Abstract
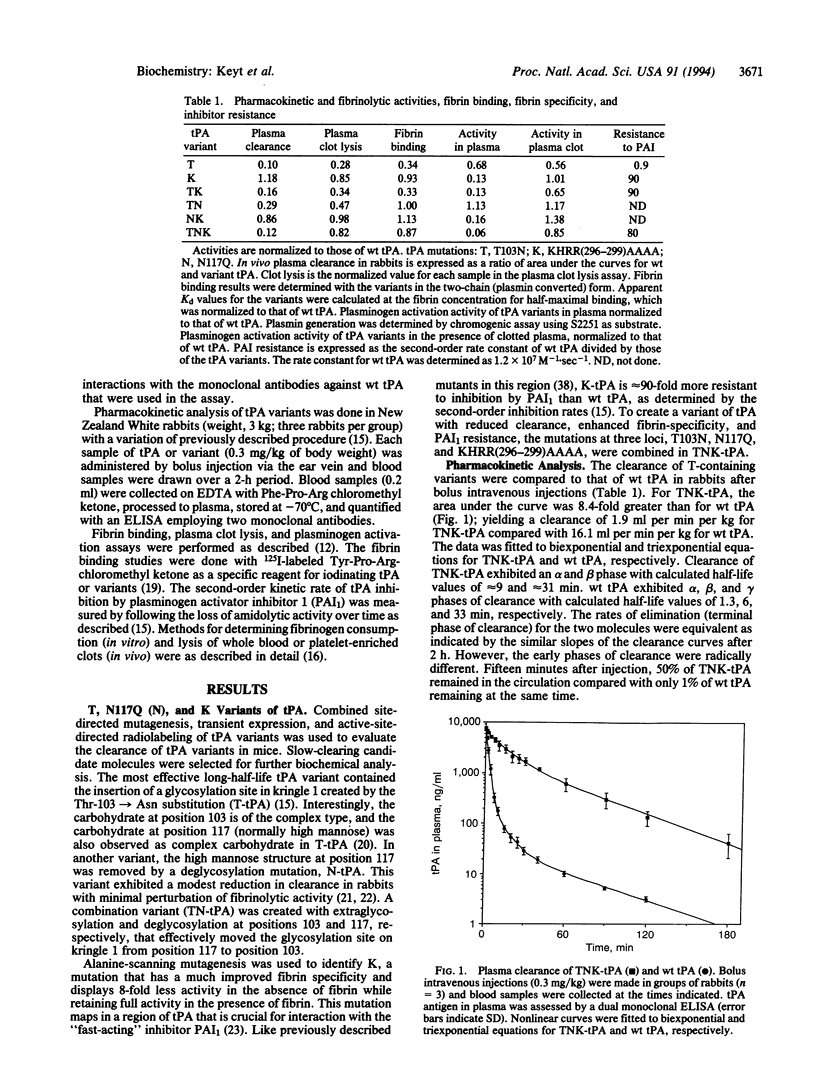
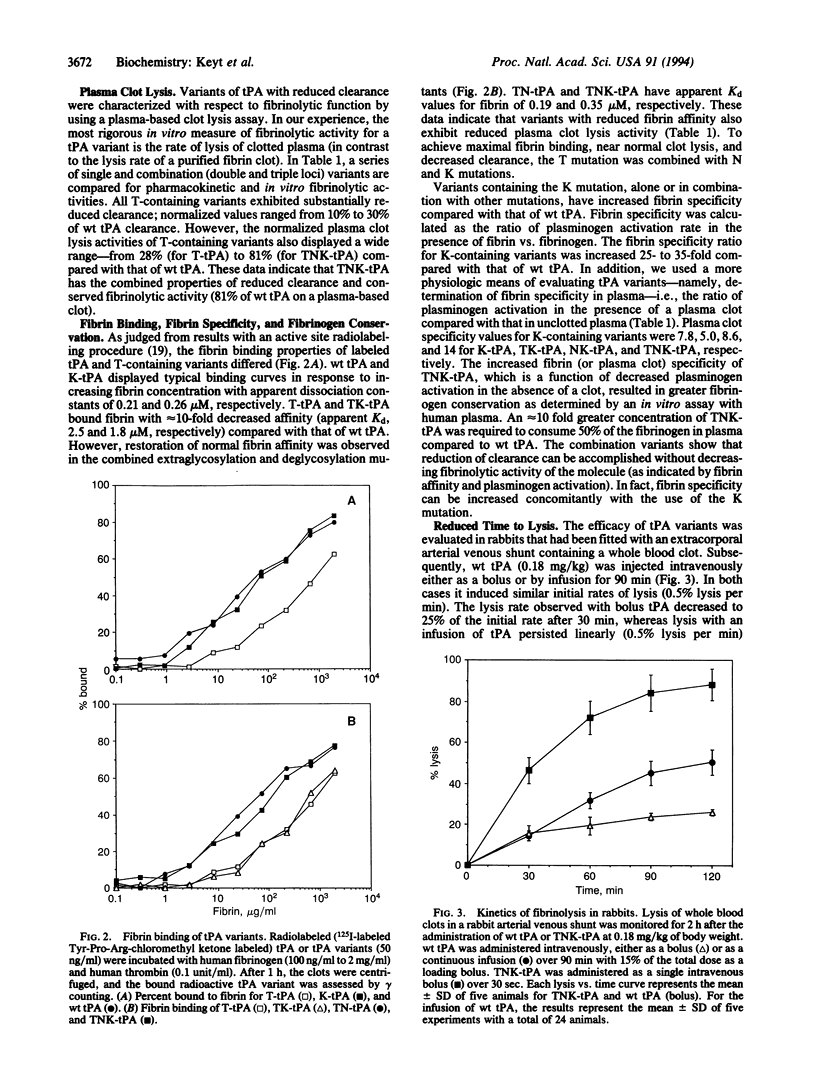
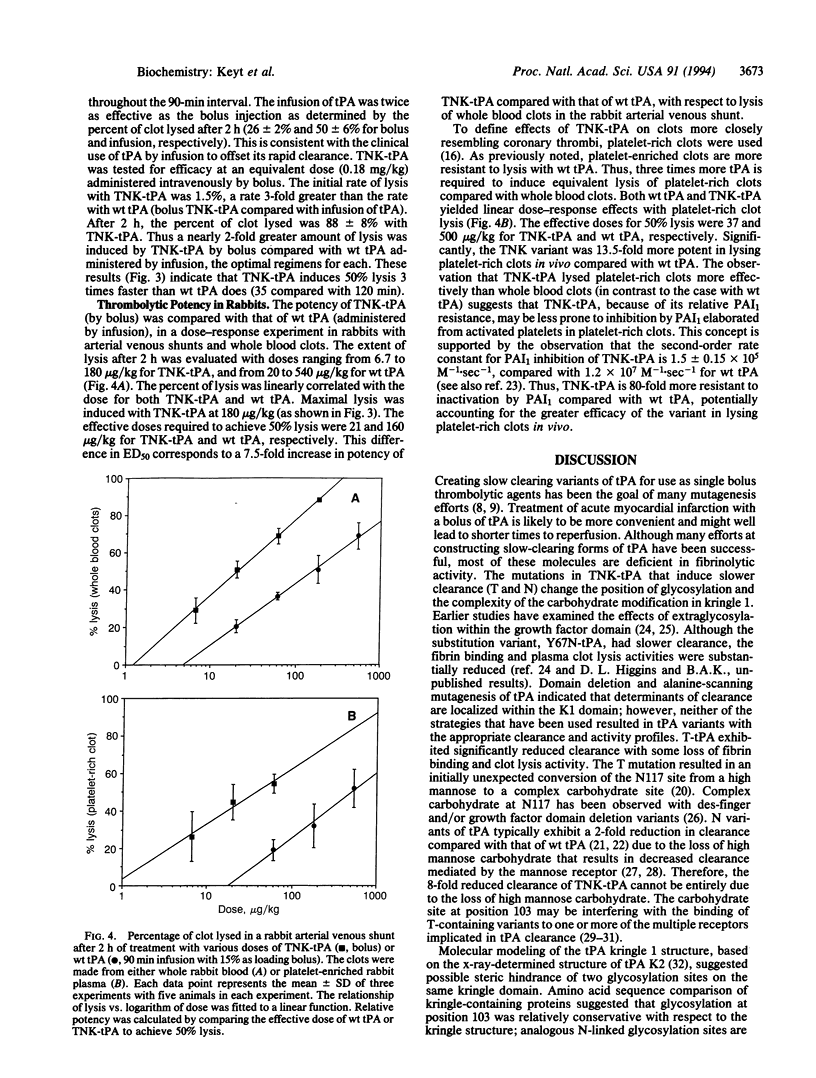
Current treatment with tissue plasminogen activator (tPA) requires an intravenous infusion (1.5-3 h) because the clearance of tPA from the circulation is rapid (t 1/2 approximately 6 min). We have developed a tPA variant, T103N,N117Q, KHRR(296-299)AAAA (TNK-tPA) that has substantially slower in vivo clearance (1.9 vs. 16.1 ml per min per kg for tPA in rabbits) and near-normal fibrin binding and plasma clot lysis activity (87% and 82% compared with wild-type tPA). TNK-tPA exhibits 80-fold higher resistance to plasminogen activator inhibitor 1 than tPA and 14-fold enhanced relative fibrin specificity. In vitro, TNK-tPA is 10-fold more effective at conserving fibrinogen in plasma compared to tPA. Arterial venous shunt models of fibrinolysis in rabbits indicate that TNK-tPA (by bolus) induces 50% lysis in one-third the time required by tPA (by infusion). TNK-tPA is 8- and 13-fold more potent in rabbits than tPA toward whole blood clots and platelet-enriched clots, respectively. TNK-tPA conserves fibrinogen and, because of its slower clearance and normal clot lysis activity, is effective as a thrombolytic agent when given as a bolus at a relatively low dose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassel-Duby R., Jiang N. Y., Bittick T., Madison E., McGookey D., Orth K., Shohet R., Sambrook J., Gething M. J. Tyrosine 67 in the epidermal growth factor-like domain of tissue-type plasminogen activator is important for clearance by a specific hepatic receptor. J Biol Chem. 1992 May 15;267(14):9668–9677. [PubMed] [Google Scholar]
- Bennett W. F., Paoni N. F., Keyt B. A., Botstein D., Jones A. J., Presta L., Wurm F. M., Zoller M. J. High resolution analysis of functional determinants on human tissue-type plasminogen activator. J Biol Chem. 1991 Mar 15;266(8):5191–5201. [PubMed] [Google Scholar]
