Abstract
Objective
Current approaches offer no cures for rheumatoid arthritis (RA). Accumulating evidence has revealed that manipulation of bone-marrow mesenchymal stem cells (BMSCs) may have the potential to treat RA. While BMSC-based therapy faces many challenges such as limited cell availability and reduced clinical feasibility, we herein demonstrate that substitution of gingival-derived mesenchymal stem cells (GMSCs) results in significantly improved therapeutic effects on established collagen-induced arthritis (CIA).
Methods
CIA has been induced with the immunization of type II collagen (CII) and CFA in DBA/1J mice. GMSCs were injected i.v. into mice on day 14 after immunization. In some experiments, injection of PC61 (anti-CD25 antibody) i.p. was used to delete Tregs in arthritic mice.
Results
Infusion of GMSCs in DBA/1J mice with CIA significantly decreased the severity of arthritis and pathology scores, and down-regulated inflammatory cytokine (IFN-γ, IL-17A) production. Infusion of GMSCs resulted in an increase in CD4+CD39+Foxp3+ cells in arthritic mice. These increases were noted early in spleen and LN and later in synovial fluid. The increased frequency of Foxp3+ Treg cells consisted of cells that were mainly Helios negative. Infusion of GMSCs partially interfered with the progress of CIA when Treg cells were depleted. Pre-treatment of GMSCs with CD39 or CD73 inhibitor significantly reversed the protective effect of GMSCs on CIA.
Conclusion
The role of GMSCs in controlling CIA pathology mostly depends upon CD39/CD73 signals and partially upon the induction of CD4+CD39+Foxp3+ Treg cells. GMSCs provide a promising approach for the treatment of autoimmune diseases.
Rheumatoid arthritis (RA) is a symmetric polyarticular arthritis that primarily affects the small diarthrodial joints of body (1). Clinical drug development for treatment of RA has progressed slowly. Currently, only about half of RA patients respond to most products such as TNF inhibitors, IL-1 antagonists, and anti-IL-6 receptor antibody. None of them are curative for RA (1). Novel approaches to cure this disease are sorely needed.
Mesenchymal stem cells (MSCs) can exhibit immunomodulatory effects. They inhibit T-cell proliferation in mixed lymphocyte cultures, prolong skin allograft survival, and decrease graft-versus-host disease (GVHD) when co-transplanted with hematopoietic stem cells (2). These properties make them well-suited to serve as a candidate for a new approach in the prevention and treatment of allograft rejection, GVHD and other autoimmune diseases. Bone marrow-derived MSCs (BMSCs) have been considered as a potential strategy in clinical cell therapy, however, there are some drawbacks and limitations for their clinical feasibility such as the difficulty in obtaining sufficient numbers for therapeutic use.
Recent study has confirmed that gingival tissue-derived MSCs (GMSCs), a population of stem cells exists in the human gingiva (3), have been shown to have several advantages over BMSCs. GMSCs are easy to isolate, they are homogenous and proliferate more rapidly than BMSCs (4). Additionally, GMSCs display stable morphological and functional characteristics at higher passage numbers and are not tumorigenic (4). Although GMSCs demonstrate beneficial effects in preventing experimental colitis (3) and mitigating chemotherapy-induced oral mucositis (5), utilization of GMSC for the treatment of autoimmune arthritis and other immune diseases has not been explored.
Recent studies have demonstrated that adoptive transfer of MSCs can upregulate CD4+CD25+Foxp3+ regulatory T cells (Tregs) in vivo (6-7). Treg cells play an important role in the prevention and control of experimental autoimmune arthritis, an animal model that shares many features of rheumatoid arthritis (8-9). It is less clear what role is played by Tregs in the suppressive effect that MSCs exhibit on immune responses. Deaglio et al (10) have shown that the co-expression of CD39 (nucleoside triphosphate diphosphohydrolase-1, NTPDase 1) and CD73 (ecto-5'-nucleotidase) in Treg cells contribute to its inhibitory function.
CD39 promotes the hydrolysis of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) to generate adenosine monophosphate (AMP), which is then hydrolyzed by CD73 to adenosine. ATP is an important signaling molecule involved in many biological processes including immune responses. While MSCs are known to express CD73, it is unclear whether they also express CD39, and also whether either of these ectoenzymes participates in their immunoregulatory function. In the present study, we demonstrate that GMSCs significantly attenuate inflammatory arthritis in CIA. The therapeutic effects of GMSCs depend mainly upon CD39/CD73 signals. We also find that their effects are at least partially dependent upon the induction and expansion of regulatory T (Treg) cells in vivo, a cell type that has been recognized as playing an important role in controlling autoimmunity (11-14). These results implicate that manipulation of GMSCs may provide a promising therapeutic approach for the treatment of patients with rheumatoid arthritis and other autoimmune diseases.
MATERIALS AND METHODS
Mice
DBA/1J mice (female, 8–10 wk old) were obtained from Jackson Laboratory (Bar Harbor, ME). C57BL/6 Foxp3gfp reporter mice were generously provided by Dr. Talil Chatilla (UCLA). DBA/1J Foxp3gfp reporter mice were produced by backcrossing C57BL/6 Foxp3gfp reporter mice with DBA/1 J mice for 8-10 generations. All experiments using mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at University of Southern California.
Induction of arthritis
Bovine type II collagen (CII) was extracted and purified from bovine articular cartilage according to established protocols. CII was emulsified with an equal volume of complete Freund's adjuvant (CFA) containing 4 mg/ml heat-denatured mycobacterium (Chondrex, LLC, Seattle, WA). DBA/1J mice or DBA/1J Foxp3gfp reporter mice were immunized via intradermal injection at the base of the tail with 50 μl of emulsion (CII 100 µg/mouse). To determine intervention effects, mice received a single intravenous injection of 2×106 GMSCs on day 14 after immunization. Alternatively, a similar dose of human dermal fibroblasts (a cell line from American Type Culture Collection, Manassas, VA) was injected intravenously as a control. To deplete CD4+CD25+Foxp3+ Tregs, mice were treated intraperitoneally with 0.25 mg of anti-CD25 antibody (clone PC61) 7 days after CII immunization.
