Abstract
Policy Points.
Despite beliefs that baby boomers are healthier than previous generations, we found no evidence that the health of baby boomers is substantially different from that of the previous or succeeding cohorts.
The effects of increased education, higher income, and lower smoking rates on improving self-rated health were nearly counterbalanced by the adverse effect of increasing body mass index (BMI).
Assumptions that baby boomers will require less health care as they age because of better education, more prosperity, and less propensity to smoke may not be realized because of increases in obesity.
Context
Baby boomers are commonly believed to be healthier than the previous generation. Using self-rated health (SRH) as an indicator of health status, this study examines the effects of age, period, and birth cohort on the trajectory of health across 4 generations: World War II (born between 1935 and 1944), older baby boomers (born between 1945 and 1954), younger baby boomers (born between 1955 and 1964), and Generation X (born between 1965 and 1974).
Methods
We analyzed Canada’s longitudinal National Population Health Survey 1994-2010 (n = 8,570 at baseline), using multilevel growth models to estimate the age trajectory of SRH by cohort, accounting for period and incorporating the influence of changes in education, household income, smoking status, and body mass index (BMI) on SRH over time.
Findings
SRH worsened with increasing age in all cohorts. Cohort differences in SRH were modest (p = 0.034), but there was a significant period effect (p = 0.002). We found marked cohort effects for increasing education, income, and BMI, and decreasing smoking from the youngest to the oldest cohorts, which were much reduced (education and smoking) or removed (income and BMI) once period was taken into account. At the population level, multivariable analysis showed the benefits of increasing education and income and declines in smoking on the trajectory of improving SRH were almost counterbalanced by the effects of increasing BMI (obesity).
Conclusions
We found no evidence to support the expectation that baby boomers will age more or less healthily than previous cohorts did. We also found that increasing BMI has likely undermined improvements in health that might have otherwise occurred, with possible implications for the need for health care. Period effects had a more profound effect than birth cohort effects. This suggests that interventions to improve health, such as reducing obesity, can be targeted to the entire, or a major portion of the, population and need not single out particular birth cohorts.
Keywords: self-rated health, age-period-cohort, socioeconomic status, obesity
A major concern for the provision of health and social services is the aging of the huge baby boomer generation and the fear that meeting their needs will overwhelm the current system. The impetus for our study was the tension between references to the health of the aging baby boomer population in the popular media and the scientific literature. In the media, the claims “60 is the new 40” and “50 is the new 40” exemplify the belief that baby boomers are healthier than their counterparts in previous generations. A Google search for these tags pulled up at least a quarter million hits for each. Typical examples are the headline “60 is the new 40” that accompanied the announcement of Oprah Winfrey's 60th birthday1 and the casual mention of “50 is the new 40” in connection with Michelle Obama's 50th birthday.2 A more substantial example is the series of summit meetings for social workers and health care professionals that led to the publication in 2005 of a book titled Baby Boomers: Can My Eighties Be Like My Fifties?3 In contrast, the general tenor in the scientific literature is concern about the magnitude of the future health care burden resulting from the deteriorating health of aging baby boomers. To date, there is little scientific evidence available addressing how the health of baby boomers compares with that of earlier generations. While it is certain that there will be a profound impact on the use of health and social services by the greater number of older people in the population, an important question for health policy and planning is whether this impact might be larger or smaller because baby boomers’ characteristics and health behaviors are different from those of their older and younger counterparts.
The Premise of Our Study
In this article we compare the self-reported health of 4 cohorts: the World War II cohort (WW2, born between 1935 and 1944), older baby boomers (OBB, born between 1945 and 1954), younger baby boomers (YBB, born between 1955 and 1964), and Generation X (GenX, born between 1965 and 1974). The WW2 cohort was born during the economically and socially difficult later years of the Depression and in the shadow of World War II. “Baby boom” refers to the increase in the birthrate after World War II, which in North America lasted for 2 decades until the mid-1960s. This resulted in an unprecedentedly large generation: according to the 2011 Canadian census, almost 30% of the population were baby boomers between the ages of 46 and 65.4 The baby boomer cohort was the first generation to grow up in an era of increasing affluence, with greater educational and employment opportunities and also greater access to health and welfare services than the previous generation had.5 As children, they were the first generation to have access to immunizations and antibiotics, and they have lived in an environment of accelerating development of diagnostic technologies and pharmacological and surgical therapies. As adults, they were the first generation to have access to oral contraceptives, which had major implications for social change, particularly in the roles of women.5 The older baby boomers, born between 1945 and 1954, spent their teenage years and early adulthood in the 1960s and early 1970s, during the era of the Beatles, Woodstock, “Flower Power,” “Women's Liberation,” the assassination of President John F. Kennedy, and the Vietnam War, to mention only a few of the key events. The older boomers were the pioneers in this time of great social and political change, and in many ways they paved the way for the younger baby boomers (born between 1955 and 1964).5 This latter cohort was born in the years leading to the peak of the economic boom but came of age after the prosperity peak had passed and entered the labor force after many of the best positions had been taken.5,6 Members of the following cohort, Generation X or GenX, were born into smaller families as a result of the low fertility rates in the late 1960s and early 1970s, likely a consequence of their parents’ increased access to contraception.5 Generation X, a term popularized by the author Douglas Coupland,7 was the first generation whose mothers were more likely to work outside the home.5 They were born at the downturn of the economic boom and reached maturity at a time of high unemployment and unfavorable income distribution. Despite GenXers being even better educated than their parents, they are said to be the first postwar generation to be economically worse off than their parents.6
Self-Rated Health
In this study, we used self-rated health (SRH) to explore cohort differences in health over time. SRH is among the most frequently used measures of health status; in fact, a PubMed search using the keywords “self-rated health” and the synonym “self-perceived health” yielded more than 5,000 English-language citations in the last decade. We chose this indicator for 2 reasons: (1) SRH has long been established as a reliable predictor of morbidity,8–13 mortality,8,14–20 and health care utilization.15,16,21–23 (2) In addition to the more traditional health outcome measures of death rates and health care use, SRH reflects an individual's perceptions and expectations of his or her health.24,25 It thus incorporates the baby boomer population's diverse perceptions of aging and health, as well as the effect that these perceptions might have on their use of health care services.
SRH is known to be affected by such determinants of health as socioeconomic status (SES) and lifestyle. In North American studies, the most frequently used indicators of SES are educational attainment and income, whereas European studies tend to use indicators of social class. Generally speaking, however SES is measured, individuals with a higher SES have better SRH.26–30 Furthermore, longitudinal studies show that a lower SES is associated with greater deterioration in SRH over time.28,30,31 In cross-sectional studies, smoking is associated with having worse SRH.32–34 Similarly, in longitudinal studies, being a smoker is associated with worsening SRH over time.9,28 While there are relatively few studies of the relationship between obesity and SRH, the literature suggests that obese individuals generally report a lower SRH and that weight gain is associated with a decrease in SRH.31,32,35–38
In North America, educational attainment has increased dramatically over time.39–41 Parallel to the increases in education were large rises in prosperity and the standard of living, fueled in part by the greater participation of women in the labor market.42,43 Both of these increases might be expected to contribute to better health in younger generations. In addition, the prevalence of smokers in Canada has declined since the 1960s, which also might be expected to be reflected in better health in the population.44 But greater prosperity and technological changes have enabled a more sedentary lifestyle and an explosion in the availability of “fast food,” which has been accompanied by a rise in obesity and overweight in the populations of many countries.45–52 The increasing proportion of younger people who are obese is thus a growing concern.38,53,54 Given the relationship between SRH and educational attainment, income, smoking, and obesity, we would expect changes in these determinants of health over time to be reflected in the health of the population.
