Significance
Even moderate doses of alcohol can cause considerable motor impairment. This effect has been linked to ethanol-induced potentiation of GABA actions at δ subunit-containing GABAA receptors (δ-GABAARs). Here, we demonstrate that the neuropeptide oxytocin selectively attenuates ethanol-induced motor impairment in rats as well as ethanol-induced potentiation of GABAergic activity at δ-GABAARs. This effect of oxytocin is shown to be independent of the oxytocin receptor (OTR) and involves a direct action at δ-GABAARs. To our knowledge, this study provides the first evidence of oxytocin having a direct, non-OTR–mediated effect on GABA–ethanol interactions. Recent preclinical and clinical studies indicate that oxytocin may also attenuate alcohol consumption, craving, and withdrawal, and the present study shows a previously unidentified mechanism through which some of these effects may occur.
Keywords: oxytocin, alcohol, GABA, motor impairment, electrophysiology
Abstract
Even moderate doses of alcohol cause considerable impairment of motor coordination, an effect that substantially involves potentiation of GABAergic activity at δ subunit-containing GABAA receptors (δ-GABAARs). Here, we demonstrate that oxytocin selectively attenuates ethanol-induced motor impairment and ethanol-induced increases in GABAergic activity at δ-GABAARs and that this effect does not involve the oxytocin receptor. Specifically, oxytocin (1 µg i.c.v.) given before ethanol (1.5 g/kg i.p.) attenuated the sedation and ataxia induced by ethanol in the open-field locomotor test, wire-hanging test, and righting-reflex test in male rats. Using two-electrode voltage-clamp electrophysiology in Xenopus oocytes, oxytocin was found to completely block ethanol-enhanced activity at α4β1δ and α4β3δ recombinant GABAARs. Conversely, ethanol had no effect when applied to α4β1 or α4β3 cells, demonstrating the critical presence of the δ subunit in this effect. Oxytocin had no effect on the motor impairment or in vitro effects induced by the δ-selective GABAAR agonist 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol, which binds at a different site on δ-GABAARs than ethanol. Vasopressin, which is a nonapeptide with substantial structural similarity to oxytocin, did not alter ethanol effects at δ-GABAARs. This pattern of results confirms the specificity of the interaction between oxytocin and ethanol at δ-GABAARs. Finally, our in vitro constructs did not express any oxytocin receptors, meaning that the observed interactions occur directly at δ-GABAARs. The profound and direct interaction observed between oxytocin and ethanol at the behavioral and cellular level may have relevance for the development of novel therapeutics for alcohol intoxication and dependence.
Extrasynaptic δ subunit-containing γ-aminobutyric acid type A receptors (δ-GABAARs) play a major role in regulating tonic inhibition in the central nervous system (CNS) (1). The δ-GABAARs also represent one of the major targets of ethanol in the CNS, particularly at relatively low ethanol concentrations, and mediate some of the rewarding and ataxic effects of ethanol (2–8). For instance, a genetic polymorphism that exaggerates the actions of ethanol at δ-GABAARs also potentiates ethanol-induced motor impairment (2). Further, knockdown of δ subunit expression in the medial shell of the nucleus accumbens reduces alcohol intake in rats (6). Finally, overstimulation and subsequent internalization of α4βδ extrasynaptic GABAARs after persistent stimulation by ethanol seem to underlie the development of rapid tolerance to the ataxic effects of ethanol (4).
The neuropeptide oxytocin (OT) is well-known for its regulatory role in mammalian sociability and various other central and peripheral physiological processes (9). Early preclinical studies suggested that OT can prevent the development of tolerance to the sedative and ataxic effects of ethanol in rodents (10) and also modulate the severity of ethanol withdrawal (11). More recent studies show that OT reduces alcohol intake in rats (12) and reduces the severity of alcohol withdrawal and craving in dependent humans during detoxification (13). However, the mechanism underlying these actions is largely uncharacterized, including whether the modulation of various alcohol-related effects by OT involves the OT receptor (OTR).
Given the role of α4βδ extrasynaptic GABAARs in mediating tolerance to the ataxic effects of ethanol (4), and the modulation of ethanol tolerance by OT (14), we hypothesized that OT might act to prevent ethanol actions directly at δ-GABAARs. Such an action would be expected to reduce alcohol-induced motor impairment. We therefore assessed whether centrally administered OT inhibits ethanol-induced motor impairment in rodents. After this experiment, we tested OT effects on ethanol-potentiated GABA-gated current in α4βδ subunit-containing recombinant GABAARs. To ascertain whether any OT effects were due to general characteristics shared by similar neuropeptides or were specific to OT, we also tested the effect of the structurally related neuropeptide arginine vasopressin (AVP) at these recombinant receptors. To determine whether the δ subunit was crucial for any ethanol and OT effects, we also assessed ethanol actions at α4β recombinant GABAARs. Finally, to determine whether the inhibitory action of OT was specific to effects induced by ethanol, we tested the impact of OT on the sedation and currents induced by the GABAAR agonist 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP), which has high selectivity for the δ subunit but at a distinct binding site to ethanol (15).
Results
To facilitate brevity and clarity of reporting, only summary statistics are presented below and also in the figure legends. Detailed results from statistical analyses are provided in Tables S1–S4.