Evaluation for clinical arthritis
Clinical signs of arthritis were evaluated to determine arthritis incidence every 2–3 days. Each paw was evaluated and scored individually using a 0 to 4 scoring system (15-17). The paw scores were summed to yield an individual mouse score, with a maximum score of 16 for each animal. Each paw score was judged as follows: 0, no signs; 1, mild swelling confined to the tarsal bones or ankle joint; 2, mild swelling extending from the ankle to the tarsal bones; 3, moderate swelling extending from the ankle to the metatarsal joints; and 4, severe swelling encompassing the ankle, foot and digits, or ankylosis of the limb.
Histopathological evaluation of joints
After the animals were sacrificed on day 60, the hind limbs were collected. Following routine fixation, decalcification and paraffin embedding, tissue sections were prepared and stained with hematoxylin and eosin. All slides were evaluated by investigators blinded to the experimental conditions. The extent of synovitis, pannus formation, and bone/cartilage destruction was determined using a graded scale, as follows: grade 0, no signs of inflammation; 1, mild inflammation with hyperplasia of the synovial lining without cartilage destruction; 2 through 4, increasing degrees of inflammatory cell infiltration and cartilage/bone destruction.
Flow cytometric analysis
Ice-cooled single-cell suspensions were prepared from trypsinized GMSC cultures, GSMCs co-cultured with mouse T cells, or mouse lymphoid organs. For GMSC phenotype identification, antibodies directed against human CD11b, CD29, CD45, CD73, CD86, CD90, MHC-II or isotype-matched control IgGs were from BD PharMingen, human CD31, CD34, CD44, CD105, MHC-1 and isotype IgG from eBioscience. Antibodies against CD4 (RM4-5), IFN-γ, IL-4, IL-17 were from eBioscience. Antibodies to Helios and CD39 were from Biolegend. Synovial fluid from two knee joints of each mouse with arthritis was collected and flushed out using 10 ml PBS via 25G needle. This method usually yields 1~6×104 cells from normal mice and 3~10×104 cells from arthritic mice. For mouse Treg cell identification in vivo, results were obtained on a BD FACS Calibur flow cytometer and analyzed using FlowJo.
Cytokine analysis
T cells were isolated from spleens and draining lymph nodes of arthritic mice at day 60 after CII immunization, then stimulated in vitro with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 5h, with brefeldin A (10 μg/ml; all from Calbiochem) for 4h, and intracellular IL-4, IL-17, IFN-γ, TNF-α, IL-2 and IL-10 expression was analyzed by flow cytometry.
Murine naïve CD4+ T cell differentiation in vitro
Naïve CD4+CD25−CD62L+ T cells were purified from spleens of DBA/1 mice via magnetic isolation (Miltenyi Biotec, Auburn, CA). GMSCs were co-cultured with naïve CD4+CD25−CD62L+ T cells (1:25) during their in vitro differentiation into T helper cells. GMSCs were allowed to adhere to plate overnight before co-culture. Naïve CD4 cells were stimulated with anti-CD3 (2 μg/ml; Biolegend) and anti-CD28 (2 μg/ml; Biolegend) in the presence of irradiated (30 cGy) syngeneic non-T cells, plus cytokines for Th1, Th2, or Th17 cell polarization differentiation as previously described (18). After 3 days in culture, differentiated cells were re-stimulated with PMA and Ionomycin for 5 hours and BFA for 4 hours, IFN-γ, IL-4 and IL-17 expression was measured by flow cytometry.
In vitro suppression assays
To examine the suppressive activity of GMSCs in vitro, mouse splenic T cells isolated with nylon wool or splenic CD4+CD25− cells isolated using magnetic isolation as above from DBA/1 mice were stimulated with anti-CD3 (0.025 μg/ml) and irradiated (30 cGy) APCs. GMSCs were plated in triplicate in 96-well plates and allowed to adhere to the plate overnight. The ratio of GMSCs to mouse CD4+CD25− T cells ranged from ratios of 1:1 to 1:200. Cells were cultured for 3 days and 1 μCi/well of 3H-thymidine was added for last 18 hours of culture as previously reported (19).
To assess the possibility that GMSCs may induce mouse T cell death, CD4+CD25− T cells labeled with CFSE (Invitrogen) were stimulated with soluble anti-CD3 (0.025 μg/ml) with irradiated non-T cells as APCs (1:1). A gradient of GMSCs were added to CD4+CD25− T responder cells (GMSC/Tresp) at a ratio of 1:1-1:200, and suppression of cycling CFSE-labeled CD4+CD25− T cells was assessed on the gate of CD4+CFSE+7-AAD− cells.
To determine the dependence of the suppressive function of GMSCs on cell contact, a Transwell system was used. Briefly, these experiments were performed in 24-well Transwell plates with 0.4 µm pore membranes (Corning Costar). 1×106 mouse CD4+CD25− cells and 1×106 irradiated APCs were seeded to the upper compartment of the chamber, while GMSCs (2×105) were seeded to the lower compartment. Cells were cultured in the presence of anti-CD3 for 72 h and analyzed as described above.
In some experiments, mouse CD4+CD25− T cells were co-cultured with GMSCs (1:25) and stimulated with anti-CD3 (0.025 μg/ml) in the presence of soluble factors including CD39 inhibitor (Sodium polyoxotungstate [POM1]; Tocris Bioscience; 100 μM), CD73 inhibitor (α,β-methylene ADP [APCP]; Sigma-Aldrich; 100 μM), selective A2A adenosine receptor competitive antagonist (SCH58261; Tocris Bioscience; 25 μM), selective A2B adenosine receptor antagonist (Alloxazine; Sigma-Aldrich; 10 μM), heme oxygenase-1 (HO-1) inducer (Hemin; Sigma-Aldrich; 50 ng/ml), selective HO-1 inhibitor (zinc protoporphyrin IX[Zn(II)PPIX]; Frontier Scientific, Inc; 50 ng/ml), selective cyclooxygenase(COX)-1 inhibitor (indomethacin; Sigma-Aldrich; 20 μM), indoamine-2,3-dioxygenase (IDO) inhibitor (1-methyl-L-tryptophan [1-MT]; Sigma-Aldrich; 500 μM), nitric oxide synthase (NOS) inhibitor ( NG-nitro-L-arginine methylester hydrochloride [L-NAME], Sigma-Aldrich; 1 mM), selective COX-2 inhibitor (NS398; Tocris Bioscience; 10 μM), anti-TGF-β (BD PharMingen; 10 μg/ml) or anti-IL-10R (R&D System; 10 μg/ml). Proliferation was determined with 3H-thymidine incorporation.
