Abstract
Kidney transplant recipients who have abnormally high creatinine levels in their blood often have allograft dysfunction secondary to rejection. Creatinine has become the preferred marker for renal dysfunction and is readily available in hospital clinical settings. We developed a rapid and accurate polymer-based electrochemical point-of-care (POC) assay for creatinine detection from whole blood to identify allograft dysfunction. The creatinine concentrations of 19 blood samples from transplant recipients were measured directly from clinical serum samples by the conducting polymer based electrochemical (EC) sensor arrays. These measurements were compared to the traditional clinical laboratory assay. The time required for detection was less than 5 minutes from sample loading. Sensitivity of the detection was found to be 0.46 mg/dL of creatinine with only 40 μL sample in the creatinine concentration range of 0 mg/dL to 11.33 mg/dL. Signal levels that were detected electrochemically correlated closely with the creatinine blood concentration detected by the UCLA Ronald Reagan Medical Center traditional clinical laboratory assay (correlation coefficient 0.94). This work is encouraging for the development of a rapid and accurate POCT device for measuring creatinine levels in whole blood.
Keywords: Conducting polymer, electrochemical sensor, creatinine, renal transplantation, point-of-care testing
Introduction
Electrochemical (EC) sensors, especially bioaffinity sensors, such as DNA hybridization biosensors or immunosensors, have gained considerable attention in recent years and have been extensively used in the clinical diagnostic laboratory for biosensing and detection1-8. Such devices exploit selective binding of specific target species by surface-confined receptor molecules for triggering informative electrical signals. Electrochemical affinity biosensors commonly rely on specific antibody-, DNA- or aptamer-recognition events. Bioelectronic assays with enzyme tracers have been extensively applied due to the signal amplification from biocatalytic reactions, and hold promise for ultrasensitive detection of nucleic acids and/or proteins. In addition, EC sensors have achieved high sensitivity and specificity and are simple with respect to sample processing and instrument operation. EC sensors have the potential to be transformed from a laboratory-based instrument to a POC device. Electrochemical biosensors have thus become a very promising area of research and development in clinical diagnostic testing 3, 9, 10.
Due to the biocompatibility, convenience in fabrication and low cost, conducting polymer based biosensors provide ideal platforms for various applications of point-of-care devices 10-13. Conducting polymer (CP) has been widely applied as an easy-fabricating and biocompatible substrate material for a diverse array of analytes14-18. Most of the CP based biosensors immobilize probes, such as an oligonucleotide or antibody and enzyme, directly onto polymer film by simply mixing them with the monomer before the electro-polymerization19, 20. In this rapid and simple procedure, the embedding of the CP matrix with the desired molecule without any labeling is very important for probe immobilization. Especially for the small molecule (chemical, hormone, drugs, et. al.) detection based on affinity, modifications are required to generate the binding site between the molecule and the surface. By applying the co-polymerization with polymer matrix, the molecule can be directly embedded into the surface without additional modification. The total reaction time is from several seconds to minutes, at room temperature with regular biocompatible buffer.
Diagnostic methods for detecting renal dysfunction of kidney transplant recipients has not changed in over 20 years. This approach involves patients coming to the hospital clinic periodically for their blood to be drawn to determine the creatinine level. Although the gold standard measurements for renal dysfunction are radiolabelled 125I-iodothalamate and inulin, these tests are difficult to perform and generally unavailable 21. Therefore, creatinine has become the preferred marker for renal dysfunction and readily available in essentially all hospital clinical settings. Creatinine is a by-product of muscle metabolism and typically remains in a steady state balanced out by renal elimination. Kidney transplant recipients who have an abnormally high creatinine level in their blood often have allograft dysfunction secondary to rejection. The recipient is then scheduled for an ultrasound-guided biopsy of the allograft to confirm tissue diagnosis. If rejection is caught early it can be easily reversed with current immunosuppressants. However, over time transplant recipients have their creatinine measured infrequently as they grow tired of the inconvenience of enduring traffic and waiting room lines for their blood to be drawn. Therefore, by the time rejection is detected it is often symptomatic, and irreversible damage has been done to the allograft. In fact, the deceased donor waiting list is burdened by 17% of candidates who have lost their previous graft to rejection and are awaiting their second, third or fourth transplantation22.
