Abstract
Background. Crotalidae Polyvalent Immune Fab (Ovine) has been the only antivenom commercially available in the US since 2007 for treatment of Crotalinae envenomation. Late coagulopathy can occur or recur after clearance of Fab antivenom, often after hospital discharge, lasting in some cases more than 2 weeks. There have been serious, even fatal, bleeding complications associated with recurrence phenomena. Frequent follow-up is required, and additional intervention or hospitalization is often necessary. F(ab')2 immunoglobulin derivatives have longer plasma half life than do Fab. We hypothesized that F(ab')2 antivenom would be superior to Fab in the prevention of late coagulopathy following treatment of patients with Crotalinae envenomation. Methods. We conducted a prospective, double-blind, randomized clinical trial, comparing late coagulopathy in snakebitten patients treated with F(ab')2 with maintenance doses [F(ab')2/F(ab')2], or F(ab')2 with placebo maintenance doses [F(ab')2/placebo], versus Fab with maintenance doses [Fab/Fab]. The primary efficacy endpoint was coagulopathy (platelet count < 150 K/mm3, fibrinogen level < 150 mg/dL) between end of maintenance dosing and day 8. Results. 121 patients were randomized at 18 clinical sites and received at least one dose of study drug. 114 completed the study. Of these, 11/37 (29.7%) in the Fab/Fab cohort experienced late coagulopathy versus 4/39 (10.3%, p < 0.05) in the F(ab')2/F(ab')2 cohort and 2/38 (5.3%, p < 0.05) in the F(ab')2/placebo cohort. The lowest heterologous protein exposure was with F(ab')2/placebo. No serious adverse events were related to study drug. In each study arm, one patient experienced an acute serum reaction and one experienced serum sickness. Conclusions. In this study, management of coagulopathic Crotalinae envenomation with longer-half-life F(ab')2 antivenom, with or without maintenance dosing, reduced the risk of subacute coagulopathy and bleeding following treatment of envenomation.
Keywords: Snakes, Antivenins, Toxinology
Introduction
Background
Snakebites are an important threat to public health. Worldwide, more than 5 million bites result in as many as 94,000 deaths annually. Almost one-fourth of global snakebite incidence occurs in America, although mortality is relatively low compared with that in Africa and Asia.1 In the US, thousands of bites are reported each year, but fewer than half a dozen are fatal.2,3 Snakebite mortality has decreased through the years and in certain regions of the world, in part because of the development of safe and effective antivenoms.4,5 Antivenoms bind and neutralize venom toxins, and facilitate redistribution away from target tissues and elimination from the body.6,7 Antivenom production involves collecting venom from a medically significant organism and inoculating a suitable domestic animal.8 Sublethal amounts generate immunity, and antibodies are obtained from the plasma of the immunized animal.9 Adverse drug reactions to early, unrefined antiserums were frequent, often severe and sometimes fatal.5,10,11 Physicians were, therefore, hesitant to use antivenom, and fear of dangerous side effects led to delays in administration and to insufficient dosing. Most modern antivenoms undergo further modification,9,12,13 including plasma fractionation, protein precipitation, ion-exchange or affinity chromatography, ultra- and nanofiltration, addition of preservative, lyophilization, and the regulation via Good Manufacturing Practices for quality control and minimization of microbial contamination. Further, many manufacturers enzymatically reduce the crude Immunoglobulin G (IgG) molecule to yield antigen-binding fragments. The Y-shaped IgG is cleaved with pepsin to yield a V-shaped F(ab')2 or with papain to generate two Fab fragments. The Fc portion (the stem of the Y), suspected for causing most of the adverse reactions, is removed along with other non-neutralizing components. Immune reactions are now markedly less problematic than those with earlier and less ameliorated preparations.4,5
Importance
However, another kind of clinical problem emerged as Fab antivenom gained widespread use in the US,7 where it has been the only commercially available antidote for pit viper (Crotalinae) envenomation since 2007.14 Rattlesnake (Crotalus and Sistrurus spp.) envenomation commonly causes thrombocytopenia and hypofibrinogenemia.15–17 These venom-induced coagulopathies usually improve, but an estimated one-third to more than one-half of them recur in the days to weeks following treatment with Fab antivenom.18,19 Recurrent coagulopathy is almost always as severe as the presenting coagulopathy and sometimes worse.20 In addition, new-onset late coagulopathies develop in many instances despite a full course of Fab antivenom treatment. When exactly and if coagulation abnormalities will develop and nadir are difficult to predict.21,22 Furthermore, coagulopathies sometimes persist, even with repeated doses of Fab antivenoms, and transfusions are ineffective.9,23 Studies in Asia and Africa conclude that more often repeated doses of Fab antivenoms are not required with antigen-binding fragment antivenoms, but that F(ab')2 is more effective than Fab at restoring blood coagulability.24,25 Frequent monitoring and follow-up are necessary with Fab antivenoms, along with additional intervention, hospitalization and cost.22 Serious bleeding complications and deaths have resulted from late coagulopathies after treatment of viper (e.g., Crotalus adamanteus∗, C. atrox, C. ruber∗, C. oreganus oreganus, C. horridus, and Bothrops alternatus∗; Viperidae) envenomation with a Fab antivenom.23,26–30 [∗ – deaths]
Goals of this investigation
Recurrence of coagulopathy appear, at least in part, to be caused by a pharmacokinetic mismatch between venom and antivenom, probably because a depot of venom remains at the bite site, distributing into the surrounding tissues and circulation according to the properties and kinetics of its various toxins.9 IgG, F(ab')2, and Fab differ in molecular mass, volume of distribution, and elimination half-life.9 A Phase 2 clinical trial explored the relationship of venom antigenemia (“venonemia”) with recurrent coagulopathy, showing a clear difference between the patterns with F(ab')2 versus Fab antivenom during the 2 weeks following treatment of envenomation.18 No recurrence was observed in patients treated with F(ab')2 in preliminary Phase 2 study, but larger studies were necessary for confirmation or negation of this finding. We hypothesized that F(ab')2 antivenom would be superior to Fab in the prevention of late coagulopathy following treatment of envenomation. To test this hypothesis, we conducted a prospective, multicenter, blinded, randomized, controlled Phase 3 clinical trial comparing F(ab')2 to Fab for the treatment of patients with Crotalinae envenomation.
