Abstract
Environmental contaminants are known to exert endocrine-disrupting effects on the reproductive axis of animals. Many of these molecules can affect steroid biosynthesis or estrogen-receptor signaling by behaving as estrogen-like molecules (“xenoestrogens”), or by exerting estrogenmodulatory effects. Exposure to some compounds has been correlated with the skewing of sex ratios in aquatic species, feminization and demasculinization of male animals, declines in human sperm counts, and overall diminution in fertility of birds, fish, and mammals. We herein devote space to several classes of endocrine-disrupting compounds (EDCs), including estrogenic substances such as bisphenol A (BPA), molecules that can behave at times anti-estrogenically while activating the aromatic hydrocarbon receptor (AHR), such as dioxins (a known human carcinogen), and novel, ubiquitous molecules such as nanoparticles, particularly gold nanoparticles (GNPs), that appear to alter the sexsteroid biosynthetic pathway.
Keywords: endocrine disruptor, xenoestrogen, dioxin, nanoparticle, fertility, ovary
INTRODUCTION AND HISTORICAL RELEVANCE
We are now well aware due to an accumulation of evidence that various environmental pollutants can act as endocrine disruptors. The term endocrine disruptor (ED) was first coined in 1993 by Dr. Theo Colborn [1] and has been defined by Kavlock et al. [2] as “an exogenous agent that interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body responsible for the regulation of homeostasis and the regulation of developmental processes”. Many of these EDs are persistent organic pollutants (POPs). A European Workshop (described in the Weybridge Report [3]) in 1996 concluded that an environmental endocrine disruptor (EED) “...causes adverse health effects in an intact organism or its progeny, secondary to changes in endocrine function”. Historical focus has been on compounds that act as estrogen agonists, termed “xenoestrogens”. A xenoestrogen is a compound that fits well into the above definitions of EEDs, particularly with respect to binding of the estrogen-receptor (ER). Xenoestrogens are capable of binding to nuclear ERs (ERα or β [4] (encoded by genes Esr1 and Esr2, respectively), or organelle (e.g., endoplasmic reticulum) membrane-bound ERs such as GPER/GPR30 that operate via ERK or other signal-transduction pathways [5]; and this binding may cooperate with estrogen-related receptors (ERRs)) (see [6]). Further, compounds have been classified as xenoestrogens if they succeed in producing estrogen-like effects in a specific bioassay (e.g., uterine weight gain) or in a reporter gene construct. And while some compounds can be ER agonists, others are estrogen-modulatory (dioxin) or antagonists (of the androgen-receptor, for example). ERβ appears to preferentially bind some EEDs more so than ERα, and both work via transcriptional co-regulators (e.g., the co-activator SRC-1) and other molecules [7]. Evidence for the existence of these endocrine disruptors in the environment and their potentially deleterious effects in animals has already been indicated in the landmark book published in 1962 by Rachel Carson [8]. September 27, 2012 marked the 50th anniversary of the publication of Rachel Carson's book entitled ‘Silent Spring’. Dr. Theo Colborn and colleagues later gave us the highly acclaimed treatise ‘Our Stolen Future’ in 1996 [9]. These books dramatically increased our awareness of the potential reproductive and developmental effects of environmental pollutants. However, there are many other environmental pollutants that modulate estrogen-receptor signaling, or estrogen synthesis, causing reproductive and developmental anomalies and we attempt to describe several of them here. This, however, is neither meant to be a completely comprehensive nor exhaustive review of the literature, but rather one that focuses on a few significant EEDs and a potentially novel one - nanoparticles.
Xenoestrogens
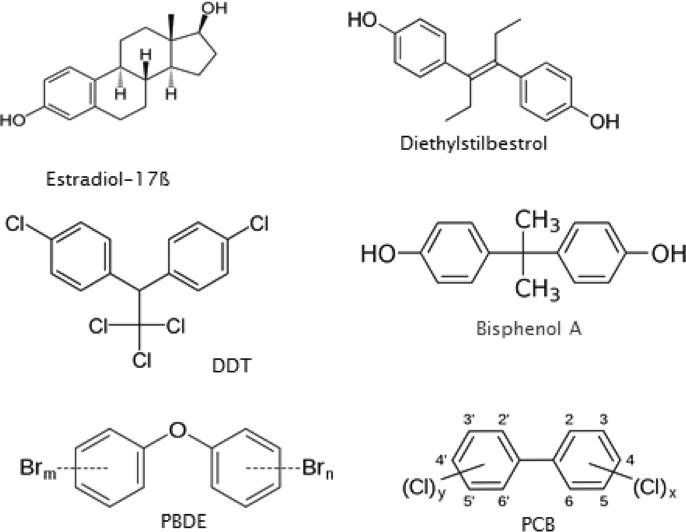
Several examples of the effects of EEDs or endocrine-disrupting compounds (EDCs) on endocrine systems/reproduction follow. These compounds range from medicines, pesticides, and legacy contaminants to everyday items such as food storage containers, clothing, and personal care products. The non-steroidal estrogen diethylstilbestrol (DES) was decades ago used by an estimated two million or more women to maintain pregnancy and prevent miscarriage, but was subsequently shown to increase the incidence of cervical dysplasia in daughters and hypospadias in sons so exposed in utero (see Fig. 1; [10], exposing up to several million offspring in total, and it is still used today emergently by women in cases of rape. DDT (banned in the US in the early 1970's) is an insecticide that is known to induce eggshell thinning, and exert untoward effects on fish and wildlife, including demasculinization of alligators [11], and may even increase the risk for childhood obesity (Fig. 1; [12])). DDT can produce developmental abnormalities in reproductive organs, and behave estrogenically on breast tissue, having been correlated in some studies with breast cancers, although this connection is contentious and controversial, and has been discounted in other publications [13]. Complicating matters is the fact that DDT is still the cheapest and most effective agent currently available to combat malaria-carrying mosquitoes worldwide. Plant or phytoestrogens have been implicated in both beneficial and detrimental estrogenic effects [14, 15, 16]. High doses of ethinyl estradiol, the active estrogen in most contraceptive pills, native estradiol-17β (E2; Fig. 1) from cycling women, and equine estrogens (e.g., Premarins) taken by post-menopausal women have been detected in waste water effluent from some metropolitan areas worldwide [17], and may be responsible for skewing the sex ratios toward females in aquatic species in several areas [18].