- Bergmann S. R., Fox K. A., Ter-Pogossian M. M., Sobel B. E., Collen D. Clot-selective coronary thrombolysis with tissue-type plasminogen activator. Science. 1983 Jun 10;220(4602):1181–1183. doi: 10.1126/science.6602378. [DOI] [PubMed] [Google Scholar]
- Bovill E. G., Terrin M. L., Stump D. C., Berke A. D., Frederick M., Collen D., Feit F., Gore J. M., Hillis L. D., Lambrew C. T. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med. 1991 Aug 15;115(4):256–265. doi: 10.7326/0003-4819-115-4-256. [DOI] [PubMed] [Google Scholar]
- Bu G., Maksymovitch E. A., Schwartz A. L. Receptor-mediated endocytosis of tissue-type plasminogen activator by low density lipoprotein receptor-related protein on human hepatoma HepG2 cells. J Biol Chem. 1993 Jun 15;268(17):13002–13009. [PubMed] [Google Scholar]
- Collen D., Lijnen H. R., Vanlinthout I., Kieckens L., Nelles L., Stassen J. M. Thrombolytic and pharmacokinetic properties of human tissue-type plasminogen activator variants, obtained by deletion and/or duplication of structural/functional domains, in a hamster pulmonary embolism model. Thromb Haemost. 1991 Feb 12;65(2):174–180. [PubMed] [Google Scholar]
- Dihanich M., Monard D. cDNA sequence of rat prothrombin. Nucleic Acids Res. 1990 Jul 25;18(14):4251–4251. doi: 10.1093/nar/18.14.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetta A. W., Basa L. J., Hancock W. S., Keyt B. A., Bennett W. F. Identification of carbohydrate structures in glycoprotein peptide maps by the use of LC/MS with selected ion extraction with special reference to tissue plasminogen activator and a glycosylation variant produced by site directed mutagenesis. Anal Chem. 1993 Nov 1;65(21):2953–2962. doi: 10.1021/ac00069a004. [DOI] [PubMed] [Google Scholar]
- Higgins D. L., Bennett W. F. Tissue plasminogen activator: the biochemistry and pharmacology of variants produced by mutagenesis. Annu Rev Pharmacol Toxicol. 1990;30:91–121. doi: 10.1146/annurev.pa.30.040190.000515. [DOI] [PubMed] [Google Scholar]