Methods
Study design
In this prospective, blinded, randomized, controlled clinical trial, prevention of late coagulopathy using F(ab')2 versus Fab antivenom was compared in treatment of Crotalinae envenomation at 18 clinical sites in the US. The Institutional Review Board at each site approved the study protocol. The study was registered at ClinicalTrials.gov, number NCT00636116.
Materials
The Fab antivenom was Crotalidae polyvalent immune Fab (ovine) (CroFab®, “Fab”), a commercial product. Immunizing venoms used to produce it are from
C. adamanteus, C. atrox, C. scutulatus, and Agkistrodon piscivorus. This preparation is purified via sodium sulfate precipitation and affinity chromatography. Protein content of each vial is “up to 1 g,” according to the manufacturer.12
The F(ab')2 antivenom was Crotalidae equine immune F(ab')2 [Anavip®, “F(ab')2”], an investigational new drug. Immunizing venoms for this product are from B. asper and C. simus. This preparation is purified via ammonium sulfate precipitation and filtration. F(ab')2 contains approximately 120 mg of protein per vial.13 A relative potency of one vial of Fab to two vials of F(ab')2 was assigned based on a Phase 2 clinical trial and mouse ED50 comparisons.18,31 Study drugs and monitoring were provided by sponsors Instituto Bioclon, SA de CV, and Rare Disease Therapeutics, Inc.
“Study drug” was whichever antivenom and/or placebo combination the subject was randomized to receive.
Selection of participants
Eligible patients were males and females aged 2–80 years presenting for emergency treatment of a Crotalinae bite and able to provide informed consent. Signs of envenomation (as judged by clinician investigators) were swelling, tenderness, redness, ecchymosis, or blebs emanating from the bite site; decreased platelets or fibrinogen; and hypotension, bleeding beyond the puncture site, refractory vomiting or diarrhea, angioedema, or neurotoxicity. Exclusion criteria included pregnancy; breast-feeding; current treatment with antivenom or treatment with antivenom within the last month; participation in a clinical trial within the previous month; allergy to horse serum, sheep serum, or papaya; no clinical indications of snakebite requiring antivenom for treatment (i.e., no local tissue injury other than tooth marks and no lab abnormalities or systemic abnormalities consistent with snake venom toxicity); and underlying medical conditions or medications that significantly alter platelet count or fibrinogen. The study took place from May 2008 through September 2011, and patients were followed for 22 days after enrollment.
Interventions
Randomization was done using Statistical Analysis Software (Cary, NC). Three treatment arms [F(ab')2 with F(ab')2 maintenance therapy, F(ab')2 with placebo maintenance therapy, and Fab with Fab maintenance therapy] were established, in a 1:1:1 ratio with a block size of 6. After informed consent was granted, a study pharmacist contacted an Interactive Voice Response System (Covance, Inc, Princeton NJ) to learn the group assignment. Study drug was reconstituted and diluted to 250 mL with normal saline (NS). Placebo consisted of 250 mL of NS only. To ensure blinding of all other participants, study drug was provided to clinicians with no reference to group assignment. Study subjects, all investigators and care providers, the IND sponsor, and study monitors remained blinded until completion of the study.
Study drug was infused intravenously over 1 hour. Initial dose(s) consisted of 10 vials of F(ab')2 or 5 vials of Fab administered every 2 hours until “Initial Control” of the envenomation was achieved, meaning that (1) the leading edge of local injury was not progressing more than 1 inch per hour, and (2) platelet count, serum fibrinogen level, prothrombin time (PT), and partial thromboplastin time (PTT) were either normal or returning toward normal. After initial control, maintenance dosing of 4 vials of F(ab')2 [for the F(ab')2/F(ab')2 group] or 250 mL of NS [for the F(ab')2/placebo group] or 2 vials of Fab (for the Fab/Fab group) was administered every 6 hours for 3 doses. At any time during the study, patients with ongoing signs of envenomation could receive an additional 4 vials of F(ab')2 or 2 vials of Fab, in accordance with group assignment, at physician discretion, maintaining the blind.
