Abstract
Background
While restrictive mitral annuloplasty (RMA) is the preferred surgical treatment for functional ischemic mitral regurgitation (FIMR), some patients with severely dilated left ventricles suffer from recurrent mitral regurgitation (MR). Consequently, new surgical strategies have been entertained to compensate for severely dilated ventricles by maximizing coaptation and reducing subvalvular tethering. Anterior leaflet augmentation (ALA) with mitral annuloplasty has been theorized to meet these goals. This study sought to compare the mechanistic effects of RMA and adjunct ALA in the setting of FIMR.
Methods and Results
Mitral valves (MV) were mounted in a clinically relevant left heart simulator. Tested conditions included control, FIMR, RMA, and true sized annuloplasty with either a small or large ALA. A2-P2 leaflet coaptation length, FMR, and strut and intermediary chordal forces were quantified. All repairs alleviated MR. Coaptation length was significantly increased from FIMR to RMA, small ALA, and large ALA (p<.001). Between repairs, large ALA created the greatest length of coaptation (p<.05). Tethering forces from the posteromedial strut chordae were reduced from the FIMR condition by all repairs (p<.001). Only large ALA reduced intermediate chordal tethering from the FIMR condition (p<.05).
Conclusions
Large ALA procedure created the greatest coaptation and reduced chordal tethering. Although all repairs abolished MR acutely, repairs that create the greatest coaptation may conceivably produce a more robust and lasting repair in the chronic stage. A clinical need still exists to best identify which patients with altered MV geometries would most benefit from an adjunct procedure or RMA alone.
Keywords: Ischemic Mitral Regurgitation, Mitral Valve, Repair, Annuloplasty, Mechanics
INTRODUCTION
Functional ischemic mitral regurgitation (FIMR) is classified as a Carpentier's type IIIb valvular dysfunction that results from post infarction left ventricular remodeling [1]. This disease poses a significant clinical challenge as FIMR severity is directly associated with patient mortality [2]. While significant knowledge of FIMR and its physiological impact exists, the surgical management of FIMR remains controversial [3-5]. Amid this controversy, restrictive mitral annuloplasty (RMA) with a complete rigid ring has been regarded as a preferred surgical repair [3, 6]. While typically effective, approximately 10-15% of patients presenting with severe left ventricular dilation suffer from recurrent mitral regurgitation, have low rates of reverse left ventricular remodeling, and exhibit poor survival [7-10].
Consequently, new adjunct surgical strategies have been attempted to compensate for severely dilated left ventricles [4]. When completed with mitral annuloplasty, these adjunct strategies aim to further alleviate chordal-leaflet tethering, improve leaflet mobility, and maximize leaflet coaptation. Of note, anterior leaflet augmentation [5, 11] has been recently demonstrated to restore leaflet coaptation and mobility without the need to unnaturally restrict the mitral annulus [10, 11] While preliminary studies have demonstrated the potential benefits of both RMA and anterior leaflet augmentation, little knowledge exists of what mechanistic improvements in valve function can be expected.
In this manuscript, we aim to elucidate the functional and mechanical benefits of anterior leaflet augmentation with true sized mitral annuloplasty as compared to isolated RMA in the setting of functional ischemic mitral regurgitation. Anterior leaflet augmentation was performed with two pericardial patch sizes to further elucidate the potential effects of procedural technique. We hypothesize that anterior leaflet augmentation will achieve comparable leaflet coaptation to RMA, while yielding significant improvements in leaflet tethering as measured by chordal forces.
MATERIALS AND METHODS
In Vitro Simulator
The extensively studied Georgia Tech left heart simulator was utilized for this study [12]. This in vitro model has been previously demonstrated to mimic the systolic MV geometry [12], leaflet coaptation , regurgitation , and anterior leaflet strain of healthy and chronic functional ischemic mitral regurgitation ovine models. In this model, ovine MVs were excised, mounted into the simulator, and tested under pulsatile left heart hemodynamics.