Fig. 1.
Several notable endocrine disruptors (from diethylstilbestrol (DES), DDT top, left to right): estradiol-17ß (E2), (dichorodiphenyltrichlorethane), bisphenol A (BPA), PBDE, (polybrominated diphenyl ethers) and PCB (polychlorinated biphenyls).
Polychlorinated biphenyls (PCBs, banned in the US in about 1976) are an example of so-called “legacy” pollutants that persist in sediment for long periods of time (Fig. 1). PCBs were used as industrial coolants and lubricants, some of which, particularly non-coplanar forms such as PCB 153, are estrogenic, and have also been correlated in humans with an increased disposition to breast or prostate cancers and childhood obesity/diabetes; thereby, in the latter case, acting potentially as “obesogens” and/or diabetogens [19]. Women in both more- and less-developed countries carry in their breast milk many polychlorinated biphenyls (PCBs) (e.g., PCB 153) and planar or co-planar PCBs (e.g., PCBs 77, 126 and 169, which are dioxin like and not estrogenic), especially due to the presence of large quantities of fish in their diets [20].
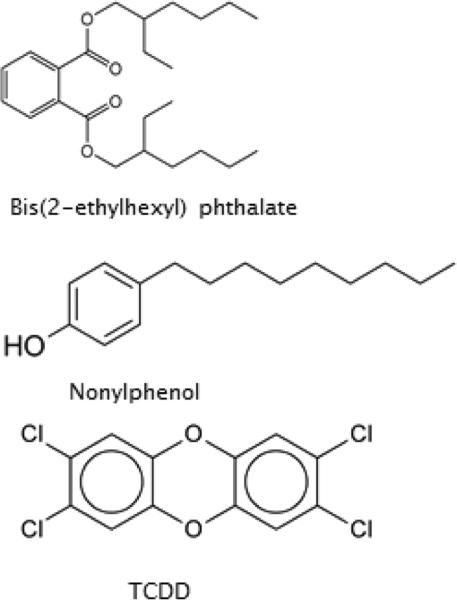
Phthalates are plasticizers used in soft toys, bottles, and medical tubing, and can alter male reproductive function (Fig. 2). At environmentally relevant concentrations, they disrupt follicle-stimulating hormone (FSH)-induced granulosa cell function in follicular aspirates as part of IVF protocols [21], modulate sperm function; and have been correlated with alterations in the timing of parturition [22, 23]. Some polybrominated diphenyl ethers (PBDEs; Fig. 1) are flame retardants that can disrupt thyroid function and serum thyroid hormone concentrations, and have been correlated with altered learning and neurodevelopment in children up to 72 months of age after the World Trade Center attacks in New York City [24]. PBDE congeners 47, 99, 100, and 153 are specifically associated with diminished fecundity in women [25]. Researchers from the University of California-Berkeley and Duke University [26] found that 41 percent of the 102 couches they tested had “foam with chlorinated Tris (a post-PBDE flame retardant), a probable human carcinogen, removed from baby pajamas in 1977”. They also discovered that 17 percent of the sofas “contained the chemical pentaBDE (a specific form of PBDE),” which has been banned globally. In 2009, the US Congress banned several phthalates due to associations with hormone disruption, developmental problems, lower IQ and impaired fertility [27].
Fig. 2.
Endocrine disruptors, including 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD).
Ingredients in our personal care products have recently been scrutinized for their potential to act as EEDs. Alkylphenols such as nonylphenol (Fig. 2) are non-ionic detergents that have been shown to skew sex ratios in fish toward almost exclusively females, and to demasculinize alligators and turtles [18]. Triclosan (which we see in 75% of bactericidal hand soaps, and which may be most effective in toothpaste vs. gingivitis) is found in municipal wastewater after chlorine treatment, and when exposed to sunlight can be metabolized to certain dioxins [28].
Finally, it is conceivable that binary or greater combinations of these molecules may act synergistically to induce even greater effects than either chemical alone, on the same or other receptor sites in target tissues, including reproductive organs. The role of EDCs has certainly galvanized the interest of the scientific and lay communities because of their potentially toxic effects; but aspects of their nature, use, effects, and mechanism of action are not fully elucidated and remain highly controversial, requiring much further investigation. The now quintessential endocrine disruptor BPA is one of these environmental chemicals that has recently been at the center of concern and initiated a heated debate. We will next focus on some specific molecular aspects and physiologic effects of this compound.
Bisphenol A
Bisphenol A exposure shows in some studies an increased risk for diabetes, mammary and prostate cancers, declining sperm count, and effects on non-human primate ovarian function (Fig. 1) [29, 30, 31]. BPA was discovered in the bloodstream of over 80% of men and women participating in IVF treatments for the first time [32], and was found in newborn infants cord blood in one study [33]. The CDC has found BPA in the urine of 93% of surveyed Americans over the age of 6 [34]. Due to the importance of ovarian hormones, especially estrogens, in regulating gametogenesis and steroidogenesis and thereby overall fertility, the role of endocrine disruptors such as BPA has piqued the interest of the scientific community. In ‘Endocrine Today’, vom Saal stated that “More research has been conducted on low doses of BPA than [on] any other chemical being used in products that people come in contact with every day” [35]. These low doses have shown adverse effects in many published studies, including behavioral changes, cancers of prostate and breast, decreased sperm counts, and even obesity. Vandenberg and associates tout non-monotonic (non-linear) dose-response effects such as U- and inverted U-curves that certainly implicate non-conventional effects of such compounds (although these effects are also quite controversial within the scientific community [36]). Some compounds may not even manifest a NAOEL (no adverse observed effects limit), meaning that even the smallest amount of a toxicant may exert some untoward effect over time. Certainly such effects on ovarian function have already been implicated [37, 38].