Measurements
Patients had the following assessments and measurements recorded at baseline: history, medications, vital signs, physical examination, and a urine or blood pregnancy test; blood was drawn for complete blood count (CBC) PT/PTT, fibrinogen, venom, and antivenom levels; the subfamily, genus, and species of the snake were identified if possible; the physician or study nurse drew a line on the patient's skin indicating the proximal leading edge of the lesion; and Snakebite Severity Score (SSS)32 was assessed. On completion of the initial infusion, the physician or study nurse drew another line on the patient's skin to compare with the previous line and to facilitate subsequent assessments of ongoing local injury. Lesion assessment and repeat labs (CBC, PT/INR (International Normalized Ratio), fibrinogen, and venom/antivenom levels) were drawn 2 hours after the start of the initial infusion and 2 hours after the start of each additional infusion until initial control was achieved. Vitals were repeated 2 hours after start of each maintenance dose, and lab tests were repeated 2 hours after start of the third maintenance dose. At each follow-up visit on days 5, 8, and 15, patients had vitals taken, lab results obtained, and an assessment for any symptoms or signs suggestive of recurrent venom effect or adverse event (AE). The reason for this periodicity of follow-up was based on the documented range of recurrence chronology. During the follow-up phone call on day 22, concomitant medications and AEs were reviewed with the patient.
Outcomes
The primary efficacy endpoint was defined as coagulopathy between the end of maintenance dosing and study day 8 (+/− 1 day). Coagulopathy was defined as platelet count less than 150,000/mm3, fibrinogen less than 150 mg/dL, or use of antivenom to treat a coagulation abnormality between the end of maintenance dosing and study day 5. Secondary efficacy endpoints included platelet counts, fibrinogen levels, and venom levels.
Safety monitoring included specific overall assessment for acute and delayed serum reactions, clinical evidence of bleeding or coagulopathy, and other individual AEs regardless of apparent cause. AE seriousness, frequency, intensity, relationship to study drug, action taken, and outcome were recorded and clinically judged by investigators. AEs were monitored for 22 days.
Data analysis
Sample size calculations were based on predicted rates of coagulopathy during follow-up of 35 percent for Fab and 5 percent for F(ab')2. This difference in proportions would yield a moderate effect size. Under these assumptions, a sample size of 36 patients per treatment group gave greater than 80% power to detect a difference in coagulopathy rates between treatments. The total number (108) was later amended to 120, to allow a reasonable power for analyses even if up to 10% of patients withdrew from the study or were eliminated because of protocol deviations. The trial ended when the enrollment goal was reached. The primary efficacy analysis was based on the intent-to-treat (ITT) population.
The null hypothesis assumed no difference in coagulopathy incidence between treatments, the alternative hypothesis is that F(ab')2 is superior to Fab. Any fibrinogen level less than 60 was analyzed as 60 mg/dL, favoring the null hypothesis. For the primary efficacy endpoint, separate pairwise comparisons between each F(ab')2 group versus the Fab group were performed using Fisher's exact test. The Bonferroni–Holm method was used to preserve a family-wise type I error rate of 0.05.33 For all other analyses, a similar method was employed. Categorical outcomes were analyzed using Fisher's exact test; continuous outcomes were analyzed using analysis of variance (ANOVA). Statistical analyses were performed using IBM SPSS Statistics for Macintosh, 21.0, Armonk, NY, and power calculation was performed using PASS 2005, NCSS, LLC, Kaysville, UT.
Results
Characteristics of study subjects
A total of 123 patients were randomized, 41 in each group, of which 121 received antivenom and 114 completed the study (Fig. 1). There were no differences in gender, ethnicity, baseline mean platelet count or fibrinogen level, SSS, or time to antivenom administration among groups (Table 1). The F(ab')2/F(ab')2 group included more children and was younger on average than the Fab/Fab group. Eighteen study sites in 8 states participated in the study: AZ, CA, FL, KS, LA, NC, NM, and TX. Protocol deviations related to study drug type or dose occurred in 6 cases. The identified snake species included C. oreganus helleri, C. scutulatus, C. mitchellii, C. atrox, C. cerastes, C. ruber, C. viridis, C. molossus, C. horridus, S. miliarius barbouri, A. contortrix, and a single A. piscivorus. Copperhead bites affected 6 in the F(ab')2/F(ab')2 group, 7 in F(ab')2/placebo group, and 8 in the Fab/Fab group. One cottonmouth bite was identified in the Fab/Fab group. The rest were rattlesnake or unidentified bites (n = 102).
Fig. 1.