OT Inhibits Ethanol-Induced Motor Impairment.
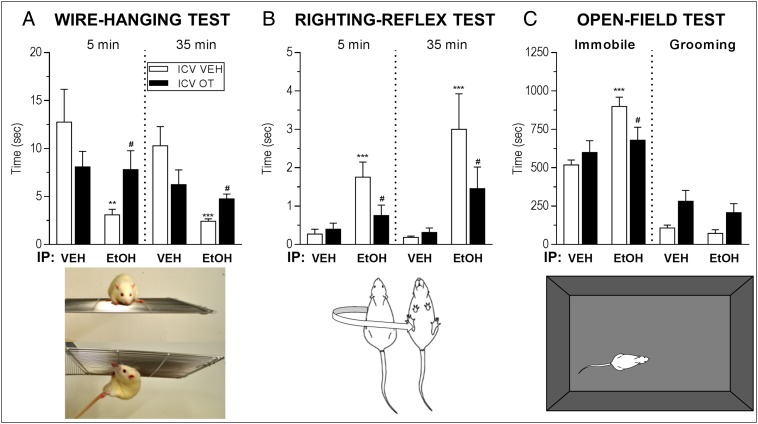
Intracerebroventricular (i.c.v.) administration of OT (1 μg/5 μL) to adult male Wistar rats immediately before i.p. injection with 1.5 g/kg ethanol attenuated the motor impairment observed in the wire-hanging test and righting-reflex test and reduced the sedation and overall inhibition of locomotor activity observed in the open-field test (Fig. 1 and Movie S1). This striking attenuation of acute ethanol effects by OT was seen both 5 and 35 min post-ethanol injection on the wire-hanging test and righting-reflex test and at 10–30 min post–ethanol injection on the open-field test (Table S1). To assess whether OT is also able to interfere with the much more pronounced motor impairment induced by a higher dose of ethanol, we conducted another experiment using 3 g/kg ethanol i.p. At this dose of ethanol, i.c.v. OT (1 μg/5 μL) did not attenuate the severe ethanol-induced motor impairments observed on the righting-reflex test and the open-field test, and it did not alter the duration of the loss of righting-reflex (Fig. S1).
Fig. 1.
Oxytocin (OT) attenuates ethanol (EtOH)-induced motor impairment in rats. (A) Rats treated with 1.5 g/kg i.p. EtOH remained suspended from an inverted platform for less time than those given vehicle (VEH) in the wire-hanging test. One microgram i.c.v. OT did not affect performance on the wire-hanging test alone (P > 0.05) but inhibited the EtOH-induced impairment of wire-hanging test performance at both 5 and 35 min post-EtOH. (B) Rats treated with EtOH took longer to right themselves in the righting-reflex test. OT did not affect performance in the righting-reflex test alone (P > 0.1) but reduced the EtOH-induced delay of the righting reflex. (C) EtOH caused an increase in the amount of time spent immobile in an open field relative to vehicle treatment. OT given alone had no effect on time spent immobile (P > 0.1) but prevented EtOH-induced increases in immobility. **P < 0.01 vs. i.c.v. VEH + i.p. VEH; ***P < 0.001 vs. i.c.v. VEH + i.p. VEH; #P < 0.05 vs. i.c.v. VEH + i.p. EtOH.
OT Prevents Ethanol Enhancement of GABA-Gated Current at δ Subunit-Containing GABAARs.
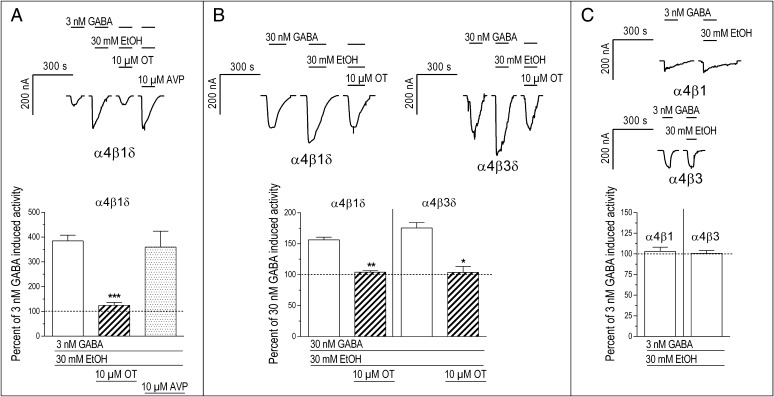
Using two-electrode voltage-clamp electrophysiology, we found that OT completely blocked ethanol-induced increases in GABAergic activity in Xenopus laevis oocytes expressing α4β1δ or α4β3δ receptors (Fig. 2 A and B). When 30 mM ethanol (the circulating concentration in adult rats after 1.5 g/kg i.p. injection of ethanol) (16) was coapplied with 300 nM GABA onto cells expressing α4β1δ receptors, there was no change in the magnitude of the current induced by GABA alone (P > 0.1). Conversely, when 30 mM ethanol was added to a lower 30 nM concentration of GABA, there was a significant increase in the magnitude of the GABA-gated current (P < 0.01) (Table S2). This ethanol-induced increase in the GABA response was completely prevented by 10 μM OT (the approximate concentration after a 1-μg central infusion based on a 90-μL cerebrospinal fluid volume in the adult rat) (17) (P < 0.01) (Table S3), such that no significant ethanol-induced increase in the GABA-gated current was observed (Fig. 2B) (P > 0.1) (Table S2). Similarly, 30 mM ethanol greatly potentiated currents elicited by 3 nM GABA by nearly 400% (P < 0.001) (Table S2). This potentiation was also completely blocked by 10 μM OT (P < 0.001) (Tables S2 and S3) (Fig. 2A; the GABA concentration dependency of ethanol’s effects is shown in Fig. 3). In contrast to OT, AVP had no effect on the ethanol-induced increases in GABAergic activity (Fig. 2A and Tables S2 and S3).