Age, Birth Cohort, and Period Effects
While there have undoubtedly been considerable changes over time that may have influenced the population's SRH, it is not clear that these differ by cohort. Cohort differences are closely linked to changes over time associated with aging, as well as influences from factors in the environment that may be changing at the same time. To unravel these differences, a statistical approach, an “age-period-cohort analysis” or “APC analysis,” is needed. Age effects are the familiar consequences of growing older, irrespective of birth cohort and calendar time.55 Differences in experiences and outcomes in individuals born and growing up in different time periods are referred to as “birth cohort effects.” These may be effects that are unique to a particular generation or that accumulate over the lifetime. In addition to cohort effects are “period effects” that result from events that take place at a particular time or during a particular period and can affect people of all ages and birth cohorts. Examples are the introduction of new policies, technologies, or health treatments or more general societal changes such as more educational opportunities, greater prosperity, or smoking cessation programs. Birth cohort and period effects have important implications for planning and providing services. A cohort effect implies that interventions may need to be targeted to specific groups in the population, and a period effect indicates that a more general approach could address the entire, or a major portion of the, population.
SRH is strongly associated with aging because it tends to deteriorate with age.9,31,32 This makes it difficult to study cohort effects in cross-sectional studies because comparing two cohorts at the same time point must mean that one is older than the other. Instead, we need to be able to compare different cohorts at the same chronological age. To do this, we require longitudinal data spanning the age difference between cohorts, or the ability to combine data from a series of cross-sectional studies.
The findings from the few studies comparing SRH across generations are mixed. A study of American women found worse SRH among the baby boomers than among the wartime birth cohort.32 In contrast, another US study using an age-period-cohort analysis with a combined data set from repeated cross-sectional studies found little evidence for cohort effects in SRH.56 A British study, also using multiple cross-sectional studies, showed no differences in SRH between the baby boomer and the wartime birth cohorts.57 But a longitudinal study in Scotland comparing individuals in 3 age groups that were followed for 20 years showed a strong association of higher SES with better SRH, with a suggestion of worse health in the more recent cohort.31 Specifically, members of the GenX cohort (individuals born in 1972) were slightly more likely to report worse SRH than were baby boomers (born in 1952) who, in turn, were slightly more likely to report worse health than those born in 1932. To date, we are aware of no studies that have used an age-period-cohort analysis to study the trajectory of SRH using longitudinal panel data.
Our study takes advantage of 16 years of Canadian longitudinal population health data. Our purpose was to examine birth cohort and period effects in the age trajectory of SRH over 16 years in 4 birth cohorts—WW2, OBB, YBB, and GenX—to see whether any birth cohort and period effects were associated with differences over the time period of this study (1994-2010) in known determinants of SRH: educational attainment, income, smoking, and obesity. To do this, we needed to take into account any birth cohort and period effects in these potential determinants. Given the changes in the population over time that we just described, we hypothesized that (1) birth cohort and/or period effects in SRH would enable younger cohorts to have better health and (2) birth cohort or period differences in SRH would be associated with cohort or period differences in educational attainment, income, smoking, and body mass index (BMI) (obesity).
Methods
Data Source
The Canadian National Population Health Survey (NPHS) is a longitudinal panel survey developed and administered by Statistics Canada and started in 1994.58 The NPHS interviewed a representative sample of the Canadian population every 2 years up to 2010, yielding 9 cycles of data. The survey's target population were household residents in the 10 Canadian provinces, excluding people living on Indian reserves and Crown lands, residents of health institutions, full-time members of the Canadian forces, and people living in some of the remote areas of Ontario and Québec. The NPHS employed a stratified 2-stage sampling design (geographic clusters and then dwellings within each cluster) based on the Canadian Labour Force Survey in all provinces except Québec where the Equête Sociale et de Santé was used.58 In the first cycle of the survey, households were randomly selected and then within each household, 1 member 12 years of age or older was chosen to be the longitudinal respondent.
In 1994 (Cycle 1), three-quarters of the interviews were conducted in person and the rest by telephone. From then on, around 95% of the interviews were conducted by telephone. Because of the inevitable attrition in longitudinal studies, the NPHS administered a special questionnaire to individuals who had moved to long-term care institutions. The deaths of respondents and the cause and date of death were confirmed using the Canadian Vital Statistics Database. (More detailed descriptions of the NPHS design and interview procedures are available from Statistics Canada.58) At the baseline (Cycle 1), the 4 birth cohorts contained 10,140 people (aged 20 to 60 years). From this time onward, 8,570 participants contributed to at least 3 cycles, which we used for our analyses of age trajectories over time.
Measures
The date of birth of each participant was recorded in Cycle 1, which we used to determine participants’ birth cohort. We calculated age in years at each cycle and we used years since survey initiation (baseline) as an indicator of period.
At each cycle, the respondents were asked the same core questions, the responses to which we used in this study. Participants were asked to rate their health with the question: “In general, would you say your health is excellent, very good, good, fair, or poor?” Their responses were recorded on a 1 to 5 scale, with 1 being excellent health and 5 being poor health. In order to use the full scale for our analyses, we recoded the data using the values developed by Diehr and colleagues.59 In this way, the value for each health state represents the approximate probability that a person will maintain his or her health state for the next 2 years. The values we used were excellent (96), very good (93), good (76), fair (35), and poor (19).59
At each cycle of interviews, participants were asked about their highest level of education, information we used to develop a measure for number of years of schooling. Questions were also asked about total annual household income: no income, less than $5,000, $5,000 to $9,999, $10,000 to 14,999, $15,000 to $19,999, $20,000 to $29,999, and then increments of $10,000 up to $80,000 or more. Because approximately 15% of the participants did not consistently report their household income in all or most cycles, we created an “income unknown” category so that we still could include these participants in our analyses. Participants were asked to report their height and weight at each cycle, which we used to calculate BMI with the formula (weight [kg]) / (height [m])2, except for pregnant women and those shorter than 0.914 m or taller than 2.108 m in height, who were omitted from the analyses (1.4% of the sample). Participants were asked a series of questions about smoking, which were used by Statistics Canada to develop a variable indicating current smoking status at each cycle in 1 of the following groups: current smoker, former smoker, and never smoker.
For respondents who did not provide information in some cycles but then resumed their participation, we interpolated missing values on SRH, income, and BMI as the mean of the preceding and following cycles. For missing values on education and smoking, we used the value from the previous cycle to impute values for missing cycles.
Modeling Strategy
As indicated, because age, period, and cohort are inextricably related, all three cannot be statistically modeled at once.60–63 Typically in APC analysis, adding the period effect is often problematic. In our study, however, cohorts were defined by a 10-year age range, and the variation associated with age and period within this 10-year span meant that we could model and examine the 3 effects simultaneously. In summary, for each of our outcomes (SRH, education, income, smoking, and BMI), the approach we used first looked at the effect of age, with the 4 cohorts combined to determine the trajectory over time. We then added birth cohort to the model to determine whether the effect of cohort was significant over and above the effect of age. We also tested for a statistical interaction to see whether the age trajectory differed by cohort. Finally, in order to look at the independent effect of period over time, we added period as a variable indicating years since baseline and examined whether the effect of period differed by cohort.