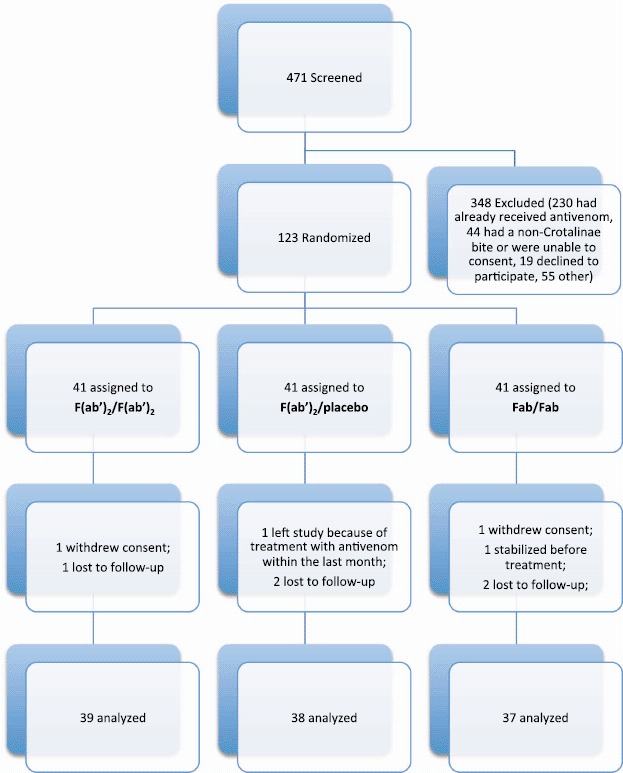
CONSORT flow diagram (colour version of this figure can be found in the online version at www.informahealthcare.com/ctx).
Table 1.
Demographic and baseline characteristics of the patients.∗
| Variable | F(ab')2/F(ab')2 | F(ab')2/Placebo | Fab/Fab |
|---|---|---|---|
| (N = 41) | (N = 40) | (N = 40) | |
| Age – yr | |||
| Mean | 32.9†± 22.26 | 40.3 ± 21.02 | 45.6 ± 16.52 |
| Median | 36 | 43 | 48 |
| Range | 2–80 | 7–77 | 5–80 |
| Age < 10 yrs – no. (%) | 12‡ (29.3) | 5 (12.5) | 1 (2.5) |
| Female gender- no. (%) | 11 (26.8) | 10 (25.0) | 12 (30.0) |
| Ethnicity- no. (%) | |||
| White | 29 (70.7) | 31 (77.5) | 26 (65.0) |
| Hispanic | 8 (19.5) | 7 (17.5) | 10 (25.0) |
| Black | 3 (7.3) | 1 (2.5) | 2 (5.0) |
| Native American | 1 (2.4) | 1 (2.5) | 2 (5.0) |
| Baseline platelet count – K/mm3 | |||
| Mean | 197.1 ± 100.7 | 223.6 ± 85.7 | 189.5 ± 79.3 |
| Median | 185.5 | 221.0 | 199.5 |
| Range | 15–400 | 26–382 | 35–348 |
| Baseline fibrinogen level – mg/dL | |||
| Mean | 275.8 ± 129.2 | 276.5 ± 98.5 | 268.4 ± 86.6 |
| Median | 256.0 | 293.0 | 267.0 |
| Range | 60–718 | 20–475 | 60–438 |
| Snakebite Severity Score31 | |||
| Mean | 3.8 ± 2.10 | 3.0 ± 1.51 | 3.8 ± 2.33 |
| Median | 3.0 | 3.0 | 3.0 |
| Range | 1–11 | 1–7 | 1–11 |
| Bite-to-antivenom start time – hrs | |||
| Mean | 6.4 ± 6.22 | 4.9 ± 3.44 | 8.7 ± 10.93 |
| Median | 4.4 | 3.5 | 4.5 |
| Range | 2.1–35.5 | 1.8–18.7 | 1.6–58.7 |
Plus-minus values are means± SD.
p < 0.05, One-way ANOVA, compared with Fab/Fab.
p < 0.05, Fisher's exact test, compared with Fab/Fab.
Efficacy
Coagulopathy was less frequent in the F(ab')2/F(ab')2 and the F(ab')2/placebo groups compared with that in the Fab/Fab group, and there was an absolute risk reduction in both F(ab')2 groups compared with that in Fab group (Table 2). The lowest platelet counts were lower in the Fab group compared with those in both the F(ab')2 groups. The lowest fibrinogen levels were lower in the Fab group compared with those in the F(ab')2/placebo group. The only patients with fibrinogen levels less than 60 mg/dL (n = 2) and platelet counts less than 50,000/mm3 (n = 2) after initial control were in the Fab group. Post-hoc review of venom and antivenom levels by Instituto Bioclon suggested that analyte degradation during transport and prolonged storage at −20 may have reduced signal intensity to such an extent that comparison of results across groups was invalidated. For this reason, these results were excluded from this analysis.
Table 2.
Efficacy endpoints.
| F(ab')2/F(ab')2
(N = 39) |
F(ab')2/placebo (N = 38) |
Fab/Fab (N = 37) |
|
|---|---|---|---|
| Experienced late coagulopathy—No. (%) | 4 (10.3)‡ | 2 (5.3)‡ | 11 (29.7) |
| Absolute risk reduction (95% Cl)∗ | 0.195 (0.014–0.367) | 0.245 (0.073–0.410) | − |
| Number needed to treat (patients)∗ | Approximately 5 | Approximately 4 | – |
| Mean platelet count, 1,000 s per mm ± SD (range) | |||
| Day 5 | 265.2 ± 81.4 (121–434) | 259.5 ± 61.4 (132–374) | 227.1 ± 70.0 (77–389) |
| Day 8 | 266.8 ± 84.7 (125–447) | 265.0 ± 73.5 (114–424) | 234.1 ± 80.8 (40–415) |
| Mean fibrinogen, mg/dL ± SD (range) | |||
| Day 5 | 369.4 ± 75.1 (223–530) | 394.7 ± 97.7 (173–650) | 364 ± 116.5 (81–564) |
| Day 8 | 344.7 ± 70.9 (208–477) | 387.1 ± 96.0 (195–693) | 334.8 ± 114.4 (105–589) |
| Lowest platelet count, 1,000 s per mm3 ± SD (range) | |||
| 253.4†± 80.3 (121–434) | 247.5†± 62.6 (114–374) | 208.9 ± 76.9 (40–389) | |
| Lowest fibrinogen, mg/dL ± SD (range) | |||
| 340.0 ± 70.0 (208–477) | 367.8†± 93.9 (173–650) | 309.0 ± 116.4 (< 60–564) |
∗Compared with Fab/Fab.
