SUMMARY
Most recent investigations into cancer etiology have identified a key role played by epigenetics. Specifically, aberrant DNA and histone modifications which silence tumor suppressor genes or promote oncogenes have been demonstrated in multiple cancer models. While the role of epigenetics in several solid tumor cancers such as colorectal cancer are well established, there is emerging evidence that epigenetics also plays a critical role in breast and prostate cancer. In breast cancer, DNA methylation profiles have been linked to hormone receptor status and tumor progression. Similarly in prostate cancer, epigenetic patterns have been associated with androgen receptor status and response to therapy. The regulation of key receptor pathways and activities which affect clinical therapy treatment options by epigenetics renders this field high priority for elucidating mechanisms and potential targets. A new set of methylation arrays are now available to screen epigenetic changes and provide the cuttingedge tools needed to perform such investigations. The role of nutritional interventions affecting epigenetic changes particularly holds promise. Ultimately, determining the causes and outcomes from epigenetic changes will inform translational applications for utilization as biomarkers for risk and prognosis as well as candidates for therapy.
Keywords: Breast cancer, Prostate Cancer, CpG (cytosine-guanine), DNA methylation, epigenetic changes, disparities
(I) INTRODUCTION
The etiology of cancer was long held as an aberration of the genome. However, over the past decade, the regulation of the genome through epigenetic change has added to the complexity. Epigenetic modification has long been known to play a key role in normal developmental functions such as imprinting and X-chromosome inactivation [1]. Only recently has the role of aberrant epigenetic modifications been revealed to play an important role in neoplasia. Formally, epigenetic changes are defined as heritable cellular information which are not genetic and can be transmitted through cell division [2]. Two major epigenetic modifications discussed in this chapter include DNA level methylation in which CpG islands in the genome undergo covalent bonding with a methyl group resulting in control of gene expression and histone modification in which histone proteins undergo deacetylation/methylation resulting in regulation of chromosomal packing [2,3].
DNA level methylation changes can be categorized into two broad types of hypomethylation and hypermethylation which significantly affect gene expression [4]. Hypomethylation results when previously methylated genes in adult DNA undergo demethylation resulting in expression of a gene. Expression of genes normally repressed, such as oncogenes HRAS and others, can lead to aberrant cellular activity and subsequent tumorogenesis[5]. Conversely, hypermethylation affecting gene transcription occurs when CpG islands in regulatory or promoter sites of a gene undergo silencing by methylation[4]. A complex series of steps take place for methylation and gene silencing to occur including recruitment of several regulatory proteins and biochemical reactions ultimately resulting in alterations in the histone state and chromosomal folding[4,6,7]. Among the most studied examples of hypermethylation in relation to cancer is silencing of hMLH1 in colorectal cancer [8,9] and BRCA1 in breast cancer [9,10]. Both result in the incapacitation of key tumor suppressor genes – in this case, proteins involved in DNA maintenance and repair [8,10].
Histone modifications have increasingly gained importance in the context of epigenetics. Histone proteins can undergo acetylation, phosphorylation, and methylation which in turn regulate chromosomal stability and packing. Acetylation results in relaxing of the chromosomal packing, allowing for transcription factors to access and initiate transcription of genes [11]. Conversely, deacetylation by histone deacetylases (HDACS) and subsequent methylation of histone residues results in the tightening of the histones, reducing access of regulatory transcriptional proteins.
Several studies have identified that a complex relationship exists between DNA level epigenetic changes and histone level changes. There is data to suggest that DNA methylation changes may precipitate histone residue modifications and chromatin packing [9,11,12]. Data to support this conclusion came from studies demonstrating that histone demethylation through inhibition of HDACs was not sufficient to reverse methylation of DNA and result in gene expression [13]. Other studies conversely argue that histone mediated chromatic modification, not DNA methylation, is not the primary driver for epigenetic mediated gene silencing [14]. The supporting data for this hypothesis was derived from studies demonstrating DNA-methylation independent gene silencing through histone modification alone [15].
Additional studies are warranted to identify the exact mechanism which predominantly contribute to signaling. Most likely, a tissue-specific pattern with complexes composed of DNA methyltransferases (DNMTs), HDACS, and cofactors (methyl CpG binding proteins) will be ultimately responsible for gene silencing [11,13]. Hence, it is paramount to examine epigenetics in multiple organ systems. While significant strides have been made in the field for colorectal cancers, gliomas, and leukemias [9], there is still a need for further investigation in other high-incidence cancers such as breast and prostate cancer. To date, our group and others have identified that epigenetic modifications (both global and gene-specific) significantly contribute to tumorogenesis and progression in both breast and prostate cancers [16-20]. This chapter will report the findings from several studies on breast and prostate cancer as well as the methods and tools to conduct further investigation. Notably, the role of epigenetics in contributing to health disparities will be highlighted. Furthermore, the most recent findings from the promise of natural compounds will also be discussed. Lastly, future directions will explore potential translational directions in the field of epigenetics.
(II) EPIGENETIC CHANGES IN BREAST AND PROSTATE CANCERS
Breast Cancer
Breast cancer is the most frequently diagnosed cancer and the second leading cause of cancer death in women[21]. In addition to genetic alterations such mutations in oncogenes and tumor suppressor genes, epigenetic alternations such as promoter methylation and histone modification could also lead to initiation, promotion, and metastasis of breast cancer [9]. Epigenetics may further play a role in drug interventions and cancer therapy thereby meriting significant focus for research to improve breast cancer outcomes.
(1) Methylation of promoter CpG islands of genes in breast cancer
Hypermethylation of promoter CpG islands represents an alternative mechanism of gene inactivation and may occur early in breast cancer development. More than 100 genes have been reported to be hypermethylated in primary breast tumors or breast cancer cell lines [10,22,23]. Many of these aberrantly methylated genes play critical roles in tumor suppression, cell cycle regulation, apoptosis, angiogenesis, tissue invasion, and metastasis [9,22].
(i). CpG island hypermethylation and breast cancer progression
Promoter CpG islands hypermethylation have been associated with breast cancer progression. recent study assessed methylation levels of tumor suppressor genes, RARβ2 and RASSF1A, MINT17, and MINT13 during key steps of breast cancer development [24]. The study identified a significant increase in the expression levels of these genes during the breast cancer development [24]. Hypermethylation of promoter CpGs of RARβ2 and RASSF1A have been confirmed to play a role in breast cancer in other studies and can be considered early epigenetic events in breast cancer. Jovanovic and colleagues found RARβ2 and RASSF1A methylation within lesions from both in situ lobular (LCIS) and ductal carcinoma (DCIS) [18]. Another study screened 57 promoter CpG loci in 20 invasive ductal carcinomas (IDC) and their paired normal breast tissues. The study demonstrated that methylation of 15 genes (DLEC1, GRIN2B, HOXA1, MT1G, SFRP4, TMEFF2, APC, GSTP1, HOXA10, IGF2, RARβ, RASSF1A, RUNX3, HIN-1 and SFRP1) increased stepwise from normal to atypical ductal hyperplasia (ADH)/flat epithelial atypia (FEA) to ductal carcinoma (DCIS) [25].
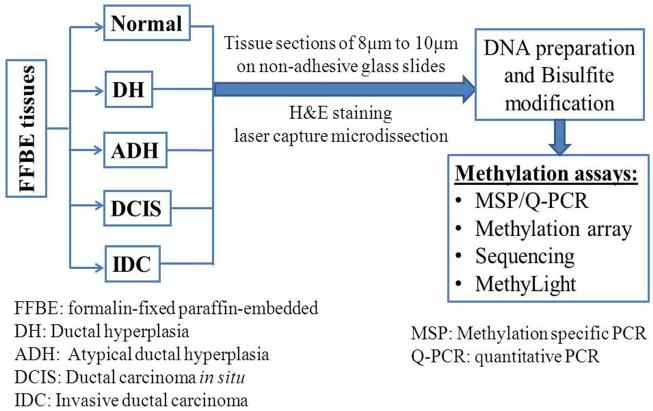
The association of promoter CpG gene hypermethylation and breast cancer progression can be assessed by comparing the methylation status in normal, pre-malignant breast lesions, and breast cancer tissues. DNA for these studies is best prepared by using formalin-fixed paraffin-embedded (FFPE) tissues with laser capture microdissection that ensures the type of tissues needed (normal, benign, tumor) is captured for use in DNA extraction and methylation assays. Figure 1 demonstrates an example schema of this process.
Figure 1.
Assessment of hypermethylation of CpG sites in gene promoters in breast cancer.
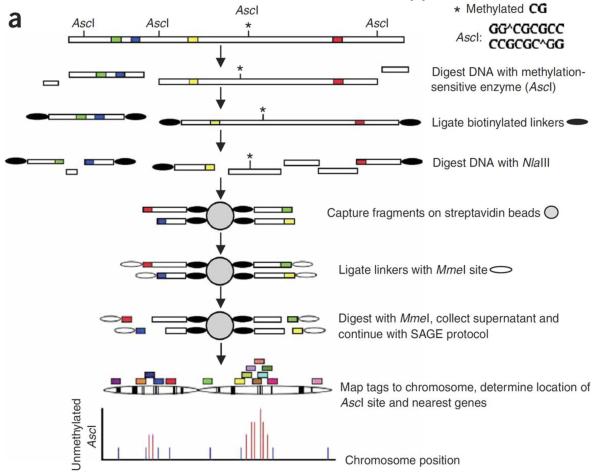
Aberrant DNA methylation has also been related to clinical and pathologic characteristics of breast cancer. Genome-wide DNA methylation analysis using methylation specific digital karyotyping (MSDK) of breast tumor tissues identified the presence of differentiation specific DNA methylation and gene expression patterns in breast carcinoma [18]. These epigenetic changes have subsequently been associated with tumor size, lymph node status, distant metastasis and hormone receptor status [18,23,26]. The MSDK is a comprehensive DNA methylation profiling technology and was developed by the K. Polyak lab at Dana Farber Cancer Institute and Harvard Medical School [27]. The MSDK method allows for comprehensive and unbiased methylation profiling. The principles and overview of this approach is provided in Figure 2.
Figure 2. Schematic of the MSDK Approach.
Reprinted by permission from Macmillan Publishers Ltd: [Nature Genetics] (37(8):899-905), copyright (2005).
(ii.) DNA methylation in hormone receptor positive and negative breast cancer
Different epigenetic profiles have also been identified between hormone receptor positive and negative tumors [23,28,29]. Although array-based methylation analysis can measure large numbers of genes simultaneously, there are some shortcomings. Array-based analysis is not able to provide quantitative measurement of CpG methylation and requires subsequent experiments to confirm and validate findings. Hence the use of new techniques, such as pyrosequencing is warranted. Pyrosequencing is a sequencing-by-synthesis method which can quantify DNA methylation at specific CpG sites within the target region of interest. Feng et al used pyrosequencing methylation analysis to identify two panels of methylation profiles which correlated with hormone receptor expression in breast cancer [28]. Specifically, the study examined 12 tumor suppressor genes (ARHI, RASSF1A, HIN-1, RARβ2, hMLH1, 14-3-3σ, RIZ1, p16, E-cadherin, RIL, CDH13 and NKD2) in 90 pairs of malignant/normal breast tissues. The data from this study showed that 5 (RIL, HIN-1, RASSF1A, CDH13 and RARβ2) out of 12 genes examined were frequently methylated in breast cancer tissues, but not in normal breast tissues. The methylation of HIN-1 and RASSF1A strongly correlated to the expression of ER and/or PR, while, the methylation of RIL and CDH13 strongly correlated to negative ER and/or PR. Subsequent studies have shown that the differences of methylation profiles between hormone receptor positive and negative breast tumors can also influence tumor response to hormonal therapy such as tamoxifen [29,30].
Approximately 70% of breast tumors are positive for ER expression at the time of diagnosis. Patients with ER+ breast cancer are candidates for tamoxifen treatment which competes with estradiol for binding to ER. Postmenopausal women with ER+ breast tumors are also candidates for treatment with aromatase inhibitors (AIs). AIs reduce the production of estrogen through inhibition of the enzyme aromatase that synthesizes estrogen from testosterone and androstenedione [31,32]. Despite the well document benefits of hormone therapy for ER+ breast cancer patients, not all patients with ER+ tumors respond to the therapy. As many as one third of ER+ breast cancer patients experience relapse within 5 years and develop drug resistant tumors [33] . Lack of ER expression and/or PR status have been identified as major causes of resistance. Interestingly, there are a number of studies showing that the 5′UTR of the ESR1 gene is methylated in ER-negative breast cancer cell lines [34,35]. The methylation of ER CpG islands was specifically confirmed in breast tumor tissues [36]. However, some recent studies [29] have shown no significant relationship between ESR1 expression levels and quantified ESR1 methylation levels when the tumors were analyzed collectively. A trend between ESR1 gene expression and ESR1 methylation levels in PR-negative tumors was, however, observed in their study [29].