Chordal Force Transducers
Miniature c-shaped force transducers have been used previously to quantify tethering forces of the MV's chordae tendineae [13]. These strain gage based transducers were manufactured, and tested within our laboratory. Calibration was performed before and after each experiment. The relationship between the calibrated load and transducer voltage output was linear with a regression coefficient (R2) between 0.98 - 1.00. The relative difference between measured and true calibrated values (accuracy) was less than 2%. Smallest measurable tension was 0.01 N [13]. Prior to each experiment, these transducers were sutured directly to selected chordae without altering the chordae's native length. The section of chordae located between the transducer's measurement arms was bisected such that all tensile loading of the chord was transferred to the transducer.
Mitral Valve Experimental Preparation
For this study, fresh ovine hearts (N = 15) were procured from a local abattoir. MVs were excised preserving their annular and subvalvular anatomy. MVs with an anterior leaflet height of 20-25 mm, type I or II PMs, and with all leaflet chordae inserting directly into each PM were selected for experimentation. Selected MVs were sutured to the simulator's annulus using a Ford interlocking stitch. During valve suturing, care was taken to place each suture just above the valve's natural hinge and not through the leaflet tissue. Additionally, normal annular-leaflet geometric relationships were respected: anterior leaflet occupying 1/3rd of annular circumference and commissures aligned in the 2 and 10 o'clock positions. After annular suturing, strut chordae inserting into the anterior leaflet (N=2) and intermediary chordae inserting into the posterior leaflet (N=2) were instrumented with chordal force transducers. Following instrumentation, each PM was attached to the positioning control rods.
Experimental Protocol
After mounting each of the instrumented MVs within the simulator, the mitral annulus was conformed to the shape of a size 30 Physio annuloplasty ring (Edwards Lifesciences, Irvine, CA). Upon establishing human pulsatile left heart hemodynamics (cardiac output: 5.0 L/min; heart rate: 70 beats/min; transmitral pressure: 120 mmHg), each PM was carefully positioned to establish the control MV geometry [14]. Mitral coaptation was inspected via echocardiography: the anterior leaflet spanned two-thirds of the A2-P2 annular diameter, 5-6 mm of coaptation were achieved, and less than 1 mm tenting was observe. If the control valve geometry conditions were successfully achieved, transmitral flow, left atrial and ventricular pressure, 3D echocardiography (Philips iE-33 Matrix, Phillips Healthcare, Andover, MA), Doppler Echocardiography, and chordal forces were acquired.
To simulate chronic FIMR due to an inferior myocardial infarction, the valve annulus was asymmetrically dilated to 150% of the control valve area. The antero-lateral papillary muscle was displaced 3 mm apically and 2 mm anteriorly, while the postero-medial papillary muscle was displaced 4 mm apically, 4 mm posteriorly, and 8 mm laterally. These changes were consistent with data from chronic functional ischemic mitral regurgitation due to inferior myocardial infarction [1]. Upon establishing these conditions, all experimental measurements were acquired.
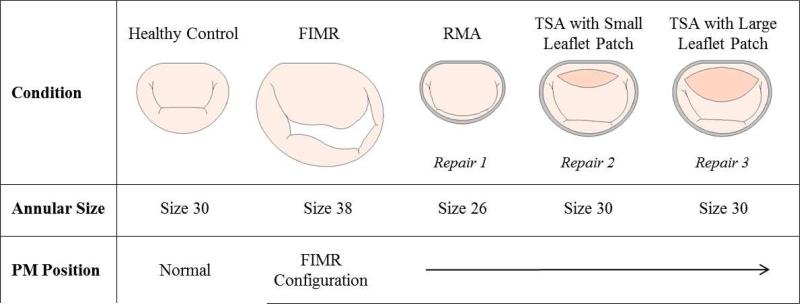
In this study, three repair strategies were evaluated that included restrictive mitral annuloplasty (N=15), true-sized annuloplasty with a “small” sized anterior leaflet augmentation (N=8), and a true-sized annuloplasty with a “large” anterior leaflet augmentation (N=7). Restrictive and true-sized mitral annuloplasty were simulated by conforming the simulator's annulus to a size 26 and size 30 Physio ring, respectively (Figure 1).