Fig. 2.
Oxytocin (OT) prevents the action of ethanol (EtOH) at δ-GABAARs. (A) The 30 mM EtOH coapplied with 3 nM GABA onto GABAA receptor α4β1δ subunit-expressing X. laevis oocytes increased the magnitude of the GABA-gated current to almost 400% of the response elicited by GABA alone (P < 0.001). This effect was prevented by coapplication of 10 μM OT with EtOH and GABA (***P < 0.001), such that no significant EtOH-induced effect was observed (P > 0.1). AVP did not affect EtOH-induced enhancement of GABA-gated currents (P > 0.1). (B) EtOH potentiation of GABA-gated currents in α4β1δ-expressing cells and its antagonism by OT (**P < 0.01) were also seen when a higher concentration of GABA (30 nM) was used, but the effects of EtOH were less pronounced. The interaction of EtOH, GABA, and OT also occurred with α4β3δ-expressing cells (*P < 0.05). (C) EtOH (30 mM) had no effect on 3 nM GABA-gated current in α4β1 (P > 0.1) or α4β3 (P > 0.1) cells.
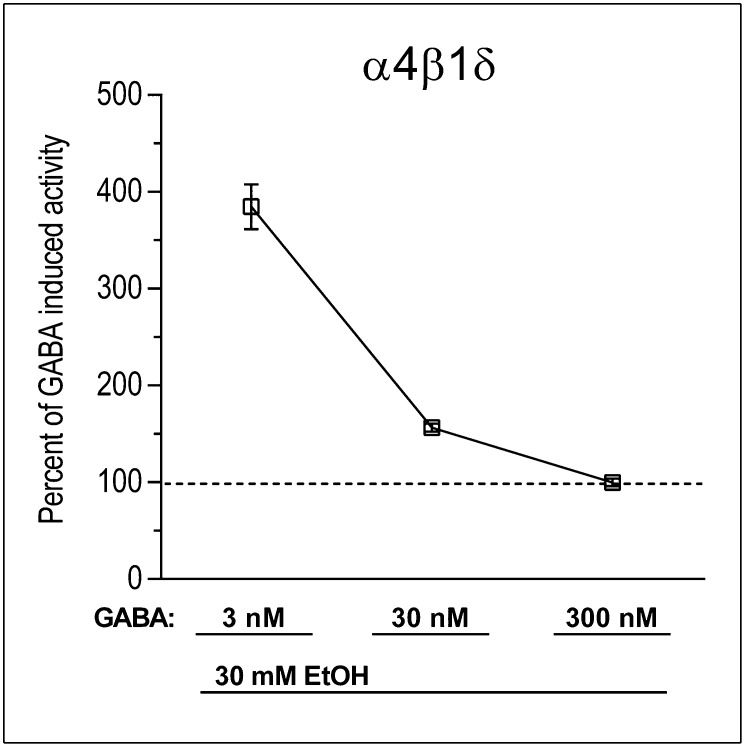
Fig. 3.
Ethanol (EtOH) potentiation of GABA-gated currents at α4βδ recombinant receptors depends on the concentration of GABA. The effectiveness of 30 mM EtOH in potentiating GABA-gated current was strongly related to GABA concentrations, being highly effective at 3 nM (P < 0.001), less effective at 30 nM (P < 0.01), and ineffective at 300 nM (P > 0.1).
The δ Subunit Is Required for OT to Influence Ethanol-Induced Effects.
To test the selectivity of OT effects for α4β1δ receptors, we repeated the experiment using cells expressing α4β3δ receptors, where ethanol also potentiated GABA-elicited currents (P = 0.013) (Table S2) and OT again prevented this potentiation (Fig. 2B) (P = 0.015) (Tables S2 and S3). To determine whether the presence of the δ subunit is crucial, we conducted parallel experiments with cells expressing α4β1 and α4β3 subunits only. Coapplication of 30 mM ethanol did not potentiate the response to 3 nM GABA at these recombinant receptors (Fig. 2C and Table S2), indicating that the δ subunit is most likely necessary for the interaction between ethanol and OT.
OT Selectively Blocks Ethanol’s in Vivo and in Vitro Effects.