Recently, Pathiraja et al reviewed several studies which utilized high-throughput DNA methylation profiling to identify DNA methylation of candidate genes that contribute to resistance [34]. Widschwendter and colleagues utilized MethyLight analysis to examine 148 tumors from patients who had received adjuvant tamoxifen therapy and found promoter methylation of ESR1 and CYP1B1 predicted response to tamoxifen treatment [29]. Another study by Martens and team examined 499 CpG sites from regulatory regions of 117 candidate genes from 200 ER and/or PR + tumors [37]. The bisulfite treated DNA was hybridized to an array of immobilized oligonucleotides reflecting the methylated (CG) and non-methylated (TG) status for each CpG position. The hybridization conditions allowed the detection of the single nucleotide differences. Of the genes analyzed, the team identified 10 genes with promoter DNA methylation status that correlated with clinical outcome and endocrine therapy resistance: PSAT1, STMN1, S100A2, SFN, PRKCD, SYK, VTN, GRIN2D, TGFBR2, and COX7A2L. Specifically, Martens et al identified that phosphoserine aminotransferase (PSAT1) was strongly correlated with poor outcome from all genes screened, and hypermethylation of the PSAT1 promoter was a good prognostic marker for outcome [37].
(iii.) DNA methylation and molecular subtypes of breast cancer
Molecular subtypes of breast cancer have also been associated with DNA methylation. Subtypes are determined by gene expression profiles and expression of ER/PR, HER2 (human epidermal growth factor receptor 2), cytokeratin 5/6(CK5/6), and/or HER1 (EGFR, human epidermal growth factor receptor 1) expression. Breast cancer can be classified as five main subtypes, Luminal A (ER/PR+/HER2−), Luminal B (ER/PR+/HER2+), HER2-enriched type (ER/PR−/HER2+), basal-like (ER/PR−/HER2−, CK5/6+ and/or HER1+)/triple negative (TNBC), and normal breast like or unclassified (breast cancers that do not fall into these four subtypes)[38,39] . Overall about 42-59% of breast cancers are Luminal A subtype, Luminal B subtypes are 6-10%, ER/PR−/HER2+ subtypes are 7-12% and TNBCs are 15-20% [40,41].
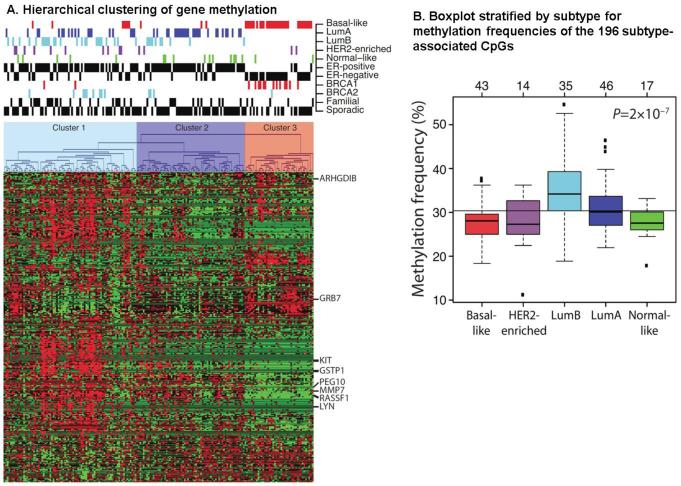
Similar to gene profiling patterns, epigenetic patterns have been identified that correlate with breast cancer subtypes in multiple studies [23,26,42,43]. Holms and colleagues used the methylation array (Illumina GoldenGate Methylation Cancer Panel I, from Illumina, San Diego, CA, USA) in their study of 1505 CpG loci corresponding to 807 cancer-related genes [42]. High methylation frequency was found among Luminal type tumors compared to basal-like tumors by array-based methylation assays. The analysis included a total of 189 fresh frozen primary breast tumors from different molecular subtypes of breast cancer and identified that subtype-specific genes were often regulated by methylation. Basal-like, Luminal A and Luminal B tumors had different methylation profiles as shown in Figure 3A. The frequency of methylation in Luminal B samples was significantly higher than in basal-like tumors (Figure 3B). Breast tumors with amplification of the HER2 gene are less likely to respond to therapy and more likely to develop metastatic disease [44]. Trastuzumab (Herceptin), an antibody targeting the HER2 receptor, has been shown to successfully treat HER2+ breast cancer and prolong the survival of patients with metastatic HER2-overexpressing breast cancer [45,46]. However, a significant portion of patients with HER2+ breast tumors will eventually become resistant to trastuzumab [47].
Figure 3. Methylation Clustering by Subtype.
Figure 3A. Hierarchical clustering of gene methylation. Heatmap shows relative methylations levels (red, more methylated; green, less methylated). Clustering resulted in three clusters: Cluster 1 (Luminal B), Cluster 2 (Luminal A), and Cluster 3 (Basal-Like). Adapted from [42].
Figure 3B. Boxplot stratified by subtype for methylation frequencies of the 196 subtype- associated CpGs. CpGs represented in the plot are more frequently methylated in Luminal B tumors and less methylated in basal-like tumors. P-value was calculated using analysis of variance. The number of tumors in each subtype is shown at the top axis. Adapted from [42].
Methylation patterns in relation to subtype have been most studied in the basal-like or Triple Negative Receptor Breast Cancer (TNBC) subtype. Branham et al. analyzed methylation of CpG islands in 69 cancer-related genes in TNBC tumors and compared the methylation profile with non-TNBC tumors [48]. The assay used for characterization of the methylation profile was Methyl-specific multiplex ligation-dependent probe amplification assay (MS-MLPA) obtained from MRC-Holland, Amsterdam, The Netherlands (www.mrc-holland.com). The MSMLPA assay extended restriction enzyme incubation time, separated ligation and restriction steps which reduced background signals. The PCR products were fluorescently labeled and separated by capillary electrophoresis (ABI-3130 sequencer, Applied Biosystems, Foster City, CA, USA). The resulting data was subsequently analyzed by GeneMarker v1.75 software (Softgenetics, State College, PA, USA) which normalized the data. The normalized peaks from the analyzed samples were compared with the normalized peaks from the control reaction. Through this method, a specific panel of genes including CDKN2B, CD44, MGMT, RB and p73 was found to be methylated in TNBC tumors, but not in non-TNBC tumors [48].
(iv.) Promoter hypermethylation of tumor suppressor genes in breast cancer
Epigenetic inactivation of the tumor suppressor genes, such as BRCA1, have been implicated as important events in sporadic breast cancer [9,10,49]. Early reports showed that one of the key mechanisms of BRCA1 expression loss was epigenetic silencing [10,50]. More recently, loss of expression of BRCA1 has been associated with basal-like/TNBC tumors [51-53]. Stefansson et al. examined BRCA1 methylation in 111 sporadic breast tumors which had previously been screened for BRCA1 germline mutations [53]. The study demonstrated that CpG island hypermethylation of BRCA1 was significantly associated with basal-like/TNBC tumors [53]. Another recent study by Hsu et al also examined methylation of BRCA1 promoters from 139 early stage breast cancer tissues using Methylation-specific PCR (MSP) and their data also demonstrated an association of BRCA1 promoter methylation and TNBC type of tumors [54].
Another key tumor repressor in breast cancer implicated in epigenetic mediated loss is PTEN [18,26,55-57] . The gene encodes PIP3 phosphatase and negatively regulates the PIP3-Akt pathway. Loss of PTEN can lead to activation of Akt pathway, suppression of apoptosis, and increased cell survival [58]. Expression of PTEN protein has been found to be lost or reduced in 38% of invasive breast cancer [55]. Promoter hypermethylation has been implicated as a key mechanism of loss of the PTEN gene in breast cancer. Khan et al examined PTEN promoter methylation from 44 invasive breast tumors using MSP. The study found that 34% tumors had hypermethylation of PTEN genes and the PTEN promoter hypermethylated tumors had loss of PTEN protein in 60% of samples [55]. Similarly, Garcia et al analyzed promoter hypermethlation of the PTEN gene in 90 invasive breast tumors and found that the PTEN promoter was hypermethylated in 48% of tumor tissues [56]. Subsequent studies have confirmed these findings and further associated PTEN hypermethylation with ERBB2 overexpression, large tumor size, and higher histologic grade [26].
(2) Methylation profile and breast cancer health disparities
The incidence and mortality of breast cancer can vary significantly among racial and ethnic groups [21]. Although the overall survival from breast cancer has improved, African-American women still show the worse outcome and increased mortality at virtually all age groups [21]. In addition to socioeconomic factors, biological factors and lifestyles may contribute to breast cancer health disparity. Studies have suggested that differences in the frequency of tumor receptor-subtypes among African-American women may potentially play a role in the reduced cancer outcomes [59] . Specifically, younger African-American women are more often diagnosed with the TNBC subtype compared to European-American/Caucasian women [40,59-62]. Notably, women with TNBC have limited treatment options since targeted receptor therapies such as tamoxifen for HR+ tumors, and trastuzumab for HER2+ tumors would not be recommended [63].
In addition to clinicopathological factors, varying epigenetic patterns may also contribute to breast cancer disparities. For example, Mehrotra and colleagues examined the frequency of promoter hypermethylation in five key genes implicated in breast cancer (HIN-1, Twist, Cyclin D2, RAR-β, and RASSF1A) from African-American and Caucasian patient tumors. Among the cohort of women with ER/PR- tumors and < 50 years old, the study identified a higher methylation frequency of HIN-1, Twist, Cyclin D2 and RASSF1A in African-American women compared with Caucasians [64]. Another study conducted by Dumitrescu et al investigated tumor suppressor gene promoter hypermethylation in breast tissue from healthy African-American and Caucasian women. The study found p16INK4 promoter hypermethylation was more frequently observed among Caucasian women with family history and BRCA1 promoter hypermethylation was more frequently observed among African American women with family history [65]. Hence, there is data to suggest that differences in the frequency of gene promoter methylation may influence the disease outcome of breast cancer among African-American and Caucasian women. Studies investigating the role of epigenetics on other populations including the Hispanic/Latino, Asian, and Native American/Pacific Islander women are needed to better understand the implications of epigenetics on disparities.
(3) DNA methylation and histone modification in breast cancer
DNA (5-cytosine)-methyltransferases (DNMTs) are enzymes that methylate the cytosine residue of CpGs. Four major types of DNMTs have been identified (DNMT1, 2, 3a and 3b), among which DNMT1, DNMT3a and DNMT3b are primarily active [66]. The demethylating drugs 5-azacytidine (Vidaza™) and 5-aza-2′-deoxycytidine (decitabine) are the most studied DNMT inhibitors [9]. These compounds incorporate into the DNA replacing cytosine during DNA replication and result in covalent trapping of DNMTs. The trapped DNMTs cannot continue methylation, hence, the cell is loses DNMT activity resulting in DNA demethylation [67]. Although DNA methylation inhibition alone can reduce gene silencing, abnormal histone modification in combination with DNA hypermethylation can frequently compound gene silencing and complicate reversal of expression. Figure 4 shows a schematic of epigenetic modification that regulates chromatin organization and gene expression. The interrelated activity of DNA level and histone level epigenetic modification suggests effective therapies to reverse silencing must apply dual targeting of both the DNA and histone level modifications [2]. The use of a 5-aza-2′-deoxycytidine and HDAC inhibitors (such as trichostatin A) separately have been evaluated clinically in multiple cancer systems, with greatest focus on leukemias and myelodysplastic syndromes [68,69]. Further investigation is warranted in solid tumor systems such as breast, prostate and colorectal cancer. In breast cancer, there is substantial preclinical data to suggest combined therapy may be efficacious. For example, a study by Wu et al investigated Caspase 8 (an apoptosis related gene) which is methylated in human breast cancer cell lines, MDA-MB231 (TNBC line), SKBR3 (HER2+), BT474 (HER2-positive), and MCF-7 (ER-positive) [16]. Treatment with 5-aza-2′-deoxycytidine inhibited DNMT3a and DNMT3b and reactivated CASP8 gene in MDA-MB231, SKBR3 and BT474, but not in MCF-7 cells. Chromatin Immunoprecipitation (ChIP) assay identified that the silencing of the CASP8 gene in MCF7 involved methylation of histone H3 (Lys27). Only through combined treatment with 5-aza-2′-deoxycytidine and the HDAC inhibitor, trichostatin A, was the gene silencing reversed and Caspase 8 expression significantly restored [16]. This approach can potentially be utilized to restore other silenced genes implicated in breast cancer tumorogenesis and resistance to disease. An excellent target would be the ESR1 gene which can be demethylated to restore expression of ER and subsequent tumor response to targeted therapy such as tamoxifen. Indeed, Fan and colleagues have confirmed that combined treatment does restore ER expression in ER- breast cancer cell lines and restores response to ER targeted therapy [70]. Additional studies to confirm these findings in primary TNBC cells lines and additional preclinical models is warranted particularly with the new sets of histone methyltransferase and demethylase inhibitors available for testing (see Table 1).
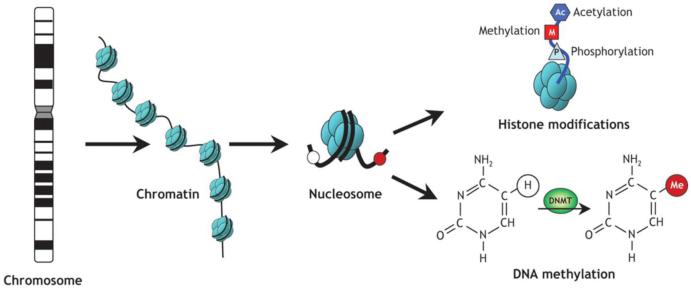
Figure 4. Schematic of epigenetic modifications that regulate chromatin organization and gene expression.