In order to appreciate the effects of EDCs such as BPA on reproductive function, we need to briefly state some aspects of ovarian physiology. Ovarian function plays a key role, of course, in the development of the female reproductive tract and in reproductive function overall. Ovaries accomplish this feat via follicles that contain the egg, or oocyte. These follicles contained in the ovary are responsible for producing the sex steroids via the steroidogenic pathway [39]. The hormones produced by the ovaries (estrogen, progesterone, and inhibin) are so vital that even slight changes induced by EDCs such as BPA can affect female physiologic functions. We now discuss BPA in more detail below.
BPA is an organic compound with two functional phenol groups [37]. BPA is a monomer of polycarbonate plastics that is used in numerous consumer products (and is found in plastics with the recycling symbol “7”, or “other” [37]), including food and water containers, baby bottles, linings of metal food and beverage cans, coatings for carbonless paper receipts, metal tubing, epoxy resins, and dental fillings [40]. More than six billion pounds of BPA are used each year to create polycarbonate plastics and epoxy resins representing more than $7 billion in sales. Small amounts of BPA can be liberated from incompletely polymerized polycarbonates or by partial hydrolysis, especially upon heating [41]. Many studies in the United States, Europe, and Japan have documented BPA levels ranging from 0.2 to 10 ng/mL (~0.5-40 nM) in adult and fetal human serum [40], as well as in breast milk [42]. Since the molecule is lipophilic, BPA can also accumulate in fat, and detectable levels of BPA have been found in half of the breast adipose tissue samples examined [43].
BPA is structurally similar to potent estrogens such as DES. Due to the fact that BPA contains two benzene rings and two (4, 4’)-OH substituents, it can bind to the estrogen receptor. BPA is a flaky white powder that, as mentioned, is present in some materials that contact food directly. This is due to the fact that the monomer can leach out of polycarbonate polymer plastics used in feeding bottles, tableware, microwavable ovenware, storage containers, milk bottles, and refillable water containers. BPA-containing epoxy-phenolic resins are used for internal protective linings for food and beverage cans and as a coating on metal lids. Biochemical assays have shown that BPA binds to both ERα and ERβ. However, the affinity of BPA for these ERs is approximately 10,000-fold weaker than that of estradiol [14]; and BPA appears to preferentially bind ERβ, although recent evidence shows even greater support for high-affinity binding to ERRγ [34, 44, 45]. Differences in the ability of ERα or ERβ to recruit co-activators (such as SRC-1 and CBP/p300) when BPA is bound may contribute to the complex tissue–specific responses observed with BPA exposure [4]. BPA was originally developed as a synthetic estrogen in the 1930's and initially used as a growth promotant in domestic animals, and because of these effects it has been recently linked to breast and prostate cancer (still controversially), cognitive and behavioral problems, reproductive failures, heart disease, diabetes, asthma and obesity [46]. BPA has been shown to cause toxic effects in many recent studies, and the use of BPA is currently under intense scrutiny. There exists a complex chronology of events regarding banning the use of BPA.
The 2008-2009 Annual Report of the U.S. President's Cancer Panel declared: “because of the long latency period of many cancers, the available evidence argues for a precautionary approach to these diverse chemicals, which include (...) bisphenol A” [47]. The U.S. Food and Drug Administration (FDA) then missed several self-imposed deadlines on apprising consumers of whether it was safe to use products made with bisphenol A. The FDA had promised that it would take a “fresh look” at the science surrounding BPA, and promised that a decision would be made by early fall of 2009 [48], but it later postponed that decision, saying that BPA levels in products were too low to achieve deleterious effects. We currently see a state-by-state approach to BPA bans, rather than a rational, nation-wide approach. For example, in June 2010 there was to be no more importation of bisphenol A-containing plastics into Wisconsin, USA and other states [49]. A 2010 report from the FDA raised further concerns regarding exposure of fetuses, infants, and young children [50]. On 17 July 2012, the FDA banned BPA from baby bottles and “sippy cups” in the United States [51]. In July 2013, the FDA stated that it would no longer authorize the use of BPA in infant formula packaging, and that this decision was based upon “abandonment” and not on safety. The FDA continues to study BPA as of the end of 2013 [52]. The US Environmental Protection Agency (EPA) has also begun to more aggressively scrutinize the use of BPA, encouraging a reduction in the release of and exposure to BPA. In 2010 and 2011, for example, the EPA began to address thermal paper coatings such as cash register receipts, which contain BPA or another similar molecule. [53]. BPA is now also being investigated as a potential obesogen [54], as some experts claim that its hormonal effects may promote obesity. A 2008 study in ‘The Journal of the American Medical Association’ also linked BPA to an increased risk for heart disease, diabetes, and liver problems [55]. Compared with people who had the lowest levels of BPA, those with the highest levels had nearly triple the odds of heart disease and were nearly 2.5 times more likely to have diabetes. However, no link to cancer was found in this study. In the journal ‘Environmental Health Perspectives’ (EHP) [56], pregnant women who had BPA levels in their blood twice that of a comparator cohort were linked to reduced total tetraiodothyronine (T4, the most-abundant thyroid hormone), and a decrease in thyroid-stimulating hormone (TSH) in their male neonates, potentially affecting fetal development [57].