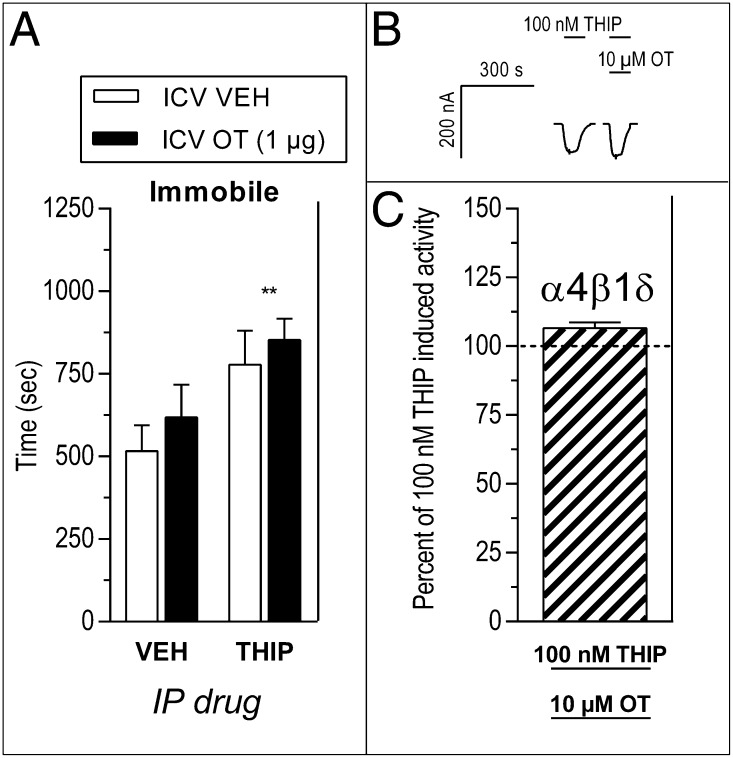
To determine whether the inhibitory action of OT at δ-GABAARs is specific to ethanol’s effects, we tested the impact of OT on the behavioral and receptor-level effects of the GABAA agonist 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP). THIP has high selectivity for the δ subunit but occupies a different binding site to ethanol (15). When injected into rats, THIP (7 mg/kg, i.p.) caused significant sedation (immobility) in the open-field test, but this sedation was unaffected by pretreatment with OT (1 μg, i.c.v.) (Fig. 4A and Table S4). Application of 100 nM THIP induced currents in α4β1δ-expressing cells, but coapplication of 10 μM OT did not affect these THIP-induced currents (Fig. 4 B and C and Table S2).
Fig. 4.
Oxytocin (OT) does not alter the in vivo or in vitro effects of the GABAA agonist 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP). (A) THIP (7 mg/kg, i.p.) increased immobility in the open-field test in rats (**P < 0.01, THIP vs. VEH), and this increased immobility was unaffected by i.c.v. OT (1 μg; P > 0.1). (B and C) Current induced in α4β1δ cells by application of 100 nM THIP was unaffected by coapplication of 10 μM OT (P > 0.05).
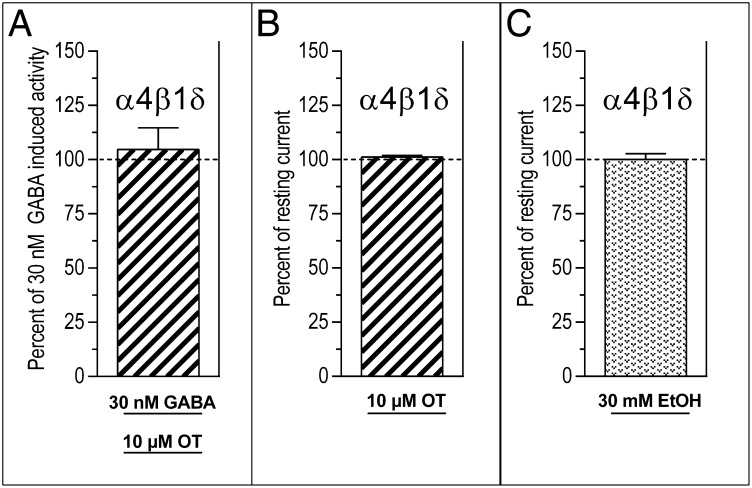
Further emphasizing that OT has a highly specific impact on ethanol actions, rather than a more general effect on the δ subunit, we found that OT did not alter the GABA-gated current induced by 30 nM GABA alone (Fig. 5A and Table S2) and induced no current when applied on its own (Fig. 5B and Table S2). Ethanol also had no effect on the cells when infused alone at a concentration of 30 mM (Fig. 5C and Table S2).
Fig. 5.
Oxytocin (OT) does not affect current induced by GABA alone and does not induce current when applied alone. (A) OT (10 μM) had no effect on the current induced by 30 nM GABA alone at α4β1δ recombinant receptors (P > 0.1). Neither 10 μM OT (B) nor 30 mM ethanol (EtOH) (C) applied alone induced any change in current in these receptors (P > 0.1).
Discussion
The present study reports a hitherto uncharacterized interaction between the neuropeptide OT and one of the most widely used recreational drugs in the world, ethanol. This finding comes at a time when there is substantial interest in the potential of OT as a therapeutic for various psychiatric problems (18), including substance-use disorders (19–23). A robust antagonistic action of OT on moderate doses of ethanol was evident in the present report at both the behavioral and cellular level of analysis. Thus, OT provided a striking attenuation of the characteristic intoxicating effects of acute injection of a moderate dose of ethanol in rats. At the same time, OT prevented the potentiating action of ethanol on GABA-gated currents at δ-GABAARs, a cellular action that is mechanistically linked to ethanol-induced motor impairment. In contrast, OT had no impact on either the behavioral impairment or in vitro effects of THIP, which has similar in vivo and in vitro effects to ethanol but binds at a distinct site on the δ subunit (15). Furthermore, OT did not alter the more profound motor impairment induced by a much higher dose of ethanol. This result would be expected based on our electrophysiological findings because high doses of ethanol exert their extreme ataxic effects primarily through a non-δ-GABAAR–mediated mechanism (7).