The traditional method for detecting creatinine is based on a modified Jaffe reaction, which is widely used in both laboratory and clinical detection. Jaffe reaction is based on the orange-red color produced by creatinine reacting with alkaline picrate. The sensitivity of Jaffe reaction is 0.5 mg/dL of creatinine in samples. The whole detection time is around 30 minutes with sample volume at least 1 mL. However, the specificity of the Jaffe reaction is limited. Several interferents in clinical samples will affect the signal readout, such as metabolites and drugs (glucose, proteins, ketones, haemoglobin, bilirubin, pyruvic acid) and cephalosporines (cefoxitin, cephalotin, cefatril, cefazolin). In addition, this detection requires expensive spectrometer and sample pretreatment, which limit the application to monitor creatinine over the long run.
There are also several promising techniques developed for monitoring creatinine in blood, including enzymatic catalysis of creatinine (direct and indirect) and antibody based affinity detection. The enzymatic catalysis of creatine usually has the enzyme for creatinine (creatinine iminohydrolase23, creatinine amidohydrolase, creatine amidinohydrolase and creatinine deiminase24). Specifically for the creatinine electrochemical sensor, the existing detections are mostly based on the electrochemical catalysis of creatinine. In this detection, creatinine amidohydrolase catalyzes the hydrolytic reaction converting creatinine to creatine, followed by the sarcosine oxidase (SOX) reaction with the mediator system13. Amperometric current is readout simultaneously with 60 μl untreated blood sample in about 90 seconds. However, this reaction also has a low specificity from the interference of creatine in the samples25. This harbours concerns that discrepant results may affect clinical management. These discrepancies may be related to elevations in hematocrit interfering with the enzymatic method.
Antibody based affinity detection is more specific for creatine measurements in body fluids complex. The reaction is usually based on competitive reaction, which measure the signal decrease with the existence of creatinine. Pioneering investigations have been done in this area. Usually a modification of creatine as the capture molecule, or an optical detector is needed, which limits the application as a point-of-care device32. In addition, multiplexing measurements are not applicable for this technology.
Development of a point-of-care testing (POCT) device to specifically measure blood creatinine levels, would allow patients the convenience of monitoring their allograft function frequently in the comfort of their own home. Besides the obvious improvement in quality-of-life, a creatinine POCT device would likely detect rejection at an earlier stage when it is more easily reversed. Ultimately this device could lessen the burden of patients that return to the waiting list by extending graft survival. The purpose of this study was to develop a rapid and accurate assay for creatinine detection from whole blood by a POCT device based on creatinine specific immunoassay.
Experimental Section
Sample collection
Freshly collected whole blood samples from renal transplant recipients were obtained under the auspices of an ongoing UCLA IRB for immune monitoring. Patient specimens were assigned a numeric code to remove any identification. Nineteen blood samples were utilized for this study. All samples were run in triplicate to assess experimental precision and human error variance.
Traditional lab assay for creatinine comparison measurement
For this diagnostic validation, the creatinine levels were measured via modified Jaffe reaction. Creatinine in the blood samples reacted with alkaline picrate and generated orange-red color products. The signal readouts were based on the spectra from the orange-read color products. All automated creatinine tests had been run on the Olympus 5400 (Olympus Diagnostic Systems) calorimetric assay. This was conducted by the routine diagnostic flow at the UCLA Ronald Reagan Medical Center Central Laboratory. The routine turnaround time for detection at the central laboratory was 4 hours and required 5 mL of blood sample.
The electrochemical sensor is based on the competitive amperometric measurement from the creatinine in the samples (Fig. 1) 2, 26.
Figure 1.