We have also previously shown reproductive deficits with other xenoestrogens. DES inhibited estrogen production by hamster ovarian cells in vitro; and even natural, native estrogens that circulate in a woman's bloodstream dramatically inhibited (by 70%) the ability of FSH to induce the activity of aromatase, the enzyme that converts androgens to estrogens [58]. Another EDC found in rocket propellants (ammonium perchlorate or AP) was shown in female rats to reduce the number of preantral and antral ovarian follicles that contain the oocytes, with T4 co-administration attenuating some of the observed deleterious effects [59]. An industrial, multiple-PCB-containing compound, Aroclor 1016, also reduced the number of preantral and small antral follicles of certain size classes in rats exposed during a critical period of development, possibly by inducing programmed cell death, or apoptosis [60]. Such studies show the numerous forms and ubiquity of endocrine-disrupting compounds, and there is no better example of this than dioxins.
Dioxins
Dioxins are produced as by-products of herbicide overuse, from paper bleaching, plastics manufacture, and waste incineration. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is often called the most potent toxicant known to mankind, and has in the past been derived from factory waste effluents and overuse of defoliants such as 2,4-D (historically also found as a contaminant in Agent Orange, the herbicide used by U.S. air forces during the Vietnam War; Fig. 2). Today, most TCDD is produced by personal and municipal waste incineration, and non-point sources such as forest fire and volcanic emissions into the atmosphere (all contain dioxins of various types) [38]. We are primarily exposed to TCDD via our diets on a daily basis by ingestion of animal tissues and by-products. TCDD has for some years been acknowledged to be a human carcinogen (the DHHS NTP Report on Carcinogens (RoC), 13th Edition, 2014 [61], listed TCDD as “known to be a human carcinogen”, upgraded to this level since 2001), but it is also a tumor progressor, teratogen, immune function suppressor, and endocrine disrupter in laboratory species and wildlife, and from the relatively abundant data from several accidental exposures, in humans [62].
TCDD has been correlated with altered fecundity and endometriosis in monkeys, and with certain cancers and reproductive deficits in animals and humans [63, 64]. Our laboratories and those of others have shown modulation of ovarian follicular function. We have demonstrated that dioxins significantly reduced circulating estrogen concentrations and ovarian follicular growth in prepubertal female rats whose mothers received TCDD during mid-gestation, without appreciably affecting GnRH, LH or FSH concentrations. TCDD also reduced estrogen secretion by rat and human ovarian granulosa cells (GC) exposed in vitro, diminished expression of steroidogenic and estrogen-signaling genes in rats and zebrafish, and reduced fertility in the latter species, posing potential transgenerational effects. The receptor for dioxin was found in rat, human and non-human primate ovarian tissues; and it modulated inhibin secretion and induced apoptosis in human granulosa cells in vitro [63, 65-76]. TCDD is known to achieve much of its effects by activating the aromatic hydrocarbon receptor (AHR).
AHR belongs to the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of proteins and is a ligand-inducible transcription factor [77]. Upon binding of TCDD, the receptor-ligand complex undergoes conformational changes (“transformation”), including dissociation of two heat-shock proteins (HSP-90s), an AHR-interacting protein (AIP, also known as XAP2, which prevents premature translocation into the nucleus [78]), and/or other protein(s), depending upon cell and tissue type. With loss of XAP2, AHR is translocated to the nucleus where it further complexes with the resident aromatic hydrocarbon receptor nuclear translocator (ARNT, or HIF1β) protein [77]. This heterodimeric complex (AHR-ARNT) is then able to bind cisgenomic dioxin-response elements (DREs), or now more commonly referred to as aromatic hydrocarbon response elements (AHREs), typically upstream from the core promotor sequence of target genes, thereby altering gene expression in a wide variety of tissues [79, 80]. Full expression of biologic effects requires AHREs and transcriptional co-regulators (e.g., SRC-1 or the retinoblastoma (RbP) or other proteins [81]).
Overall, the evidence strongly supports the ovary as a major target of TCDD action [38]. Other investigators have also shown that TCDD may interfere with the positive feedback of ovarian estrogen in ovulation and/or reduce ovarian sensitivity to gonadotropins [82]. Because the ovary (including GC) possesses AHR, we now describe the action of TCDD on ovarian estrogen biosynthesis.
Mechanism for TCDD action
From the standpoint of the potential molecular mechanism of action, Mutoh et al. [83] showed that dioxin inhibits cholesterol translocation and sex steroid synthesis in murine species by modulating LH synthesis in fetal brain, and thereby attenuates expression and activity of testicular STAR. Whether this same situation holds true for females is not known, but we do know that AHRE sequences function as transcriptional enhancers at various genes in females also [84], and that dioxin-activated AHR/ARNT can recruit ER and co-activator p300 to estrogen-responsive elements (EREs), leading to transactivation and estrogenic effects in the absence [85] or presence of estrogenic ligand [86]; and appears to act as a ligand-dependent E3 ubiquitin ligase [87]. This dioxin-dependent ubiquitination may then be responsible for degrading ERs. We are currently evaluating in a zebrafish model [76, 88] the in-vivo effects of dioxin, and have observed that chronic dietary exposure to TCDD reduces the numbers of ovarian follicles overall (by increased apoptosis, or atresia) and the proportion of vitellogenic follicles (i.e., preovulatory or Graafian follicles in mammals), the number of oocytes ovulated, the number of healthy offspring produced; and that TCDD achieves this possibly by affecting several enzyme genes in the steroidogenic pathway (cyp11a1, cyp19a1), and in the ER-signaling pathway (esr1) [76, 88].
Lastly, we move to a novel class of molecules that is ubiquitous in our world, but of which we know very little, and that may, in fact, exert disruptive effects on reproductive endocrine function.
Nanoparticles
Nanoparticles (NPs) are materials of approximately 1-100 nm in length/width. Interest in nanoparticles has increased due to their current and anticipated use in industrial and medical applications [89]. However, nanotoxicologic research exploring the potential adverse effects of nanoparticles on mammalian physiology, especially female reproductive function, is severely lacking.