Strands of DNA are wrapped around (A) Schematic of epigenetic modifications. Strands of DNA are wrapped around histone octamers, forming nucleosomes, which organize chromatin. Reversible and site-specific histone modifications occur at multiple sites through acetylation, methylation and phosphorylation. DNA methylation occurs at 5-position of cytosine residues in a reaction catalyzed by DNA methyltransferases (DNMTs). Together, these modifications provide a unique epigenetic signature that regulates chromatin organization and gene expression. Adapted from Luong P., Basic Principles of Genetics [Connexions Web site]. March 2, 2014. Available at: http://cnx.org/content/m26565/1.1/.
Table 1.
Examples of Histone Methyltransferases and Demethylase inhibitors.
| Inhibitor Name | Major Targeted Enzyme | Histone Targets |
|---|---|---|
| Chaetocin1 | Suv39h1, G9a | H3K9 |
| Clorgyline | LSD1 | H3K4 |
| DZNep | EZh1 | H3K27 |
| N-oxalylglycine | JMJD2A, JMJD2C | H3K9, H3K36 |
| Novobiocin | SMYD3 | H3K4 |
| Pargyline | LSD1 | H3K4 |
| Polyamine analogs | LSD1 | H3K4 |
| Transcylpromine | LSD1 | H3K4 |
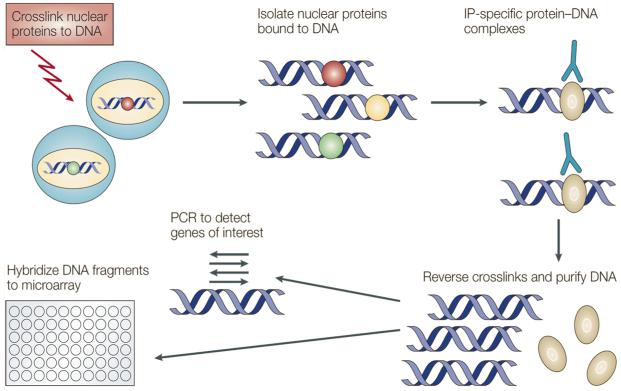
Further studies investigating the efficacy of these compounds could benefit from the use of the ChiP assay. The ChiP assay is a powerful tool for determining histone modification status. Figure 5 shows the assay principles and major steps. When performing the ChIP assay, cells are first reversibly fixed (such as with formaldehyde) which serves to crosslink or “preserve” the protein-DNA interactions occurring in the cell nucleus. Cells are then lysed and chromatin is harvested and fragmented using either sonication or enzymatic digestion. The chromatin is then subjected to immunoprecipitation using antibodies specific to a particular protein or histone modification. Any DNA sequences that are associated with the protein or histone modification of interest will co-precipitate as part of the cross-linked chromatin complex and the relative amount of that DNA sequence will be enriched by the immunoselection process. After immunoprecipitation, the protein-DNA cross-links are reversed and the DNA is purified. The enrichment of a particular DNA sequence or sequences can then be detected by a number of different methods, such as PCR, q-PCR, immunoblotting, or assessing on DNA hybridized array.
Figure 5. Principles of Chromatin Immunoprecipitation assay (ChiP).
Reprinted by permission from Macmillan Publishers Ltd: [Nature Reviews Cancer] (Jul;4(7):562-8), copyright (2004).
Summary
Aberrant epigenetic modifications in breast cancer impact a multitude of cell functions including cell cycle regulation, DNA repair, hormone regulation (ERα, PR), cell adhesion, invasion, angiogenesis, and cellular growth-inhibitory signaling related genes. A summary of genes with CpG hypermethylation in breast cancer and methods used for identification is summarized in Table 2. Although breast tumors are also frequently hypomethylated on genome-wide scale, the number of genes reported as hypomethylated in breast cancer is relative small. When assessing epigenetic changes, both DNA and histone level modifications must be examined, particularly if modified genes are therapeutic targets.
Table 2.
Summary of Genes Hypermethylated in Breast Cancer
| Gene | Official Gene Name | Methods of Identification |
|---|---|---|
|
| ||
| 14–3–3sigma | Stratifin (SFN) | MSP+ Bisulfite sequencing |
| ABCB1 | ATP-binding cassette, sub-family B, member 1 | MethyLight |
| AK5 | AK5 adenylate kinase 5 | MSP+ Bisulfite sequencing |
| AMN | Amnion associated transmembrane protein | MSP+ Bisulfite sequencing |
| APC | Adenomatous Polyposis Coli | MSP |
| BCL2 | B-cell CLL/lymphoma 2 | MethyLight |
| BRCA1 | Breast Cancer 1, early onset | MSP+ Bisulfite sequencing |
| CALCA | Calcitonon-related polypeptide alpha | MethyLight |
| CASP8 | Caspase-8, Apoptosis-related cysteine | MSP |
| CCND2 | Cyclin D2 | MSP |
| CDCP1 | CUB domain containing protein 1 | Bisulfite sequencing |
| CDH1 | Cadherin 1, E-cadherin | MSP+ Bisulfite sequencing |
| CDH13 | Cadherin 13, H-cadherin | MSP |
| CDKN1C | P57 | MSP |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A (p16, p14ARF) | MethyLight |
| CEBPD | CCAAT/enhancer binding protein | MSP |
| CLCA2 | Chloride channel, calcium activated, family member 2 | Bisulfite sequencing |
| CST6 | Cystatin E/M | MSP+ Bisulfite sequencing |
| Cx26 | Connexin 26 | MSP |
| CYP1B1 | Cytochrome P450, family 1, subfamily B, polypeptide 1 | MethyLight |
| DAB2 | Disabled homolog 2, mitogen-responsive phosphoprotein | Bisulfite sequencing |
| DAL1 | Erythrocyte membrane protein band 4.1-like 3 | MSP |
| DAPK | Death Associated Protein Kinase 1 | MethyLight |
| DCC | Deleted in colorectal carcinoma | MSP+ Bisulfite sequencing |
| DLC1 | Deleted in liver cancer 1 | MSP+ Bisulfite sequencing |
| DSC3 | Desmocollin 3 | Bisulfite sequencing |
| ESR1 | Estrogen Receptor 1 | MethyLight |
| ESR2 | Estrogen Receptor 2 | MethyLight |
| FHIT | Fragile histidine triad gene | MSP |
| FOXA2 | Forkhead box A2 | MSP+ Bisulfite sequencing |
| GPC3 | Glypican 3 | MSR + Southen Blot |
| GREM1 | Gremlin 1 | MSP |
| GSTP1 | Glutathione S-transferase pi | MethyLight; MSP |
| HIC-1 | Hypermethylated in cancer 1 | MSR* + Southen Blot |
| HOXA5 | Homeobox A5 | MSP+ Bisulfite sequencing |
| HOXD11 | Homeobox D11 | MSP+ Bisulfite sequencing |
| HRAS | Harvey rat sarcoma viral oncogene homolog | MethyLight |
| HS3ST2 | Heparan sulfate 3-O-sulfotransferase 2 | MSP |
| HSD17B4 | Hydroxysteroid (17-beta) dehydrogenase 4 | MethyLight |
| hTERT | Telomerase reverse transcriptase | MethyLight; MSP |
| ID4 | Inhibitor of DNA binding 4 | MSP |
| IGFBP3 | Insulin-like growth factor binding protein 3 | MSP |
| KLK10 | Kallikrein-related peptidase 10 (NES1) | MSP+ Bisulfite sequencing |
| KLK6 | Kallikrein-related peptidase 6 | Bisulfite sequencing |
| LAMA3 | Laminin, alpha 3 | MSP |
| LAMB3 | Laminin, beta 3 | MSP |
| LAMC2 | Laminin, gamma 2 | MSP |
| LATS1/LATS2 | Large tumor suppressor, homolog 1/2 | MSP |
| NKD2 | Naked cuticle homolog 2 | Pyrosequencing |
| SERPINB5 | Maspin, mammary serine protease inhibitor | MSP |
| MGMT | O-6-methylguanine-DNA methyltransferase | MethyLight |
| MLH1 | MutL homolog 1, colon cancer, nonpolyposis type 2 | MethyLight |
| MYOD1 | Myogenic differentiation | MethyLight |
| PAX5 | Paired box 5 | MSP+ Bisulfite sequencing |
| PCDH10 | Protocadherin 10 | MSP+ Bisulfite sequencing |
| PGR | Progesterone receptor | MSR + Southen Blot |
| PLAGL1 | Pleiomorphic adenoma gene-like 1 | MSP |
| PTEN | Phosphatase and tensin homolog | MSP |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (Cox-2) | MethyLight |
| RAD9 | RAD9 homolog A | Bisulfite sequencing |
| RAR-β | Retinoic acid receptor, beta | MSP+ Bisulfite sequencing |
| RARRES1 | Retinoic acid receptor responder 1 | MSP |
| RASSF1A | Ras associated domain family 1 | MSP |
| RBP1 | Retinol binding protein 1 | MSP |
| RIZ1 | PR domain containing 2, with ZNF domain (PRDM2) | MSP |
| RNR1 | Mitochondrially encoded 12S RNA | MSP+ Bisulfite sequencing |
| ROBO1 | Roundabout, axon guidance receptor, homolog 1 | Bisulfite sequencing |
| RUNX3 | Runt-related transcription factor 3 | MSP+ Bisulfite sequencing |
| SCGB3A1 | Secretoglogin, family 3A, member 1 (HIN-1) | MSP |
| SERPINB5 | Serpin peptidase inhibitor, clade B, member 5 | Bisulfite sequencing |
| SFRP1 | Secreted frizzled-related protein 1 | MSP+ Bisulfite sequencing |
| SIM1 | Single-minded homolog 1 | MSP+ Bisulfite sequencing |
| SLIT2 | Slit homolog 2 | MSP |
| SOCS1 | Suppressor of cytokine signalling 1 | MSP+ Bisulfite sequencing |
| SRBC | Protein kinase C, delta binding protein (PRKCDBP) | Bisulfite sequencing |
| SULT1A1 | Sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1 | Bisulfite sequencing |
| SYK | Spleen tyrosine kinase | MSP |
| TERT | TERT telomerase reverse transcriptase | MethyLight |
| TDH | L-threonine dehydrogenase | MSP+ Bisulfite sequencing |
| TFF1 | Trefoil factor 1 | MSP+ Bisulfite sequencing |
| TGF-β R2 | Transforming growth factor β receptor 2 | MSP+ Bisulfite sequencing |
| THBS1 | Thrombospondin 1 | MSP+ Bisulfite sequencing |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | MSP+ Bisulfite sequencing |
| TMEFF2 | Transmembrane with EGF-like and two follistatin-like domains 2 | MSP |
| TMS1 | PYD and CARD domain containing (PYCARD) | MSR + Southern Blot |
| TNFRSF12 | Tumour necrosis factor receptor superfamily, member 25 | MSP+ Bisulfite sequencing |
| TPM1 | Tropomyosin 1 | Bisulfite sequencing |
| TSC1 | Tuberous sclerosis 1 | MSP |
| TSC2 | Tuberous sclerosis 2 | MSP |
| TSLC1 | tumor suppressor in lung cancer 1 | MSP |
| TSPAN-2 | Tetraspanin 2 | MSP+ Bisulfite sequencing |
| TWIST1 | Twist homolog 1 | MSP |
| TYMS | Thymidylate synthetase | MSP+ Bisulfite sequencing |
| WIF1 | WNT inhibitory factor 1 | MSP+ Bisulfite sequencing |
| WRN | Werner syndrome | MSP |
| XT3 | Solute carrier family 6, member 20 (SLC6A20) | MSP+ Bisulfite sequencing |
Prostate cancer
(1.) DNA methylation in prostate cancer
Prostate cancer is the most frequently diagnosed cancer and second leading cause of cancer death in men in United States and Western countries [21]. Prostate cancer is one of the most common human malignancies that arises through genetic and epigenetic alterations. Overall there are numerous genes that undergo aberrant hypermethylation in prostate cancer. These genes include classic and putative tumor-suppressor genes involved in a number of cellular pathways such as hormonal responses, tumor-cell invasion and/or tumor architecture, cell cycle control, and DNA damage repair [71-73] . For many of these genes, promoter hypermethylation is often the main mechanism underlying their functional loss. Silencing of these genes can contribute to cancer initiation, progression, invasion, and metastasis. Hypermethylation of genes in prostate cancer has been correlated in several studies with pathologic grade, clinical stage, androgen independence, as well as outcome [74,75]. A better understanding of the epigenetic changes in prostate cancer is likely to contribute to improved diagnosis, clinical management, and better outcome.
(i.) Hypermethylation of CpG islands of genes for risk prediction of prostate cancer
Aberrant DNA methylation patterns may be the earliest somatic genome changes in prostate cancer. Studies have suggested methylation of CpG sites of gene promoters may predict risk of prostate cancer [72]. Studies assessing predictive methylation patterns compared methylation status between cancer tissues and non-cancer tissues. One study by Yegnasubramanian et al using real-time methylation-specific PCR (Q-MSP) assessed CpG island hypermethylation of 16 genes from seven prostate cancer cell lines (LNCaP, PC-3, DU-145, LAPC-4, CWR22Rv1, VCaP, and C42B), normal prostate epithelial cells, normal prostate tumor cells, 73 primary prostate cancers, 91 metastatic prostate cancers, and 25 noncancerous prostate tissues [76]. The study identified that CpG islands at GSTP1, APC, RASSF1a, PTGS2, and MDR1 were hypermethylated in more than 85% of prostate cancers and cancer cell lines. CpG islands at EDNRB, ESR1, CDKN2a, and hMLH1 exhibited low to moderate rates of hypermethylation in prostate cancer tissues and cancer cell lines but were entirely unmethylated in normal tissues. Furthermore, hypermethylation of the CpG island in EDNRB was correlated with the grade and stage of primary prostate cancers and PTGS2 CpG island hypermethylation portended an increased risk of recurrence [76].