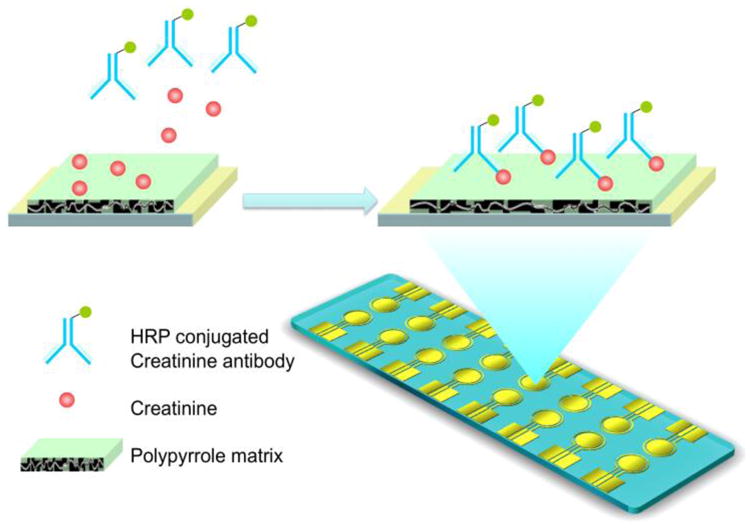
Illustration of the direct measurement of creatinine from blood sample via conducting polymer electrochemical sensor. The reaction is based on the amperometric measurement of the HRP-conjugated creatinine antibody bound with creatinine.
EC sensors and reader
The EC sensor is an array of 16 bare gold electrode chips (GeneFluidics, USA). Each unit of the array has a working electrode, a counter electrode, and a reference electrode. The 3-electrodes are bare gold before the reaction, and the specimens are immobilized on the working electrode (Fig. 2A). Electrochemical current is measured between the working electrode and counter electrode under the potential between the working electrode and the reference electrode. The potential profile could be a constant value, a linear sweep or a cyclic square wave. 16 array of plastic wells separate each three-electrode set, which avoid the cross contamination between different sensors. A conducting polymer was deposited on the working electrodes as the supporting film. The 16-channel EC reader (GeneFluidics Inc.) controls the electrical field applied onto the 16 array sensors and reports the amperometric current simultaneously.
Figure 2.
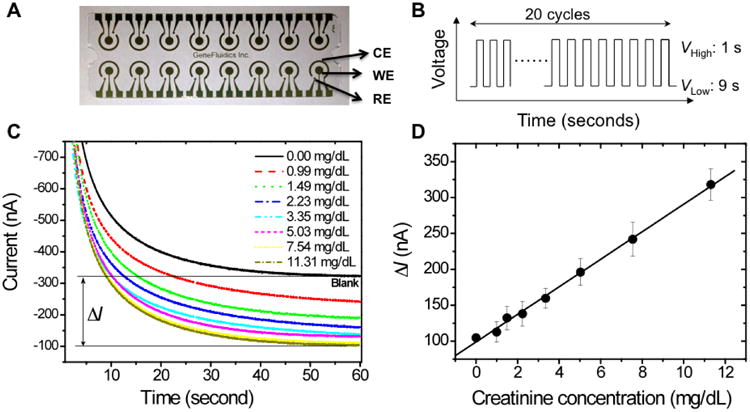
Electrochemical sensor array for the creatinine measurements and the calibration curves. (A) 16-array bare gold sensor chip with counter electrode (CE), working electrode (WE) and reference electrode (RE). (B) Cyclic square-wave electric potential applied during the polymerization and the reaction. For EC polymerization, the low voltage was + 350 mV and the high voltage was +950 mV. For the surface recognition, the low voltage was -200 mV and the high voltage was +300 mV. (C) Amperometric curves of the spiked creatine into blood sample. Current signal for calibration is the difference between the sample signal and the blank control signal. (D) sensitivity of the EC sensor for creatinine detection of blood samples with linear fit (R2=0.98). The linear fitting equation is ΔI (nA) =98.98+19.17×creatinine concentration (mg/dL). Mean value and standard deviation are both illustrated with triplet experiments.
All the electrical potentials in the following steps are referred to the gold reference electrode, which was determined to be +218 mV vs. SCE by measuring cyclic voltammetric curves of 0.1 mM [Fe(CN)6]3-/4-. For the experiments, solutions were loaded onto the whole area of the three-electrode region including the working, counter, and reference electrodes, which were confined and separated by the 16 array of plastic wells. After each step, the EC sensors were rinsed with ultrapure water (18.3 MΩ·cm) then dried under pure N2.