It has been estimated that the nanotechnology market may exceed $1 trillion by 2015 [90]. NPs such as quantum dots, dendrimers, silica, carbon nanotubes and fullerenes (C60, C80), oxides (e.g., titanium oxide), metals (e.g., gold, silver, aluminum), and liposomes exhibit unique physicochemical phenomena (size, morphology, surface area) that enable novel application in the manufacture of consumer products such as sports accessories, tires, water-resistant and odor-absorbing clothing, sunscreens, toothpastes, cosmetics, and electronics as well as a host of other industrial and medical applications [89, 91]. One such nanoparticle is gold, which has gained considerable attention for its potential novel use in targeted drug- and gene-delivery systems [92, 93]; e.g., transmucosal insulin-delivery systems [94], molecular diagnostics [95], bio-imaging [96], cancer diagnostics [97], diabetes [98], and atherosclerosis [99]; and even in more mundane applications such as microbicides in toothpastes and toothbrushes. However, despite these advances toward putative therapeutic benefits of GNPs, concern has arisen as to the possible toxicologic consequences (including reproductive) of these molecules within biologic systems and in the environment [100].
We have demonstrated that 10 nm nanogold particles (GNPs) enter rat ovarian granulosa cells (GC), and can localize in lipid droplets and mitochondria, alter mitochondrial morphology, and can modulate GC estrogen production [101]; and that certain enzyme genes in the steroid biosynthetic pathway show subtle alterations in our intact ovarian culture model. In the latter, 10 nm GNPs appear to affect expression of rat Star, Cyp11a1, and Hsd3b1 upstream of progesterone (P4) accumulation [102]. These effects might modulate reproductive function as ovarian steroids are important for maintaining ovarian health and female fertility. Other evidence suggests that GNPs can alter gene expression and induce cellular states of oxidative stress, apoptosis, necrosis, autophagy, and inflammation [103, 104]; however, responses are variable among cell and nanoparticle types [105]. Such variability in the reported cellular response to NPs warrants continued investigation. The fact that GNPs can be distributed in vivo via the bloodstream, enter rat testis [106], and potentially impact cellular gene expression and disrupt cellular function (e.g., apoptosis and oxidative stress [107-108], and signaling pathways [109]) suggests that GNPs may be capable of disrupting complex cellular functions such as ovarian steroidogenesis and/or follicular development viaan oxidative stress- and/or apoptosis-mediated mechanism in vivo. Research has also shown that NPs may target mitochondria [101, 110], causing release of cytochrome c oxidase from the outer mitochondrial membrane (related to programmed cell death or apoptosis); increase calcium uptake and organelle damage [111; and cause mitochondrial DNA damage specifically [110]. The resulting oxidative stress is shown to induce apoptosis [108, 112], inhibit steroidogenesis [113], and disrupt cellular function [114]. Importantly, no models of female fertility and nanoparticle exposure exist currently, and therefore this nascent area deserves greatly increased investigation.
As our studies point to the ovary as a target of nanoparticle action in vivo, additional studies will aid in determining possible female reproductive pathologies upon nanogold exposure. Importantly, such reports will further educate our society regarding the potentially harmful effects of engineered NPs, and, in turn, should increase public reproductive health awareness.
SUMMARY
Although it is tempting to speculate that all EEDs exert a negative impact on the reproductive physiology of animals and humans, the actual situation is often much more complex. The effects observed are many times subtle and depend upon the compound, its concentration, and how it is administered, whether the molecule is found singly or in a mixture, the animal model, etc. In summary, we believe that some of the reproductive deficits observed from exposure to endocrine-disrupting environmental contaminants may be attributable to the modulation of steroid synthetic and signaling pathways, and that nanoparticles may constitute a novel endocrine disruptor of the female reproductive axis.
ACKNOWLEDGEMENTS
Supported in part by NIH grants ES008342, ES011569 and ES006807 (to RJH), 5P51RR000167 (to the Wisconsin National Primate Research Center); and ES004184 (to the NIEHS Center at UWM and the Children's Research Institute, Milwaukee, WI).
ABBREVIATIONS
- ESR1
estrogen receptor-α
- ESR2
estrogen receptor-ß
- GPER/GPR30
G protein-coupled estrogen receptor 1/G protein-coupled receptor 30
- ERK
extracellular signal-regulated kinases
- SRC-1
steroid receptor co-activator 1
- DDT
dichlorodiphenyltrichloroethane
- IVF
in-vitro fertilization
- CDC
U.S. Centers for Disease Control and Prevention
- IQ
intelligence quotient
- nM
nanomolar
- CBP/p300
paralogs that both bind cyclic AMP response element binding protein (CREB)
- DHHS
U.S. Department of Health and Human Services
- NTP
U.S. National Toxicology Program
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- XAP2
hepatitis B virus X-associated protein
- HIF1ß
hypoxia-inducible factor 1-beta
- STAR
steroidogenic acute regulatory protein/ gene/mRNA
- CYP11A1
cytochrome P450, family 11, subfamily A, polypeptide 1 (side-chain cleavage)
- CYP19A1
cytochrome P450, family 19, subfamily A, polypeptide 1 (aromatase)
- HSD3B1
hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 (3ß hydroxysteroid dehydrogenase)
- CYP1A1
cytochrome P450, family 1, subfamily A, polypeptide 1
- mRNA
messenger ribonucleic acid
Footnotes
CONFLICT OF INTEREST STATEMENT
All authors declare that we have no conflicts of interest to disclose concerning the topics included in this paper.