†p < 0.05, One-way ANOVA, compared to Fab/Fab.
‡p < 0.05, Fisher's exact test, compared to Fab/Fab.
None of the children < 10 years old (n = 18; 15.8%) experienced late coagulopathy. Six extra doses were given to F(ab')2/F(ab')2 recipients, eleven to F(ab')2/placebo recipients, and eighteen to Fab/Fab recipients. Of 6 patients treated with F(ab')2 who experienced late coagulopathy, all manifested at a single site in inland southern California; the species, identified by the first author, were C. oreganus helleri (n = 2), C. mitchellii, C. ruber, C. atrox, and one unknown Crotalus sp. Late coagulopathy occurred in Fab patients at 7 sites: in California (n = 8), Arizona (n = 5), New Mexico (n = 2), Florida (n = 1), and North Carolina (n = 1). Removal of protocol deviation cases from the ITT analysis did not affect significance of results.
Adverse events and safety
Overall, fewer AEs were reported in the F(ab')2/placebo group than in the F(ab')2/F(ab')2 or Fab/Fab (Table 3). The majority of events reported in all three of the study groups were assessed as mild.
Table 3.
Adverse events (Safety population).
| Event | F(ab')2/F(ab')2∗ (N = 43) |
F(ab')2/Placebo∗ (N = 37) |
Fab/Fab (N = 41) |
|---|---|---|---|
| Number of AEs | 130 | 72 | 137 |
| Patients reporting at least 1 AE—No. (%) | 35 (81.4) | 24 (64.9) | 33 (80.5) |
| Itching (pruritus)—No. (%) | 20 (46.5) | 14 (37.8) | 20 (48.8) |
| Easy bruising, gingival bleeding, petechiae or melena—No. (%) | 4 (9.3) | 3 (8.1) | 10 (24.4) |
| Nausea, vomiting—No. (%) | 8 (18.7) | 6 (16.2) | 7 (17.0) |
| Rash —No. (%) | 5 (11.6) | 5 (13.5) | 5 (12.2) |
| Arthralgia—No. (%) | 4 (9.3) | 4 (10.8) | 7 (17.1) |
| Myalgia—No. (%) | 3 (7.0) | 3 (8.1) | 8 (19.5) |
| Dehydration—No. (%) | 2 (4.7) | 0 (0.0) | 4 (9.8) |
| Chest pain—No. (%) | 0 (0.0) | 1 (2.7) | 2 (4.9) |
| Other (fever, headache, cellulitis, diarrhea, pain, fatigue or blisters)—No. (%) | 18 (41.9) | 11 (29.7) | 17 (41.5) |
| Serious adverse events—No. (%) | 6 (14.6) | 1 (2.7) | 2 (4.9) |
| Immune reactions | |||
| Acute serum reaction—No. (%) | 1 (2.3) | 1 (2.7) | 1 (2.4) |
| Serum sickness—No. (%) | 1 (2.3) | 1 (2.7) | 1 (2.4) |
∗No pairwise comparison with Fab/Fab was significant using Fisher's exact test.
Nine patients experienced Serious AEs (SAEs), all assessed as unrelated to study drug. One patient in the F(ab')2/F(ab')2 group died on Day 7 from injuries related to a traffic collision. One SAE in the Fab/Fab group was related to multicomponent coagulopathy recurrence and persistence, with major extension of ecchymosis on day 2, bleeding from an intrajugular intravenous site on day 6, and a 10-g drop in hemoglobin during an 11-day hospital course.
One patient in each arm of the study experienced an acute serum reaction; however, all three tolerated subsequent doses after resolution of the AEs. One patient in each group experienced a pattern of symptoms suggestive of serum sickness during follow-up. No immune reaction was severe.
Mean antivenom dose in terms of vials was similar for the F(ab')2/placebo (16.1 ± 7.89, range: 5–38) and Fab/Fab (14.2 ± 5.66, range: 7–38) groups, but greater in the F(ab')2/F(ab')2 (27.2 ± 7.25, range: 22–46) group. Maximum total protein exposure was 3.5–6 times greater in the Fab group than that in either of the F(ab')2 groups.
Discussion
This study supports the hypothesis that management of US Crotalinae envenomation using an F(ab')2 antivenom reduces subacute coagulopathies following treatment in comparison with that using Fab antivenom. An absolute risk reduction of 19.5–24.5 percent would suggest that 4–5 patients would need to be treated with F(ab')2 to result in fewer cases of late coagulopathy. Serious bleeding complications after treatment with Fab occurred in our study, consistent with past reports.23,26–30 This implies that use of F(ab')2 antivenom could reduce medically significant late bleeding after snakebite, and that the need for repeated blood testing after treatment could be reduced. In addition, our study suggests that maintenance dosing is not required with F(ab')2 to prevent late coagulopathy.