Figure 1.
Experimental conditions (annulus and anterior leaflet patch sizes) are shown in this schematic representation of the experimental protocol.
Anterior leaflet augmentations were randomized and completed in two sizes using a bovine pericardium lightly fixed in 0.5% gulteraldeyhde. For the large augmentation, the, the patch dimensions were: length: 2.68 ± 0.25 cm; height: 1.07 ± 0.10 cm; area: 2.26 ± 0.36 cm2. For the small augmentation, the patch dimensions were: (length: 1.77 ± 0.10 cm; height: 0.65 ± 0.14 cm; area: 0.86 ± 0.29 cm2). An incision was made near the base of the anterior leaflet and parallel to the mitral annulus. This created an oblong shaped opening in the anterior leaflet to which the patch was sutured. The length of the incisions corresponded to the length of the desired patch dimensions. With either augmentation repair, a concomitant true sized annuloplasty procedure was performed. The pericardial patch was positioned in place with 4 interrupted knots in the 3, 6, 9, and 12 o'clock positions. A running mattress suture was used to secure the pericardium to the leaflet and create a leak free seal.
Data Analysis
All hemodynamic and chordal force data were ensemble averaged over 10 cardiac cycles. Mitral regurgitation was quantified by integrating the negative (or reverse) flow over the systolic portion of the cardiac cycle. The presence of MR was confirmed with Doppler echocardiography. Philips Qlab (Phillips Healthcare, Andover, MA) was used to quantify MV leaflet coaptation length measured at the A2-P2 coaptation line. Chordal force data is presented as the difference between peak systolic and peak diastolic force. Percent improvements in coaptation length (increase in coaptation length) and chordal force (reduction in force) data were calculated between postoperative repair and FIMR groups.
Measured endpoints were checked for normality using the Anderson-Darling test. Independent samples T-Test and a Two-Way ANOVA were used when appropriate. A Tukey post hoc test was used to further investigate significance. All statistical analyses were completed using Minitab 16 (Minitab Inc, State College, PA). All data are reported as mean ± 1 standard deviation.
RESULTS
Functional ischemic Mitral Regurgitation
Functional ischemic mitral regurgitation was successfully simulated with asymmetric annular dilation and papillary muscle displacement for all experiments (3+ MR grade: N = 4; 4+ MR Grade: N = 11). Large, asymmetric regurgitant jets were observed via color Doppler echocardiography. Leaflet tethering was observed and coaptation length was significantly decreased from control (Control: 4.8 ± 0.5 mm; FIMR: 3.2 ± 0.5 mm; p < 0.005) at the central A2-P2 plane (Table 1).
Table 1.
Coaptation length and tethering forces for each chordae across all conditions are shown.
| Healthy | FIMR | RMA | Small Patch | Large Patch | ||
|---|---|---|---|---|---|---|
| Sample Size | 15 | 15 | 15 | 8 | 7 | |
| Coaptation Length [mm] | 4.8 ± 0.5 | 3.2 ± 0.5* | 4.7 ± 0.6 | 4.8 ± 0.5 | 5.8 ± 1.0† | |
| Chordal Forces [N] | ||||||
| Posterior Papillary Muscle | Anterior Strut | 0.71 ± 0.31 | 1.23 ± 0.44‡ | 0.89 ± 0.33 | 0.91 ± 0.37 | 0.74 ± 0.31 |
| Posterior Intermediate | 0.28 ± 0.13 | 0.55 ± 0.29§ | 0.51 ± 0.26 | 0.39 ± 0.18 | 0.45 ± 0.25 | |
| Anterior Papillary Muscle | Anterior Strut | 0.62 ± 0.44 | 0.80 ± 0.52 | 0.66 ± 0.39 | 0.78 ± 0.43 | 0.94 ± 0.60 |
| Posterior Intermediate | 0.49 ± 0.34 | 0.71 ± 0.51 | 0.54 ± 0.33 | 0.46 ± 0.24 | 0.63 ± 0.45 | |
Statistical comparisons were made between experimental conditions for each quantitative measurement (each row in the table).