REFERENCES
- 1.Colborn T, vom Saal FS, Soto AM. Environ. Health. Perspect. 1993;101:378. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. Environ. Health Perspect. 1996;104(S4):715. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Commission European Workshop on the Impact of Endocrine Disrupters on Human Health and Wildlife. Proceedings from a workshop; Weybridge, UK. December 2-4, 1996; 1996. Report reference EUR 17549. Brussels, Belgium: European Commission. http://www.scribd.com/doc/117997922/The-Impacts-of-Endocrine-Disrupters. [Google Scholar]
- 4.Routledge EJ, White R, Parker MG, Sumpter JP. J. Biol. Chem. 2000;275:35986. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 5.Dong S, Terasaka S, Kiyama R. Environ. Pollut. 2011;159:212. doi: 10.1016/j.envpol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Environ. Health Perspect. 2008;116:1642. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durrer S, Ehnes C, Fuetsch M, Maerkel K, Schlumpf M, Lichtensteiger W. Environ. Health Perspect. 2007;15(Suppl. 1):42. doi: 10.1289/ehp.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson R. Silent Spring. Houghton Mifflin; Boston, MA: 1962. [Google Scholar]
- 9.Colborn T, Dumanoski D, Meyeres JP. Our Stolen Future: Are We Threatening Our Fertility, Intelligence and Survival? A Scientific Detective Story. Dutton; New York: 1996. [Google Scholar]
- 10.Glaze GM. J. Am. Osteopath. Assoc. 1984;83:435. [PubMed] [Google Scholar]
- 11.Guillette LJ, Jr., Pickford DB, Crain DA, Rooney AA, Percival HF. Gen. Comp. Endocrinol. 1996;101:32. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- 12.Cupul-Uicab LA, Klebanoff MA, Brock JW, Longnecker MP. Environ. Health Perspect. 2013;121:1103. doi: 10.1289/ehp.1205901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE, Scinicariello F. Regul. Toxicol. Pharmacol. 2013;67:421. doi: 10.1016/j.yrtph.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, Van der Burg B, Gustafsson JA. Endocrinology. 1998;139:4252. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 15.Patisaul HB, Todd KL, Mickens JA, Adewale HB. Neurotoxicology. 2009;30:350. doi: 10.1016/j.neuro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC. Steroids. 2005;70:364. doi: 10.1016/j.steroids.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V, Johnson AC, Nakada N, Yamashita N, Tanaka H. J. Hazard. Mater. 2012;227-228:49. doi: 10.1016/j.jhazmat.2012.04.078. [DOI] [PubMed] [Google Scholar]
- 18.White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Endocrinology. 1994;135:175. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- 19.Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, van Gaal L. Obesity (Silver Spring) 2011;19:709. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- 20.Sun SJ, Kayama F, Zhao JH, Ge J, Yang YX, Fukatsu H, Iida T, Terada M, Liu DW. Chemosphere. 2011;85:448. doi: 10.1016/j.chemosphere.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 21.Huang XF, Li Y, Gu YH, Liu M, Xu Y, Yuan Y, Sun F, Zhang HQ, Shi HJ. PLoS One. 2012;7:e50465. doi: 10.1371/journal.pone.0050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, Herrick R, Swan SH. Am. J. Epidemiol. 2009;169:1015. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, Perera FP, Andrews H, Just AC, Hoepner L, Tang D, Hauser R. Pediatrics. 2009;124:e1213. doi: 10.1542/peds.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Environ. Health Perspect. 2010;118:712. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman JE, Biesemeier JA, Johnson GT, Harbison C, Harbison RD, Zhu Y, Lee RV, Silberberg H, Hardy M, Stedeford T. Environ. Health Perspect. 2010;118:a330. doi: 10.1289/ehp.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Environ. Sci. Technol. 2012;46:13432. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. http://www.gpo.gov/fdsys/pkg/BILLS-112hr2715enr/pdf/BILLS-112hr2715enr.pdf and http://www.cpsc.gov//PageFiles/129663/cpsia.pdf.
- 28.Venkatesan AK, Pycke BF, Barber LB, Lee KE, Halden RU. J. Hazard. Mater. 2012;229-230:29. doi: 10.1016/j.jhazmat.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Proc. Natl. Acad. Sci. USA. 2012;109:17525. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso-Magdalena P, Ropero AB, Soriano S, Quesada I, Nadal A. Hormones (Athens) 2010;9:118. doi: 10.1007/BF03401277. [DOI] [PubMed] [Google Scholar]
- 31.Rochester JR. Reprod. Toxicol. 2013;42:132. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Bloom MS, vom Saal FS, Kim D, Taylor JA, Lamb JD, Fujimoto VY. Environ. Toxicol. Pharmacol. 2011;32:319. doi: 10.1016/j.etap.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Sun H, Kannan K. Environ. Sci. Technol. 2013;47:4686. doi: 10.1021/es303808b. [DOI] [PubMed] [Google Scholar]
- 34.Lakind JS, Naiman DQ. J. Expo. Sci. Environ. Epidemiol. 2008;18:608. doi: 10.1038/jes.2008.20. [DOI] [PubMed] [Google Scholar]
- 35.vom Saal FS. Endocrine Today. 2010 Jan; [Google Scholar]
- 36.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Endocr. Rev. 2012;33:378. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers DE, Hutz RJ. Gen. Dent. 2011;59:262. [PubMed] [Google Scholar]
- 38.Hutz RJ. J. Reprod. Develop. 1999;45:1. [Google Scholar]
- 39.Hutz RJ, Dierschke DJ, Wolf RC. J. Med. Primatol. 1990;19:553. [PubMed] [Google Scholar]
- 40.Welshons WV, Nagel SC, vom Saal FS. Endocrinology. 2006;147:856. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 41.Le HH, Carlson EM, Chua JP, Belcher SM. Toxicol. Lett. 2008;176:149. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuruto-Niwa R, Tateoka Y, Usuki Y, Nozawa R. Chemosphere. 2007;66:1160. doi: 10.1016/j.chemosphere.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez MF, Arrebola JP, Taoufiki J, Navalon A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Reproductive Toxicol. 2007;24:259. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Environ. Health Perspect. 2008;116:32. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y. J. Biochem. 2007;142:517. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, Liu Y, Bi Y, Lai S, Ning G. J. Clin. Endocrinol. Metab. 2012;97:E223. doi: 10.1210/jc.2011-1989. [DOI] [PubMed] [Google Scholar]
- 47.National Cancer Institute Annual Report for 2008-2009. http://deainfo.nci.nih.gov/advisory/pcp/annu alReports/pcp08-09rpt/PCP_Report_08-09_508.pdf.