Another study conducted by Vanaja et al investigated use of CpG sites as molecular markers to distinguish indolent and aggressive prostate tumors [77]. The study examined the methylation status of 8 genes, including FLNC, EFS, ECRG4, RARB2, PITX2, GSTP1, PDLIM4, and KCNMA1 in 32 non-recurrent, 32 recurrent primary prostate tumors, and 32 benign prostate tissues using EpiTYPER™ technology (Sequenom, Inc, San Diego, CA). CpG site hypermethylation of FLNC, EFS, ECRG4, PITX2, PDLIM4, and KCNMA1 genes were found to predict local and systemic recurrence of prostate cancer. Specific CpG site hypermethylation of RARβ2 and GSTP1 CpG sites were found to be useful for diagnosis of prostate cancer [77].
Additional studies conducted by Mahapatra and colleagues used microarray analysis to compare promoter global hypermethylation profiles from prostate cancer vs. normal tissues. They identified 25 genes which were significantly methylated in prostate cancer tissues compared with normal tissues : AOX1, CYBA, EDG3, ELF4, EPB41L3, FLJ12056, FLJ90650, FLT4, GAS6, GRASP, GSTP1, HAAO, HIF3A, HOXC11, LEP, MGC39606, MOBKL2B, RAB34, RARβ2, RHCG, RND2, SLC34A2, SPATA6, TPM4 and ZNF154. The results were confirmed by pyrosequencing. The distinct profiles identified by this study could be used as biomarkers to identify subjects who may be at high risk to develop aggressive prostate cancer [78].
(ii.) Methylation profile in localized and advanced prostate cancer
Mahapatra and colleagues also investigated the role of epigenetics on progression of prostate cancer. Specifically, the study utilized microarray analysis with DNA samples from prostate cancer and normal adjacent tissues from 238 patients. DNA methylation analysis was conducted using the Illumina™ Infinium™ Human Methylation 27 Bead Chip. The bead chip allowed the interrogation of 27,578 CpG sites representing 14,495 protein-coding gene promoters, which includes about 1,000 cancer-associated genes. Four distinct methylation profiles emerged for: (i) normal vs. tumors, (ii) recurrence vs. non-recurrence, (iii) clinical recurrence vs. biochemical recurrence, and (iv) systemic recurrence vs. local recurrence. The normal vs. tumors profile was described earlier above. Notably, a 25 gene profile was identified which was associated with recurrent prostate cancer including the following genes: ACTL6B, AEBP1, AMID, CD8A, CRIP1, FLJ30934, FLNC, FMOD, FOXE3, GAS7, GDPD5, HS3ST2, LOC349136, NEUROG1, PLTP, PTGER2, RASGRF2, RUNX3, SIX6, SLC9A3, SPSB4, SRD5A2, SUSD3, SYT10 and TMEM74. Validation of genes by pyrosequencing from group 1 (GSTP1, HIF3A, HAAO, and RARβ2), group 2 (CRIP1, FLNC, RASGRF2, RUNX3, and HS3ST2), group 3 (PHLDA3, RASGRF2, and TNFRSF10D), and group 4 (BCL11B, POU3F3, and RASGRF2) confirmed the microarray results.
The study results revealed an expanded list of genes as which can be used both for biomarkers for diagnosis and prognosis of prostate cancer. Interestingly, alterations in methylation that were identified in early stage tumors were very homogeneous whereas methylation patterns in tumors from recurrent patients were more heterogeneous. This can be explained by the increasing number of epigenetic aberrations that accumulate during tumor progression, and tumor cells acquire increasingly unique epigenetic changes. Therefore, no single gene can likely predict the progression of all advanced prostate tumors. The altered methylation of genes associated with cancer recurrence should be combined with histopathologic workup for a more robust prediction of prostate cancer progression.
In a similar study, Lin and colleagues examined 20 benign prostate tissues, 16 prostate cancers and 8 advanced prostate cancers (both metastatic and castration-resistant) using MassARRAY™ EpiTYPER™ assay (Sequenom, San Diego, CA). Matrix-assisted laser desorption ionization/time-of light mass spectrometry was utilized. Their data suggested that most DNA methylation changes occurred in the context of allele-specific methylation and the variations in tumor epigenetic landscape of individuals may be partly mediated by genetic differences that may affect prostate cancer disease progression [79].
(iii.) Methylation patterns between androgen receptor positive and androgen receptor negative prostate cancer
A study from the Vadgama lab characterized the promoter methylation profile of 82 genes in three prostate cancer cell lines (LNCaP, PC3, and DU145) and two normal prostate cell lines (RWPE1 and RWPE2) using TranSignal Promoter Methylation Array (Panomics Inc., CA, USA) [17]. The data showed that >50% of the genes were hypermethylated in prostate cancer cells compared with 13% in normal cell lines. Among hypermethylated genes were genes for tumor suppressors (RB, TMS1, DAPK, RBL1, PAX6, and FHIT), cell cycle (p27KIP1 and CDKN2A), transporters (MDR1, MLC1, and IGRP), and transcription factors (STAT1, CIITA, MYOD, and NPAT) genes. Relative methylation patterns show that most of these genes were methylated from 5-fold to >10-fold compared with normal prostate cells. In addition, the study identified promoter methylation for the first time in genes such as RIOK3, STAT5, CASP8, SRBC, GAGE1, and NPAT. Furthermore, a significant difference in methylation pattern was observed between AR-sensitive versus AR-negative cancer cells for the following genes: CASP8, GPC3, CD14, MGMT, IGRP, MDR1, CDKN2A, GATA3, and IFN. Identification of differences in methylation pattern between androgen-sensitive and androgen-independent prostate cells provided further insight into potential target genes relevant in the diagnosis, prognosis, and treatment of prostate cancers.
The use of the TranSignal Promoter Methylation Array from Panomics was key for screening multiple gene promoters. The TranSignal Array consists of immobilized probes of known gene promoters on a nitrocellulose membrane. Methylation binding protein-purified methylated DNA was hybridized on the membrane and detected by the chemiluminescence method.
(iv.) Histone modification and androgen receptor activity
Chromatin remodeling and histone post-translational modifications also play an important role in deregulation of gene expression in prostate cancer [80]. Figure 6 shows an example of histone acetylation at specific lysine (K) residues and the effect of chromatin modification on gene transcription [81]. Korkmaz and colleagues investigated the role of histone modification in relation to androgen receptor status [82]. They examined whether histone acetylation can influence AR transcriptional activity by using histone deacetylase (HDAC) inhibitors (HDACIs) trichostatin A (TSA), sodium butyrate (Na-But) and depsipeptide (FR901228). The study found that inhibition of HDAC activity significantly increased endogenous AR activity, as well as the prostate-specific antigen (PSA) in LNCaP cells. An increase in histone acetylation of target genes, such as CREB-binding protein may be essential for mediating AR transcriptional activity in prostate cancer cells. Histone acetylation seems to be intimately involved in AR activity regulation [82].
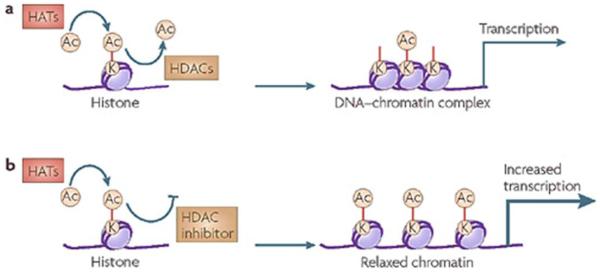
Figure 6. Example of Histone Acetylation.
a. Levels of histone acetylation at specific lysine (K) residues are determined by concurrent reactions of acetylation (AC) and deacetylation, which are mediated by histone acetylases (HATs) and histone deacetylases (HDACs). This histone acetylation is vital for establishing the conformational structure of DNA-chromatin complexes, and subsequently transcriptional gene expression. b. By blocking the deacetylation reaction, HDAC inhibitors change the equilibrium of histone acetylation levels, leading to increased acetylation, chromatin modification to relax confirmation and transcription upregulation. Reprinted by permission from Macmillan Publishers Ltd: [Nat Rev Drug Discov] (Oct;7(10):854-68), copyright (2008).
(2) Methylation profile and health disparities in prostate cancer
(i.) Methylation profiles differ among African American, Caucasian and Asian men with prostate cancer
The incidence and mortality of prostate cancer is 2-fold higher in African-American than that in Caucasian men in the US [21]. A complex combination of environment, socioeconomic and genetic variations may all contribute to the disparities of incidence and mortality in prostate cancer. Aberrant hypermethylation in regulatory genes in prostate tissues may precede and predispose to developed malignancy [73]. Differences in the distribution of aberrant methylation may contribute to differential cancer health disparities among varying cohorts. An early study from Woodson and colleagues investigated differences in DNA hypermethylation of GSTP1, CD44 and E-cadherin in archival tumor tissues from African-American (n=47) and Caucasian (n=64) men using real-time methylation –sensitive PCR [83]. The study identified GSTP1 hypermethylation in 84% of prostate cancer tissues, but no difference was identified between African American and Caucasian men. E-cadherin was not methylated in any of the tumors. The data did, however, show that the frequency of CD44 methylation was higher in African-American men compared with Caucasian even though the CD44 methylation was less prevalent overall (in 32% of tumors) [83].
A study by Enokida et al used a similar approach with methylation-specific PCR to analyze 291 prostate cancer (African American =44, Caucasian=77 and Asian=170) and 172 benign prostate hypertrophy samples (African American=38, Caucasian=38 and Asian=96) [84]. CpG methylation of GSTP1 was found in 65.6% of prostate cancer tissues, and 24.5% of benign prostate hypertrophy samples. The frequency of GSTP1 methylation was significantly higher in prostate cancer in each ethnic group. Compared to Caucasian and Asian men, African-American men had a higher hazard ratio of GSTP1 hypermethylation based on logistic regression. The methylation of GSTP1 was significantly associated with higher Gleason score in Asian men, but not in African-American men - possibly due to small sample size of African-Americans in their study [84].
Recently another study by Kwabi-Addo and colleagues used pyrosequencing to quantitatively measure the methylation status of GSTP1, AR, RARβ2, SPARC, TIMP3 and NKX2-5 in prostate cancer and matched normal tissues from 39 African American and 67 Caucasian men [85]. Their data showed that overall there was significant methylation in prostate cancer tissues from African-American compared to Caucasian men. In agreement with Enokida et al., Kwabi-Addo and colleagues found that the higher frequency of methylation in African-American samples was not correlated to disease aggressiveness since Gleason score was highest in tissues from Caucasian men. In addition, data from Kwabi-Addo’s study demonstrated higher methylation of NKX2-5 and TIMP3 in normal prostate tissues from African-American compared to Caucasian men. Hence, analysis of gene methylation may increase sensitivity for detecting prostate cancer tumor activity in different ethnic groups [85].
(3) Summary-Gene frequently methylated in prostate cancer
Promoter hypermethylation is a significant mechanism of gene silencing. To date more than 50 genes with common aberrant hypermethylation have been identified in prostate cancer (Table 3). These genes encompass many cellular functions including cell cycle control, apoptosis, hormone response, DNA repair and damage prevention, signal transduction, tumor invasion and tumor suppression. Frequent promoter methylation of genes such as APC, CCND2, GSTP1, RARβ2, RASSF1A and PTGS2, were found. The frequency of RARβ2 methylation has been reported in 60%-95% of tissues and the frequency of MDR1 methylation has been reported at 51%-100% in tissues. Methods for the identification of epigenetic changes implicated in prostate cancer included MSP, quantitative-MSP (QMSP), MethyLight PCR, Combined Bisulfite Restriction Analysis (COBRA), and Combination of methylated DNA precipitation and restriction enzyme digestion (COMPARE) assay. The MSP and MethyLight analysis have been introduced previously. The principles of COBRA and COMPARE analysis should also be noted for future utility.
Table 3.