To illustrate the findings graphically, we plotted the predicted age trajectory of our outcomes for each cohort. We centered age at 52 years (the median of the age distribution for the 4 cohorts at baseline [1994]) so that the intercept could be interpreted as the average outcome (eg, SRH) at age 52 years. This meant that we could directly compare differences between the cohorts at the same ages. To look at the relative contribution of period to any cohort differences, we set the value of period to zero in order to eliminate the period effect. We then plotted the predicted age trajectories with and without a period effect to see what the age trajectory by cohort would have been had there not been a period-related contribution (Figure 1).
Figure 1.
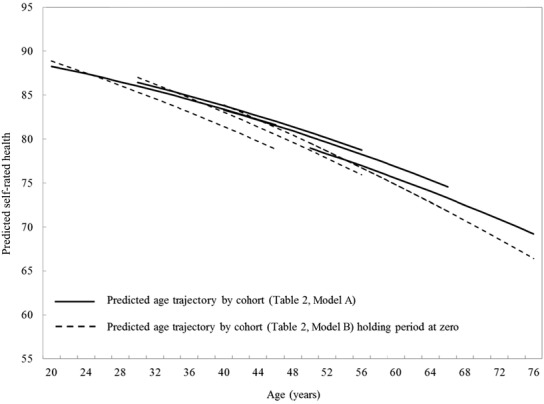
Predicted Age Trajectories of SRH by Birth Cohort: Canadian National Population Health Survey, 1994-2010
To address Hypothesis 2, we added education, income, smoking, and BMI to the model for SRH. Although education and income were modestly correlated cross-sectionally, we were able to add education and income separately, given their different pattern of age trajectory by cohort (Figure 2). To show the impact of changes in education, income, smoking, and BMI on our findings, we plotted predicted age trajectories for SRH holding these variables constant. In this way we could illustrate the relative contribution of period effects on these variables to SRH.
Figure 2.
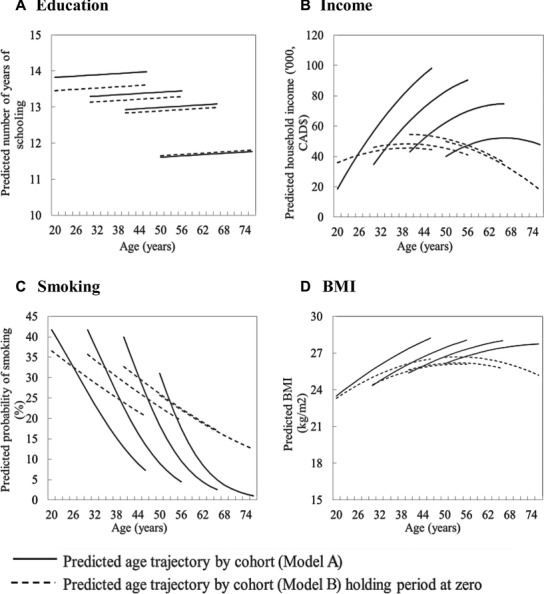
Predicted Age Trajectories of Education, Income, Proportion of Smokers, and BMI by Birth Cohort: Canadian National Population Health Survey, 1994-2010
A more statistical description of our modeling technique is as follows: We used multilevel growth models to examine age, period, and birth cohort effects on SRH. Growth modeling allowed us to estimate simultaneously how SRH evolved over the life course (age trajectory) and how the trajectory was affected by other variables. Using the SAS version 9.3 PROC GLIMMIX, we fitted a 2-level random coefficient model, with multiple observations nested within persons over time, using data from individuals who contributed at least 3 cycles of data.64 We estimated the models, including incomplete cases up to the point at which individuals had been lost to follow-up or had died, using restricted maximum likelihood estimators, which adjust for missing data assuming that they were missing at random. The statistical significance of variables was assessed using a Wald test. We modeled SRH, education (measured as years of schooling), household income, and BMI as continuous outcomes using linear models. We used a logistic model for smoking status, which was modeled as a binary outcome (current smokers versus former and nonsmokers). To test Hypothesis 2, we introduced education, income, smoking status, and BMI to the base SRH model to examine their unique contributions to SRH. To simplify the presentation of findings for these analyses, we used categorical values for education, income, and BMI and grouped years of schooling as fewer than 12, 12 to 15, and 16 years or more. Income was grouped based on quartiles of the overall distribution, with a separate “income unknown” category. Five categories were used for BMI: underweight (<18.5), normal weight (18.5 to 24.9), overweight (25 to 29.9), obese I (30.0 to 34.9), and obese II (≥35.0).
Sensitivity Analysis
Because recoding SRH as a binary score (fair/poor versus excellent/very good/good) may give a slightly different perspective on the findings,65 we repeated all the analyses with logistic models using SRH as a binary outcome. In order to examine the effect of missing data due to dropouts and mortality on the results, (1) we adjusted for dropouts and mortality by including indicator variables to identify participants who had dropped out or died before the end of the study; (2) we imputed a value of zero (ie, extremely poor health) to SRH for those who had died; and (3) for those who had dropped out, we imputed the last value (worst-case) until the end of the survey with a value of zero to SRH for those who had died. For all these analyses, we compared parameter estimates and their significance level with those from prior analyses.
Results
At baseline there were 1,631 participants in the WW2 cohort, 2,167 in the OBB cohort, 2,660 in the YBB cohort, and 2,112 in the GenX cohort who had at least 3 cycles of data. At the end of the study, 5,931 respondents remained; 24 participants had been institutionalized but had not died; 558 participants had died; and 2,057 had dropped out. Men and those of low SES (income or education) were less likely to remain in the study, which in each case was particularly true for the WW2 cohort. By Cycle 9 (up to age 76 years), 16% and nearly 18% of the WW2 cohort (aged 50 to 60 years at baseline) had dropped out or died, respectively (Table 1). Those in the youngest cohorts reported better SRH than those in the older cohort at baseline. In addition, in each birth cohort, SRH declined over time.
Table 1.
Characteristics of Participants in Each Birth Cohort: Canadian National Population Health Survey, 1994-2010a
| WW2 | OBB | YBB | GenX | |||||
|---|---|---|---|---|---|---|---|---|
| (Born 1935-1944) | (Born 1945-1954) | (Born 1955-1964) | (Born 1965-1974) | |||||
| Cycle 1 | Cycle 9 | Cycle 1 | Cycle 9 | Cycle 1 | Cycle 9 | Cycle 1 | Cycle 9 | |
| 1994/1995 | 2010/2011 | 1994/1995 | 2010/2011 | 1994/1995 | 2010/2011 | 1994/1995 | 2010/2011 | |
| Number | 1,631 | 1,069 | 2,167 | 1,587 | 2,660 | 1,896 | 2,112 | 1,379 |
| Mean age | 54.6 | 70.4 | 44.6 | 60.5 | 34.7 | 50.7 | 25.0 | 40.9 |
| % women | 54.1 | 55.8 | 51.8 | 53.0 | 54.4 | 56.4 | 54.4 | 55.0 |
| Mean self-rated health | 76.4 | 75.6 | 82.5 | 78.4 | 84.9 | 81.8 | 86.4 | 83.3 |
| Mean number of years of schooling | 11.6 | 12.1 | 13.0 | 13.3 | 13.2 | 13.7 | 13.4 | 14.1 |
| Mean household income (000s)b | 48.3 | 54.7 | 54.7 | 74.6 | 49.5 | 87.2 | 42.4 | 89.1 |
| % current smokers | 30.6 | 11.4 | 35.3 | 19.5 | 38.3 | 22.3 | 39.6 | 24.6 |
| Mean BMI | 26.6 | 27.3 | 25.9 | 27.7 | 25.2 | 27.6 | 24.4 | 27.3 |
| % missing: dropout | – | 16.1 | – | 19.2 | – | 25.8 | – | 32.9 |
| % missing: dead | – | 17.7 | – | 7.3 | – | 2.7 | – | 1.8 |
WW2 = World War II cohort, OBB = Older baby boomer, YBB = Younger baby boomer, GenX = Generation X.