Statistical analysis
For comparison of treatment groups, we performed unpaired t-tests (Mann-Whitney), paired t-tests, and one-way or two-way ANOVA (where appropriate) methods. Percent comparisons were done using the chi-square test. All statistical analyses were performed using GraphPad Prism Software (version 4.01). The p<0.05 is considered as statistically significant.
RESULTS
GMSCs suppressed mouse T cell proliferation and differentiation through CD39/CD73 signals
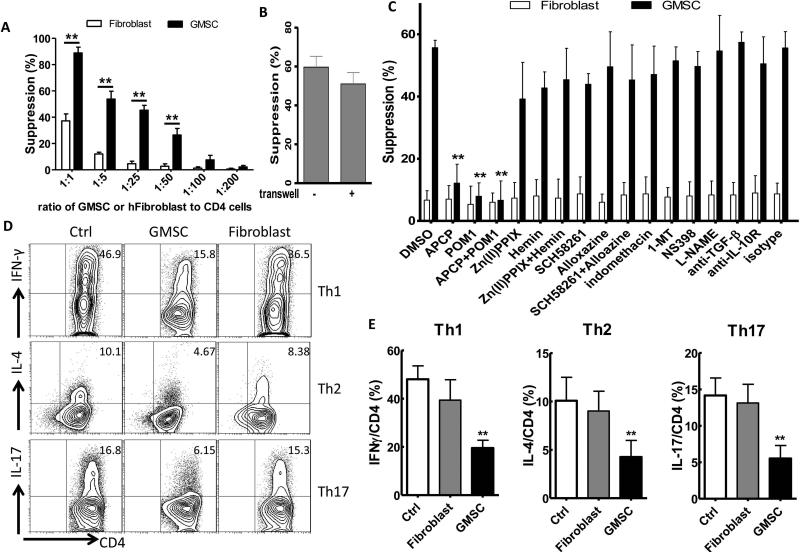
We and others have recently shown that GMSCs display similar immunomodulatory properties like human BMSCs (hBMSCs) including the inhibition of human T cell activation and proliferation (3-4, 20-21). To determine whether GMSCs have immunosuppressive effects on mouse CD4+ T lymphocytes in response to TCR stimulation in vitro, we co-cultured these cells and found that the GMSCs inhibited the proliferation of mouse CD4+CD25- T cells in a dose dependent fashion (Figure 1A, Figure S1A,B). Control human fibroblast cells showed significantly less suppression than GMSC in vitro (Figure 1A). When using a Transwell system in which GMSCs and CD4+CD25- T cells were physically separated, GMSCs still inhibited mouse T cell proliferation (Figure 1B, Figure S1A), which suggests that the soluble factor(s) secreted by GMSCs play a main role in the suppressive function of GMSCs.
Figure 1.
GMSCs inhibit mouse CD4+ T lymphocytes proliferation and differentiation in vitro. A and B, Co-culture with or without gradient doses of GMSCs (or a human fibroblast cell line), CFSE-labeled mouse CD4+ T cells were stimulated with soluble anti-CD3 and irradiated non-T cells for 72h (A). Transwell assays were used with a 1:5 ratio of GMSC to mouse CD4+ T cells (B). The proliferation (CFSE dilution) of CD4+ T cells was analyzed by flow cytometry. Cells were gated on live cell (CFSE+7-AAD− cells). C, CFSE-labeled mouse CD4+ T cells were co-cultured with GMSCs (1:25) in the presence of soluble factors as indicated in the panel and proliferation was determined as before. The data are presented as the mean ± SEM from three separate experiments. D, Naïve CD4+ T cells, with or without GMSCs or human fibroblast cells (1:25) were co-cultured for 3 days under Th1, Th2, Th17 polarization conditions. Intracellular cytokines were analyzed by flow cytometry. Data are representative of three separate experiments. E, Summarized data of IFNγ, IL-4, IL-17 expression on CD4 cells as in panel D. Data are presented as the mean ± SEM. **P< 0.001 versus control group.
To explore what mechanisms are responsible for GMSC-mediated suppression, we analyzed several potential candidates. To this end, we demonstrated that GMSCs inhibited mouse T cell proliferation via a process that is dependent on CD73 and CD39 signals. We also observed that the TGF-β, indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2) pathways were not involved (Figure 1C, Figure S1C). As a control to determine if any fibroblast cell can mediate this suppression, we have used a human epidermal fibroblast cell line that is also differentiated from mesenchymal stem cells (22). We observed that fibroblast did not inhibit T cell proliferation in vitro, though they express CD73 but they do not express CD39 (Figure 1C, Figure S2).
In order to rule out the possibility that the human-derived gingival cells might kill the murine T cells to non-specifically suppress T cell responses, we labeled the latter with CFSE and measured the inhibition of proliferation (CFSE dilution) of responder T cells by gating on CD4+CFSE+7-AAD− live cells. We found a 50% of suppression against CD4+ cell proliferation at a ratio of 1:25 (GMSC to T responder cells) (Figure 1A), suggesting that cell killing was not involved. Furthermore, GMSCs but not fibroblast cell also significantly inhibited mouse Th1, Th2, Th17 cell differentiation in vitro (Figure 1D and E).