Summary of Genes Hypermethylated in Prostate Cancer
| Pathway | Gene | Official gene name | Methods of identification |
|---|---|---|---|
|
| |||
| Hormonal response |
AR | Androgen receptor | MSP |
| ESR1 | Estrogen receptor 1 | MSP | |
| ESR2 | Estrogen receptor 2 | MSP, Bisulfite sequencing | |
| RARβ2 | Retinoic acid receptor β2 | MSP, MethyLight PCR | |
| RARRES1 | Retinoic acid receptor responder 1 | MSP,QMSP | |
|
| |||
| Cell cycle control | CCND2 | Cyclin D2 | MSP, QMSP |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A (p16) | MSP, QMSP | |
| RPRM | Reprimo | QMSP | |
| SFN | Stratifin (14-3-3 sigma) | QMSP | |
| CDC2 | Cell division cycle 2 | TranSignal array | |
| CDKN1B | Cyclin-dependent kinase inhibitor 1B (p27kip) | TranSignal array | |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21) | TranSignal array | |
|
| |||
| Signal transduction |
DKK3 | Dickkopf 3 | MSP+Bisulfite sequencing |
| EDNRB | Endothelin receptor type B | MSP, QMSP, Southern blot, MethyLight | |
| RASSF1A | Ras association domain family protein 1 isoform A | MSP, QMSP | |
| RUNX3 | Runt-related transcription factor 3 | MSP | |
| SFRP1 | Secreted frizzled-related protein 1 | MSP+ Bisulfite sequencing | |
|
| |||
| Tumor invasion | APC | Familial adenomatous polyposis | MSP, Bisulfite sequencing, MethyLight |
| CAV1 | Caveolin 1 | MSP, Bisulfite sequencing | |
| CHD1 | E-cadherin | MSP, COBRA | |
| CHD13 | Cadherin 13 | MSP, QMSP | |
| CD44 | Cluster differentiation antigen 44 | MSP | |
| LAMA3 | α-3 laminin | MSP | |
| LAM C2 | γ-3 laminin | MSP, QMSP, TranSignal array | |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | MSP | |
|
| |||
| Tumor suppressor |
RB | Retinoblastoma | TranSignal array |
| TMS1 | Target of methylation-induced silencing 1 | TranSignal array | |
| DAPK | Death-associated protein kinase | TranSignal array | |
| RBL1 | Retinoblastoma-like 1 | TranSignal array | |
| PAX6 | Paired box gene 6 | TranSignal array | |
| FHIT | fragile histidine triad | TranSignal array | |
|
| |||
| DNA damage repair |
GSTM1 | Glutathione S-transferase M1 | MSP+ Bisulfite sequencing |
| GSTP1 | Glutathione S-transferase P1 | MSP, QMSP, MethyLight Bisulfite | |
| GPX3 | Glutathione peroxidase 3 | sequencing, | |
| MGMT | O-6-methylguanine DNA methyltransferase | MSP, QMSP | |
| ASC | Apoptosis-associated Speck-like protein containing a CARD |
MSP, QMSP | |
|
| |||
| Apoptosis | BCL2 | B cell lymphoma 2 | MSP, COBRA, QMSP |
| DAPK | Death-associated kninase | MSP, QMSP | |
| CASP8 | Caspase 8, Apoptosis-Related Cysteine | TranSignal array, MSP | |
| CD14 | monocyte differentiation antigen CD14 | TranSignal array | |
| MDR1 | Multidrug resistance receptor 1 | QMSP, MSP+COBRA, COMPARE, TranSignal array |
|
| MSP, MethyLight | |||
| MSP | |||
|
| |||
| Others | PTGS2 | Prostaglandin endoperoxidase synthase 2 | TranSignal array |
| HIC | Hypermethylated in cancer | TranSignal array | |
| GLUT4 | Solute carrier insulin transport | TranSignal array | |
| GATA3 | Gata binding protein 3 | TranSignal array | |
| SYBL1 | Synaptobrevin-like 1 | TranSignal array | |
| ATF2 | Activating transcription factor 2 | TranSignal array | |
| MYOD | Myogenic differentiation 1 | TranSignal array | |
| SIM2 | Single-minded homologue 1 | TranSignal array | |
| WT1 | Wilms tumor 1 | TranSignal array | |
| CIITA | Class 2 MHC transcription 5A | TranSignal array | |
MSP: Methylation Sensitive PCP, QMSP: Quantitative Methylation Sensitive PCP, COBRA: Combined Bisulfite Restriction Analysis, COMPARE: Combination of methylated DNA precipitation and restriction enzyme digestion
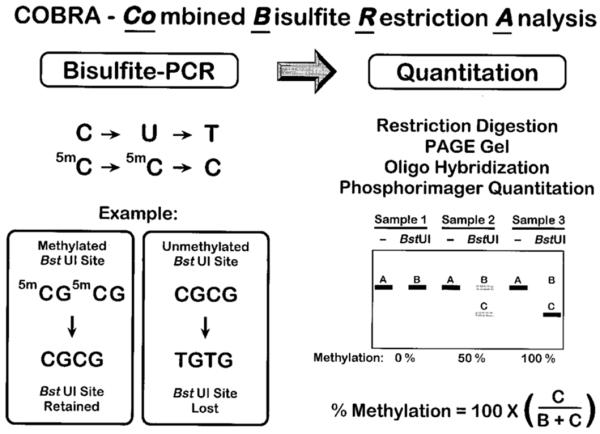
The COBRA assay has been described by Xiong and Laird in 1997 [86]. COBRA consists of a standard sodium bisulfite PCR treatment followed by restriction digestion and a quantitation step. The purified bisulfite-PCR products are further digested with a restriction enzyme with a recognition sequence containing a CpG site in the original unconverted DNA. Cleavage will occur only if the CpG sequence has been retained during the bisulfite conversion by a methylation of the cytosine residue. It is essential to ensure that the bisulfite conversion is complete. Therefore, a control digest is performed with an enzyme such as Hsp92II, which has a recognition sequence (CATG) that should be destroyed by the bisulfite conversion. Any cleavage by Hsp92II would indicate either non-CpG DNA methylation or incomplete sodium bisulfite conversion. The digested PCR products are then separated on an 8% denaturing polyacrylamide gel and transferred to Zetabind charged membrane (American Bioanalytical) by electroblotting. The membranes are hybridized with 5′-end-labeled oligonucleotides and quantified with a Molecular Dynamics PhosphorImager. The principle and workflow of COBRA is shown in Figure 7.
Figure 7. Workflow of COBRA assay.
Reprinted by permission from Oxford University Press: [Nucleic Acids Res] (Jun 15;25(12):2532-4), copyright (1997).
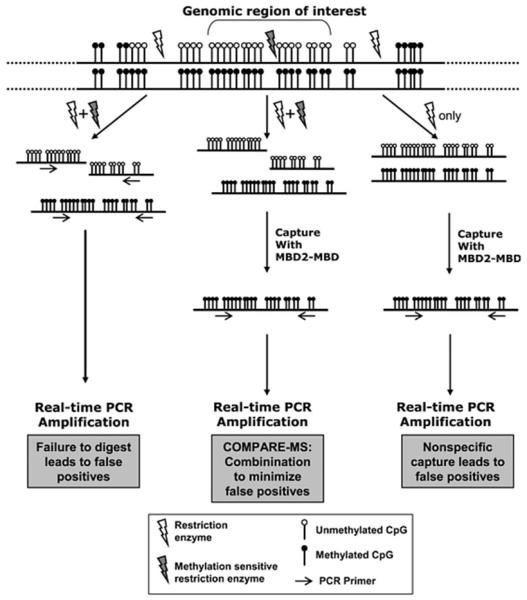
Details of the COMPARE assay are described by Yegnasubramanian and colleagues [87]. The key of the COMPARE assay is that the methylated genomic DNA fragments are captured and enriched by magnetic-bead immobilized recombinant methyl-binding domain polypeptides. SYBR Green-based real-time PCR is subsequently performed. The Methylation Index (MI) for each sample is be determined as follows: MIsample = (Q-untreated)/(QM.SssI), where Q-untreated is the amount of m ethylation at a given locus for the sample of interest, QM.SssI is the amount of methylation at the given locus for the equivalent amount of M.SssI methylated sample of interest. The MI is an estimate of the fraction of alleles that are methylated in a given sample. Figure 8 is the workflow of COMPARE.
Figure 8. Workflow of COMPARE assay.
Reprinted by permission from Oxford University Press: [Nucleic Acids Res] (Feb 9;34(3):e19.), copyright (2006).
In summary evidence suggests that epigenetic alternations could be an early event in prostate carcinogenesis and many factors such as diet and environmental factors may all contribute to the alternations.
(III) METHODS AND TECHNOLOGIES USED FOR EPIGENETIC CHANGES DETECTION
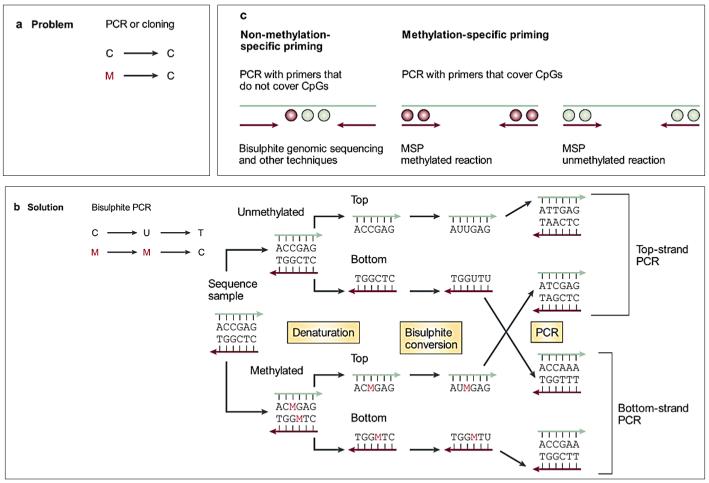
The sodium bisulfite modification method was established to investigate methylation patterns in DNA in the 1990’s. The method used uses sodium bisulfite modification which coverts unmethylated cytosines to uracil, with methylated cytosines remaining unchanged [88,89]. This method sensitized detection of methylation patterns from only small quantities of DNA and allows one to distinguish methylated from unmethylated DNA via PCR amplification. During PCR amplification, unmethylated cytosines are amplified as thymine and methylated cytosines are amplified as cytosines (Figure 9) [89,90]. Most of the methods for DNA methylation analysis at specific loci are based on this approach.
Figure 9. Principles of Bisulphite Conversion and Methylation Specific PCR.
Standard molecular biology techniques to analyze individual gene loci, such as polymerase chain reaction (PCR) and biological cloning, erase DNA methylation information, leaving the investigator oblivious to the epigenetic information that was present in the original genomic DNA (panel a). 5-methylcytosine residues are indicated as red Ms. The solution to this problem is to modify the DNA in a methylation-dependent way before amplification. This can be achieved either by digestion with a methylation-sensitive restriction enzyme (not shown), or by treating the genomic DNA with sodium bisulphite (panel b), which converts unmethylated cytosines to uracil residues. As a consequence, the converted DNA is no longer self-complementary, and amplification of either the top or bottom DNA strand requires different primers. Priming can be either universal, or methylation specific (panel c). MSP, methylation-specific PCR. Reprinted by permission from Macmillan Publishers Ltd: [Nat Rev Cancer] (Apr;3(4):253-66), copyright (2003).
To determine DNA promoter CpG methylation, the modified DNA can be further analyzed using different approaches according to the goals of studies: global or locus-specific methylation analysis. High-performance liquid chromatography (HPLC) is a classical method to quantify global methylation [89]. This method is highly reproducible, but it requires large amount of high quality genomic DNA and is not suitable for high-throughput analysis. More recently, new methods have been developed for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements [91]. This approach requires little DNA and can be applied to paraffin-embedded tissues [92].
Global methylation could provide an overall view of methylation profile, however, it will need to have gene-specific methylation analysis to characterize candidate genes and link such changes to functional outcomes. The gene-specific methylation analysis can be achieved by (1) methylation sensitive PCR (MSP); (2) bisulfite sequencing or bisulfite-pyrosequencing; (3) matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). The most frequent used methods are MSP and bisulfite-sequencing/ pyrosequencing. The keys for those methods are (1) bisulfite modification of DNA; (2) primer design. Recently more high-throughput array base analysis have been developed by different companies. These arrays are designed upon promoter and CpG islands and are described in further detail below.
(1.) Methylation Arrays
The following array based analytical methods are currently in use for measuring DNA methylation.
(i.) HumanMethylation450 BeadChip™ (Illumina Inc, San Diego, CA USA)
This is a beadchip based array and it offers a unique combination of comprehensive, expert-selected coverage, including 99% of RefSeq genes, 96% of CpG islands, and other content categories selected by methylation experts. The Illumina’s Infinium Methylation Assay™ provides quantitative methylation measurement at the single-CpG-site level which offers the highest resolution for understanding epigenetic changes. With Infinium Methylation Assays™, researchers can quantitatively interrogate methylation sites at single-nucleotide resolution, profiling 12 samples in parallel to deliver high-throughput power while minimizing the cost per sample. However, the array requires an additional module to run. The analysis of Infinium methylation data requires the GenomeStudio Methylation Module™ (Illumina Inc). This program enables two basic types of methylation data analysis: calculating methylation levels within an individual sample, and determining whether methylation levels have changed between a reference group and another experimental group.
(ii.) Human DNA Methylation Microarray™ (Agilent Technologies, Inc. Santa Clara, CA, USA)
Agilent’s Human DNA Methylation Microarrays™ consist of 27,627 expanded CpG islands and 5,081 UMR regions CpG islands. The array enables comprehensive analysis of the relative methylation levels in a genomic DNA sample. Each glass slide is formatted with one high-definition 244K array and costs ~$629. However, it requires Agilent equipment to conduct and analyze the array.