- Hotchkiss A., Refino C. J., Leonard C. K., O'Connor J. V., Crowley C., McCabe J., Tate K., Nakamura G., Powers D., Levinson A. The influence of carbohydrate structure on the clearance of recombinant tissue-type plasminogen activator. Thromb Haemost. 1988 Oct 31;60(2):255–261. [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Keyt B. A., Berleau L. T., Nguyen H. V., Bennett W. F. Radioiodination of the active site of tissue plasminogen activator: a method for radiolabeling serine proteases with tyrosylprolylarginyl chloromethyl ketone. Anal Biochem. 1992 Oct;206(1):73–83. doi: 10.1016/s0003-2697(05)80013-2. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Collen D. Strategies for the improvement of thrombolytic agents. Thromb Haemost. 1991 Jul 12;66(1):88–110. [PubMed] [Google Scholar]
- Lucore C. L., Fry E. T., Nachowiak D. A., Sobel B. E. Biochemical determinants of clearance of tissue-type plasminogen activator from the circulation. Circulation. 1988 Apr;77(4):906–914. doi: 10.1161/01.cir.77.4.906. [DOI] [PubMed] [Google Scholar]
- Madison E. L., Goldsmith E. J., Gerard R. D., Gething M. J., Sambrook J. F., Bassel-Duby R. S. Amino acid residues that affect interaction of tissue-type plasminogen activator with plasminogen activator inhibitor 1. Proc Natl Acad Sci U S A. 1990 May;87(9):3530–3533. doi: 10.1073/pnas.87.9.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison E. L., Goldsmith E. J., Gerard R. D., Gething M. J., Sambrook J. F. Serpin-resistant mutants of human tissue-type plasminogen activator. Nature. 1989 Jun 29;339(6227):721–724. doi: 10.1038/339721a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Neuhaus K. L., Feuerer W., Jeep-Tebbe S., Niederer W., Vogt A., Tebbe U. Improved thrombolysis with a modified dose regimen of recombinant tissue-type plasminogen activator. J Am Coll Cardiol. 1989 Nov 15;14(6):1566–1569. doi: 10.1016/0735-1097(89)90399-9. [DOI] [PubMed] [Google Scholar]