- 48.Erler C, Novak J. J. Pediatr. Nurs. 2010;25:400. doi: 10.1016/j.pedn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 49. http://latimesblogs.latimes.com/greenspace/2011/10/bpa-ban-signed-by-california-governor-jerry-brown.html.
- 50.Update on Bisphenol A for Use in Food Contact Applications U.S. Food and Drug Administration January 2010, U.S. Food and Drug Administration. http://www.fda.gov/downloads/NewsEvents/PublicHealthFocus/UCM197778.pdf.
- 51. http://www.gpo.gov/fdsys/pkg/FR-2012-07-17/html/2012-17366.htm.
- 52. http://www.fda.gov/newsevents/publichealth focus/ucm064437.htm#current.
- 53. http://www.epa.gov/oppt/dfe/pubs/projects/bpa/
- 54.Gruen F, Blumberg B. Mol. Cell. Endocrinol. 2009;304:19. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melzer D, Rice NE, Lewis C, Henley WH, Galloway TS. PLoS One. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, Harley KG. Environ. Health Perspect. 2013;121:138. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newbold RR, Jefferson WN, Padilla-Banks E. Environ. Health Perspect. 2009;117:879. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutz RJ, Gold DA, Dierschke DJ. Cell Tissue Res. 1987;248:531. doi: 10.1007/BF00216480. [DOI] [PubMed] [Google Scholar]
- 59.Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. Reprod. Toxicol. 2004;19:155. doi: 10.1016/j.reprotox.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. Reprod. Toxicol. 2003;17:567. doi: 10.1016/s0890-6238(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 61. http://ntp.niehs.nih.gov/pubhealth/roc/roc13/index.html.
- 62.Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Environ. Health Perspect. 2011;119:1700. doi: 10.1289/ehp.1103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho HM, Ohshima K, Watanabe G, Taya K, Strawn EY, Hutz RJ. J. Reprod. Dev. 2006;52:523. doi: 10.1262/jrd.18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rier SE, Turner WE, Martin DC, Morris R, Lucier GW, Clark GC. Toxicol. Sci. 2001;59:147. doi: 10.1093/toxsci/59.1.147. [DOI] [PubMed] [Google Scholar]
- 65.Trewin AL, Woller MJ, Wimpee BA, Conley LK, Baldridge MG, Hutz RJ. J. Reprod. Dev. 2007;53:765. doi: 10.1262/jrd.18101. [DOI] [PubMed] [Google Scholar]
- 66.Heiden TK, Carvan MJ, 3rd, Hutz RJ. Toxicol. Sci. 2006;90:490. doi: 10.1093/toxsci/kfj085. [DOI] [PubMed] [Google Scholar]
- 67.Heiden TK, Hutz RJ, Carvan MJ., 3rd Toxicol. Sci. 2005;87:497. doi: 10.1093/toxsci/kfi201. [DOI] [PubMed] [Google Scholar]
- 68.Baldridge MG, Hutz RJ. Am. J. Primatol. 2007;69:681. doi: 10.1002/ajp.20381. [DOI] [PubMed] [Google Scholar]
- 69.Dasmahapatra AK, Wimpee BA, Trewin AL, Wimpee CF, Ghorai JK, Hutz RJ. Mol. Cell Endocrinol. 2000;164:5. doi: 10.1016/s0303-7207(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 70.Chaffin CL, Trewin AL, Hutz RJ. Chem. Biol. Interact. 2000;124:205. doi: 10.1016/s0009-2797(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 71.Heimler I, Rawlins RG, Owen H, Hutz RJ. Endocrinology. 1998;139:4373. doi: 10.1210/endo.139.10.6264. [DOI] [PubMed] [Google Scholar]
- 72.Heimler I, Trewin AL, Chaffin CL, Rawlins RG, Hutz RJ. Reprod. Toxicol. 1998;12:69. doi: 10.1016/s0890-6238(97)00101-9. [DOI] [PubMed] [Google Scholar]
- 73.Chaffin CL, Trewin AL, Watanabe G, Taya K, Hutz RJ. Biol. Reprod. 1997;56:1498. doi: 10.1095/biolreprod56.6.1498. [DOI] [PubMed] [Google Scholar]
- 74.Chaffin CL, Heimler I, Rawlins RG, Wimpee BA, Sommer C, Hutz RJ. Endocrine. 1996;5:315. doi: 10.1007/BF02739065. [DOI] [PubMed] [Google Scholar]
- 75.Chaffin CL, Peterson RE, Hutz RJ. Biol. Reprod. 1996;55:62. doi: 10.1095/biolreprod55.1.62. [DOI] [PubMed] [Google Scholar]
- 76.Liu Q, Rise ML, Spitsbergen JM, Hori TS, Mieritz M, Geis S, McGraw JE, Goetz G, Larson J, Hutz RJ, Carvan MJ., 3rd Aquat. Toxicol. 2013;140-141:356. doi: 10.1016/j.aquatox.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beischlag TV, Wang S, Rose DW, Torchia J, Reisz-Porszasz S, Muhammad K, Nelson WE, Probst MR, Rosenfeld MG, Hankinson O. Mol. Cell. Biol. 2002;22:4319. doi: 10.1128/MCB.22.12.4319-4333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hollingshead BD, Petrulis JR, Perdew GH. J. Biol. Chem. 2004;279:45652. doi: 10.1074/jbc.M407840200. [DOI] [PubMed] [Google Scholar]
- 79.Dolwick KM, Swanson HI, Bradfield CA. Proc. Natl. Acad. Sci. USA. 1993;90:8566. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McLane KE, Whitlock JP., Jr. Receptor. 1994;4:209. [PubMed] [Google Scholar]
- 81.Ge NL, Elferink CJ. J. Biol. Chem. 1998;273:22708. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 82.Petroff BK, Roby KF, Gao X, Son D, Williams S, Johnson D, Rozman KK, Terranova PF. Toxicology. 2001;158:91. doi: 10.1016/s0300-483x(00)00367-x. [DOI] [PubMed] [Google Scholar]