The motor impairment induced by ethanol was clearly evident across three different behavioral models and at two different time points. Strikingly, OT (1 μg/5 μL, i.c.v.) administered before an intoxicating, moderate dose of ethanol (1.5 g/kg, i.p.) markedly antagonized the ataxia induced by ethanol in the wire-hanging test and in the righting-reflex test and reversed ethanol-induced hypoactivity in an open-field test. Observation of behavior on the latter test (Movie S1) suggests a remarkable “sobering-up” effect of OT in ethanol-treated rats.
At a molecular level, OT completely prevented the characteristic enhancement of GABA-gated currents by ethanol at recombinant δ-GABAARs. The δ-GABAARs, sometimes called the “one-glass-of-wine” receptors (24), are located in several brain regions, including the thalamus, cerebellum, hippocampus, and striatum (25, 26), and show high sensitivity to relatively low doses of ethanol (2–8, 27). A number of previous studies demonstrate the involvement of δ-GABAARs in a range of ethanol-induced functional effects, including ataxia, sedation, and reward (2–8). We established that OT blocked ethanol enhancement at both α4β1δ and α4β3δ subunit-containing receptors. However, ethanol had no effect on currents when applied to α4β1 or α4β3 subunit-containing receptors, indicating that the δ subunit is likely to be the key site of OT–ethanol interactions.
Specificity was also shown, in that AVP, a closely related neuropeptide to OT, did not influence ethanol-induced potentiation of GABA-induced currents in α4β1δ-expressing cells. OT and AVP are nonapeptides with a similar molecular mass (OT, 1,007 Da; AVP, 1,084 Da) and differ by only two amino acids (third and eighth position). The absence of an AVP effect on ethanol’s actions at δ-GABAARs suggests that the key structural features allowing OT to inhibit ethanol actions at these receptors may involve the isoleucine (third position) or leucine (eighth position) segments of the OT molecule (i.e., those amino acids that differ from AVP).
Of additional interest in the present study was the observation that 30 mM ethanol enhanced GABA-gated current at δ-GABAARs only in the presence of low (3 nM) or moderate (30 nM) concentrations of GABA. When 300 nM GABA was applied, no potentiating effects of ethanol were observed, most likely due to a ceiling effect whereby maximal stimulation was achieved by the high dose of GABA alone. Two populations of δ-GABAARs with unknown stoichiometry have been identified—one with a high-potency GABA-binding site and the other with a lower potency GABA-binding site (28, 29). Therefore, both ethanol effects and OT–ethanol interactions likely occur at the high-potency subpopulation of δ-GABAARs that respond to low nM concentrations of GABA. Therefore, the inability of some studies to observe potentiation of GABA-gated current at δ-GABAARs by low and moderate concentrations of ethanol (e.g., ref. 30) may be explained by the clear GABA concentration dependency of ethanol effects demonstrated in the present study, highlighting the importance of GABA concentration dependency for future cellular studies of ethanol effects and OT-ethanol interactions at δ-GABAARs.
OT exerted its striking antagonism of ethanol effects at δ-GABAARs via a mechanism that seems independent of the OTR. X. laevis oocytes do not endogenously express OTRs (31), and OTR RNA was not coexpressed into the oocytes because we injected cells with RNA only for the α4, β, and δ GABAAR subunits. This finding is consistent with recent studies demonstrating that a range of OT functional effects rely on receptors other than the OTR (32–36). OT has, however, been found to alter the expression and function of GABAARs, albeit via an OTR-mediated mechanism (37–39). Specifically, GABA is the primary excitatory neurotransmitter in immature neurons, but, shortly before parturition, OT, via an OTR-mediated action, triggers a transient reduction in intracellular chloride concentration that switches GABA actions from excitatory to inhibitory (38, 39).
The effects of OT at both the behavioral and cellular level highlighted in the present study bear a striking similarity to those reported with the benzodiazepine inverse agonist Ro15-4513, which is a competitive antagonist and an antidote to ethanol. This compound reverses the ataxic effects of ethanol (40) and blocks the effects of ethanol at δ-GABAARs (3, 8). Initially there were hopes that Ro15-4513 would have a significant impact on the clinical treatment of alcohol-use disorders. However, due to its actions as a benzodiazepine inverse agonist, Ro15-4513 was found to be anxiogenic (41) and to potentiate seizures during and after alcohol withdrawal (42). In contrast, OT has a well-characterized anxiolytic effect (9) and, when dosed at a specific level, powerfully reduces seizures and lethality during ethanol withdrawal in mice (11). In a recent preliminary clinical study, OT also attenuated symptoms of alcohol withdrawal in dependent humans to the point where a standard clinical intervention for anxiety and seizures (lorazepam) was not required (13).