Conducting polymer sensor fabrication for Creatinine
A cyclic square-wave electrical field (csw E-field) was applied for the electropolymerization and surface-recognition processes, which provides more effective and versatile way to control the assay (Fig. 2B). With the csw E-field, both the hybridization and protein binding are finished on the same chip within minutes, while previously these processes have to be completed separately and the incubation time varies from 1-24 hours. The positive potential in the csw E-field help to accumulate the molecules onto the working electrode, while the negative potential removes the weak non-specific binding which generate high specificity. The flapping between positive and negative potential also provides good mixing during the incubation, which accelerates the binding process as well. Each cycle of square-wave consisted of 9 s at low voltage and 1 s at high voltage. For EC polymerization, the low voltage was + 350 mV and the high voltage was +950 mV. For the surface recognition, the low voltage was -200 mV and the high voltage was +300 mV. In total, 20 cycles of square-waves were applied for each surface reaction, which lasted for 200 s.
The 16-array gold electrochemical sensor is first coated with creatinine embedded in the polyrrole conducting polymer. For electropolymerization, the 20 mg/dL creatinine (Abnova, USA) was diluted together with pyrrole (Sigma, USA) in 1×PBS (pH 7.5, Invitrogen, USA) in a volume ratio of 1:50. Potassium chloride was added at a final concentration of 300 mM to achieve high ionic strength. The final concentration of pyrrole was 10 mM. After loading of the mixture onto the gold electrode, a csw E-field was applied for electropolymerization. Each square-wave consisted of 9 s at a potential of +350 mV and 1 s at +950 mV, and 20 cycles of square-waves were applied. The whole process lasted for 200 s. After the polymerization, the electrode was rinsed with ultrapure water (18.3 MΩ•cm) then dried under pure N2.
Amperometric Creatinine measurement
The final concentration is reported based on the individual calibration curve for each sample. The antibody is a sheep polyclonal antibody against Creatinine (Abnova, USA). The immunogen is creatinine conjugated with BSA. HRP (Horseradish peroxidase) was conjugated to the antibody at our lab with the NH2-biotin-label kit, following the enclosed instruction from the company (Dojindo, USA). The final concentration of the HRP-anti-creatinine antibody is 250 μg/ml.
10 μl of HRP conjugated anti-creatinine antibody was first mixed with 40 μl raw blood sample solution at room temperature. Then the mixture was transferred onto the electrodes for competitive reaction between the creatinine in solution and on the polymer matrix. 20 cycles of square-wave consisted of 9 s at a potential of -300 mV and 1 s at +200 mV were applied. The whole process lasted for 200 s. After that, the sensor was washed with water and then dried under pure N2. Then the amperometric measurements were carried out in the presence of 3,3-,5,5- tetramethylbenzidine (TMB/H2O2, Neogen Corp., USA) low-activity substrate at -200 mV. The decreased current of the HRP-antibody is proportional to the level of creatinine in the samples (Fig. 2C). In our experiments, the electrochemical signal was the current generated by the redox cycles between TMB, the HRP reporter enzyme, and H2O2. All experiments were performed at room temperature.
For each individual sample a calibration curve was obtained as well as the signal from the sample. In the calibration step, different concentrations of creatinine standard were spiked into each individual sample. The concentration ranged from 0 mg/dL to 11.3 mg/dL, which is the typical range of creatinine in the human body. The concentration of the creatinine in each sample was calculated according to its specific calibration curve. The total detection time from sample loading was less than 5 minutes. The sample volume requirement was 40 μL.
Results
Calibration curve for Creatinine standards
In order to get an accurate readout of the creatinine concentration, calibration curves were obtained for each individual sample. In the calibration experiment, creatinine standards were spiked into the blood samples at different concentrations from 0 mg/dL to 11.3 mg/dL by serials dilution, which covers the dynamic range of blood creatinine level. For each dilution, the dilution ratio is 1:1.5 from 11.3 mg/dL. The signals result in a linear relationship to the spiked creatinine concentration. By a linear regression fitting process, the accurate concentration of the blood sample was interpreted according to this calibration curve (Fig. 2D). The linear fitting equation is ΔI(nA) =98.98+19.17×creatinine concentration (mg/dL). The limit-of-detection (LOD) for the electrochemical sensor was calculated based on the two standard deviation (SDV) cutoff from the calibration curves. According to the calibration curve, the LOD for creatinine was around 0.46 mg/dL. The overall estimation of all the calibration curves from the 19 samples demonstrates very similar fitting parameters. The average slope is 19.35±1.58. In the future a single universal calibration curve could be generated that would simplify the process for clinical application.