In Canadian dollars and not corrected for inflation.
SRH: Age, Birth Cohort, and Period Effects (Hypothesis 1)
Table 2 shows the results of the fitted growth models with a quadratic age function for SRH. Both linear and quadratic age terms were statistically significant (p < 0.0001). Although small, the net birth cohort differences between members of the WW2 and GenX cohorts were statistically significant (p = 0.032) over and above the age effects (Table 2, Model A). Model B shows the results for the age and birth cohort effects after accounting for period effects; there was a significant (p = 0.019) period effect on SRH, suggesting a linear increase in SRH from 1994 to 2010 (Table 2, Model B) across all cohorts. The age and cohort coefficients in Model B represent the effect of these variables after accounting for the effect of period.
Table 2.
Age, Cohort, and Period Effects on SRH: Results From Growth Models: Canadian National Population Health Survey, 1994-2010a
| Outcome: Self-Rated Health | ||||
|---|---|---|---|---|
| Model A: | Model B: | |||
| Age | Age, Period, | |||
| and Cohort | and Cohort | |||
| β | S.E. | β | S.E. | |
| Intercept | 80.06*** | 0.45 | 76.52*** | 1.52 |
| Gender (ref: men) | ||||
| Women | −0.71* | 0.33 | −0.721* | 0.33 |
| Linear ageb | −0.33*** | 0.01 | −0.46*** | 0.06 |
| Quadratic age | −0.002*** | 0.001 | −0.002*** | 0.001 |
| Birth cohort (ref: GenX)c | ||||
| WW2 | −1.38* | 0.59 | 2.46 | 1.69 |
| OBB | −0.08 | 0.51 | 2.47* | 1.17 |
| YBB | 0.40 | 0.47 | 1.66* | 0.70 |
| Periodd | 0.14* | 0.06 | ||
p < 0.05
p < 0.01
p < 0.0001.
Growth model with random intercept and age.
Age was centered at 52 years.
WW2 = World War II cohort, OBB = Older baby boomer, YBB = Younger baby boomer, GenX = Generation X.
Years since baseline.
Figure 1 shows the predicted age trajectories of SRH by birth cohort (solid line). Overall, SRH worsened with increasing age in all birth cohorts (Model A). The vertical displacement of the lines illustrates the cohort effects: at the same ages the predicted SRH is slightly better (higher) for younger cohorts. The dotted line shows the predicted trajectory for SRH from Model B, but holding period fixed at zero. Clearly, there is a substantial difference between the solid and the dotted lines, demonstrating the importance of the period effect on the trajectory of SRH. The results suggest that particularly in the younger cohorts, without period effects, SRH would be somewhat worse than that actually reported.
Birth Cohort Differences in Education, Income, Smoking, and BMI
Table 3 shows the results of the models for education and income, and Table 4 shows the results for smoking and BMI. The solid lines in Figure 2(A) represent the predicted age trajectories by cohort for education (average years of schooling). As expected, the younger cohorts had higher levels of education compared with those of the WW2 cohort. We found a small but significant period effect; when this was accounted for, the birth cohort differences among OBB, YBB, and GenX remained essentially unchanged (dotted line). Figure 2(B) shows the predicted age trajectories by cohort for average household income. Again, younger cohorts had higher average incomes (solid line) and steeper predicted increases in income with age than did older cohorts. But when we accounted for period effects, in effect controlling for inflation, these differences disappeared (dotted line). Moreover, the resulting overall trajectory of income and age reflects the dynamics of income growth over the life course, peaking in middle age, with a decline in older ages.
Table 3.
Age, Cohort, and Period Effects on Education (Years of Schooling) and Household Income: Results From Growth Models: Canadian National Population Health Survey, 1994-2010a
| Outcome: Years | Outcome: Household | |||||||
|---|---|---|---|---|---|---|---|---|
| of Schooling | Income | |||||||
| Model A: | Model B: | Model C: | Model D: | |||||
| Age | Age, Period, | Age | Age, Period, | |||||
| and Cohort | and Cohort | and Cohort | and Cohort | |||||
| β | S.E. | β | S.E. | β | S.E. | β | S.E. | |
| Intercept | 14.02*** | 0.07 | 13.65*** | 0.18 | 107.71*** | 0.76 | 41.46*** | 3.09 |
| Linear ageb | 0.01*** | 0.001 | −0.01 | 0.01 | 1.31*** | 0.02 | −0.66*** | 0.11 |
| Quadratic age | 0.0001*** | 0.0001 | −0.001*** | 0.0001 | −0.05*** | 0.001 | −0.03*** | 0.002 |
| Birth cohort (ref: GenX)c | ||||||||
| WW2 | −2.39*** | 0.10 | −1.99*** | 0.21 | −64.85*** | 1.09 | 7.24* | 3.41 |
| OBB | −1.01*** | 0.10 | −0.74*** | 0.16 | −42.32*** | 0.97 | 9.00*** | 2.54 |
| YBB | −0.59*** | 0.09 | −0.38** | 0.12 | −21.83*** | 0.93 | 2.71 | 1.67 |
| Period | 0.01 | 0.006 | 2.72*** | 0.13 | ||||
| Period by birth cohort (ref: GenX)d | ||||||||
| WW2 | 0.009* | 0.004 | −1.32*** | 0.14 | ||||
| OBB | 0.001* | 0.003 | −0.77*** | 0.10 | ||||
| YBB | −0.004 | 0.002 | −0.14*** | 0.06 | ||||
p < 0.05
p < 0.01
p < 0.0001.
Growth model with random intercept and gender as a fixed effect.
Age was centered at 52 years.
WW2 = World War II cohort, OBB = Older baby boomer, YBB = Younger baby boomer, GenX = Generation X.
Years since baseline.
Table 4.