Because OT lacks the benzodiazepine inverse agonist effects of Ro15-4513 and the molecules have considerable structural differences, OT may have a distinct binding site to both Ro15-4513 and ethanol at α4βδ GABAARs. However, given that Ro15-4513 and OT share considerable similarity in their antagonism of ethanol effects at the level of both behavior and receptor pharmacology, it is also possible that they share the same binding site or an overlapping binding site. Future work could explore these possibilities.
The present study extends early and more recent observations of OT–ethanol interactions. The capacity of centrally administered OT to delay tolerance to the sedative and ataxic effects of ethanol (10, 14) has been known since the late 1980s. The present study suggests that this effect of OT on the development of tolerance may reflect the antagonistic action of OT on acute ethanol-induced sedation and ataxia and its blockade of ethanol potentiation of GABA-gated current at δ-GABAARs. It is through overstimulation and subsequent internalization of these receptors that rapid tolerance to ethanol’s effects seems to develop (4). It is also possible that the effects described here are relevant to the inhibitory action of OT on ethanol consumption (12, 19). A regionally specific knockdown of δ subunits in the medial shell of the nucleus accumbens reduces ethanol consumption in rats (6), whereas δ knockout mice are known to consume less ethanol than wild types (5). Future studies will hopefully determine whether the anticraving actions of OT across a range of addiction-relevant behaviors (12, 13) reflect binding to δ-GABAARs in reward-relevant areas such as the nucleus accumbens. The present study demonstrates that OT has a profound inhibitory effect on the acute behavioral and cellular effects of ethanol.
Methods
All work involving rats was approved by the University of Sydney Animal Ethics Committee and the Committee on Animal Health and Care of the Government of Oberpfalz, Germany. All work involving X. laevis was approved by the University of Sydney Animal Ethics Committee. All work was conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (43) and the Guide for the Care and Use of Laboratory Animals (44).
Behavioral Experiments.
Subjects.
Adult male Albino Wistar rats weighing 328–414 g and aged 10–12 wk at the time of testing were used for the OT–ethanol (N = 28, n = 7) and the OT–THIP (N = 32, n = 8) behavioral experiments. Subjects were group housed on arrival under standard laboratory conditions (12:12 h light:dark cycle, 22 °C, 60% humidity, food and water available ad libitum). After surgery, rats were transferred to observation cages and remained single-housed for the remainder of the experiment. All behavioral testing took place from 2 to 5 h into the light cycle. Rats were randomly assigned to experimental conditions.
Surgery.
For intracerebroventricular (i.c.v.) drug infusion, guide cannulae were stereotaxically implanted 1 mm posterior and 1.6 mm lateral to Bregma and at a depth of 1.8 mm. Rats were anesthetized using isoflurane and injected with an antibiotic (oxytetracycline, 10 mg/kg, i.p.) at the conclusion of surgery. The i.c.v. guide cannula (21 G, 12-mm length) was fixed to the skull using jewelers’ screws and dental cement and was closed by a stainless steel stylet (25 g). After surgery, rats were handled daily (stroking, holding, cleaning of stylets) for five days to allow adequate recovery and to minimize nonspecific stress responses during the experiments.
I.c.v. infusions and i.p. injections.
For the OT–ethanol and the OT–THIP experiments, rats received an i.c.v. infusion of either synthetic OT (1 µg/5 µl; AusPep Ltd.) or vehicle (5 µL of sterile Ringer’s solution) via an infusion cannula that extended 2 mm beyond the guide cannula and that was connected via polyethylene tubing to a Hamilton syringe. The infusion cannula was left in place for 30 s after the infusion. Immediately after the i.c.v. infusion, rats received an i.p. injection of either ethanol (1.5 g/kg; 15% wt/vol) or vehicle (equivalent volume of isotonic saline). We replicated the OT–ethanol experiment (with slight modifications) using a higher dose of ethanol (3 g/kg; 20% wt/vol) (Fig. S1). For the OT–THIP experiment, rats received either THIP (7 mg/kg, i.p.), or vehicle (an equivalent volume of isotonic saline). The dose of THIP was based on pilot experiments comparing the inhibition of locomotor activity induced by various doses of THIP relative to that induced by 1.5 g/kg ethanol (Fig. S2). The 7 mg/kg THIP dose caused similar sedation to 1.5 g/kg ethanol and is a dose that has selectivity for the δ subunit (15). One rat was excluded from the OT–THIP experiment due to a blocked guide cannula. Rats were euthanized using CO2, and India ink (5 µL) was infused i.c.v. before removal of the brain to allow visualization of infusion sites. Brains were sliced coronally on a cryostat to confirm staining of the ventricle.
Wire-hanging test.
The wire-hanging test is a standard behavioral assay of motor impairment and is sensitive to ethanol-induced deficits (45–47). It is particularly useful for assessing the effect of chemical substances on muscle strength. At the start of each trial, rats were placed standing on a wire mesh platform. The platform was briefly shaken by the experimenter to cause the rat to grip onto the bars before the platform’s being inverted and placed 750 mm above a landing box that was filled with wood shavings to prevent any injury to the rat from the fall. The length of time that the rat was able to hang from the inverted platform was recorded by the experimenter. If a duration of 60 s was reached, then the trial was ended and a time of 60 s recorded. Rats were tested on three consecutive trials at two time points (5 and 35 min after i.p. injection of ethanol or vehicle), with the average time across the trials at each time point used for analysis.