Denotes significance of coaptation length between control and FIMR.
Denotes significance of coaptation length between large patch and control.
Denotes significance between anterior strut chordal force from the anterior PM between FIMR and control.
Denotes significance between posterior intermediate chordal force from the posterior PM between FIMR and control.
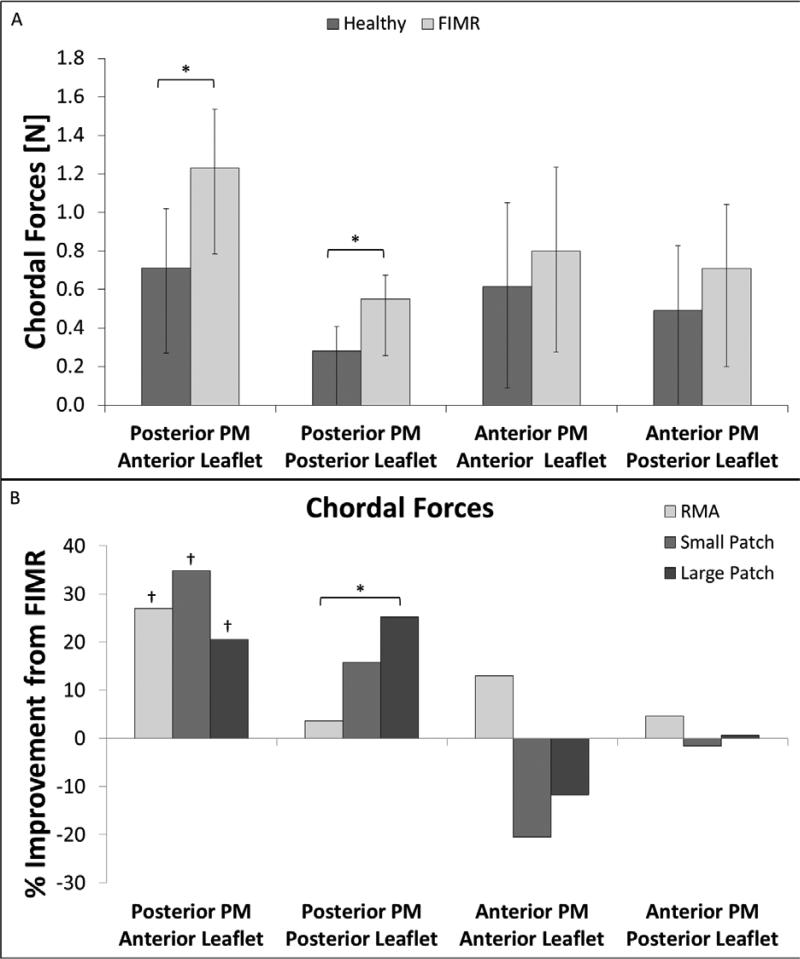
For the posteromedial PM, the tethering force in the anterior strut chordae was significantly increased compared to control (Control: 0.71 ± 0.31 N; FIMR: 1.23 ± 0.44 N; p = 0.001) (Table 1, Figure 2). Similarly, the force in the posterior intermediate chordae was significantly increased compared to control (Control: 0.28 ± 0.13 N; FIMR: 0.55 ± 0.29 N; p = 0.006) (Table 1, Figure 2).
Figure 2.
(A) Chordal forces are shown for all four chordae in the healthy an FIMR conditions. (B) Improvement (% reduction) in chordal force as compared to FIMR is shown for all four chordae for the RMA, small, and large patch repairs.