- Neuhaus K. L., von Essen R., Tebbe U., Vogt A., Roth M., Riess M., Niederer W., Forycki F., Wirtzfeld A., Maeurer W. Improved thrombolysis in acute myocardial infarction with front-loaded administration of alteplase: results of the rt-PA-APSAC patency study (TAPS) J Am Coll Cardiol. 1992 Apr;19(5):885–891. doi: 10.1016/0735-1097(92)90265-o. [DOI] [PubMed] [Google Scholar]
- Orth K., Madison E. L., Gething M. J., Sambrook J. F., Herz J. Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7422–7426. doi: 10.1073/pnas.89.16.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter M., Zocková P., Kuiper J., van Berkel T. J., Barrett-Bergshoeff M. M., Rijken D. C. Isolation and characterization of the mannose receptor from human liver potentially involved in the plasma clearance of tissue-type plasminogen activator. Hepatology. 1992 Jul;16(1):54–59. doi: 10.1002/hep.1840160111. [DOI] [PubMed] [Google Scholar]
- Paoni N. F., Chow A. M., Peña L. C., Keyt B. A., Zoller M. J., Bennett W. F. Making tissue-type plasminogen activator more fibrin specific. Protein Eng. 1993 Jul;6(5):529–534. doi: 10.1093/protein/6.5.529. [DOI] [PubMed] [Google Scholar]
- Paoni N. F., Keyt B. A., Refino C. J., Chow A. M., Nguyen H. V., Berleau L. T., Badillo J., Peña L. C., Brady K., Wurm F. M. A slow clearing, fibrin-specific, PAI-1 resistant variant of t-PA (T103N, KHRR 296-299 AAAA). Thromb Haemost. 1993 Aug 2;70(2):307–312. [PubMed] [Google Scholar]
- Paoni N. F., Refino C. J., Brady K., Peña L. C., Nguyen H. V., Kerr E. M., Johnson A. C., Wurm F. M., van Reis R., Botstein D. Involvement of residues 296-299 in the enzymatic activity of tissue-type plasminogen activator. Protein Eng. 1992 Apr;5(3):259–266. doi: 10.1093/protein/5.3.259. [DOI] [PubMed] [Google Scholar]
- Patthy L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 1985 Jul;41(3):657–663. doi: 10.1016/s0092-8674(85)80046-5. [DOI] [PubMed] [Google Scholar]
- Potter van Loon B. J., Rijken D. C., Brommer E. J., van der Maas A. P. The amount of plasminogen, tissue-type plasminogen activator and plasminogen activator inhibitor type 1 in human thrombi and the relation to ex-vivo lysibility. Thromb Haemost. 1992 Jan 23;67(1):101–105. [PubMed] [Google Scholar]