(iii.) EpiTect Methyl II PCR Arrays™ (QIAGEN, USA)
EpiTect Methyl II PCR Arrays™ is a qPCR based array. Genes are selected based on their reported methylation status in a variety of experimental settings. These arrays allow correlation of CpG island methylation status with biological phenotypes and disease outcomes. The array allows the simultaneous DNA methylation profiling of a panel of 22 or 94 gene promoters in either disease or pathway focused genes. The method is based on the detection of remaining input DNA after cleavage with a methylation-sensitive restriction enzyme (MSRE) and/or a methylation-dependent (MDRE) restriction enzyme. These enzymes will digest unmethylated and methylated DNA, respectively. Following digestion, the remaining DNA is quantified by real-time PCR in each individual enzyme reaction using primers that flank a promoter (gene) region of interest. The relative fractions of methylated and unmethylated DNA are subsequently determined by comparing the amount in each digest with that of a mock (no enzymes added) digest using the ΔCt method. The reliability and simplicity of the procedure make this technology highly suited for semi-high-throughput DNA methylation profiling and biomarker discovery for various research fields, such as stem cell differentiation and development. The EpiTect Methyl II Signature PCR Array™ (22 genes) is available in 96-well format and EpiTect Methyl II Complete PCR Array™ (94 genes) is available in 384-well formats. The EpiTect Methyl II PCR Arrays™ from QIAGEN are affordable and easy to perform since it is a qPCR based assay. These arrays are useful for obtaining methylation profiles in different diseases or specific pathways quickly, but no quantified information. In addition, since the genes arranged in the array are not duplicated and qPCR is extremely sensitive, the slightest mistakes can have significant influence on the final results.
(iv.) TaqMan Human DNA Methylation Array™ (Applied Biosystems, USA)
The Applied Biosystems TaqMan Array Human DNA Methylation™ and Transcriptional Repression 96-well Plate™ contain 28 assays for DNA methylation and transcriptional repression-associated genes and 4 assays for candidate endogenous control genes. All assays are plated in triplicate. The Gene Signature Plates are 96-well plates that are pre-configured with the most appropriate TaqMan Gene Expression Assays™ (PCR primers and TaqMan® probe sets) for a specific biological process, pathway, or disease state. Each plate contains predefined assays and endogenous controls dried-down in the wells, ready for accurate assessment of an entire gene signature in one simple experiment. Since this array is a TaqMan based detection assay, it is therefore an improved assay in terms of sensitivity and specificity compared to EpiTect Methyl II PCR Arrays™ (SYBR-green based detection) from QIAGEN. However, this array is at a slightly higher cost compared to EpiTect Methyl II PCR Array™ and the plates have been only validated for use on Applied Biosystems® 7000, 7300, 7500 and 7900HT Fast Real-Time PCR Systems.
(v.) TranSignal Promoter Methylation Arrays (Panomics Inc, USA)
Panomics now is part of Affymetrix Inc (Huston TX, USA). The TranSignal Promoter Methylation Arrays™ designed by Panomics use genomic DNA that is digested with a restriction enzyme to produce small fragments of methylated DNA. The digests are purified and annealed to linkers and subsequently incubated with MBP (methylation binding protein) to form protein-DNA complexes. These complexes are isolated, purified, and subsequently labeled with biotin-dCTP. The final step is hybridization of the labeled DNA on the array. The signals are detected by using Strepavidin-HRP, which eliminates the use of hazardous radioactive chemicals associated with traditional Southern blotting techniques. A single experiment using the Panomics array takes two days to complete and permits analysis of 82 different promoter regions from each sample. The Panomics TranSignal Promoter Methylation Arrays™ are relatively easy to use since the assay uses enzyme digestion to produce methylation DNA instead bisulfide modification. The data obtained from these arrays are relatively consistent and reproducible, but the exposure time for visualizing the chemiluminiscent signals is very critical for maintaining the consistent results from array to array. The arrays are also re-usable, however, the stripping step is quite important, and background signals may significantly affect results.
(3.) Summary
Continued rapid improvement of the current technology makes the study of DNA methylation more and more accessible. The method or approach should be selected based upon the goal, scope, and design of the individual study. Therefore, it is important to understand the type of information provided by different approaches, and the potential for bias associated with the different methods. In addition, the cost, availability of sample, and supplemental instruments needed for analysis should be considered when selecting a method for methylation studies.
(IV) FACTORS THAT INFLUENCE THE EPIGENETIC CHANGES IN CANCER
There is growing evidence that environmental and dietary factors can affect the epigenome. These events can start as early as prenatal or early postnatal development. Moreover, the changes can be sustained throughout life, leading to long term modification of phenotypes and contribute to development of abnormalities including cancers.
(1.) Nutritional factors that influence the methylation outcomes in cancer
Nutrients such as folic acid, Vitamin B, and S-adenosylmethionine (SAM) are key components of the methyl-metabolism pathway. DNA methylation occurs at 5′position of the cytosine residues within CpG dinucleotides through addition of a methyl group to form 5-methylcytosine. Dietary factors can modify DNA methylation by influencing the supply of methyl groups for the formation of SAM; or by modifying the utilization of methyl groups through processes inducing shifts in DNA methyltransferase activity [66].
As indicated before, the DNA methyltransferase (DNMTs) family of enzymes catalyze the transfer of a methyl group to DNA. The DNMTs use SAM as the methyl donor. Three active DNA methyltransferases have been identified in mammals:DNMT1, DNMT3a and DNMT3b. DNMT1 is primarily involved in the maintenance of DNA methylation after replication and DNMT3a and DNMT3b are the de novo methyltransferases that set up DNA methylation patterns early in development. Dietary factors can modify the availability of methyl donors, including folate, choline, and methionine, as well as the activity of DNMTs. An early animal study from the Poirier group showed that dietary methyl deficiency of folate, choline and methionine altered hepatic DNA methylation patterns and induced liver cancer in the absence of carcinogen [93]. In addition, dietary factors may be also able to regulate DNA demethylation activity [66]. The hypermethylation of CpGs in promoters of tumor suppressor genes can lead to gene transcriptional silencing and cause malignant transformation in cancers including breast, colon, lung, and prostate cancers.
Moreover, recent studies have linked obesity overall with epigenetic modifications associated with cancer. A recent study by Uriarte and colleagues have found that high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats [94]. Adipocytes act as regulators of energy balance and glucose homeostasis, and aberrations lead to the emergence of obesity. In addition, epigenetic modifications of obesogenic genes such as leptin may further exacerbate the risk of obesity [95]. It is well established that obesity is associated with increased risk of the multiple types of cancers, including postmenopausal breast cancer, pancreatic cancer, and colorectal cancer to name a few [96].
(2) Role of nutrition on epigenetic alternations - Bioactive dietary components for cancer prevention by modification of epigenetics
Epigenetics has been shown to play a role in cancer development and can be modified by nutritional factors [66] . Hence, utilization of dietary compounds in additional to pharmacological drugs to target epigenetic modification may be a valuable strategy for cancer prevention and treatment [97]. Several decades of studies have provided evidence that bioactive dietary components play important roles in regulation of epigenetics changes predominantly by: (1) inhibition of DNMTs (Figure 10); and, (2) inhibition of histone modifications (Figure 11). These bioactive dietary components may modify cancer risk as well as tumor progression. Below, the role of some common bioactive dietary compounds and their effects on epigenetics are described in detail.
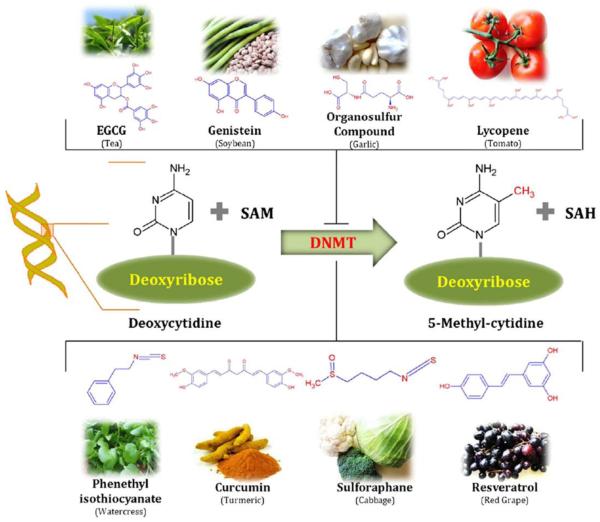
Figure 10. Dietary inhibitors of DNA methylation.
DNA methylation is a biochemical process that is essential for development. Some dietary phytochemicals are reported to inhibit the methylation of cytosine. Hypermethylation of cytidine by DNMTs usually results in transcriptional gene silencing and gene inactivation. Several phytochemicals derived from different food source such as: resveratrol from grapes and berries, curcumin from turmeric, tea phenols from tea leaves, genistein from soybeans, sulforaphane from broccoli, phenethyl isothiocynate from cauliflower, organosulfur compounds from garlic, quercetin from citrus fruits, and lycopene from tomato act as dietary inhibitors of DNA methyltransferases. These compounds also alter gene expression via epigenetic mechanisms. Reprinted from Pharmacol Ther. 2013 Apr;138(1):1-17. Sharmila Shankar, Dhruv Kumar, Rakesh K. Srivastava, Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition, Pages No.1-17, Copyright (2013), with permission from Elsevier.
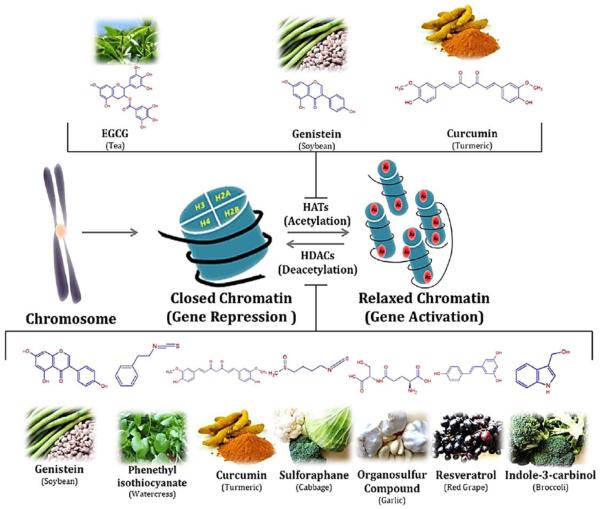
Figure 11. Examples of dietary inhibitors of histone modifications.
Representation of histonemodifications (acetylation and deacetylation) by the phytochemicals derived from different food sources. Phytochemicals like EGCG, genistein and curcumin play important role in inhibition of histone acetylation by inactivating histone acetyl transferase enzyme. Some other phytochemicals like sulforaphane, curcumin, genistein, phenyl isothiocynate, organosulfur compound, resveratrol and indol-3-carbinol inhibits the deacetylation of relaxed chromatine by inactivating histone deacetylase enzyme. Reprinted from Pharmacol Ther. 2013 Apr;138(1):1-17. Sharmila Shankar, Dhruv Kumar ,Rakesh K. Srivastava, Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition, Pages No.1-17, Copyright (2013), with permission from Elsevier.
(i.) Tea polyphenols
Tea is consumed worldwide. Epidemiologic and laboratory studies have indicated that polyphenolic compounds present in tea and green tea may play a role in cancer prevention [97] and progression [98]. The most abundant chemical compounds are catechins, which consist of (−)-epigallocatechin (EC), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatechin-3-gallate (EGCG) [97]. EGCG is the most abundant and accounts for more than 50% of total polyphenols . EGCG has been identified as most effective constituent of green tea [99] and has been extensively studies as a potential demethylating agent. Early studies have shown that EGCG can inhibit DNMT activity by forming hydrogen bonds with Pro1223, Glu1265, Cys1225, Ser1229, and Arg1309 in the catalytic pocket of DNMT [100]. The inhibition of DNMTs by EGCG can lead to the reactivation RARβ, p16INK4α, MGMT (o6-methylguanine methyltransferase) and hMLH1 genes in colon, esophageal, and prostate cancers cells. Besides direct inhibition of DNMT by EGCG, it has been reported that EGCG could decrease S-adenosyl-L-methionine (SAM) and increase s-adenosyl-L-homocysteine (SAH), a potent inhibitor of DNA methylation. Together, these data provide evidence of indirect inhibition of DNA methylation by EGCG [97].
Recently, EGCG has also been discovered to have strong HAT inhibitory activity. Choi et al found that EGCG reversed the acetylation of histone H3 and H4 on chromatin and suppressed p300/CBP-mediated p65 acetylation. This resulted in inhibition of lymphoma. However, in this study EGCG did not change HDACs [101]. Combination of green tea polyphenol and histone deacetylase inhibitor has been showed synergistic epigenetic reactivate estrogen receptor-α (ERα) in the ER-negative breast cancer cell line, MBA-MB231 [102]. Inhibition of tumorigenesis by EGCG or green tea polyphenols has also been showed in vivo studies [97]. However, the effects on epigenetic mechanisms and epigenome in vivo have not been well defined yet.
(ii.) Curcumin
Curcumin is a yellow pigment present in the spice turmeric. It has been shown to have anti-inflammatory, anti-angiogenic, wound-healing, antioxidant, and anti-cancer properties. Specifically, curcumin has been shown to inhibit DNMT activity by covalently blocking the catalytic thiolate of C1226 of DNMT1 [103]. Evidence also shows that curcumin may be a potent DNA hypomethylating agent. Besides, curcumin also can function as a HDAC and HAT inhibitor and play a role in histone modification in cancer cell models. However, the issue with using curcumin is its insolubility and instability in water that results in low bioavailability. It has been suggested that the bioavailability of curcumin can be enhanced by utilizing rubusoside (found in Chinese blackberry extract) or phosphatidylcholine (found in soy and egg yolks) [97].