Clinical samples measurement
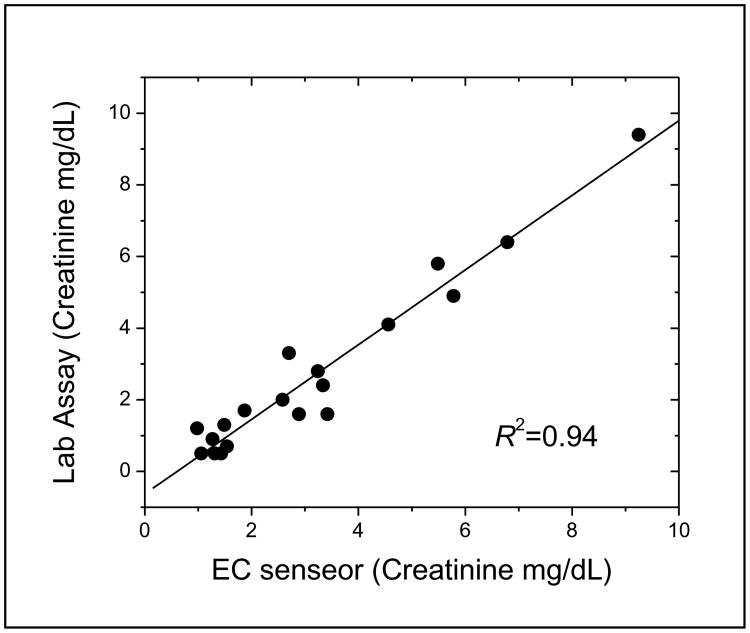
Clinical blood samples were tested with the electrochemical sensor. For each sample, 40 μL volume was utilized during the test. 10 μl of HRP conjugated anti-creatinine antibody was first mixed with 40 μl raw blood sample solution at room temperature. Then the mixture was transferred onto the electrodes for competitive reaction between the creatinine in solution and on the polymer matrix. Each measurement took less than 5 minutes from the sample loading by pipetting the mixture of HRP conjugated antibody and blood sample onto the electrode. For comparison, the creatinine concentration was also measured by traditional Jaffe methodology at the UCLA Ronald Reagan Clinical Laboratory. Figure 3 lists the creatinine concentration from the 19 clinical samples based on electrochemical sensor and the Jaffe reaction. The linear correlation between the two methods is also presented in Fig. 3. The EC sensor has very comparable sensitivity with the traditional Jaffe reaction (correlation coefficient R2 is 0.94). Of note, the EC sensor required only 40 μL of blood sample compared to the 1000 μL required by the Jaffe reaction. Additionally, the measurement based on the EC sensor took less than 5 minutes to complete compared to over 1 hour for the Jaffe reaction.
Figure 3.
Data correlation between EC sensor and the Ronald Reagan UCLA Medical Center traditional Jaffe based assay on paired clinical samples. The linear correlation is illustrated (R2=0.94).
Discussion
Point-of-care testing (POCT) testing is defined as medical testing at the site of patient care. The glucometer-POCT device has revolutionized the quality of life and insulin regulation for diabetic patients. The rapid and portable detection of creatinine would have a tremendous impact on renal transplant recipients' quality of life, while enabling rejection to be determined at an earlier stage when it is more easily reversed. This technology could be potentially applied to oncology and radiology specialties where the rapid detection of creatinine is important prior to the induction of chemotherapy and to avoid contrast induced nephropathy.
Four promising POCT devices to measure whole blood creatinine have already been brought to the marketplace. Namely IRMA TRUpoint (ITC, Edison, NJ), Radiometer ABL800 FLEX (Radiometer A/S, Bronshoj, Denmark), StatSensor (Nova Biomedical, Waltham, MA), and i-STAT (Abbott Diagnostics, East Windsor, NJ). While TRUpoint and Radiometer are larger multifunctional bench top analyzers; StatSensor and i-STAT (crea-cartridge) are handheld POCT devices that can rapidly measure whole blood creatinine. Both i-STAT and StatSensor utilize similar enzymatic chemical reactions to detect creatinine electrochemically 27, 28. The i-Stat device has been available for almost a decade and has seen some slow adoption by radiology and emergency departments. Detractors of this device report consistent overestimation of creatinine, and the 100 μL volume requirement, which makes it improbable for finger, prick sampling 27-29. StatSensor is a new device with considerable potential as it is simple to use and can measure creatinine from a fingerprick sample. Unfortunately at least 2 previous studies have reported that the StatSensor did not meet expectations, was imprecise and consistently underestimated creatinine levels 27, 30. Shephard et al., concluded that the StatSensor needs urgent improvement 30.