Age, Cohort, and Period Effects on Smoking and BMI: Results From Growth Models: Canadian National Population Health Survey, 1994-2010a
| Outcome: Current Smokers | Outcome: BMI | |||||||
|---|---|---|---|---|---|---|---|---|
| Model A: | Model B: | Model C: | Model D: | |||||
| Age | Age, Period, | Age | Age, Period, | |||||
| and Cohort | and Cohort | and Cohort | and Cohort | |||||
| β | S.E. | β | S.E. | β | S.E. | β | S.E. | |
| Intercept | −3.25*** | 0.10 | −1.55*** | 0.36 | 28.79*** | 0.11 | 26.63*** | 0.44 |
| Linear ageb | −0.12*** | 0.004 | −0.03* | 0.01 | 0.11*** | 0.002 | 0.02 | 0.02 |
| Quadratic age | −0.001*** | 0.0000 | −0.001 | 0.0000 | −0.002*** | 0.0001 | 0.003*** | 0.0001 |
| Birth cohort (ref: GenX)c | ||||||||
| WW2 | 2.21*** | 0.14 | 0.42 | 0.40 | −2.44*** | 0.16 | −0.07 | 0.49 |
| OBB | 1.51*** | 0.12 | 0.44 | 0.31 | −1.91*** | 0.15 | −0.45 | 0.35 |
| YBB | 0.69*** | 0.10 | 0.26 | 0.20 | −1.12*** | 0.14 | −0.44 | 0.23 |
| Period | −0.05** | 0.02 | 0.07*** | 0.02 | ||||
| Period by birth cohort (ref: GenX)d | ||||||||
| WW2 | −0.09*** | 0.02 | 0.02 | 0.01 | ||||
| OBB | −0.04* | 0.02 | 0.02* | 0.01 | ||||
| YBB | −0.02 | 0.01 | 0.01 | 0.01 | ||||
p < 0.05
p < 0.01
p < 0.0001.
Growth model with random intercept and gender as a fixed effect.
Age was centered at 52 years.
WW2 = World War II cohort, OBB = Older baby boomer, YBB = Younger baby boomer, GenX = Generation X.
Years since baseline.
Figure 2(C) shows the estimated age trajectory of the proportion of smokers for each cohort. The proportion of smokers declined sharply with increasing age in all birth cohorts, with a lower proportion of smokers in the younger cohorts (Figure 2[C], solid line). Again, there was a significant period effect in the proportion of smokers (Table 4). This is illustrated in Figure 2(C) (dotted line), where we see that the birth cohort difference of the estimated proportion of smokers and the decline in smoking with age was much reduced once the effect of period was accounted for. The predicted age trajectories of BMI by birth cohort are shown in Figure 2(D) (solid line). The BMI in the 4 cohorts increased with age over the study period. The overall birth cohort differences in BMI levels were significant, with members of the younger cohorts having higher BMI levels than their older counterparts. A substantial period effect on BMI was found, with an overall increase in BMI from 1994 to 2010. When we accounted for the effect of period, the birth cohort differences largely disappeared (dotted line), suggesting that the higher BMIs in the successively younger cohorts were mainly due to period effects.
Influence of Education, Income, Smoking, and BMI
Building on the baseline models presented in Table 2, Table 5 shows the results of the models for education, income, smoking, and BMI as predictors of SRH, adjusting for age, cohort, and period. The findings highlight the significant effect of the 4 predictors on SRH. The positive coefficients for education, income, and smoking indicate that those with more education or a higher income reported better SRH compared with those in the lowest education and income categories and that nonsmokers reported better health than current smokers (SRH difference = 2.42, p < 0.0001). The opposite effect was found for BMI, with individuals with a higher BMI reporting worse SRH than those with a BMI in the normal range (eg, mean SRH difference = –6.5, p < 0.0001 for obese II compared with normal). This suggests that in the population overall, the positive effects of improvements in education, income, and not smoking are to some degree offset by increases in BMI. The age effects for SRH were unchanged after the inclusion of these indicators, but cohort and period effects were no longer significant (bottom half of Table 5).
Table 5.
Effect of Education, Income, Smoking, and BMI on SRH: Results From a Growth Model: Canadian National Population Health Survey, 1994-2010a
| β | S.E. | |
|---|---|---|
| Predictors | ||
| Years of schooling (ref: <12 years) | ||
| 16 years or above | 7.39*** | 0.45 |
| 12-15 years | 4.90*** | 0.35 |
| Household income (ref: bottom quartile) | ||
| Missing | 1.27 | 0.27 |
| Top quartile (Q4) | 4.47*** | 0.24 |
| Q3 | 3.61*** | 0.22 |
| Q2 | 2.87*** | 0.20 |
| Smoking status (ref: current smokers) | ||
| Nonsmoker (never) | 2.42*** | 0.29 |
| Former | 1.24 | 0.22 |
| BMI categories (ref: normal) | ||
| Obese II | −6.50*** | 0.40 |
| Obese I | −2.72*** | 0.27 |
| Overweight | −0.55 | 0.19 |
| Underweight | −3.23*** | 0.62 |
| Age, Period, and Cohort Effects | ||
| Intercept | 72.11*** | 1.35 |
| Gender (ref: men) | ||
| Women | −0.79** | 0.28 |
| Linear ageb | −0.36*** | 0.05 |
| Quadratic age | −0.002*** | 0.001 |
| Birth cohort (ref: GenX)c | ||
| WW2 | 1.15 | 1.47 |
| OBB | 1.26 | 1.01 |
| YBB | 1.05 | 0.60 |
| Periodd | 0.04 | 0.05 |
p < 0.05
p < 0.01
p < 0.0001.
Growth model with random intercept and age.
Age was centered at 52 years.
WW2 = World War II cohort, OBB = Older baby boomer, YBB = Younger baby boomer, GenX = Generation X.
Years since baseline.
To illustrate the impact of changes over time in education, income, smoking, and BMI in a semiquantitative way, we recalculated the age trajectory of changes in SRH for each of the cohorts, but this time with education, income, and BMI as continuous variables and smoking as a binary variable. Using this model, we computed and graphed 2 more trajectories with the following assumptions (Figure 3). First, we computed a trajectory with values for education, income, and smoking chosen to illustrate what the SRH age trajectory would have been if the cohorts had not experienced improvements over time. For this trajectory, years of schooling was fixed at 10 years (the median of the distribution for the oldest age group) and household income at $40,000 (the median at baseline), and we also assumed that the proportion of current smokers was the same as at the baseline assessment. We found that the trajectory for each cohort had shifted down toward worse SRH (dotted line, Figure 3). Second, we computed trajectories to illustrate what the age trajectory of SRH would have been if BMI had remained stable at the recommended normal weight. For these trajectories, we assumed that the average BMI remained constant at the midpoint of the normal range (23 kg/m2). We found that the trajectory had shifted up toward better health (dashed line, Figure 3), so that if there had been no period effect of increasing BMI over time, SRH would have improved. As can be seen from Figure 3, these findings suggest that the effect of more education, higher income, and smoking cessation on improving health over time has been partially offset by the effect of increasing BMI. Further exploration showed that education and income had the greatest effect on SRH and that adding smoking status made little difference.
Figure 3.
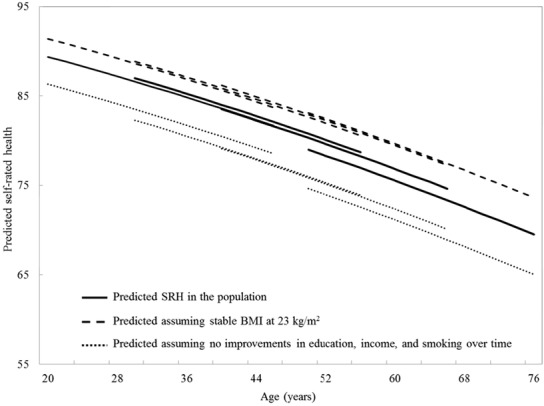
Predicted Age Trajectories of SRH by Birth Cohort: Canadian National Population Health Survey, 1994-2010
Trajectories are as predicted by a growth model with random intercept and age. Model includes linear and quadratic age terms, sex, education, income, smoking status, and BMI.