(iii.) Selenium (Se)
Se is a nutrient found in Brazil nuts, chicken, game meat and beef [97]. Se is an essential element with antioxidant, proapoptotic, DNA repair and anticancer properties. An early study from Vadgama et al. showed that supplementation of Selenium enhanced chemotherapeutic effect of Taxol and Doxorubicin in different cancers cell lines beyond that seen with the chemotherapeutic drugs used alone [104]. The role of Se in epigenetics has been investigated by several groups. Xiang and colleagues showed that selenite reactivates a silenced gene, GSTP1, by decreasing DNMT1 and DNMT3a, and modulating histones in human prostate cancer cells [105]. The histone modification by Se involves decreasing histone deacetylase activity and increasing acetylatation of histone H3. In vivo studies from Davis et al. showed that Se deficiency caused global hypomethylation of liver and colon [106]. In rodents, the depletion of methyl groups by dietary restriction leads to a decrease in SAM those results in liver carcinogenesis and in liver hypomethylation, which precedes tumor development. Overall further studies in vitro and in vivo need to be conducted for validating the effect of Se on the epigenome.
(iv.) Genistein
Genistein is an isoflavone belonging to the flavonoid group of compounds and is found in plants including fava beans, soybeans, lupin, kudzu and psoralea. Genistein has found to have ani-cancer and anti-angiogenic properties in many cancers. It has been indicated to inhibit prostate, cervix, brain, breast and colon cancers [97]. The mechanisms of the anticancer effect of genistein may involve regulation of gene transcription or silencing activity by modulating epigenetic changes, such as DNA methylation and/or chromatin modifications. It has been suggested that genistein can act as a DNMT inhibitor. Within this role, it can cause demethylation of CpG islands in the promoter of genes and subsequently activate tumor suppressor genes, p16, RARβ2 and MGMT at least in renal cancer [107], breast cancer [108], and prostate cancers cells [109]. Specifically, genistein can mediate histone acetylation and induce tumor suppressor genes p16, p21, PTEN, CYLD, p53 and FOXO3 in prostate cancer cells [110]. Mechanistically, genistein can modulate histone H3-Lysine 9 (H3-K9) methylation and deacetylation [97] hence its use as a dietary compound may have significant effects on global epigenetic changes.
(v.) Resveratrol
Resveratrol is a dietary polyphenol derived from grapes and is most commonly consumed in the form of red wine. The anti-cancer properties of resveratrol have been supported by its ability to inhibit proliferation of a wide variety of human cancer cells [111]. Its best characterized epigenetic roles include affecting the histone H3K9 acetylation which significantly regulates activity of the BRCA1 promoter [97]. Resveratrol has less DNMT inhibitory activity compared to EGCG and was unable to reverse the methylation of certain tumor suppressor genes. However, resveratrol has been associated with activation of the type III HDAC inhibitors, sirtuin 1(SIRT1) and p300 [112]. The SIRT1 has been reported to mediate BRCA1 signaling in human breast cancer cells by altering H3 acetylation [112].
(3.) Summary
There is substantial evidence in the literature to demonstrate that dietary factors may play an important role in the epigenetic process, including histone modification. Certain bioactive dietary components such as tea phenols, curcumin, selenium, genistein, and resveratrol may have great potential not only in the prevention but also in the therapy of a wide variety of cancers by altering various epigenetic modifications. Furthermore, additional in vivo studies are needed to validate the data obtained from in vitro studies. Specifically, clinical studies evaluating the efficacy of these interventions should analyze the safety profile of doses, route of administration, organ specificity, as well as bioavailability of these bioactive components in human subjects. Epigenetic alterations affected by these compounds should also be assessed.
(V) Future directions
In this chapter, the role of epigenetics as key mediators of gene expression has been discussed. Specifically, evidence was presented to highlight the role of epigenetic aberrations in cancer with an emphasis in breast and prostate cancer. Studies have shown that epigenetic signatures can serve as biomarkers for early detection as well as prognosis and outcomes [71,89].
In breast, this chapter gave example of methylation of the estrogen receptor gene (ESR1) as a potential mechanism for both emergence of triple-negative breast cancer as well as tumors that eventually form resistance to standard targeted therapy [18]. Studies identified that ESR1 promoter methylation was correlated with both ER and PgR protein expression levels [113]. Loss of the ER receptor, a key target for treatment of 80% of breast cancers by well-established therapies like tamoxifen, can result in more aggressive breast phenotype and reduced treatment options.
Another key biomarker in breast cancer implicated by aberrant epigenetic changes is BRCA1. Although, BRCA1 promoter methylation was not common in overall breast cancers, there was a significantly higher frequency in basal-like and/or TNBCs. This pattern was further correlated with reduced breast cancer outcome implicating BRCA1 methylation in both the development of TNBC/aggressive tumors as well as patient survival [49,53,54]. These data warrant clearer confirmation in breast tumors from larger and more diverse ethnic cohorts.
A detailed list of well-established as well as new biomarkers in breast cancer epigenetics are shown in Table 2 and includes CASP8, CCND2, CDH3 (E-cadherin), CDKN2A, RARβ, RASSF1A, and SERPINB5. Combined, these biomarkers represent regulation of crucial cellular processes in breast cancer development and progression including tumor suppression, proliferation, and cell adhesion. Accumulating pre-clinical evidence points to potential future utilization of these biomarkers in standard tumor evaluation, and ultimately may inform treatment options in breast cancer. Particularly, evaluation through immunohistochemical methods as performed in many of the studies reviewed in this chapter show efficacy in detection. Careful validation in additional pre-clinical and clinical models is necessary prior to use; however, the long term promise of identifying high risk patients for more vigilant clinical evaluation is highly necessary [18].
Studies investigating the epigenetics in prostate cancer have similarly revealed several biomarkers which have been associated with both risk and outcome. The methylation of the glutathione S-transferase PI (GSTP1) in particular has yielded promise [71]. The differential methylation patterns of GSTP1 in prostate cancer vs. normal prostate tissue, combined with the vital role in detoxification that glutathione S-transferase plays renders this a high-priority biomarker. Preclinical studies have already shown that GSTP1 closely correlated with prostate cancer risk as well as reoccurrence [77]. Evaluation of GSTP1 in a standard clinical context has yet to be established, however, several groups are in the process of developing and testing screens for hypermetylation from both tumor samples [114] as well as urine samples for less invasive assessment [115]. A more detailed list of genes is provided in Table 3. Key genes include GSTP1, AR, RARβ2, SPARC, TIMP3 and NKX2-5. Future studies in prostate cancer and epigenetics should also investigate the role of epigenetic patterns as biomarkers in diverse populations. The presence of a new series of high resolution and high through-put tools will facilitate larger screening and association studies needed to understand the mechanisms and targets for epigenetics in prostate cancer.
Preclinical studies to date have yielded promising response to DNA and histone level reversal of modifications. As the complex interplay of DNA and histone modifications and their accompanying enzymes proteins (DNMTs, HDACs, etc) are better understood, targeting of aberrant epigenetic activity along various points in these pathways offer options for potential intervention. Of note, combined demethylating agents (5-aza-2′-deoxycytidine) with HDAC inhibitors (trichostatin A) conceptually offer the most rational approach to tackling both DNA level and histone level aberrations [13,69]. Although these approaches have resulted in some progress in clinical application (such as with leukemias), the application in solid tumors must still be explored and clarified further. Combined translational approaches investigating both preclinical and clinical applications must include larger and more diverse cohorts to reduce variances from disparities and produce results more globally applicable. Furthermore, earlier investigations ruling out efficacies of epigenetic biomarkers and/or anti-methylation treatment merit some reconsideration in light of new, more specific assays available to carry out rigorous epigenetic study.
Acknowledgments
Grant Funding: Support from NIH (Grant numbers: NCI U56CA101599-01; CA15083-25S3; U54CA14393; NIMHD U54MD007598; and NCATS CTSI UL1TR000124 to J.V.V, and a NIMHD-CRECD R25MD007610 to Y.W).
REFERENCES
- 1.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 3.Urnov FD, Wolffe AP. Above and within the genome: epigenetics past and present. J Mammary Gland Biol Neoplasia. 2001;6:153–167. doi: 10.1023/a:1011304606604. [DOI] [PubMed] [Google Scholar]
- 4.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983;111:47–54. doi: 10.1016/s0006-291x(83)80115-6. [DOI] [PubMed] [Google Scholar]
- 6.Jones PA. DNA methylation and cancer. Oncogene. 2002;21:5358–5360. doi: 10.1038/sj.onc.1205597. [DOI] [PubMed] [Google Scholar]
- 7.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 8.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 10.Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 11.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 12.Kass SU, Pruss D, Wolffe AP. How does DNA methylation repress transcription? Trends Genet. 1997;13:444–449. doi: 10.1016/s0168-9525(97)01268-7. [DOI] [PubMed] [Google Scholar]
- 13.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 14.Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat Rev Cancer. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachman KE, Park BH, Rhee I, Rajagopalan H, Herman JG, et al. Histone modifications and silencing prior to DNA methylation of a tumor suppressor gene. Cancer Cell. 2003;3:89–95. doi: 10.1016/s1535-6108(02)00234-9. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Alvarez M, Slamon DJ, Koeffler P, Vadgama JV. Caspase 8 and maspin are downregulated in breast cancer cells due to CpG site promoter methylation. BMC Cancer. 2010;10:32. doi: 10.1186/1471-2407-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra DK, Chen Z, Wu Y, Sarkissyan M, Koeffler HP, et al. Global methylation pattern of genes in androgen-sensitive and androgen-independent prostate cancer cells. Mol Cancer Ther. 2010;9:33–45. doi: 10.1158/1535-7163.MCT-09-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jovanovic J, Ronneberg JA, Tost J, Kristensen V. The epigenetics of breast cancer. Mol Oncol. 2010;4:242–254. doi: 10.1016/j.molonc.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian J, Lee SO, Liang L, Luo J, Huang CK, et al. Targeting the unique methylation pattern of androgen receptor (AR) promoter in prostate stem/progenitor cells with 5-aza-2′-deoxycytidine (5-AZA) leads to suppressed prostate tumorigenesis. J Biol Chem. 2012;287:39954–39966. doi: 10.1074/jbc.M112.395574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M, Park JY. DNA methylation in promoter region as biomarkers in prostate cancer. Methods Mol Biol. 2012;863:67–109. doi: 10.1007/978-1-61779-612-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 22.Hinshelwood RA, Clark SJ. Breast cancer epigenetics: normal human mammary epithelial cells as a model system. J Mol Med (Berl) 2008;86:1315–1328. doi: 10.1007/s00109-008-0386-3. [DOI] [PubMed] [Google Scholar]
- 23.Bediaga NG, Acha-Sagredo A, Guerra I, Viguri A, Albaina C, et al. DNA methylation epigenotypes in breast cancer molecular subtypes. Breast Cancer Res. 2010;12:R77. doi: 10.1186/bcr2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Hoesel AQ, Sato Y, Elashoff DA, Turner RR, Giuliano AE, et al. Assessment of DNA methylation status in early stages of breast cancer development. Br J Cancer. 2013;108:2033–2038. doi: 10.1038/bjc.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SY, Kwon HJ, Lee HE, Ryu HS, Kim SW, et al. Promoter CpG island hypermethylation during breast cancer progression. Virchows Arch. 2011;458:73–84. doi: 10.1007/s00428-010-1013-6. [DOI] [PubMed] [Google Scholar]
- 26.Klajic J, Fleischer T, Dejeux E, Edvardsen H, Warnberg F, et al. Quantitative DNA methylation analyses reveal stage dependent DNA methylation and association to clinico-pathological factors in breast tumors. BMC Cancer. 2013;13:456. doi: 10.1186/1471-2407-13-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 28.Feng W, Shen L, Wen S, Rosen DG, Jelinek J, et al. Correlation between CpG methylation profiles and hormone receptor status in breast cancers. Breast Cancer Res. 2007;9:R57. doi: 10.1186/bcr1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, et al. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Antona C, Gomez A, Karlgren M, Sim SC, Ingelman-Sundberg M. Molecular genetics and epigenetics of the cytochrome P450 gene family and its relevance for cancer risk and treatment. Hum Genet. 2010;127:1–17. doi: 10.1007/s00439-009-0748-0. [DOI] [PubMed] [Google Scholar]
- 31.Brodie AM, Njar VC. Aromatase inhibitors in advanced breast cancer: mechanism of action and clinical implications. J Steroid Biochem Mol Biol. 1998;66:1–10. doi: 10.1016/s0960-0760(98)00022-3. [DOI] [PubMed] [Google Scholar]
- 32.Johnston SR, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003;3:821–831. doi: 10.1038/nrc1211. [DOI] [PubMed] [Google Scholar]