The specific F(ab')2 and Fab antivenoms compared in this study differ in many ways, most obviously not only in the structure and mass of the molecule but also in the venom immunogens used, affinity purification, protein content, and pharmacokinetics. Each of these variables could have influenced efficacy, and so each is considered. First, Fab is produced using venoms from snake species indigenous to the US, whereas venoms for manufacturing F(ab')2 come from Latin American species. Venom composition and antigenicity can vary considerably with species34 and geographic35 origin of a snake; it is commonly assumed that venom immunogens derived from local species produce more regionally effective antivenom.24 Our study showed the opposite, suggesting that this was less important than other factors. Second, Fab is affinity purified while F(ab')2 is not. Effective affinity purification should favor the per-mg potency and efficacy of Fab, again in contradistinction to our results, which favored the lower-protein, non-affinity-purified F(ab')2 antivenom. Finally, Phase 2 data showed that clearance of Fab is associated with recurring venom antigenemia and an accompanying drop in platelet count and fibrinogen levels, in contrast with F(ab')2, which cleared more slowly and was not associated with recurrent venom effects.18 This difference is consistent with our Phase 3 findings. Therefore, we conclude that differences in incidence of coagulopathy are principally related to antivenom kinetics. This is consistent with findings in a comparative study of F(ab')2 and Fab antivenoms for Echis ocellatus envenomations in Nigeria25 and in a comparison of F(ab')2 to Fab for restoration of blood coagulability after Daboia russellii in Sri Lanka.24
We found no tradeoffs in safety related to immune reactions or miscellaneous AEs. The plasma source for Fab is ovine, whereas for F(ab')2 it is equine. It has been postulated that immunizing sheep rather than horses could reduce risk associated with exposure to equine-produced allergenic components,36 but immune reactions in all groups were low in this study, without discernible differences between the two antivenoms. Safety as it relates to coagulopathy, however, was better with F(ab')2, consistent with results from Phase 2.18
Limitations
Limitations of the study arose from the inclusion of a disproportionate number of children in the F(ab')2/F(ab')2 group compared with that in the Fab/Fab group. None of the 17 children treated with F(ab')2 experienced late coagulopathy; however, late coagulopathies, some with medically significant late bleeding, have been reported in children less than 10 years old, suggesting that the confounding effect of this group should be minimal.26,37 This study was not powered to demonstrate the interaction of different snake venoms or site-specific populations with the primary endpoint, but the difference in geographic distribution of coagulopathic cases suggests that future studies should consider the effects of heterologous antivenom(s) on many US Crotalinae species.
Conclusions
For envenomation by North American Crotalinae snakes capable of causing unexpected bleeding, this study found that management with longer-half-life F(ab')2 antivenom reduces the risk of post-treatment recurrence and late-onset coagulopathies following treatment when compared with management with Fab. Until such time as F(ab')2 is commercially utilized in the US, patients with Crotalinae envenomation will be at continued risk for medically significant late bleeding complications (including death) in the days and weeks following treatment with Fab.
Acknowledgments
We thank Joanne Mallie, who assisted with study administration and coordinated data management from 4 sites in Tucson; Lisbeth Gaf, who set up and coordinated central data management; Pieter D'Arnaud, who assisted with statistical analysis; Angela Padilla-Jones, RN, research nurse and study coordinator at Banner Good Samaritan Medical Center; Frank Watkins, study coordinator at Vidant Medical Center; “Team Venom” at Loma Linda University Medical Center, Susan D. Smith, Tammy H. Phan, Sarah R. Pearl, Sarang Kim, and Alyssa Flores-Cuevas; and Ramona Gee.
In memoriam: The authors acknowledge with respectful memory the work of John F. Haynes, Jr., MD, whose participation as clinical investigator was essential to this study.
Declaration of interest
Rare Disease Therapeutics, Inc. (RDT) sponsored this study through contracts with the authors' affiliated institutions, and Instituto Bioclon, SA de CV manufactures the F(ab')2 antivenom in this study. Drs. Bush, Ruha, and Quan report receiving remuneration for educational presentations related to the medical management of North American Crotalinae bites with Fab antivenom from BTG international Inc (BTG). Drs. Bush, Ruha, and Seifert participated in an expert panel sponsored by BTG that developed a treatment algorithm for pit viper envenomation. Drs. Bush, Ruha, and Siefert report being investigators in phase 2 and/or 3 clinical trials of a black widow spider antivenom manufactured by Instituto Bioclon, and their institution(s) contracted with Rocky Mountain Poison & Drug Center (RMPDC) to conduct these studies. Drs. Ruha and Boyer report being investigators in phase 2 and/or 3 clinical trials of a scorpion antivenom manufactured by Instituto Bioclon. Dr. Ruha reports having received remuneration from RDT for providing presentations about scorpion antivenom. Dr. Alagón reports that he had grant support from Instituto Bioclon at the time of the study, as well as past consultancy for Instituto Bioclon. Dr. Garcia reports being a full-time employee of Instituto Bioclon. Dr. Boyer reports that she had grant support from RDT for management of patients in the study, as well as a grant from Instituto Bioclon for development of North African antivenom. The authors alone are responsible for the content and writing of the paper.