Solid line: Average trajectory in the population was obtained from model with age terms and cohort effect. Dashed line: Trajectories were obtained assuming that there were no increases on BMI over time: average BMI is stable at 23kg/m2.
Dotted line: Trajectories were obtained assuming that there were no improvements over time in education, income, and smoking: the average years of schooling is stable at 10 years, the average income at $40,000, and the distribution of smokers as in 1994.
Sensitivity Analyses
Our results using a dichotomous variable for SRH were similar to those just presented. This suggests that the impact of education, income, smoking, and BMI on the trajectory of SRH is not confined to the fair/poor categories of health. Our models, adjusting for dropouts and mortality, showed, as expected, a steeper trajectory of declining SRH with age, particularly at older ages. But there were no significant differences between the cohorts. The findings for the relative effects of education, income, smoking cessation, and BMI were similar as well. In other words, our sensitivity analyses did not change our conclusions.
Discussion
Building on the literature, which shows that SRH is a predictor of health care utilization, we focused on whether the baby boomer generation's need for health care would be greater or less than might be expected based on the size of this cohort. Using data from a 16-year longitudinal study, we found no evidence that the health of baby boomers is substantially different from that of the previous or succeeding cohorts. As noted earlier, it is commonly believed that baby boomers expect better health as they age than did previous generations. Insofar as SRH incorporates beliefs about health, we found no evidence that these differ among cohorts. Our findings held when we took into account improvements in education and income, declines in smoking, and increasing BMI over time. As expected, all these variables were strongly associated with SRH. We found that the trajectory of SRH was such that at the population level, the effect of more education, higher income, and reduced smoking on improving SRH was almost counterbalanced by increasing BMI, resulting in no overall improvements in health during this study's time frame (1994-2010). Given the link between SRH and health care use,15,16,21–23 these findings suggest that the effects of increasing education and income on improving health and thus reducing baby boomers’ and GenXers’ future need for health care as they age may not be realized because of the increase of obesity in the population.
Our study is consistent with previous studies indicating that SRH declines with age, and it extends previous research by examining cohort differences after accounting for period and age effects.31,32 We did not find net cohort differences in SRH, unlike a US study of women that found that midadult to older baby-boomer women reported worse SRH than did pre-baby-boomer women.32 Instead, our findings are in line with the UK studies, which reported only marginal cohort differences. 31,57 None of these previous studies examined period effects.
The importance of SES to health is well established.26,27,31 In our study, SES was represented by education and income. Higher educational attainment and/or higher income were strongly associated with better SRH, and changes over time in education and income explained a significant portion of the small cohort differences in SRH that we observed. In particular, education has the advantage of being established early in life and is not affected by subsequent declines in health, thereby strengthening inferences about a fundamental link between SES and SRH. These findings are compatible with those of previous studies.26,31,66
The deleterious effect of smoking on health and on SRH is well known. Our results showing that the prevalence of smoking declines with age and that it has fallen over time are similar to the findings reported in the literature.33,34,67 Efforts to control smoking have been successful in many countries. Health Canada has had a National Strategy for Tobacco Control since 1999,68 making Canada one of the most successful countries in lowering smoking rates. Canada has legislation regulating the sale, labeling, and promotion of tobacco products and stringent restrictions on smoking in public.68 It was the first country in the world to have picture-based health warnings on cigarette packages, and efforts to continue to reduce smoking are ongoing. These initiatives are reflected in a decline in the proportion of smokers in the Canadian population from about 50% in 1965 to less than 25% in 2011.44
Studies have consistently shown increasing obesity rates in recent decades in Canada as well as in most developed countries.45–52 As in our study, some research has reported period effects in these BMI increases.50 Although differences in obesity between generations is a period, not a cohort, effect, it remains true that the younger generations will have longer to live with obesity than older generations did (and do) and therefore will have a greater lifetime risk of developing obesity-related health conditions. Social changes and prosperity have also increased the variety and availability of food and changed eating patterns, which, researchers suggest, have contributed to the obesity epidemic.69,70 The development of public health strategies to curb obesity is in its infancy. Similar to other countries, Canada is beginning to use clinical practice guidelines for health care professionals71 but, so far, has only outlined public health policies targeted to the population as a whole.72
In addition to changes affecting income, education, smoking, and BMI, we might have expected other developments, such as new treatments and technologies, to have influenced SRH. The death rates in many countries have steadily declined, especially those from cardiovascular disease (CVD),73–75 with an associated increase in life expectancy.73 The average life expectancy at birth in most high-income countries now exceeds 80 years.76,77 For example, life expectancy at age 40, the midpoint age of our sample, rose between 1995/1997 and 2009/2010 (roughly the time span of the study) from 37.4 to 40.9 years and from 42.4 to 44.6 years for men and women, respectively. Given that having poor SRH is a predictor of death, even after controlling for morbidity, health behaviors, and access to health services,15,16 we might anticipate that this increase in life expectancy would be reflected in improvements in SRH. We did not find this, however. Studies directed to understanding why mortality is being postponed have concentrated mainly on the year immediately preceding death and have focused on populations aged 65 or older.78,79 Our study examined the adult population aged between 20 and 60 years at baseline: At the end of the study, the mean age in our oldest cohort (WW2) was only 70 years, so the factors potentially delaying death may be not yet relevant for most of our population. One postulated reason for increasing longevity is that people are reaching old age in better health, partly related to increases in education and prosperity.79,80 Our findings suggest that more education, higher income, and declines in smoking may indeed have contributed to better health, had it not been for the increase in obesity in the population. In this study we did not attempt to link this to mortality.
Three major theories explain the impact of greater life expectancy on the health of the population. The first theory, a compression of morbidity, draws on the notion that the same influences that lower mortality should also result in improvement in health and a decrease in chronic illness.81,82 The opposite theory, an expansion of morbidity, suggests that a decline in the death rate should be associated with worse health because of the greater survival of people with health problems.83,84 The third theory is that of a dynamic equilibrium, such that increased survival is associated with a better control of chronic diseases, so that the overall proportion of life lived in good health is unchanged.85 Studies investigating these theories have included various morbidity indicators, most frequently chronic health conditions and disability, with few studies including SRH. We found 1 study that included all 3 indicators and showed the effect of SRH as similar to that of other indicators.86 Overall, the evidence for any of these theories is mixed,78,79 with major variations in findings between countries.77,79 Unfortunately, few studies have explicitly concerned Canada.79
The main reason for the increase in life expectancy in the population is a decline in mortality from CVD.73–75 Approximately half the reduction in cardiovascular deaths is thought to be attributable to changes in the population, such as the reduction in risk factors like smoking, high blood pressure, and high cholesterol, with the further reduction due to improvements in treatment.87 It has been hypothesized that the increase in obesity in the population will halt or reverse the decline in CVD rates.88,89 This idea is in keeping with our finding of potential improvements in SRH over time being offset by the increase in obesity. A recent study of coronary heart disease death rates in European Union countries testing this hypothesis had inconclusive results, however, although the authors noted that increases in preventable risk factors for heart disease might have an impact on mortality in the future.89 In high-income countries such as Canada and the United States, the Global Burden of Disease Study has shown that the gain in years of life lived because of decreasing CVD death rates and other causes has been offset by years of life lived with disability.75 Part of the increase in life expectancy might be a result of interventions, which means that people are living longer only to develop chronic, non-life-threatening disabling conditions like arthritis.78 Certainly, the prevalence of some chronic conditions such as diabetes and asthma has grown at a greater rate than might be expected, given the aging of the population,75,90,91 and this also is likely to have affected SRH. How these different factors play out to contribute to our finding of no difference in SRH among the cohorts is beyond the scope of this article and remains to be explored.