- Refino C. J., Paoni N. F., Keyt B. A., Pater C. S., Badillo J. M., Wurm F. M., Ogez J., Bennett W. F. A variant of t-PA (T103N, KHRR 296-299 AAAA) that, by bolus, has increased potency and decreased systemic activation of plasminogen. Thromb Haemost. 1993 Aug 2;70(2):313–319. [PubMed] [Google Scholar]
- Sobel B. E., Sarnoff S. J., Nachowiak D. A. Augmented and sustained plasma concentrations after intramuscular injections of molecular variants and deglycosylated forms of tissue-type plasminogen activators. Circulation. 1990 Apr;81(4):1362–1373. doi: 10.1161/01.cir.81.4.1362. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Hagiya M., Nishizawa T., Seki T., Shimonishi M., Shimizu S., Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Werf F., Ludbrook P. A., Bergmann S. R., Tiefenbrunn A. J., Fox K. A., de Geest H., Verstraete M., Collen D., Sobel B. E. Coronary thrombolysis with tissue-type plasminogen activator in patients with evolving myocardial infarction. N Engl J Med. 1984 Mar 8;310(10):609–613. doi: 10.1056/NEJM198403083101001. [DOI] [PubMed] [Google Scholar]
- Wilhelm J., Lee S. G., Kalyan N. K., Cheng S. M., Wiener F., Pierzchala W., Hung P. P. Alterations in the domain structure of tissue-type plasminogen activator change the nature of asparagine glycosylation. Biotechnology (N Y) 1990 Apr;8(4):321–325. doi: 10.1038/nbt0490-321. [DOI] [PubMed] [Google Scholar]
- Wurm F. M. Integration, amplification and stability of plasmid sequences in CHO cell cultures. Biologicals. 1990 Jul;18(3):159–164. doi: 10.1016/1045-1056(90)90002-h. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M. H., Kelley R. F., Padmanabhan K., Tulinsky A., Westbrook M. L., Kossiakoff A. A. Crystal structure of the kringle 2 domain of tissue plasminogen activator at 2.4-A resolution. Biochemistry. 1992 Jan 14;31(1):270–279. doi: 10.1021/bi00116a037. [DOI] [PubMed] [Google Scholar]