- 83.Mutoh J, Taketoh J, Okamura K, Kagawa T, Ishida T, Ishii Y, Yamada H. Endocrinology. 2006;147:927. doi: 10.1210/en.2005-1125. [DOI] [PubMed] [Google Scholar]
- 84.Lo R, Celius T, Forgacs AL, Dere E, MacPherson L, Harper P, Zacharewski T, Matthews J. Toxicol. Appl. Pharmacol. 2011;257:38. doi: 10.1016/j.taap.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Nature. 2003;423:545. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 86.Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. Cancer Res. 2006;66:2224. doi: 10.1158/0008-5472.CAN-05-1619. [DOI] [PubMed] [Google Scholar]
- 87.Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S. Nature. 2007;446:562. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- 88.Heiden TC, Struble CA, Rise ML, Hessner MJ, Hutz RJ, Carvan MJ., 3rd Reprod. Toxicol. 2008;25:47. doi: 10.1016/j.reprotox.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Br. J. Pharmacol. 2007;150:552. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andre N, Xia T, Mädler L, Li N. Science. 2006;311:622. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 91.Homberger M, Simon U. Philos. Transact. A Math. Phys. Eng. Sci. 2010;368:1405. doi: 10.1098/rsta.2009.0275. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y, Yu F, Park YS, Wang J, Shin MC, Chung HS, Yang VC. Biomaterials. 2010;31:9086. doi: 10.1016/j.biomaterials.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghosh P, Han G, De M, Kim CK, Rotello VM. Adv. Drug Deliv. Rev. 2008;60:1307. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 94.Joshi HM, Bhumkar DR, Joshi K, Pokharkar V, Sastry M. Langmuir. 2006;22:300. doi: 10.1021/la051982u. [DOI] [PubMed] [Google Scholar]
- 95.Lee SK, Han MS, Asokan S, Tung CH. Small. 2011;7:364. doi: 10.1002/smll.201001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarit S, Rana S, Park M, Kim CK, You C, Rotello VM. Adv. Drug Deliv. Rev. 2010;62:316. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knelpp J, Knelpp H, McLaughlin M, Brown D, Knelpp K. Nano Lett. 2006;6:2225. doi: 10.1021/nl061517x. [DOI] [PubMed] [Google Scholar]
- 98.Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. Pharm. Res. 2007;24:1415. doi: 10.1007/s11095-007-9257-9. [DOI] [PubMed] [Google Scholar]
- 99.Antoniades C, Psarros C, Tousoulis D, Bakogiannis C, Shirodaria C, Stefanadis C. Curr. Drug Deliv. 2010;7:303. doi: 10.2174/156720110793360586. [DOI] [PubMed] [Google Scholar]
- 100.Stern ST, McNeil SE. Toxicol. Sci. 2008;101:4. doi: 10.1093/toxsci/kfm169. [DOI] [PubMed] [Google Scholar]
- 101.Stelzer R, Hutz RJ. J. Reprod. Dev. 2009;55:685. doi: 10.1262/jrd.20241. [DOI] [PubMed] [Google Scholar]
- 102.Larson JK, Carvan MJ, 3rd, Teeguarden JG, Watanabe G, Taya K, Krystofiak E, Hutz RJ. Nanotoxicology. 2014;8:856. doi: 10.3109/17435390.2013.837208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chuang SM, Lee YH, Liang RY, Roam GD, Zeng ZM, Tu HF, Wang SK, Chueh PJ. Biochim. Biophys. Acta. 2013;1830:4960. doi: 10.1016/j.bbagen.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 104.Li JJ, Hartono D, Ong C, Bay B, Yung LL. Biomaterials. 2010;31:5996. doi: 10.1016/j.biomaterials.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 105.Maiti S. J. Biomed. Nanotechnol. 2011;7:65. doi: 10.1166/jbn.2011.1203. [DOI] [PubMed] [Google Scholar]
- 106.De Jong WH, Borm PJA. Nanomed. 2008;3:133. [Google Scholar]
- 107.Jia HY, Liu Y, Zhang XJ, Han L, Du LB, Tian Q, Xu YC. J. Am. Chem. Soc. 2009;131:40. doi: 10.1021/ja808033w. [DOI] [PubMed] [Google Scholar]
- 108.Choudhury D, Xavier PL, Chaudhari K, John R, Dasgupta AK, Pradeep T, Chakrabarti G. Nanoscale. 2013;5:4476. doi: 10.1039/c3nr33891f. [DOI] [PubMed] [Google Scholar]
- 109.Nishanth RP, Jyostsna RG, Schlager JJ, Hussain SM, Reddanna P. Nanotoxicology. 2011;5:502. doi: 10.3109/17435390.2010.541604. [DOI] [PubMed] [Google Scholar]
- 110.Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Brandau W, Simon U, Jahnen-Dechent W. Small. 2009;18:2067. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 111.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Nano Lett. 2006;6:1794. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 112.Bayir H, Kagan VE. Crit. Care. 2008;12:206. doi: 10.1186/cc6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Reprod. Domest. Anim. 2012;47:344. doi: 10.1111/j.1439-0531.2011.01891.x. [DOI] [PubMed] [Google Scholar]
- 114.Gagner JE, Lopez MD, Dordick JS, Siegel RW. Biomaterials. 2011;32:7241. doi: 10.1016/j.biomaterials.2011.05.091. [DOI] [PubMed] [Google Scholar]