A strength of our study is that we used data from a large representative sample of Canadians who were assessed every 2 years for 16 years. We were able to examine age, period, and cohort effects simultaneously because of the wide range of ages at baseline within each birth cohort. The limitations are that the data were self-reported and there was attrition due to dropouts and mortality, particularly in the older cohort. Nevertheless, our sensitivity analyses exploring the impact of these losses on the results did not change our conclusions. We did not examine the presence of health conditions in this research because our focus was on understanding the effects of education, income, smoking, and BMI. Because all these have been found to be risk factors for developing health conditions, we can view health conditions as mediators (intermediate variables) between these determinants and SRH. Controlling for health conditions might therefore mask the extent of their contribution to SRH. We considered only the overall impact of our predictor variables; further work is needed to explore potential differential effects by SES and gender.
Conclusion
Although a recurrent theme in the public media has been the expectation that baby boomers might age more healthily than did previous generations, our study found no evidence to support this. Nor did we find evidence that baby boomers are less healthy than the previous generation. In any case, our study underscores the positive impact that improved education, greater prosperity, and smoking control have had on SRH. It also suggests that potential improvements in health have likely been undermined by increased BMI across the cohorts, with implications for the future need for health care. This highlights the importance of measures to control obesity and promote healthy weight in the population. Moreover, the findings suggest that the increase in BMI over time is due to societal changes affecting all generations. Accordingly, interventions targeted to the population as a whole have the potential to improve SRH and possibly decrease the need for health care and its associated costs.
Acknowledgments
We thank the Statistics Canada Research Data Centres (RDC) Program for providing access to the data file.
Funding/Support
This study was supported by a CIHR Operating Grant–Secondary Analysis of Databases (SEC 117113).
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No disclosures were reported.
References
- Rothman M.Oprah Winfrey on aging: “60 is the new 40.” 2014. http://abcnews.go.com/Entertainment/oprah-winfrey-aging-60-40/story?id=23255533. Accessed June 6, 2014.
- Wallace K.Michelle Obama “fabulous” at 50: has the big birthday changed? 2014. http://www.cnn.com/2014/01/16/living/michelle-obama-turns-50-parents./ Accessed June 6, 2014.
- Mellor MJ, Rehr H.Baby Boomers: Can My Eighties Be Like My Fifties? New York, NY: Springer; 2005. [Google Scholar]
- Statistics Canada. Generations in Canada. Ottawa: Statistics Canada; 2011. Catalog no. 98–311–X20111003. [Google Scholar]
- Owram D.Born at the Right Time: A History of the Baby–Boom Generation. Toronto, ON: University of Toronto Press; 1996. [Google Scholar]
- Cheung E. Baby Boomers, Generation X and Social Cycles. Vol. 1: North American Long Waves. Toronto, ON: Longwave Press; 2007. [Google Scholar]
- Coupland D. Generation X: Tales for an Accelerated Culture. New York, NY: St. Martin's Press; 1991. [Google Scholar]
- Branch L, Ku L.Transition probabilities to dependency, institutionalization, and death among the elderly over a decade. J Aging Health. 1989;1(3):370–408. [Google Scholar]
- Cott CA, Gignac MA, Badley EM.Determinants of self rated health for Canadians with chronic disease and disability. J Epidemiol Community Health. 1999;53(11):731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyselka PT, Turunen JH, Ahonen RS, Kumpusalo EA.Chronic pain and poor self–rated health. JAMA. 2003;290(18):2435–2442. [DOI] [PubMed] [Google Scholar]
- Mulsant BH, Ganguli M, Seaberg EC.The relationship between self–rated health and depressive symptoms in an epidemiological sample of community–dwelling older adults. J Am Geriatr Soc. 1997;45(8):954–958. [DOI] [PubMed] [Google Scholar]
- Perruccio AV, Power JD, Badley EM.Arthritis onset and worsening self–rated health: a longitudinal evaluation of the role of pain and activity limitations. Arthritis & Rheumatism. 2005;53(4):571–577. [DOI] [PubMed] [Google Scholar]
- Reyes–Gibby CC, Aday L, Cleeland C.Impact of pain on self–rated health in the community–dwelling older adults. Pain. 2002;95(1–2):75–82. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Ein–Dor T, Ginzburg K, Solomon Z.Trajectories of self–rated health among veterans: a latent growth curve analysis of the impact of posttraumatic symptoms. Psychosom Med. 2009;71(3):345–352. [DOI] [PubMed] [Google Scholar]
- DeSalvo KB, Fan VS, McDonell MB, Fihn SD.Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40(4):1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominick KL, Ahern FM, Gold CH, Heller DA.Relationship of health–related quality of life to health care utilization and mortality among older adults. Aging Clin Exp Res. 2002;14(6):499–508. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y.Self–rated health and mortality: a review of twenty–seven community studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- Idler EL, Kasl S.Health perceptions and survival: do global evaluations of health status really predict mortality? J Gerontol. 1991;46(2):S55–S65. [DOI] [PubMed] [Google Scholar]
- Kondo N, Sembajwe G, Kawachi I, van Dam RM, Subramanian SV, Yamagata Z.Income inequality, mortality, and self rated health: meta–analysis of multilevel studies. BMJ. 2009;339(7731):1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski W, Mor V, Hiris J.The association of self–rated health with two year mortality in a sample of well elderly. J Aging Health. 1991;2:527–545. [Google Scholar]
- Geitona M, Zavras D, Kyriopoulos J.Determinants of healthcare utilization in Greece: implications for decision–making. Eur J Gen Pract. 2007;13(3):144–150. [DOI] [PubMed] [Google Scholar]
- Kennedy BS, Kasl SV, Vaccarino V.Repeated hospitalizations and self–rated health among the elderly: a multivariate failure time analysis. Am J Epidemiol. 2001;153(3):232–241. [DOI] [PubMed] [Google Scholar]
- Menec V, Chipperfield J.A prospective analysis of the relation between self–rated health and health care use among elderly Canadians. Can J Aging. 2001;20(3):293–306. [Google Scholar]
- Bailis DS, Segall A, Chipperfield JG.Two views of self–rated general health status. Soc Sci Med. 2003;56(2):203–217. [DOI] [PubMed] [Google Scholar]
- Hurst DF, Boswell DL, Boogaard SE, Watson MW.The relationship of self–esteem to the health–related behaviors of the patients of a primary care clinic. Arch Fam Med. 1997;6(1):67–70. [DOI] [PubMed] [Google Scholar]
- Ellaway A, Benzeval M, Green M, Leyland A, MacIntyre S.“Getting sicker quicker”: does living in a more deprived neighbourhood mean your health deteriorates faster? Health and Place. 2012;18(2):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foraker RE, Rose KM, Chang PP, et al. Socioeconomic status and the trajectory of self–rated health. Age and Ageing. 2011;40(6):706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano GN, Lindstrom M.The impact of changes in different aspects of social capital and material conditions on self–rated health over time: a longitudinal cohort study. Soc Sci Med. 2010;70(5):700–710. [DOI] [PubMed] [Google Scholar]
- Gunasekara FI, Carter K, Blakely T.Change in income and change in self–rated health: systematic review of studies using repeated measures to control for confounding bias. Soc Sci Med. 2011;72(2):193–201. [DOI] [PubMed] [Google Scholar]
- Martel L, Belanger A, Berthelot JM, Carriere Y.Healthy today, healthy tomorrow? Findings from the National Population Health Survey. Component of Statistics Canada Catalogue no. 82–618–MWE2005004. 2006.