Righting-reflex test.
The righting-reflex test is a widely used assay of motor coordination and sedation (48). Rats were placed inside a tub with corncob bedding and were placed on their back by the experimenter. The time taken to right (defined as time taken to place all four paws on the ground after release by the experimenter) was recorded. If subjects were unable to right by 60 s after being placed on their back, a time of 60 s was recorded and the animal was returned to all fours. Righting reflex was assessed immediately after the final trial of the wire-hanging test. The average of three trials at each time point was used for analysis.
With the moderate dose of ethanol (1.5 g/kg, i.p.), a delay in the righting reflex was observed but not a total loss. However, when we administered the higher dose of ethanol (3 g/kg, i.p.) (Fig. S1), a total loss of righting reflex was observed in the vast majority of rats. Rats were defined as having a loss of the righting reflex when they were unable to right within 30 seconds after being placed on their back. The duration of the loss of the righting reflex was also assessed in the higher ethanol dose experiment (Fig. S1) by measuring how long after ethanol administration it took rats to regain the ability to right within 30 s after being turned on their back.
Open-field test.
The open-field test was used to examine general locomotor activity and is sensitive to the effects of both stimulants and depressants (49). For 3 d before the test session, rats were placed individually in a rectangular test arena [200 mm (height) × 400 mm (width) × 800 mm (length)] made of marine plywood and painted matt black for 20 min to habituate them to the arena and to minimize stress effects. On test day, rats were placed in the arena 10–30 min after i.p. injection of ethanol or vehicle. Test sessions were recorded, and videos were scored by an observer who was blind to experimental conditions for time spent immobile (a measure of sedation) and time spent grooming. Immobility was defined as a rat being completely stationary: i.e., engaging in no body, head, or limb movement. For the OT–THIP experiment, the open-field test was run as described above.
Statistics.
Behavioral data were analyzed using a 2 × 2 ANOVA with planned contrast analysis. The assumption of homogeneity of variance was violated for the wire-hanging tests and 35-min righting-reflex test data (Levene’s test, P < 0.05). As such, these data were log transformed for analysis (50), which restored homogeneity of variance.
Electrophysiology.
Electrophysiological procedures with GABAARs were as recently reported (51) and are described briefly below.
Expression of recombinant GABAA receptors in X. laevis oocytes.
cDNAs containing the α4, β1, β3, and δ subunits were subcloned into suitable vectors as previously described (28). Plasmids containing the α4, β1, and δ subunits were linearized using the restriction enzyme NotI, and the β3 subunits were linearized using NheI. mRNA for injection into the oocytes was transcribed using the T7 mMessage machine kit and poly-A tailed using the poly-A tailing kit (Life Technologies). Gel electrophoresis was used to visualize RNA before and after poly-A tailing, and RNA concentrations were quantified using UV/Vis spectrophotometry with a Nanodrop 2000 (Nanodrop Instruments). Staged V–VI oocytes were microinjected with 0.5–5 ng of mRNA using the following ratios of mRNA: α4β1δ (1:1:5); α4β3δ (1:1:5); α4β1 (1:1); and α4β3 (1:1). After injection, oocytes were maintained at 18 °C in ND96 wash solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM hemosodium salt augmented with 2.5 mM sodium pyruvate, 0.5 mM theophylline, 50 μg/mL gentamycin and tetracycline).
Two-electrode voltage-clamp recordings.
Whole-cell currents were measured 3–5 d after injection of cRNA by two-electrode voltage clamp with a Digidata 1200, Geneclamp 500B amplifier together with a Powerlab/200 and Chart version 3.5 (AD Instruments). Oocytes were voltage-clamped at −60 mV. The recording microelectrodes were filled with 3 M KCl and had resistance between 0.2 and 1 MΩ. A minimum of four cells were recorded for each recombinant receptor type. Substances were bath applied until a plateau was reached and the peak currents were measured. A 7-min wash with ND96 between successive applications was performed to prevent recordings being compromised by the effects of desensitization.
Statistics.
One-sample t tests were used to assess the significance of percent change in peak current from baseline (test value = 100). Paired-sample t tests were used to compare percent change in current between different combinations of substances (e.g., GABA + ethanol vs. GABA + ethanol + OT).
Supplementary Material
Acknowledgments
This research was supported by National Health and Medical Research Council grants (to M.C. and I.S.M.) and fellowships (M.T.B and I.S.M.), a Deutscher Akademischer Austausch Dienst/Group of Eight grant (to I.S.M. and I.D.N.), and Deutsche Forschungsgemeinschaft grants (to I.D.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416900112/-/DCSupplemental.