Decreased severity of experimental arthritis following treatment with GMSCs
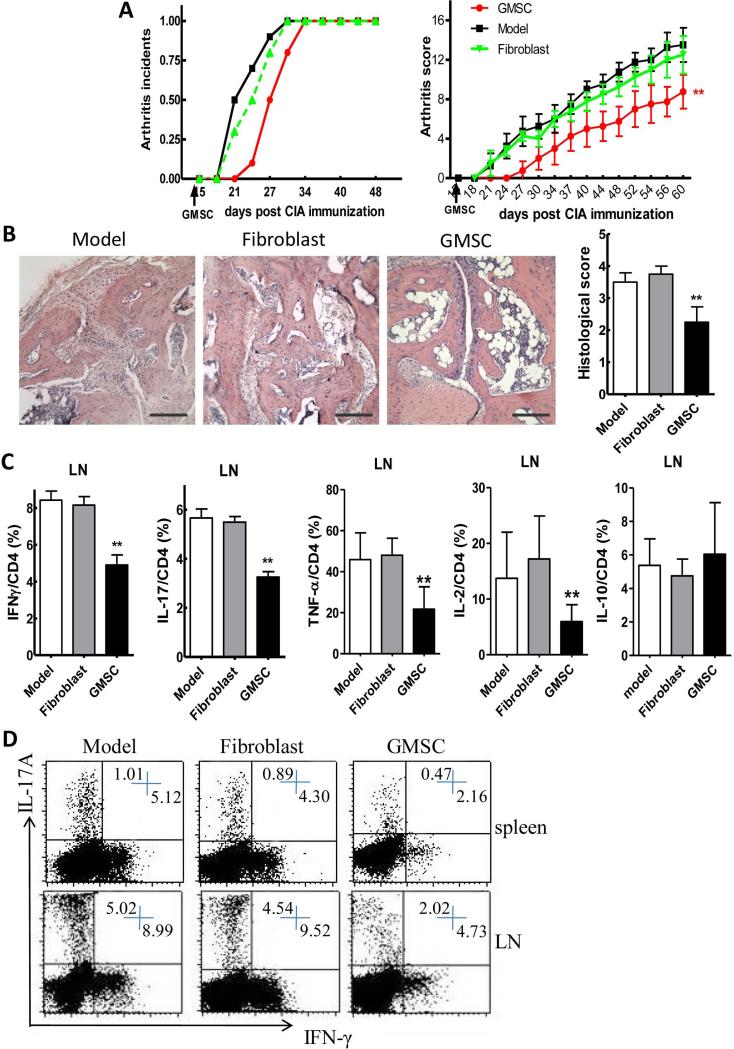
To determine the immunomodulatory role of GMSCs in the context of autoimmune arthritis, we relied on the CIA model. We observed a significant delay in disease onset and a decrease in severity scores following a single injection of GMSCs on day 14 after CII/CFA immunization (Figure 2A). Histological and quantitative analysis of whole ankle joints demonstrated a significant decrease in synovitis, pannus formation and destruction of bone and cartilage in GMSC treated mice compared with controls (Figure 2B). Because mouse skin fibroblasts have been shown to suppress the inflammatory response in a mouse model of autoimmune arthritis (23), we chose human skin fibroblast as a control for the human derived gingival stem cells. The human skin fibroblasts exhibited no protective effect in mouse CIA model (Figure 2A and B).
Figure 2.
GMSCs protect DBA/1 mice from collagen-induced arthritis when administered before onset of inflammation. DBA/1 mice were immunized with collagen II (CII) emulsified with CFA. At day 14 after CII immunization, 2×106 GMSCs or human skin fibroblast cells were injected into mice via tail vein. GMSCs were obtained from different donors and used after 2-6 passages. A, Incidence of arthritis and clinical arthritis scores. B, Hematoxylin and eosin (H&E)-stained sections and evaluation of synovitis, pannus, and erosion of ankle joints in CIA mice 60 days after primary immunization. Scale bar, 100 μm. The pathology scores of HE stained section were shown in right panel. Six mice were included in each group and data were combined from two independent experiments. C, Expression of cytokines, including IFNγ, IL-17, TNF-α, IL-2, IL-10, on CD4 positive cells in the draining LNs of CIA mice. Data are presented as the mean ± SEM from two independent experiments (n=6). **P<0.01, versus the fibroblast or model group. D, Representative data of IFNγ and IL-17 Expression gated on CD4 positive cells in draining LN.
Down-regulation of the inflammatory responses in CIA following treatment with GMSCs
We next investigated the mechanisms underlying the decrease in severity of CIA following administration of GMSCs. GMSC injection significantly reduced the percentage of cells secreting proinflammatory cytokines IFN-γ, IL-17, TNF-α in the draining lymph node in CIA mice (Figure 2C). GMSC treated mice produced consistently lower percentages of Th1 and Th17 cells (Figure 2C and D). In addition, GMSC treatment also decreased IL-2 production from mouse CD4+ T effector cells but did not significantly change IL-10 production (Figure 2C). In contrast, the frequency of cells producing Th2-type cytokines IL-4, IL-5 and IL-13 was almost undetectable in this model and GMSC treatment did not alter their levels (data not shown).
Promotion of Treg cells in CIA following treatment with GMSCs
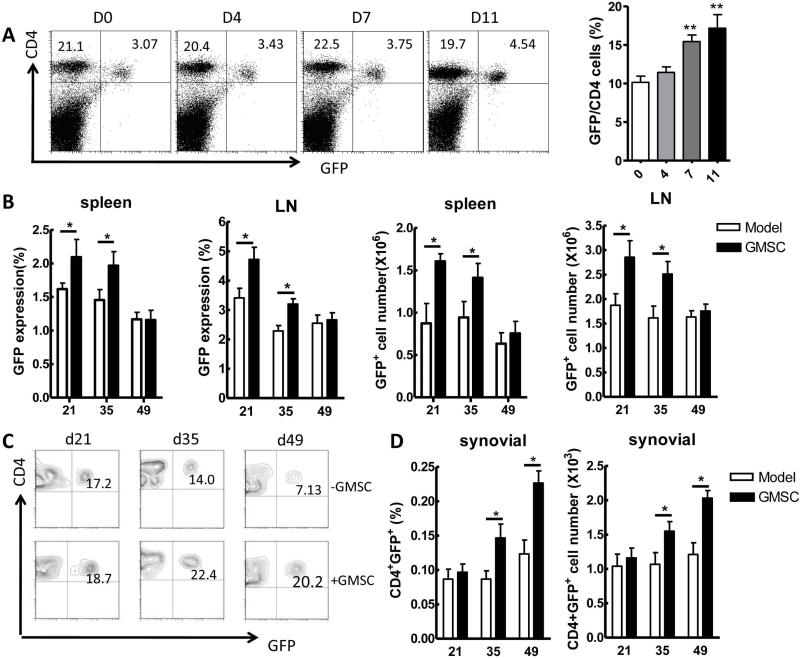
Several studies have indicated that Treg cells confer significant protection against CIA by decreasing the activation and joint homing of autoreactive Th1 cells, and inhibiting osteoclastogenesis (9, 24-26). To determine the relationship of GMSCs with Treg cells in vivo, we first infused GMSCs to naive DBA/1 Foxp3gfp reporter mice. As shown in Figure 3A, GMSCs significantly increased CD4+Foxp3+ cell frequency in the spleens and LNs 1 week after injection in these mice. Treg cell frequency reached a peak on day 11 after GMSC infusion. However, Treg levels returned to baseline values 2 weeks after GMSC injection in naive mice (data not shown).