No statistically significant changes between control and FIMR conditions were observed for the anterior strut (Control: 0.62 ± 0.44 N; FIMR: 0.80 ± 0.52 N) and posterior intermediate chordae (Control: 0.49 ± 0.34 N; FIMR: 0.71 ± 0.51 N) originating from the anterolateral PM (Table 1, Figure 2).
Restrictive Annuloplasty versus Anterior Leaflet Augmentation with True-Sized Annuloplasty
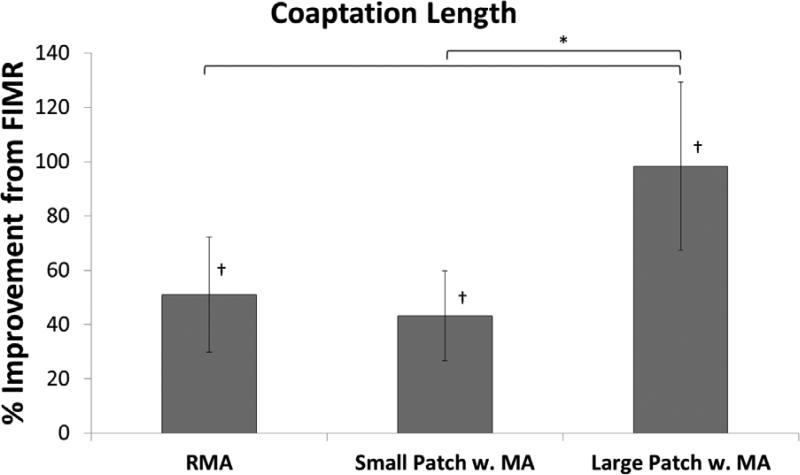
All surgical repairs alleviated mitral regurgitation; no regurgitant jets were observed postoperatively. Additionally, all repairs significantly (p < 0.005) increased coaptation length from FIMR back to or greater than healthy levels (Table 1, Figure 3). The large patch procedure with true-sized annuloplasty resulted in the best improvement in coaptation length (98.3 ± 31.0 % increase) as compared to FIMR (Figure 3). Restrictive mitral annuloplasty (51.0 ± 21.3 % increase) and the small patch with true-sized annuloplasty (43.2 ± 16.5 % increase) treatments resulted in comparable improvements in coaptation length as compared to FIMR (Figure 3). Further, postoperative coaptation length for the large patch procedure was significantly greater than the restrictive mitral annuloplasty (p = 0.006) and the small (p = 0.003) patch repairs (Table 1).
Figure 3.
Improvement (% increase) in coaptation length as compared to FIMR is shown for the RMA, small and large patch repairs.
For the posteromedial PM, the tethering force in the anterior strut chordae was significantly reduced postoperatively by all three repairs (Table 1). Compared to the FIMR condition, the force in the posterior intermediate chordae was moderately reduced by the small (15.8 ± 23.5 % reduction) and large (25.2 ± 18.0 % reduction) patch repairs. Moreover, the large patch repair significantly reduced posterior intermediate chordal force compared to the restrictive mitral annuloplasty treatment (3.7 ± 23.9 % reduction).
For the anterolateral PM, anterior strut chordal tethering force was significantly increased for both the small (20.5 ± 69.4 % increase) and large (11.8 ± 27.2 % increase) patch treatments as compared to FIMR. Restrictive mitral annuloplasty showed a moderate improvement (13.0 ± 19.1 % reduction) in chordal force as compared to FIMR. The tethering force in the posterior intermediate chordae originating from the anterolateral PM was minimally affected by all of the repair procedures (RMA: 4.7 ± 48.4 % reduction; Small Patch: 1.5 ± 48.4 % increase; Large Patch: 0.7 ± 88.1 % reduction) as compared to FIMR.
DISCUSSION
Long term postoperative repair success for restrictive mitral annuloplasty to treat functional ischemic mitral regurgitation remains elusive [7-10, 15]. Recent data suggest preoperative patient characteristics are predictive of repair failure. Of these, severely decreased coaptation [16, 17] and grossly enlarged ventricular dimensions [6] are easily measured parameters that are indicative of a patient population that may benefit from an adjunct surgical procedure. Additionally, increased leaflet tethering [7, 10l, 15] following late ventricular remodeling is a mechanism of recurrent FIMR for restrictive mitral annuloplasty repairs.