Usually with the enzymatic procedure multiple steps of catalysis are required. The final step being the measurement of small chemicals generated from the previous reaction. Interference with analogues found in body fluids, such as glucose, fructose, ketone bodies, ascorbic acid, and cephalosporins31, 32, may affect the accuracy of this final measurement. By using an antibody mediated amperometric system rather than an enzymatic mediated amperometric system, it is our hope we can avoid the interfering signals faced by these other devices when working with a complex matrix such as whole blood. This is especially important in renal transplant recipients who often have hyperglycemia and are taking multiple immunosuppressant medications that could act as confounders.
The creatinine EC sensor in this study provides a rapid and accurate way for serum creatinine measurement. The detection time was less than 5 minutes from the sample loading. Sensitivity of the detection was found to be 0.46 mg/dL of creatinine with only 40 μL sample in the creatinine concentration range of 0 mg/dL to 11.3 mg/dL. This is the ideal clinical range for detecting graft dysfunction.
However, converting our POCT device for immediate clinical application would be limited by sample volume fluctuations. Standardization in the sample processing step is necessary for different sample volumes affect the signal readout of our device. Therefore an additional sample-processing accessory would need to be developed, so that 40 μL of blood can consistently be delivered to the sensor, in a manner that is “user-friendly” for the patient.
Conclusions
This rapid assay for creatinine detection by point-of-care testing (POCT) was able to produce consistent signal levels that accurately detected the creatinine concentrations from clinical blood samples. The creatinine sensor covered the desired clinical range for detecting allograft dysfunction (0 mg/dL to 11.3 mg/dL). Signal levels that were detected electrochemically correlated closely with the creatinine blood concentrations reported by the Ronal Reagan UCLA Medical Center traditional clinical laboratory assay (R2=0.94). With the development of an accessory that could consistently deliver 40 μL of blood to the sensor, this device could become a prominent clinical diagnostic tool for measuring allograft dysfunction in renal transplantation.
Acknowledgments
This research was supported by SOD Faculty Seed Grants from the UCLA School of Dentistry (441901-69749-FWEI-FY11DR), and the Jean Perkins Foundation (JV).
References
- 1.Wei F, Lillehoj PB, Ho CM. Pediatric Research. 2009;67:458–468. doi: 10.1203/PDR.0b013e3181d361c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei F, Patel P, Liao W, Chaudhry K, Zhang L, Arellano-Garcia M, Hu S, Elashoff D, Zhou H, Shukla S, Shah F, Ho CM, Wong DT. Clinical Cancer Research. 2009;15:4446–4452. doi: 10.1158/1078-0432.CCR-09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei F, Wang JH, Liao W, Zimmermann BG, Wong DT, Ho CM. Nucleic Acids Research. 2008;36:e65. doi: 10.1093/nar/gkn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bange A, Halsall HB, Heineman WR. Biosensors & Bioelectronics. 2005;20:2488–2503. doi: 10.1016/j.bios.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Chaubey A, Malhotra BD. Biosensors & Bioelectronics. 2002;17:441–456. doi: 10.1016/s0956-5663(01)00313-x. [DOI] [PubMed] [Google Scholar]