Figure 3.
GMSCs induce Treg cells in vivo. A, Foxp3gfp reporter DBA/1 naïve mice were injected intravenously with GMSCs (2×106). CD4+Foxp3+(GFP+) Tregs were counted in the spleens on day 0, 4, 7, 11 after GMSC injection. Each group in each time points has five mice. Data are representative of two separate experiments and mean ± SEM of each group is shown. **P<0.01 versus the control group. B-D, Foxp3gfp reporter DBA/1 mice were immunized with CII and CFA. GMSCs (2×106) were injected to mice via tail vein at day 14 after immunization. Six mice were included in each time-point and experiment was repeated twice. Percentages and absolute numbers of Foxp3+(GFP+) in spleen and LNs at day 21, 35, 49 after immunization (B). Representative flow data of CD4+Foxp3+ frequency in joint synovial fluid of GMSC-treated CIA mice (C). Frequency and total numbers of CD4+Foxp3+ in joint synovial fluid of GMSC-treated mice (D). Data in B and D are presented as the mean ± SEM of two separate experiments (n=6). *P<0.05 versus untreated group.
We next investigated the dynamics of Treg cells in CIA mice using Foxp3gfp reporter mice on the DBA/1J background. In line with other reports that GMSC treatment increases the expression of Foxp3 in inflamed colon tissues in DSS-induced experimental colitis mice (3), our results revealed that GMSCs were also able to induce Treg responses in CIA mice (Figure 3B). The percentage of cells expressing Foxp3 in the spleens and draining LNs was significantly increased at 1 week and 3 weeks after GMSC injection. However, the increased Foxp3+ cell frequency in spleens and draining LNs gradually declined to levels that were similar to control groups by 5 weeks following cell infusion (Figure 3B). Interestingly, we began to observe a significant upregulation of Foxp3+ cell frequency in the synovial fluid of CIA mice 3 weeks after GMSC infusion although this increase was not observed in early stages (Figure 3C and D).
iTreg but not nTreg cells increased after GMSC treatment
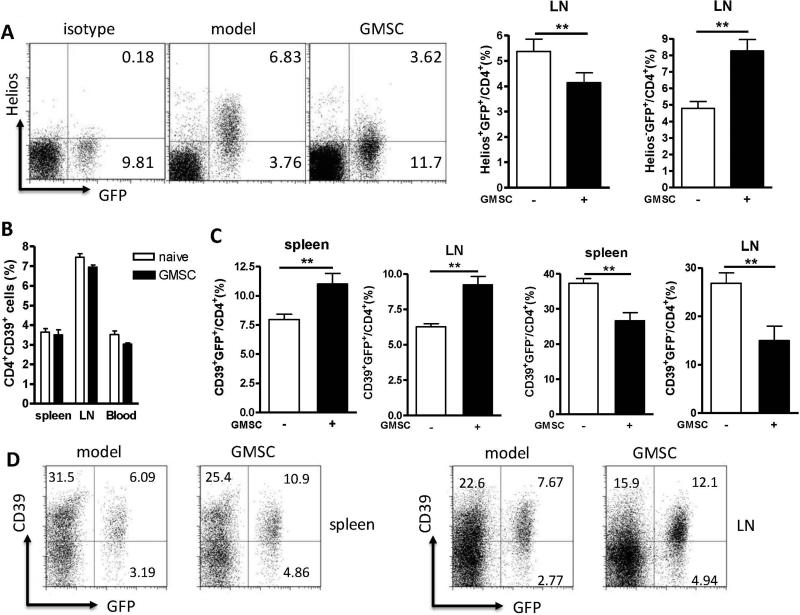
A study has recently revealed that expression of Helios, an Ikaros transcription factor family member, might distinguish thymus-derived natural Treg cells (nTreg) from induced Treg cells (iTreg) (27-29). To identify the phenotypes of increased Foxp3+ cells in GMSC-treated CIA mice, we showed that the majority of the expanded Treg cell population was Helios negative (Figure 4A). Similarly, most of the Foxp3+ cells in the synovial fluid also did not express Helios (data not shown), suggesting that GMSC treatment may induce the generation of new iTreg cells rather than the expansion of endogenous nTreg cells in CIA.
Figure 4.
GMSCs increase the frequency of iTregs but not nTregs in CIA model, most of which are CD4+Foxp3+(GFP+)CD39+Helios− Treg cells. Foxp3gfp reporter DBA/1 mice were immunized with CII and CFA. 2×106 GMSCs were injected to mice via tail vein on 14 days after CII immunization. Mice were sacrificed after a week. Each experiment includes five mice per group and experiment was repeated twice. A, Representative flow cytometric data of the Heilos expression in draining LNs. Cells were gated on CD4 positive cells. The frequency of CD4+Foxp3+Helios+ cells in draining LNs is shown in the right panel. B, Frequency of CD4+CD39+ cells in the spleens, LNs and blood. C, Frequency of CD4+Foxp3+CD39+ or CD4+Foxp3−CD39+ cells in the spleens and LNs. D, Representative flow cytometric data of CD39+Foxp3+ cell frequency gated on CD4+ cells in the spleens and LNs. Values in A, B and C were mean ± SEM of two separate experiments (n=5). *P<0.05, **P<0.01 versus the untreated group.
Given that a population of CD4+CD39+ cells comprised of TGF-β-producing Foxp3−CD39+CD4+ T cells and IL-10-producing Foxp3+CD39+CD4+ T cells has been shown to have a regulatory function in CIA model (30), we sought to investigate whether CD4+CD39+ T cells were affected by GMSC treatment in CIA model. We found that there was no alteration of the percentages and total numbers of CD4+CD39+ T cells after GMSC treatment (Figure 4B). CD4+Foxp3+ Treg cells expressed higher CD39 levels than CD4+Foxp3− non-Treg cells, while the expression of CD39 on Helios+ or Helios− Treg cells was the same (Figure S3). Nonetheless, GMSC treatment increased the CD39 positive population in CD4+Foxp3+ cells while it suppressed CD39 expression in CD4+Foxp3− cells (Figure 4C and D). Taken together, it is likely that GMSC treatment can selectively induce the CD4+Foxp3+CD39+Helios− Treg subset in CIA.