For these reasons, anterior leaflet augmentation with true sized mitral annuloplasty has emerged as an additive technique for FIMR [5, 11]. In the present study, anterior leaflet augmentation was performed in two sizes for comparison against restrictive mitral annuloplasty. All three repairs comparably alleviated functional ischemic mitral regurgitation with no postoperative MR jets detected. Additionally, all three repairs restored coaptation to physiological levels, with significant increases from FIMR pathology. Moreover, the large patch procedure resulted in an additional 1 mm gain in coaptation length, as the large patch significantly increased the anterior leaflet surface area available for coaptation. Overall, this created further redundancy to maintain a proper valvular seal, which has the potential to withstand late ventricular remodeling.
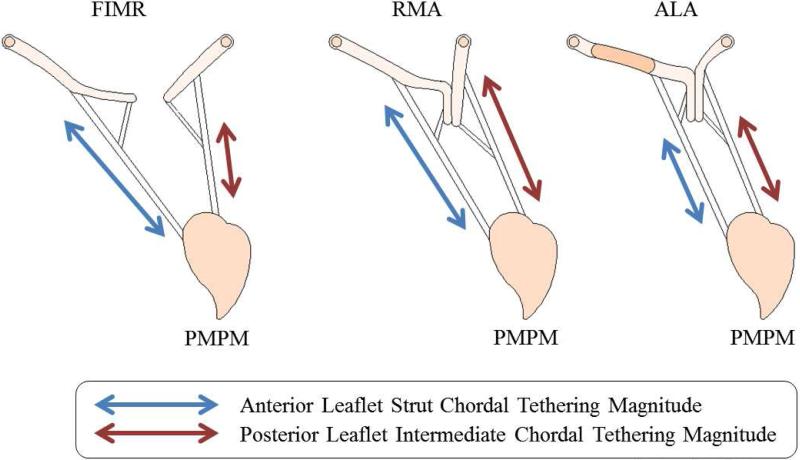
It was expected that the benefits of extra coaptation length with the large patch might result in increased cyclic chordal forces due to the larger exposed leaflet surface area. However, this was not observed for the anterior strut and posterior intermediate chordae originating from the posterior papillary muscle. Conversely, augmentation resulted in the best combined reduction in tethering force for chordae originating from the displaced posterior papillary muscle. This was most likely due to two mechanisms (Figure 4): (i) leaflet insertion points of the anterior strut chordae were displaced apically, towards the papillary muscles, by use of the patch. This effectively shorted the distance the chorda had to traverse between diastole and systole to maintain coaptation. (ii) Echocardiography showed the anterior strut chordae were moved into or near to the coaptation zone. This site is known to experience relatively low chordal forces as it is not actively engaged in resisting transmitral pressure [18].
Figure 4.
Schematic representation of the mechanisms which effect chordal forces in the FIMR and repair conditions. (A) Chordal tethering is a result of annular dilation and papillary muscle displacement and pressure forces. (B) Posterior tethering force is a balance between pressure force and the opposing displacements of the papillary muscles and annulus reshaping. (C) Anterior strut tethering is reduced by displacing the chordal insertion apically and into the coaptation zone.
Posterior intermediate chordae originating from the posterior papillary muscle experienced a reduction in tethering forces as well. Three mechanisms are suspected (Figure 4): (i) the posterior annulus was not hoisted directionally away from the pathological papillary muscle as was done with restrictive annuloplasty [15]. This effectively shortens the distance between the leaflet insertion point and the restraining papillary muscle, limiting extension of the chordae. (ii) The increased anterior leaflet mobility reduced the overall loading of the posterior leaflet, such that the posterior leaflet did not traverse as far to oppose the anterior leaflet. This effectively limits the elongation of the chordae from diastole to systole. (iii) More of the posterior leaflet was involved with coaptation, effectively displacing the posterior intermediate chordae near to the coaptation zone, a site known to experience lower chordal forces.