- 33.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 34.Pathiraja TN, Stearns V, Oesterreich S. Epigenetic regulation in estrogen receptor positive breast cancer--role in treatment response. J Mammary Gland Biol Neoplasia. 2010;15:35–47. doi: 10.1007/s10911-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, et al. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 36.Lapidus RG, Nass SJ, Butash KA, Parl FF, Weitzman SA, et al. Mapping of ER gene CpG island methylation-specific polymerase chain reaction. Cancer Res. 1998;58:2515–2519. [PubMed] [Google Scholar]
- 37.Martens JW, Nimmrich I, Koenig T, Look MP, Harbeck N, et al. Association of DNA methylation of phosphoserine aminotransferase with response to endocrine therapy in patients with recurrent breast cancer. Cancer Res. 2005;65:4101–4117. doi: 10.1158/0008-5472.CAN-05-0064. [DOI] [PubMed] [Google Scholar]
- 38.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 39.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 41.Potemski P, Kusinska R, Watala C, Pluciennik E, Bednarek AK, et al. Prognostic relevance of basal cytokeratin expression in operable breast cancer. Oncology. 2005;69:478–485. doi: 10.1159/000090986. [DOI] [PubMed] [Google Scholar]
- 42.Holm K, Hegardt C, Staaf J, Vallon-Christersson J, Jonsson G, et al. Molecular subtypes of breast cancer are associated with characteristic DNA methylation patterns. Breast Cancer Res. 2010;12:R36. doi: 10.1186/bcr2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bardowell SA, Parker J, Fan C, Crandell J, Perou CM, et al. Differential methylation relative to breast cancer subtype and matched normal tissue reveals distinct patterns. Breast Cancer Res Treat. 2013;142:365–380. doi: 10.1007/s10549-013-2738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milani A, Montemurro F, Gioeni L, Aglietta M, Valabrega G. Role of trastuzumab in the management of HER2-positive metastatic breast cancer. Breast Cancer (Dove Med Press) 2010;2:93–109. doi: 10.2147/BCTT.S6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 46.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 47.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 48.Branham MT, Marzese DM, Laurito SR, Gago FE, Orozco JI, et al. Methylation profile of triple-negative breast carcinomas. Oncogenesis. 2012;1:e17. doi: 10.1038/oncsis.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, et al. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:R38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancini DN, Rodenhiser DI, Ainsworth PJ, O’Malley FP, Singh SM, et al. CpG methylation within the 5′ regulatory region of the BRCA1 gene is tumor specific and includes a putative CREB binding site. Oncogene. 1998;16:1161–1169. doi: 10.1038/sj.onc.1201630. [DOI] [PubMed] [Google Scholar]
- 51.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 52.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 53.Stefansson OA, Jonasson JG, Olafsdottir K, Hilmarsdottir H, Olafsdottir G, et al. CpG island hypermethylation of BRCA1 and loss of pRb as co-occurring events in basal/triple-negative breast cancer. Epigenetics. 2011;6:638–649. doi: 10.4161/epi.6.5.15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu NC, Huang YF, Yokoyama KK, Chu PY, Chen FM, et al. Methylation of BRCA1 promoter region is associated with unfavorable prognosis in women with early-stage breast cancer. PLoS One. 2013;8:e56256. doi: 10.1371/journal.pone.0056256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan S, Kumagai T, Vora J, Bose N, Sehgal I, et al. PTEN promoter is methylated in a proportion of invasive breast cancers. Int J Cancer. 2004;112:407–410. doi: 10.1002/ijc.20447. [DOI] [PubMed] [Google Scholar]
- 56.Garcia JM, Silva J, Pena C, Garcia V, Rodriguez R, et al. Promoter methylation of the PTEN gene is a common molecular change in breast cancer. Genes Chromosomes Cancer. 2004;41:117–124. doi: 10.1002/gcc.20062. [DOI] [PubMed] [Google Scholar]
- 57.Muggerud AA, Ronneberg JA, Warnberg F, Botling J, Busato F, et al. Frequent aberrant DNA methylation of ABCB1, FOXC1, PPP2R2B and PTEN in ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res. 2010;12:R3. doi: 10.1186/bcr2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Y, Lin YZ, LaPushin R, Cuevas B, Fang X, et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 1999;18:7034–7045. doi: 10.1038/sj.onc.1203183. [DOI] [PubMed] [Google Scholar]
- 59.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 60.Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women : Implications for breast cancer screening recommendations. Cancer. 2011;117:2747–2753. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stead LA, Lash TL, Sobieraj JE, Chi DD, Westrup JL, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lund MJ, Butler EN, Hair BY, Ward KC, Andrews JH, et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes: a population-based study and first report. Cancer. 2010;116:2549–2559. doi: 10.1002/cncr.25016. [DOI] [PubMed] [Google Scholar]
- 63.Network NCC NCCN Clinical Practice Guidelines in Oncology - BREAST CANCER. Version 3.2013 ed.
- 64.Mehrotra J, Ganpat MM, Kanaan Y, Fackler MJ, McVeigh M, et al. Estrogen receptor/progesterone receptor-negative breast cancers of young African-American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin Cancer Res. 2004;10:2052–2057. doi: 10.1158/1078-0432.ccr-03-0514. [DOI] [PubMed] [Google Scholar]
- 65.Dumitrescu RG. Epigenetic markers of early tumor development. Methods Mol Biol. 2012;863:3–14. doi: 10.1007/978-1-61779-612-8_1. [DOI] [PubMed] [Google Scholar]
- 66.Huang S. Histone methyltransferases, diet nutrients and tumour suppressors. Nat Rev Cancer. 2002;2:469–476. doi: 10.1038/nrc819. [DOI] [PubMed] [Google Scholar]
- 67.Juttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci U S A. 1994;91:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- 69.Jabbour E, Issa JP, Garcia-Manero G, Kantarjian H. Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies. Cancer. 2008;112:2341–2351. doi: 10.1002/cncr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan J, Yin WJ, Lu JS, Wang L, Wu J, et al. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J Cancer Res Clin Oncol. 2008;134:883–890. doi: 10.1007/s00432-008-0354-x. [DOI] [PubMed] [Google Scholar]
- 71.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perry AS, Watson RW, Lawler M, Hollywood D. The epigenome as a therapeutic target in prostate cancer. Nat Rev Urol. 2010;7:668–680. doi: 10.1038/nrurol.2010.185. [DOI] [PubMed] [Google Scholar]
- 73.Dobosy JR, Roberts JL, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol. 2007;177:822–831. doi: 10.1016/j.juro.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 74.Seligson DB, Horvath S, Shi T, Yu H, Tze S, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 75.Yu J, Cao Q, Mehra R, Laxman B, Yu J, et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 76.Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, et al. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 77.Vanaja DK, Ehrich M, Van den Boom D, Cheville JC, Karnes RJ, et al. Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest. 2009;27:549–560. doi: 10.1080/07357900802620794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahapatra S, Klee EW, Young CY, Sun Z, Jimenez RE, et al. Global methylation profiling for risk prediction of prostate cancer. Clin Cancer Res. 2012;18:2882–2895. doi: 10.1158/1078-0432.CCR-11-2090. [DOI] [PubMed] [Google Scholar]
- 79.Lin PC, Giannopoulou EG, Park K, Mosquera JM, Sboner A, et al. Epigenomic alterations in localized and advanced prostate cancer. Neoplasia. 2013;15:373–383. doi: 10.1593/neo.122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J Natl Cancer Inst. 2005;97:103–115. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- 81.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 82.Korkmaz CG, Fronsdal K, Zhang Y, Lorenzo PI, Saatcioglu F. Potentiation of androgen receptor transcriptional activity by inhibition of histone deacetylation--rescue of transcriptionally compromised mutants. J Endocrinol. 2004;182:377–389. doi: 10.1677/joe.0.1820377. [DOI] [PubMed] [Google Scholar]
- 83.Woodson K, Hayes R, Wideroff L, Villaruz L, Tangrea J. Hypermethylation of GSTP1, CD44, and E-cadherin genes in prostate cancer among US Blacks and Whites. Prostate. 2003;55:199–205. doi: 10.1002/pros.10236. [DOI] [PubMed] [Google Scholar]
- 84.Enokida H, Shiina H, Urakami S, Igawa M, Ogishima T, et al. Ethnic group-related differences in CpG hypermethylation of the GSTP1 gene promoter among African-American, Caucasian and Asian patients with prostate cancer. Int J Cancer. 2005;116:174–181. doi: 10.1002/ijc.21017. [DOI] [PubMed] [Google Scholar]
- 85.Kwabi-Addo B, Wang S, Chung W, Jelinek J, Patierno SR, et al. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin Cancer Res. 2010;16:3539–3547. doi: 10.1158/1078-0432.CCR-09-3342. [DOI] [PubMed] [Google Scholar]
- 86.Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yegnasubramanian S, Lin X, Haffner MC, DeMarzo AM, Nelson WG. Combination of methylated-DNA precipitation and methylation-sensitive restriction enzymes (COMPARE-MS) for the rapid, sensitive and quantitative detection of DNA methylation. Nucleic Acids Res. 2006;34:e19. doi: 10.1093/nar/gnj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, et al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3:253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Bailey V, Puleo CM, Easwaran H, Griffiths E, et al. DNA methylation analysis on a droplet-in-oil PCR array. Lab Chip. 2009;9:1059–1064. doi: 10.1039/b821780g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lisanti S, Omar WA, Tomaszewski B, De Prins S, Jacobs G, et al. Comparison of methods for quantification of global DNA methylation in human cells and tissues. PLoS One. 2013;8:e79044. doi: 10.1371/journal.pone.0079044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mikol YB, Hoover KL, Creasia D, Poirier LA. Hepatocarcinogenesis in rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1983;4:1619–1629. doi: 10.1093/carcin/4.12.1619. [DOI] [PubMed] [Google Scholar]
- 94.Uriarte G, Paternain L, Milagro FI, Martinez JA, Campion J. Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J Physiol Biochem. 2013;69:601–611. doi: 10.1007/s13105-012-0231-6. [DOI] [PubMed] [Google Scholar]
- 95.Milagro FI, Campion J, Garcia-Diaz DF, Goyenechea E, Paternain L, et al. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 2009;65:1–9. doi: 10.1007/BF03165964. [DOI] [PubMed] [Google Scholar]
- 96.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 97.Shankar S, Kumar D, Srivastava RK. Epigenetic modifications by dietary phytochemicals: implications for personalized nutrition. Pharmacol Ther. 2013;138:1–17. doi: 10.1016/j.pharmthera.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang P, Vadgama JV, Said JW, Magyar CE, Doan N, et al. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J Nutr Biochem. 2014;25:73–80. doi: 10.1016/j.jnutbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thakur VS, Gupta K, Gupta S. The chemopreventive and chemotherapeutic potentials of tea polyphenols. Curr Pharm Biotechnol. 2012;13:191–199. doi: 10.2174/138920112798868584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 101.Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69:583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Yuan YY, Meeran SM, Tollefsbol TO. Synergistic epigenetic reactivation of estrogen receptor-alpha (ERalpha) by combined green tea polyphenol and histone deacetylase inhibitor in ERalpha-negative breast cancer cells. Mol Cancer. 2010;9:274. doi: 10.1186/1476-4598-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Medina-Franco JL, Lopez-Vallejo F, Kuck D, Lyko F. Natural products as DNA methyltransferase inhibitors: a computer-aided discovery approach. Mol Divers. 2011;15:293–304. doi: 10.1007/s11030-010-9262-5. [DOI] [PubMed] [Google Scholar]
- 104.Vadgama JV, Wu Y, Shen D, Hsia S, Block J. Effect of selenium in combination with Adriamycin or Taxol on several different cancer cells. Anticancer Res. 2000;20:1391–1414. [PubMed] [Google Scholar]
- 105.Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–2181. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davis CD, Uthus EO. Dietary folate and selenium affect dimethylhydrazine-induced aberrant crypt formation, global DNA methylation and one-carbon metabolism in rats. J Nutr. 2003;133:2907–2914. doi: 10.1093/jn/133.9.2907. [DOI] [PubMed] [Google Scholar]
- 107.Majid S, Dar AA, Ahmad AE, Hirata H, Kawakami K, et al. BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis. 2009;30:662–670. doi: 10.1093/carcin/bgp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.King-Batoon A, Leszczynska JM, Klein CB. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ Mol Mutagen. 2008;49:36–45. doi: 10.1002/em.20363. [DOI] [PubMed] [Google Scholar]
- 109.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, et al. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–7041. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 110.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123:552–560. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- 111.Srivastava RK, Unterman TG, Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem. 2010;337:201–212. doi: 10.1007/s11010-009-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang RH, Zheng Y, Kim HS, Xu X, Cao L, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gaudet MM, Campan M, Figueroa JD, Yang XR, Lissowska J, et al. DNA hypermethylation of ESR1 and PGR in breast cancer: pathologic and epidemiologic associations. Cancer Epidemiol Biomarkers Prev. 2009;18:3036–3043. doi: 10.1158/1055-9965.EPI-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosenbaum E, Hoque MO, Cohen Y, Zahurak M, Eisenberger MA, et al. Promoter hypermethylation as an independent prognostic factor for relapse in patients with prostate cancer following radical prostatectomy. Clin Cancer Res. 2005;11:8321–8325. doi: 10.1158/1078-0432.CCR-05-1183. [DOI] [PubMed] [Google Scholar]
- 115.Cairns P, Esteller M, Herman JG, Schoenberg M, Jeronimo C, et al. Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin Cancer Res. 2001;7:2727–2730. [PubMed] [Google Scholar]
- 116.Hojfeldt JW, Agger K, Helin K. Histone lysine demethylases as targets for anticancer therapy. Nat Rev Drug Discov. 2013;12:917–930. doi: 10.1038/nrd4154. [DOI] [PubMed] [Google Scholar]