The suppressive effect of GMSCs on CIA was partially dependent on Treg cells in vivo
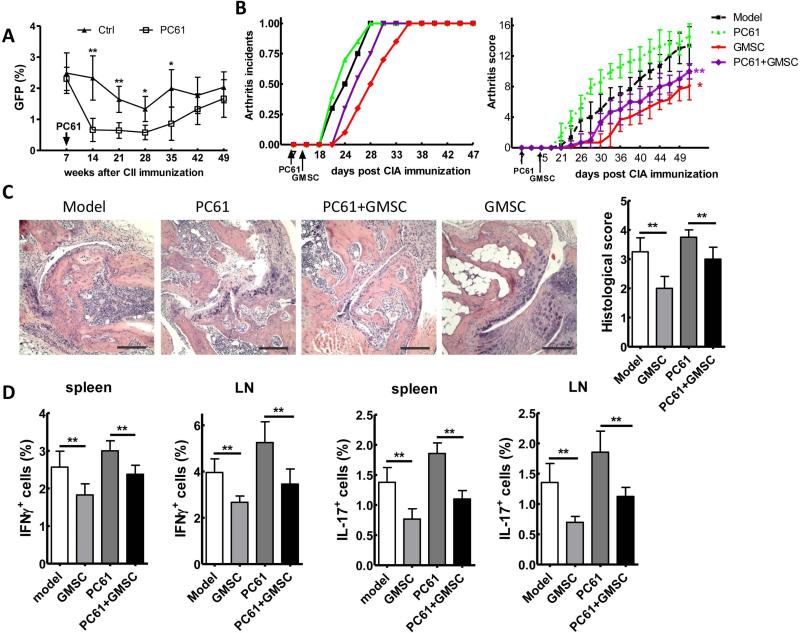
Due to the important role of Treg cells in controlling CIA progress, we next asked whether GMSC treatment is dependent on Treg function. To determine this, Tregs were depleted in arthritic mice by i.p. administration of PC61, an anti-CD25 antibody. CD4+Foxp3+ Tregs in the spleens decreased significantly 1 week after PC61 administration. The Treg depletion was maintained for 4 weeks until the Treg levels started to restore after 5 weeks of PC61 treatment (Figure 5A).
Figure 5.
GMSCs attenuate the inflammation of arthritis, which partially depends on the regulatory T cells. A, DBA/1 Foxp3gfp reporter mice were immunized with CII/CFA. At day 7, mice were injected i.p. with PC61 (anti-CD25 monoclonal antibody) 250 μg/mouse or control PBS. CD4+Foxp3+ cell frequency (mean ± SEM) was counted in the spleens on time-points as indicated. Each experiment includes five mice per group and experiment was repeated twice. *P<0.05, **P<0.01 versus the PC61 treatment group. B-D, DBA/1 mice were immunized with CII/CFA, and/or followed by PC61 i.p. injection on day 7 and/or followed by i.v. 2×106 GMSC infusion on day 14. Incidence of arthritis and clinical arthritis scores (B). H&E stained sections and evaluation of synovitis, pannus formation, and erosion of tarsal joints in CIA mice. Scale bar, 200 μm. Pathology scores of H&E sections in each group were shown in the right panel (C). Average frequency of IFNγ+ and IL-17+ cells in the spleens and draining LNs (D). Data in B, C and D are presented as the mean ± SEM of two separate experiments (n=6). *P<0.05, **P<0.01 versus the GMSCs+PC61 group, or versus the PC61 group.
We immediately determined the role of Treg cells in GMSC-mediated CIA suppression. Treatment with PC61 resulted in accelerated incidence of arthritis (Figure 5B), significantly increasing arthritis severity scores (Figure 5B) and a severe histological damage of joint (Figure 5C). In addition to PC61 treatment, GMSC injection still delayed the disease onset and reduced the arthritis severity scores compared with PC61 treatment alone (Figure 5B). However, compared to GSMCs alone treatment, GMSC and PC61 treatment group showed significantly higher arthritis severities (Figure 5B), suggesting that the depletion of Treg cells in vivo has partially attenuated the protective effect of GMSC treatment. Similarly, GMSC treated with PC61 together produced lower frequencies of Th1 and Th17 cells compared to PC61 alone treatment, and significantly higher percentages of IFN-γ and IL-17 producing cells compared to GMSC alone treatment (Figure 5D). Taken together, these data suggest that GMSC treatment can still protect mice from arthritis after Treg depletion, but the therapeutic effect of GMSCs is significantly less in Treg-depleted than in Treg-intact group. Thus, the GMSCs-mediated immunoregulatory function in vivo may be attributed to Treg cells but also to other mechanisms.