From these experimental results, there was no measured change in posterior intermediate tethering forces for the restrictive mitral annuloplasty surgical repair. An increase in force was expected due to the hoisting of the posterior annulus (Figure 4). However, it is likely this was offset by the creation of a functionally unicuspid valve, which resulted in minimal posterior leaflet area exposed to a transmitral pressure gradient. Nonetheless, posterior chordal forces remained elevated at pathological levels.
Anterior strut chordae originating from the anterior papillary muscle did not benefit from anterior leaflet augmentation. Contrarily, there was a slight increase in tethering force for this chordal grouping. This suggests that significant papillary muscle displacement (as seen with the posterior papillary muscle) has a compensatory effect on tethering forces in the presence of leaflet augmentation. Without papillary muscle displacement, the leaflet freely bellowed into the left atrium, which further exposed surface area to transmitral pressure. In this setting, restrictive mitral annuloplasty resulted in a modest improvement in tethering force, which is contributed to the smaller mitral orifice.
None of the repairs had an effect on posterior intermediate chordae originating from the anterior papillary muscle. Without significant papillary muscle displacement, hoisting of the posterior annulus did not exacerbate posterior leaflet tethering. The leaflet remained mobile and spanned more of the mitral orifice to oppose the anterior leaflet.
Limitations
Although the in vitro model used in this study simulates physiological function, hemodynamics, and mechanics of a native mitral valve, the rigid ventricle did not mimic dynamic annular and ventricular motion. However, mitral annuloplasty, as performed in the repair conditions, is known to reduce annular dynamics [19]. Although, ventricular wall motion was not simulated, the distance between the PMs and the annular plane has been shown to remain relatively constant throughout the cardiac cycle [20]. Moreover, relative papillary muscle displacements were informed from clinical data, and the left heart simulator has been shown to mimic the systolic function and mechanics of an established chronic FIMR ovine model [12].
CONCLUSIONS
This study demonstrated that in the setting of functional ischemic mitral regurgitation due to severe LV geometric distortion, anterior leaflet augmentation is a possible surgical option within the limitation of study design. The data from each patch size suggest that augmentation dimensions do not need to be precisely tailored as sufficient repair is achievable within a range. An oversized patch can be targeted as it provided the best gains in coaptation length and reduction in chordal tethering. Although all repairs abolished MR acutely, repairs that create the greatest coaptation may conceivably produce a more robust and lasting repair in the chronic stage. However, a clinical need still exists to best identify which patients with altered MV geometries would most benefit from an adjunct procedure over a simple restrictive mitral annuloplasty.
ACKNOWLEDGEMENTS
This study was partially supported by a research grant awarded from the National Institute of Health (R01 HL090661-02). We would like to additionally acknowledge the contributions of Steven A. Touchton Jr., Eric L. Pierce, and Joan Fernandez Esmerates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tibayan FA, et al. Geometric Distortions of the Mitral Valvular-Ventricular Complex in Chronic Ischemic Mitral Regurgitation. Circulation. 2003;108(90101):II–116-121. doi: 10.1161/01.cir.0000087940.17524.8a. [DOI] [PubMed] [Google Scholar]
- 2.Grigioni F, et al. Ischemic Mitral Regurgitation : Long-Term Outcome and Prognostic Implications With Quantitative Doppler Assessment. Circulation. 2001;103(13):1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 3.Bax JJ, et al. Restrictive Annuloplasty and Coronary Revascularization in Ischemic Mitral Regurgitation Results in Reverse Left Ventricular Remodeling. Circulation. 2004;110(11 suppl 1):II–103-II-108. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]