References
- 1.Brickley SG, Mody I. Extrasynaptic GABAA receptors: Their function in the CNS and implications for disease. Neuron. 2012;73(1):23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8(3):339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA. 2006;103(22):8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang J, et al. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27(45):12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mihalek RM, et al. GABA(A)-receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25(12):1708–1718. [PubMed] [Google Scholar]
- 6.Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic δ-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci USA. 2011;108(11):4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100(25):15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanchar HJ, et al. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci USA. 2006;103(22):8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Puciłowski O, Kostowski W, Trzaskowska E. The effect of oxytocin and fragment (MIF-I) on the development of tolerance to hypothermic and hypnotic action of ethanol in the rat. Peptides. 1985;6(1):7–10. doi: 10.1016/0196-9781(85)90067-1. [DOI] [PubMed] [Google Scholar]
- 11.Szabó G, Kovács GL, Telegdy G. Effects of neurohypophyseal peptide hormones on alcohol dependence and withdrawal. Alcohol Alcohol. 1987;22(1):71–74. [PubMed] [Google Scholar]
- 12.Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS ONE. 2011;6(11):e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen CA, et al. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res. 2013;37(3):484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovács GL, Sarnyai Z, Szabó G. Oxytocin and addiction: A review. Psychoneuroendocrinology. 1998;23(8):945–962. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- 15.Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J Neurophysiol. 2011;106(4):2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2009;91(4):560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davson H. The cerebrospinal fluid. In: Lajtha A, editor. Handbook of Neurochemistry. Springer; New York: 1969. pp. 23–48. [Google Scholar]
- 18.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 19.McGregor IS, Bowen MT. Breaking the loop: Oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61(3):331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Zanos P, et al. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology. 2014;39(4):855–865. doi: 10.1038/npp.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buisman-Pijlman FT, et al. Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacol Biochem Behav. 2014;119:22–38. doi: 10.1016/j.pbb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Sarnyai Z. Oxytocin as a potential mediator and modulator of drug addiction. Addict Biol. 2011;16(2):199–201. doi: 10.1111/j.1369-1600.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- 23.McGregor IS, Bowen MT. Oxytocin and addiction: Recent preclinical advances and future clinical potential. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge Univ Press; Cambridge, UK: 2013. pp. 270–287. [Google Scholar]
- 24.Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: The “one glass of wine” receptors. Alcohol. 2007;41(3):201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12(3):1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundstrom-Poromaa I, et al. Hormonally regulated α(4)β(2)δ GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5(8):721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karim N, et al. Low nanomolar GABA effects at extrasynaptic α4β1/β3δ GABA(A) receptor subtypes indicate a different binding mode for GABA at these receptors. Biochem Pharmacol. 2012;84(4):549–557. doi: 10.1016/j.bcp.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Eaton MM, et al. γ-aminobutyric acid type A α4, β2, and δ subunits assemble to produce more than one functionally distinct receptor type. Mol Pharmacol. 2014;86(6):647–656. doi: 10.1124/mol.114.094813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borghese CM, et al. The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316(3):1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 31.Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- 32.Ramos L, et al. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: Involvement of the V1A receptor. Neuropsychopharmacology. 2013;38(11):2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sala M, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biol Psychiatry. 2011;69(9):875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Bowen MT, McGregor IS. Oxytocin and vasopressin modulate the social response to threat: A preclinical study. Int J Neuropsychopharmacol. 2014;17(10):1621–1633. doi: 10.1017/S1461145714000388. [DOI] [PubMed] [Google Scholar]
- 35.Song Z, et al. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suraev AS, et al. Adolescent exposure to oxytocin, but not the selective oxytocin receptor agonist TGOT, increases social behavior and plasma oxytocin in adulthood. Horm Behav. 2014;65(5):488–496. doi: 10.1016/j.yhbeh.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Koksma JJ, et al. Oxytocin regulates neurosteroid modulation of GABA(A) receptors in supraoptic nucleus around parturition. J Neurosci. 2003;23(3):788–797. doi: 10.1523/JNEUROSCI.23-03-00788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyzio R, et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314(5806):1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 39.Tyzio R, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343(6171):675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 40.Suzdak PD, et al. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234(4781):1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- 41.Harris CM, Benjamin D, Lal H. Anxiety-like subjective effect of ethanol antagonist RO 15-4513 demonstrated in pentylenetetrazol discrimination. Neuropharmacology. 1987;26(10):1545–1547. doi: 10.1016/0028-3908(87)90178-x. [DOI] [PubMed] [Google Scholar]
- 42.Mhatre MC, Gonzalez LP. Increased Ro15-4513-induced seizures following multiple ethanol withdrawals. Pharmacol Biochem Behav. 1999;63(1):93–99. doi: 10.1016/s0091-3057(98)00257-3. [DOI] [PubMed] [Google Scholar]
- 43.NHMRC . Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. 7th Ed National Health and Medical Research Council, Canberra, ACT; Australia: 2004. [Google Scholar]
- 44.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 45.Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33(6):615–624. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 46.Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006;187(4):455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- 47.Crabbe JC, et al. Strain differences in three measures of ethanol intoxication in mice: the screen, dowel and grip strength tests. Genes Brain Behav. 2003;2(4):201–213. doi: 10.1034/j.1601-183x.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 48.Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20(8):1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 49.Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res Bull. 2003;62(3):197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Keene ON. The log transformation is special. Stat Med. 1995;14(8):811–819. doi: 10.1002/sim.4780140810. [DOI] [PubMed] [Google Scholar]
- 51.Absalom N, et al. α4βδ GABA(A) receptors are high-affinity targets for γ-hydroxybutyric acid (GHB) Proc Natl Acad Sci USA. 2012;109(33):13404–13409. doi: 10.1073/pnas.1204376109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.