GMSCs suppressed CIA that was dependent on CD39 and CD73 signals
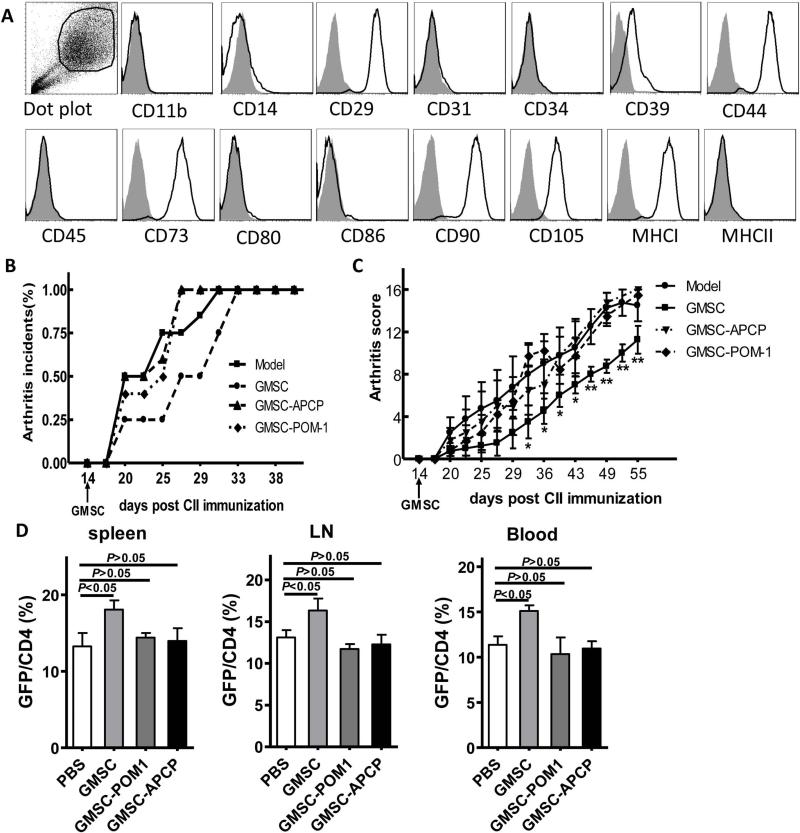
We next ask what kind of mechanism(s) was involved in GSMC function. Consistent with previous reports, we showed that GMSC cells were homogenously positive for mesenchymal marker CD29, CD44 and CD105; negative for hematopoietic marker CD34 and CD45; endothelial cell markers CD31; negative for co-stimulating molecules CD86 (31-32). Furthermore, CD39 and CD73 are also phenotypic characteristics of GMSCs (Figure 6A). We have also shown that GMSCs inhibited mouse T cell proliferation via CD39 and CD73 signals (Figure 1B). It has been reported that pretreatment of GMSCs with the COX inhibitor indomethacin significantly reversed their inhibitory effect on DC differentiation and cytokine secretion in vitro, and that these cells lost their capacity to attenuate contact hypersensitivity after injection into mice in vivo (21). Overnight treatment of indomethacin can maintain its inhibition on GMSC for at least one week (21). Considering the expression of CD39 and CD73 in mouse T cells (33-34), it is not feasible to treat mice with CD39 and CD73 inhibitors due to their pleiotropic effects. Instead, to determine whether CD39 and/or CD73 signals are involved in the mechanism of GMSC protection for CIA, we pre-treated GMSCs with POM-1 (CD39 inhibitor, 100 μM) or APCP (CD73 inhibitor, 100 μM) overnight in vitro, and then injected these cells to arthritic mice. We found that the expression of CD39 or CD73 on GMSC cells were significantly reduced after their inhibitor POM-1 or APCP treatment separately. The cell avidity of GMSCs did not change following APCP and POM-1 treatment (Figure S4). Furthermore, blocking the activity of CD39 or CD73 in GMSCs significantly reversed the protective effect of GMSCs on CIA progression, inducing accelerated incidences of arthritis (Figure 6B) and increased arthritis severity scores (Figure 6C), suggesting that both CD39 and CD73 are important for GMSCs in controlling T cell-mediated diseases.
Figure 6.
GMSCs attenuate inflammation responses in CIA mice via CD39 and/or CD73 signals. A, Analysis of GMSCs surface proteins by Flow cytometry. Fifth-passage GMSCs were stained with antibodies as indicated. B-D, DBA/1 mice were immunized with CII/CFA. 2×106 GMSCs pretreated with or without APCP (100 μM) or POM-1 (100 μM) overnight have been injected i.v. into DBA/1 mice on day 14 after CII immunization (n=6 each group and experiment was repeated twice). Incidences of arthritis of DBA/1 mice (B). Clinical arthritis scores in the indicated groups. The data are presented as the mean ± SEM. *P<0.05, **P<0.01 versus the model group (C). Foxp3+GFP+ cells in spleen, LN, Blood were examined by flow cytometry after 1 week of GMSC injection. Data are presented as the mean ± SEM of two separate experiments (n=6) (D).
We next investigated the role of CD39 and CD73 signals in the GMSC-mediated up-regulation Treg in vivo. To address this problem, we treated GMSC with CD39 or CD73 inhibitors overnight and then infused GMSC to CIA mice. We found that Foxp3+ Treg frequencies in GMSC treated group were identical with those in control mice one week following cell infusion, indicating that GMSC pre-treatment with CD39 or CD73 inhibitors results in the abrogation of their ability to increase Treg frequencies and protection from CIA (Figure 6D).
DISCUSSION
Mesenchymal stem cells (MSCs) are a variety of stromal cells that have the potential to self-renew and differentiate. The MSC's immunosuppressive function is ascribed to their inhibitory effects on T cells and other immune cells including DCs and NK cells in vitro (35-36). It has been reported that human BMSCs strongly suppress T-lymphocyte proliferation via mechanisms that do not involve the induction of T cell apoptosis but are likely due to the production of soluble factors, including hepatocyte growth factor (37), PGE2 (38), heme oxygenase-1 (HO-1) (39), TGF-β1 and NO (7). In this study, we observed that GMSCs suppress mouse T cell responses and that cell contact is not necessary for this suppression, suggesting that soluble factors are involved in this mechanism. However, to our knowledge it is the first time to show that GSMCs inhibit mouse T cell proliferation via CD39 and/or CD73 signals but not IL-10, NO, IDO, PGE2 and TGFβ1 in vitro. The species used as a source of cells may lead to the different results.
Extra- and/or immediate pericellular accumulation of adenosine, released by damaged cells as an indicator of trauma and cell death elicits immunosuppressive cellular responses that is mediated through several type 1 purinergic (adenosine) receptors, including A2A adenosine receptor (10, 40). The activation of A2A-mediated signals can attenuate T cell-mediated experimental colitis by suppressing the expression of proinflammatory cytokines in a manner independent of both IL-10 and TGF-β (41). Recent reports show that co-expression of CD39 and CD73 on the CD4+CD25+Foxp3+ regulatory T cells, that catalyses the sequential generation of adenosine by degradation of extracellular ATP/ADP to 5’-AMP (CD39) and conversion of 5’-AMP to adenosine (CD73), leads to strong down-regulation of T cell proliferation and a decreased secretion of proinflammatory cytokines (10, 34, 40). Here, we identified that GMSCs express CD39 and CD73 supporting the generation of adenosine and thereby promoting strong immunosuppression of effector T cells in vitro and in vivo. Not only can GSMCs promote the Foxp3+ Treg cell frequencies and possible migration in inflammatory disease in vivo, these cells also share part of mechanisms of immune suppression functions indirectly via adenosine.
GMSCs may directly or indirectly suppress CIA. As GMSCs express CD39 and CD73 and both 5’-AMP and adenosine have a potent immunosuppressive activity, it is reasonable that GMSCs suppress CIA in a CD39 or CD73 dependent manner. However, GMSCs may also promote Tregs through CD39 and CD73 signaling since pretreatment of GMSC with CD39 or CD73 inhibitors abrogates GMSC-mediated Treg upregulation. We have demonstrated that the suppressive effects of GMSCs on CIA is at least in part dependent upon Tregs, supporting the theory that GMSCs exert their immunosuppressive function via direct suppression of inflammatory cell responses and indirect immunoregulation function via increased induced Treg cells.