- 6.Ivnitski D, Abdel-Hamid I, Atanasov P, Wilkins E. Biosensors & Bioelectronics. 1999;14:599–624. doi: 10.1016/s0956-5663(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 7.Walcarius A. Chemistry of Materials. 2001;13:3351–3372. [Google Scholar]
- 8.Wilson R, Turner APF. Biosensors & Bioelectronics. 1992;7:165–185. [Google Scholar]
- 9.Soldatkin AP, Montoriol J, Sant W, Martelet C, Jaffrezic-Renault N. Talanta. 2002;58:351–357. doi: 10.1016/s0039-9140(02)00283-7. [DOI] [PubMed] [Google Scholar]
- 10.Subrahmanyam S, Piletsky SA, Piletska EV, Chen BN, Karim K, Turner APF. Biosensors & Bioelectronics. 2001;16:631–637. doi: 10.1016/s0956-5663(01)00191-9. [DOI] [PubMed] [Google Scholar]
- 11.Lakshmi D, Prasad BB, Sharma PS. Talanta. 2006;70:272–280. doi: 10.1016/j.talanta.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Li TJ, Chen PY, Nien PC, Lin CY, Vittal R, Ling TR, Ho KC. Analytica Chimica Acta. 2012;711:83–90. doi: 10.1016/j.aca.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Madaras MB, Buck RP. Analytical Chemistry. 1996;68:3832–3839. doi: 10.1021/ac960239r. [DOI] [PubMed] [Google Scholar]
- 14.Cosnier S. Biosensors & Bioelectronics. 1999;14:443–456. doi: 10.1016/s0956-5663(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 15.Cosnier S. Electroanalysis. 2005;17:1701–1715. [Google Scholar]
- 16.Cosnier S. Analytical Letters. 2007;40:1260–1279. [Google Scholar]
- 17.Fan CH, Wang S, Hong JW, Bazan GC, Plaxco KW, Heeger AJ. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6297–6301. doi: 10.1073/pnas.1132025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanaviciene A, Ramanavicius A. Critical Reviews in Analytical Chemistry. 2002;32:245–252. [Google Scholar]
- 19.Wei F, Liao W, Xu Z, Yang Y, Wong DT, Ho CM. Small. 2009;5:1784–1790. doi: 10.1002/smll.200900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerard M, Chaubey A, Malhotra BD. Biosensors & Bioelectronics. 2002;17:345–359. doi: 10.1016/s0956-5663(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 21.Baracskay D, Jarjoura D, Cugino A, Blend D, Rutecki GW, Whittier FC. Clinical Nephrology. 1997;47:222–228. [PubMed] [Google Scholar]
- 22.Organ Procurement and Transplantation Network by special request as of 02/08/2011.
- 23.Chou NH, Chou JC, Sun TP, Hsiung SK. Ieee Sensors Journal. 2009;9:665–672. [Google Scholar]
- 24.Lad U, Khokhar S, Kale GM. Analytical Chemistry. 2008;80:7910–7917. doi: 10.1021/ac801500t. [DOI] [PubMed] [Google Scholar]
- 25.Straseski JA, Lyon ME, Clarke W, DuBois JA, Phelan LA, Lyon AW. Clinical Chemistry. 2011;57:1566–1573. doi: 10.1373/clinchem.2011.165480. [DOI] [PubMed] [Google Scholar]
- 26.Wei F, Liao W, Xu Z, Yang Y, Wong DT, Ho CM. Small. 2009 doi: 10.1002/smll.200900369. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korpi-Steiner NL, Williamson EE, Karon BS. American Journal of Clinical Pathology. 2009;132:920–926. doi: 10.1309/AJCPTE5FEY0VCGOZ. [DOI] [PubMed] [Google Scholar]
- 28.Gault MH, Seymour ME, Howell WE. Nephron. 2001;88:178–182. doi: 10.1159/000045982. [DOI] [PubMed] [Google Scholar]
- 29.Nichols JH, Bartholomew C, Bonzagi A, Garb JL, Jin L. Clinica Chimica Acta. 2007;377:201–205. doi: 10.1016/j.cca.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Shephard M, Peake M, Corso O, Shephard A, Mazzachi B, Spaeth B, Barbara J, Mathew T. Clinical Chemistry and Laboratory Medicine. 2010;48:1113–1119. doi: 10.1515/CCLM.2010.238. [DOI] [PubMed] [Google Scholar]
- 31.Lo SC, Tsai KS. Clinical Chemistry. 1994;40:2326–2327. [PubMed] [Google Scholar]
- 32.Benkert A, Scheller FW, Schoessler W, Micheel B, Warsinke A. Electroanalysis. 2000;12:1318–1321. [Google Scholar]