10.1890/11-1901.1. [DOI] [PubMed] [Google Scholar]
- 25.Schotthoefer AM, Labak KM, Beasley VR. Ribeiroia ondatrae cercariae are consumed by aquatic invertebrate predators. J Parasitol. 2007;93(5):1240–1243. doi: 10.1645/GE1129R.1. [DOI] [PubMed] [Google Scholar]
- 26.Griffin JN, Byrnes JEK, Cardinale BJ. Effects of predator richness on prey suppression: A meta-analysis. Ecology. 2013;94(10):2180–2187. doi: 10.1890/13-0179.1. [DOI] [PubMed] [Google Scholar]
- 27.Bruno JF, Cardinale BJ. Cascading effects of predator richness. Front Ecol Environ. 2008;6(10):539–546. [Google Scholar]
- 28.Finke DL, Snyder WE. Conserving the benefits of predator biodiversity. Biol Conserv. 2010;143(10):2260–2269. [Google Scholar]
- 29.Vance-Chalcraft HD, Rosenheim JA, Vonesh JR, Osenberg CW, Sih A. The influence of intraguild predation on prey suppression and prey release: A meta-analysis. Ecology. 2007;88(11):2689–2696. doi: 10.1890/06-1869.1. [DOI] [PubMed] [Google Scholar]
- 30.Rohr JR, et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455(7217):1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- 31.Rohr JR, Crumrine PW. Effects of an herbicide and an insecticide on pond community structure and processes. Ecol Appl. 2005;15:1135–1147. [Google Scholar]
- 32.Corbett PS. Dragonflies: Ecology and Behavior of Odonata. Cornell Univ Press; Ithaca, NY: 1999. [Google Scholar]
- 33.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412(6842):72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 34.Rigby MC, Jokela J. Predator avoidance and immune defence: Costs and trade-offs in snails. Proc R Soc Lond Ser B-Biol Sci. 2000;267(1439):171–176. doi: 10.1098/rspb.2000.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohr JR, Swan A, Raffel TR, Hudson PJ. Parasites, info-disruption, and the ecology of fear. Oecologia. 2009;159(2):447–454. doi: 10.1007/s00442-008-1208-6. [DOI] [PubMed] [Google Scholar]
- 36.Koprivnikar J, Forbes MR, Baker RL. On the efficacy of anti-parasite behaviour: A case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Can. J. Zool.- Rev Can Zool. 2006;84(11):1623–1629. [Google Scholar]
- 37.Thiemann GW, Wassersug RJ. Patterns and consequences of behavioural responses to predators and parasites in Rana tadpoles. Biol J Linn Soc Lond. 2000;71(3):513–528. [Google Scholar]
- 38.Rohr JR, Madison DM, Sullivan AM. On temporal variation and conflicting selection pressures: A test of theory using newts. Ecology. 2003;84:1816–1826. [Google Scholar]
- 39.Rohr JR, Madison DM. Dryness increases predation risk in efts: Support for an amphibian decline hypothesis. Oecologia. 2003;135(4):657–664. doi: 10.1007/s00442-003-1206-7. [DOI] [PubMed] [Google Scholar]
- 40.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100(2):567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostfeld RS, Keesing F. Biodiversity and disease risk: The case of lyme disease. Conserv Biol. 2000;14(3):722–728. [Google Scholar]
- 42.Szuroczki D, Richardson JML. The behavioral response of larval amphibians (Ranidae) to threats from predators and parasites. PLoS ONE. 2012;7(11):e49592. doi: 10.1371/journal.pone.0049592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decaestecker E, De Meester L, Ebert D. In deep trouble: Habitat selection constrained by multiple enemies in zooplankton. Proc Natl Acad Sci USA. 2002;99(8):5481–5485. doi: 10.1073/pnas.082543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson RM, May RM. Regulation and stability of host-parasite population interactions: 1. Regulatory processes. J Anim Ecol. 1978;47(1):219–247. [Google Scholar]
- 45.Johnson PTJ, et al. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc Natl Acad Sci USA. 2007;104(40):15781–15786. doi: 10.1073/pnas.0707763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson PTJ, et al. Living fast and dying of infection: Host life history drives interspecific variation in infection and disease risk. Ecol Lett. 2012;15(3):235–242. doi: 10.1111/j.1461-0248.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- 47.Sears BF, Snyder PW, Rohr JR. Host life-history and host-parasite syntopy predict behavioral resistance and tolerance of parasites. J Anim Ecol. 2015 doi: 10.1111/1365-2656.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbosa P, et al. Associational resistance and associational susceptibility: Having right or wrong neighbors. Annu Rev Ecol Evol Syst. 2009;40:1–20. [Google Scholar]
- 49.Schotthoefer AM, et al. Effects of wetland vs. landscape variables on parasite communities of Rana pipiens: Links to anthropogenic factors. Ecol Appl. 2011;21(4):1257–1271. doi: 10.1890/10-0374.1. [DOI] [PubMed] [Google Scholar]
- 50.Briggs CJ, Borer ET. Why short-term experiments may not allow long-term predictions about intraguild predation. Ecol Appl. 2005;15(4):1111–1117. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.