Author contributions and responsibilities
LVB, WG-U, and AA designed the study; SPB, A-MR, LVB, SAS, DLM, BJL, TCA, RFC, WJM, EAT, SWB, GRF, DRS, FMS, RW, IDC, and DQ collected the data; SPB, LVB, A-MR, SAS, RDG analyzed the data; SPB, LVB, A-MR, and SAS were the primary authors of the manuscript with final editing and approval by all coauthors, and all authors vouch for the accuracy and completeness of the reported analyses.
Notice of Correction
Since the online publication of this article the following has been corrected in Table 1, “3.Oil.51” has been changed to “3.0±1.51.
References
- 1.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna A, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronstein AC, Spyker DA, Cantilena LR, Jr, Rumack BH, Dart RC. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila) 2012;50:911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 3.Langley RL, Morrow WE. Deaths resulting from animal attacks in the United States. Wilderness Environ Med. 1997;8:8–16. doi: 10.1580/1080-6032(1997)008[0008:drfaai]2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Boyer L, Degan J, Ruha AM, Mallie J, Mangin E, Alagón A. Safety of intravenous equine F(ab')2: insights following clinical trials involving 1534 recipients of scorpion antivenom. Toxicon. 2013;76:386–393. doi: 10.1016/j.toxicon.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Dart RC, McNally J. Efficacy, safety, and use of snake antivenoms in the United States. Ann Emerg Med. 2001;37:181–195. doi: 10.1067/mem.2001.113372. [DOI] [PubMed] [Google Scholar]
- 6.Norris RL, Bush SP, Smith J. Bites by venomous reptiles in Canada, the U.S. and Mexico. In: Auerbach PS, editor. Wilderness Medicine. 6th ed. Philadelphia: Saunders; 2012. pp. 1011–1039. [Google Scholar]
- 7.Gold BS, Dart RC, Barish RA. Bites of venomous snakes. NEJM. 2002;347:347–356. doi: 10.1056/NEJMra013477. [DOI] [PubMed] [Google Scholar]
- 8.Landon J, Smith DS. Merits of sheep antisera for antivenom manufacture. J Toxicol. 2003;22:15–22. [Google Scholar]
- 9.WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. Available at: http://www.who.int/neglected_diseases/diseases/snakebites/en/ (Accessed June 19, 2014). [DOI] [PubMed]
- 10.Litovitz TL, Schmitz BF, Bailey KM. 1989 annual report of the American Association of Poison Control Centers National Data Collection System. Am J Emerg Med. 1990;8:394. doi: 10.1016/0735-6757(90)90234-q. [DOI] [PubMed] [Google Scholar]
- 11.Litovitz TL, Holm KC, Bailey KM, Schmitz BF. 1991 annual report of the American Association of Poison Control Centers National Data Collection System. Am J Emerg Med. 1992;10:452. doi: 10.1016/0735-6757(92)90075-9. [DOI] [PubMed] [Google Scholar]
- 12.CroFab® . Prescribing Information. Brentwood, TN 37027: Protherics Inc.; 2012. [Google Scholar]
- 13.Anavip® . Prescribing Information [pending FDA approval at the time of this writing] Mexico D.F., Mexico: Instituto Bioclon S.A. de CV; [Google Scholar]
- 14.Lavonas EJ, Schaeffer TH, Kokko J, Mlynarchek SL, Bogdan GM. Crotaline Fab antivenom appears to be effective in cases of severe North American pit viper envenomation: An integrative review. BMC Emergency Medicine. 2009;9:13. doi: 10.1186/1471-227X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer LV, Seifert SA, Clark RF, McNally JT, Williams SR, et al. Recurrent and persistent coagulopathy following pit viper envenomation. Arch Intern Med. 1999;159:706–710. doi: 10.1001/archinte.159.7.706. [DOI] [PubMed] [Google Scholar]
- 16.Boyer LV, Seifert SA, Cain JS. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann Emerg Med. 2001;37:196–201. doi: 10.1067/mem.2001.113134. [DOI] [PubMed] [Google Scholar]
- 17.Seifert SA, Boyer LV. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 1. Pharmacokinetics and pharmacodynamics of immunoglobulin antivenoms and related antibodies. Ann Emerg Med. 2001;37:189–195. doi: 10.1067/mem.2001.113135. [DOI] [PubMed] [Google Scholar]
- 18.Boyer LV, Chase PB, Degan JA, Figge G, Buelna-Romero A, Luchetti C, Alagón A. Subacute coagulopathy in a randomized, comparative trial of Fab and F(ab')2 antivenoms. Toxicon. 2013;74:101–108. doi: 10.1016/j.toxicon.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Ruha AM, Curry SC, Beuhler M, Katz K, Brooks DE, Graeme KA, et al. Initial post marketing experience with Crotalidae polyvalent immune Fab for treatment of rattlesnake envenomation. Ann Emerg Med. 2002;39:609–615. doi: 10.1067/mem.2002.123698. [DOI] [PubMed] [Google Scholar]
- 20.Bush SP, Seifert SA, Oakes J, Smith SD, Phan TH, Pearl SR, Reibling ET. Continuous IV Crotalidae Polyvalent Immune Fab (Ovine) (FabAV) for selected North American Rattlesnake bite patients. Toxicon. 2013;69:29–37. doi: 10.1016/j.toxicon.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Ruha AM, Curry SC, Albrecht C, Riley B, Pizon A. Late hematologic toxicity following treatment of rattlesnake envenomation with Crotalidae polyvalent immune Fab antivenom. Toxicon. 2011;57:53–59. doi: 10.1016/j.toxicon.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Lavonas EJ, Ruha AM, Banner W, Bebarta V, Bernstein JN, Bush SP, et al. Rocky Mountain Poison and Drug Center, Denver Health and Hospital Authority Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2. doi: 10.1186/1471-227X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bush SP, Seifert SA, Pearl SR. Continuous infusion of crotalidae polyvalent Fab (Ovine) A Patient With Rattlesnake Envenomation. 2013. Presented at Biology of the Rattlesnakes, Tucson AZ, 2011. Published in Bush SP: Case study – rattlesnake envenomation. July 2012. In: Crotaline Snakebite Management: The role of CroFab Crotalidae Polyvalent Immune Fab (Ovine). BTG International, Inc.