- 4.Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. The Annals of Thoracic Surgery. 2002;74(2):600–601. doi: 10.1016/s0003-4975(02)03749-9. [DOI] [PubMed] [Google Scholar]
- 5.Hines MH, et al. Anterior leaflet augmentation for ischemic mitral regurgitation in patients with anomalous left coronary artery from the pulmonary artery and preserved left ventricular function. The Journal of thoracic and cardiovascular surgery. 2011 doi: 10.1016/j.jtcvs.2011.01.025. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 6.Braun J, et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. European Journal of Cardio- Thoracic Surgery. 2005;27(5):847–853. doi: 10.1016/j.ejcts.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Hung J, et al. Mechanism of Recurrent Ischemic Mitral Regurgitation After Annuloplasty. Circulation. 2004;110(11 suppl 1):II–85-II-90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 8.Matsunaga A, Tahta SA, Duran CMG. Failure of Reduction Annuloplasty for Functional Ischemic Mitral Regurgitation. The Journal of Heart Valve Disease. 2004;13(3):390–7. [PubMed] [Google Scholar]
- 9.McGee JEC, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. Journal of Thoracic and Cardiovascular Surgery. 2004;128(6):916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Kuwahara E, et al. Mechanism of Recurrent/Persistent Ischemic/Functional Mitral Regurgitation in the Chronic Phase After Surgical Annuloplasty: Importance of Augmented Posterior Leaflet Tethering. Circulation. 2006;114(1_suppl):I–529-534. doi: 10.1161/CIRCULATIONAHA.105.000729. [DOI] [PubMed] [Google Scholar]
- 11.Kincaid EH, et al. Anterior leaflet augmentation for ischemic mitral regurgitation. The Annals of Thoracic Surgery. 2004;78(2):564–568. doi: 10.1016/j.athoracsur.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Siefert AW, et al. In Vitro Mitral Valve Simulator Mimics Systolic Valvular Function of Chronic Ischemic Mitral Regurgitation Ovine Model. The Annals of Thoracic Surgery. 2013;95(3):825–830. doi: 10.1016/j.athoracsur.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen SL, et al. Miniature C-Shaped Transducers for Chordae Tendineae Force Measurements. Annals of Biomedical Engineering. 2004;32(8):1050–1057. doi: 10.1114/b:abme.0000036641.69903.62. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez J, et al. Effects of a Saddle Shaped Annulus on Mitral Valve Function and Chordal Force Distribution: An In Vitro Study. Annals of Biomedical Engineering. 2003;31(10):1171–1181. doi: 10.1114/1.1616929. [DOI] [PubMed] [Google Scholar]
- 15.Zhu F, et al. Mechanism of Persistent Ischemic Mitral Regurgitation After Annuloplasty: Importance of Augmented Posterior Mitral Leaflet Tethering. Circulation. 2005;112(9 suppl):I–396-I-401. doi: 10.1161/CIRCULATIONAHA.104.524561. [DOI] [PubMed] [Google Scholar]
- 16.Kongsaerepong V, et al. Echocardiographic Predictors of Successful Versus Unsuccessful Mitral Valve Repair in Ischemic Mitral Regurgitation. The American Journal of Cardiology. 2006;98(4):504–508. doi: 10.1016/j.amjcard.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 17.Roshanali F, et al. A Prospective Study of Predicting Factors in Ischemic Mitral Regurgitation Recurrence After Ring Annuloplasty. The Annals of Thoracic Surgery. 2007;84(3):745–749. doi: 10.1016/j.athoracsur.2007.04.106. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez J, et al. Effects of papillary muscle position on chordal force distribution: an in-vitro study. the Journal of Heart Valve Disease. 2005;14(3):295–302. [PubMed] [Google Scholar]
- 19.Rausch M, et al. Mitral Valve Annuloplasty. Annals of Biomedical Engineering. 2012;40(3):750–761. doi: 10.1007/s10439-011-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joudinaud TM, et al. The papillary muscles as shock absorbers of the mitral valve complex. An experimental study. European Journal of Cardio-Thoracic Surgery. 2007;32(1):96–101. doi: 10.1016/j.ejcts.2007.03.043. [DOI] [PubMed] [Google Scholar]