Multiple reports have shown that the immunoregulatory function of MSCs is associated with upregulated Treg cells in vivo (6-7, 42). Recently a population of CD4+CD39+ T cells was identified as having a regulatory function in the CIA model. This subset is composed of TGF-β-producing Foxp3−CD39+CD4+ T cells and IL-10-producing Foxp3+CD39+CD4+ T cells, each of which plays an important role in autoimmune diseases (30). Our results suggest that GMSCs selectively promote the production of Foxp3+CD39+CD4+ Treg subset in naïve mice and in the pro-inflammatory CIA disease model. Although it is arguable whether Helios can distinguish nTreg from iTreg, our data suggest that increased Foxp3+CD39+Helios− cells are a new cell population that may have been induced in CIA. Although the frequency of Treg is increased temporally in naïve mice, it is notable that GMSCs sustain the increased CD39+Foxp3+ Treg cells in CIA. It is unknown whether the inflammatory environment affects the function of GMSCs. Interestingly, whereas increased Treg frequency in the spleen and LN gradually declined, increased frequencies of Foxp3+ cells were observed in the synovial fluid in CIA 3 weeks after GMSC treatment. As MSCs may have difficulty in obtaining access to the joints, it is possible that soluble factors secreted by GMSCs may regulate Treg induction in the joints or promote the increased frequency of Treg cells in the periphery, resulting in Treg migration into synovial fluid in CIA.
In conclusion, we have demonstrated for the first time that GMSCs can inhibit T cell responses and T cell-mediated diseases via CD39/CD73 signals. GMSCs exert immunoregulatory functions in the CIA model directly and/or indirectly. GMSCs promote the induction of CD39+Foxp3+ Treg cells and these cells play a role in the GMSC-mediated suppression in CIA. These findings further support the notion that GMSCs, a unique population of MSCs with functional similarities to BMSCs, are a promising cell source for stem cell-based therapies of inflammatory diseases and transplantation.
Supplementary Material
Acknowledgments
Supported by the National Institute of Health (AR059103 and AI084359), ACR Within Our Reach Fund, Arthritis Foundation and Wright Foundation, National Nature Science Foundation of China (No. 30972951); Science and Technology Planning Project of Guangdong Province, China (No. 2010B031600200) and Science and Technology Committee Project of Shanghai Pudong new area (PKJ2009-Y41). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Zheng had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Zheng, Le, He, Huang.
Acquisition of data. Chen, Su, Lin, Guo, Wang, Zhang.
Analysis and interpretation of data. Chen, Lin, Guo, Huang, Liu, Brand, Ryffel.
Reference
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(5):321–34. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183(12):7787–98. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393(3):377–83. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P, Krasieva TB, et al. Three-Dimensional Spheroid Culture of Human Gingiva-Derived Mesenchymal Stem Cells Enhances Mitigation of Chemotherapy-Induced Oral Mucositis. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60(4):1006–19. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Mu R, Wang S, Long L, Liu X, Li R, et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12(6):R210. doi: 10.1186/ar3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey O, Reichel A, Bonhagen K, Morawietz L, Rauchhaus U, Kamradt T. Regulatory T cells control the transition from acute into chronic inflammation in glucose-6-phosphate isomerase-induced arthritis. Ann Rheum Dis. 2010;69(8):1511–8. doi: 10.1136/ard.2009.123422. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185(5):2675–9. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172(9):5213–21. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 13.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr., Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172(3):1531–9. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 14.Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Guo Zheng S. Induced Foxp3+ regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4(1):22–8. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2(5):1269–75. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 16.Kong N, Lan Q, Chen M, Wang J, Shi W, Horwitz DA, et al. Antigen-specific TGF-beta-induced regulatory T cells but not natural Tregs ameliorate autoimmune arthritis by shifting the balance of Th17 toward Treg cells. Arthritis Rheum. 2012 doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong N, Lan Q, Chen M, Zheng T, Su W, Wang J, et al. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35(4):622–32. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Wang J, Shi W, Brand DD, Liu Z, Fan H, et al. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J Mol Cell Biol. 2010;2(3):164–9. doi: 10.1093/jmcb/mjq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29(11):1849–60. doi: 10.1002/stem.738. [DOI] [PubMed] [Google Scholar]
- 22.Shamis Y, Hewitt KJ, Carlson MW, Margvelashvilli M, Dong S, Kuo CK, et al. Fibroblasts derived from human embryonic stem cells direct development and repair of 3D human skin equivalents. Stem Cell Res Ther. 2011;2(1):10. doi: 10.1186/scrt51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouffi C, Bony C, Jorgensen C, Noel D. Skin fibroblasts are potent suppressors of inflammation in experimental arthritis. Ann Rheum Dis. 2011;70(9):1671–6. doi: 10.1136/ard.2010.143297. [DOI] [PubMed] [Google Scholar]
- 24.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52(7):2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 25.Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68(5):744–50. doi: 10.1136/ard.2007.086066. [DOI] [PubMed] [Google Scholar]
- 26.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50(9):2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 27.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elkord E. Comment on “expression of helios in peripherally induced foxp3+ regulatory T cells”. J Immunol. 2012;189(2):500. doi: 10.4049/jimmunol.1290033. [DOI] [PubMed] [Google Scholar]
- 29.Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188(3):976–80. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 30.Kochetkova I, Thornburg T, Callis G, Pascual DW. Segregated regulatory CD39+CD4+ T cell function: TGF-beta-producing Foxp3- and IL-10-producing Foxp3+ cells are interdependent for protection against collagen-induced arthritis. J Immunol. 2011;187(9):4654–66. doi: 10.4049/jimmunol.1100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, et al. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev. 2011;20(12):2093–102. doi: 10.1089/scd.2010.0523. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P, Krasieva TB, et al. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012;21(6):937–47. doi: 10.1089/scd.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9(10):2303–11. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5'-adenosine monophosphate to adenosine. J Immunol. 2006;177(10):6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13(3):263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 36.Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184(10):5885–94. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 37.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 39.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–4. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 40.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 41.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177(5):2765–9. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 42.Park MJ, Park HS, Cho ML, Oh HJ, Cho YG, Min SY, et al. Transforming growth factor beta-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum. 2011;63(6):1668–80. doi: 10.1002/art.30326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.