- 24.Ariaratnam CA, Sjostrom L, Raziek Z, Kularatne SA, Arachchi RW, Sheriff MH, et al. An open, randomized comparative trial of two antivenoms for the treatment of envenoming by Sri Lankan Russell's viper (Daboia russelii russelii) Trans Roy Soc Trop Med and Hyg. 2001;95:74–80. doi: 10.1016/s0035-9203(01)90339-6. [DOI] [PubMed] [Google Scholar]
- 25.Meyer WP, Habib AG, Onayade AA, Yakubu A, Smith DC, Nasidi A, et al. First clinical experiences with a new ovine Fab Echis ocellatus snake bite antivenom in Nigeria: a randomized comparative trial with Institute Pasteur Serum (IPSER) Africa antivenom. Am J Trop Med Hyg. 1997;56:291–300. doi: 10.4269/ajtmh.1997.56.291. [DOI] [PubMed] [Google Scholar]
- 26.Lavonas EJ, Khatri V, Daugherty C, Bucher-Bartelson B, King T, Dart RC. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63:71–78. e1. doi: 10.1016/j.annemergmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Kitchens C, Eskin T. Fatality in a case of envenomation by Crotalus adamanteus initially successfully treated with polyvalent ovine antivenom followed by recurrence of defibrinogenation syndrome. J Med Toxicol. 2008;4:180–183. doi: 10.1007/BF03161198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaral versus Loma Linda University [CIV S801200] Superior Court of San Bernadino County, CA: 2011. [Google Scholar]
- 29.Balow versus University of Cincinatti 2009-Ohio-5448.
- 30.Levine M, Ruha AM, Padilla-Jones A, Gerkin R, Thomas SH. Bleeding following rattlesnake envenomation in patients with pre-envenomation use of antiplatelet or anticoagulant medications. Acad Emerg Med. 2014;21:301–307. doi: 10.1111/acem.12333. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez EE, Galan JA, Perez JC, Rodriguez-Acosta A, Chase PB, Perez JC. The efficacy of two antivenoms against the venom of North American snakes. Toxicon. 2003;41:357–365. doi: 10.1016/s0041-0101(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 32.Dart RC, Hurlbut KM, Garcia R, Boren J. Validation of a severity score for the assessment of Crotalid snakebite. Ann Emerg Med. 1996;27:321–326. doi: 10.1016/s0196-0644(96)70267-6. [DOI] [PubMed] [Google Scholar]
- 33.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 34.de Roodt AR, Clement H, Dolab A, Litwin S, Hajos SE, Boyer L, Alagón A. Protein content of antivenoms and relationship with their immunochemical reactivity and neutralization assays. Clinical Toxicology. 2014;52:594–603. doi: 10.3109/15563650.2014.925561. [DOI] [PubMed] [Google Scholar]
- 35.French WJ, Hayes WK, Bush SP, Cardwell MD, Bader JO, Rael ED. Mojave toxin in venom of Crotalus helleri (Southern Pacific Rattlesnake): molecular and geographic characterization. Toxicon. 2004;44:781–791. doi: 10.1016/j.toxicon.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Dart RC, Seifert SA, Carroll L, Clark RF, Hall E, Boyer-Hassen LV, et al. Affinity-purified, mixed monospecific crotalid antivenom ovine Fab for the treatment of crotalid venom poisoning. Ann Emerg Med. 1997;30:33–39. doi: 10.1016/s0196-0644(97)70107-0. [DOI] [PubMed] [Google Scholar]
- 37.Miller AD, Young MC, DeMott MC, Ly BT, Clark RF. Recurrent coagulopathy and thrombocytopenia in children treated with crotalidae polyvalent immune fab: a case series. Pediatr Emerg Care. 2010;26:576–582. doi: 10.1097/PEC.0b013e3181ea722b. [DOI] [PubMed] [Google Scholar]