- Benzeval M, Green MJ, Leyland AH.Do social inequalities in health widen or converge with age? Longitudinal evidence from three cohorts in the west of Scotland. BMC Public Health. 2011;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, Kasen S.Cohort differences in self–rated health: evidence from a three–decade, community–based, longitudinal study of women. Am J Epidemiol. 2007;166(4):439–446. [DOI] [PubMed] [Google Scholar]
- Chen X, Lin F, Stanton B, Zhang X.APC modeling of smoking prevalence among US adolescents and young adults. Am J Health Behav. 2011;35(4):416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek D, Kraus L, Muller S, Pabst A.To what extent do age, period, and cohort patterns account for time trends and social inequalities in smoking? Sucht. 2010;56(5):361–371. [Google Scholar]
- Andersen FK, Christensen K, Frederiksen H.Self–rated health and age: a cross–sectional and longitudinal study of 11,000 Danes aged 45–102. Scand J Public Health. 2007;35(2):164–171. [DOI] [PubMed] [Google Scholar]
- Potkanowicz ES, Hartman–Stein P, Biermann JS.Behavioral determinants of health aging revisited: an update on the good news for the baby boomer generation. Online J Issues Nurs. 2009;14(3). [PubMed] [Google Scholar]
- Zack MM, Moriarty DG, Stroup DF, Ford ES, Mokdad AH.Worsening trends in adult health–related quality of life and self–rated health—United States, 1993–2001. Public Health Rep. 2004;119(5):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A, Burgard SA.Body weight and health from early to mid–adulthood: a longitudinal analysis. J Health Soc Behav. 2010;51(1):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USC Bureau. Educational Attainment: 1940–2012. 2013. [updated April 11, 2013]. http://www.census.gov/hhes/socdemo/education/data/ps/istorical/ndex.html. Accessed June 6, 2014.
- Corbeil JPCanadian social trends: update on education. Statistics Canada; 2003. http://publications.gc.ca/collections/Collection-R/Statcan/11-008-XIE/0030311-008-XIE.pdf. Accessed June 6, 2014.
- Conference Board of Canada. How Canada performs: university completion. 2013. http://www.conferenceboard.ca/hcp/details/education/university-completion.aspx. Accessed June 6, 2014.
- Roberts L, Clifton RA, Ferguson B, Kampen K, Langlois S.Recent Social Trends in Canada, 1960–2000. Montreal, QC: McGill/Queen's University Press; 2005. [Google Scholar]
- Shaw DJ.Canada's productivity and standard of living: past, present and future. Statistics Canada; 2002. http://publications.gc.ca/Collection-R/LoPBdP/BP/prb0223-e.htm. Accessed June 6, 2014.
- Reid JL, Hammond D, Burkhalter R, Rynard VL, Ahmed R.Tobacco Use in Canada: Patterns and Trends. Waterloo, ON: Propel Centre for Population Health Impact, University of Waterloo; 2013. [Google Scholar]
- Burke MA, Heiland FW, Nadler CM.From “overweight” to “about right”: evidence of a generational shift in body weight norms. Obes. 2010;18(6):1226–1234. [DOI] [PubMed] [Google Scholar]
- Diouf I, Charles MA, Ducimetière P, Basdevant A, Eschwege E, Heude B.Evolution of obesity prevalence in France: an age–period–cohort analysis. Epidemiol. 2010;21(3):360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille SG, Wee CC, Iezzoni LI.Trends in obesity and arthritis among baby boomers and their predecessors, 1971–2002. Am J Public Health. 2005;95(9):1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C, Corey PN, Young TK.Divergent body mass index trajectories between aboriginal and non–aboriginal Canadians 1994–2009—an exploration of age, period, and cohort effects. Am J Hum Biol. 2012;24(2):170–176. [DOI] [PubMed] [Google Scholar]
- Nooyens ACJ, Visscher TLS, Verschuren WMM, et al. Age, period and cohort effects on body weight and body mass index in adults: the Doetinchem Cohort Study. Public Health Nutr. 2009;12(6):862–870. [DOI] [PubMed] [Google Scholar]
- Reither EN, Hauser RM, Yang Y.Do birth cohorts matter? Age–period–cohort analyses of the obesity epidemic in the United States. Soc Sci Med. 2009;69(10):1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WR, Utz RL, Keyes KM, Martin CL, Yang Y.Birth cohort effects on abdominal obesity in the United States: the Silent Generation, Baby Boomers and Generation X. Int J Obes. 2013;37(8):1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Colditz GA, Kuntz KM.Forecasting the obesity epidemic in the aging U.S. population. Obes. 2007;15(11):2855–2865. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Lange K, Wittert GA.Chronic disease trends due to excess body weight in Australia. Obes Rev. 2009;10(5):543–553. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Mason C.Prevalence of class I, II and III obesity in Canada. CMAJ. 2006;174(2):156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzer G. Blackwell Encyclopedia of Sociology. New York, NY: Blackwell Publishing; 2007. [Google Scholar]
- Martin LG, Freedman VA, Schoeni RF, Andreski PM.Health and functioning among baby boomers approaching 60. J Gerontol B Psychol Sci Soc Sci. 2009;64(3):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice NE, Lang IA, Henley W, Melzer D.Baby boomers nearing retirement: the healthiest generation? Rejuvenation Res. 2010;13(1):105–114. [DOI] [PubMed] [Google Scholar]
- Statistics Canada. Information about the National Population Health Survey. Statistics Canada; 2011. http://www.statcan.gc.ca/pub/82f0068x/82f0068×1997001-eng.htm. Accessed June 6, 2014.
- Diehr P, Patrick D, Hedrick S, et al. Including deaths when measuring health status over time. Med Care. 1995;33(Suppl. 4):AS164–AS172. [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, DuToit S.Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Utz RL, Robinson W, Li G.What is a cohort effect? Comparison of three statistical methods for modeling cohort effects in obesity prevalence in the United States, 1971–2006. Soc Sci Med. 2010;70(7):1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RM.The age–period–cohort conundrum as two fundamental problems. Quality and Quantity. 2011;45(6):1429–1444. [Google Scholar]
- O’Brien RM, Hudson K, Stockard J.A mixed model estimation of age, period, and cohort effects. Sociol Methods Res. 2008;36(3):402–428. [Google Scholar]
- SAS Institute Inc. The GLIMMIX Procedure. In: SAS/STAT 9.3 User's Guide. Cary, NC: SAS Institute Inc.; 2013:chap. 43. [Google Scholar]
- Manderbacka K, Kareholt I, Martikainen P, Lundberg O.The effect of point of reference on the association between self–rated health and mortality. Soc Sci Med. 2003;56(7):1447–1452. [DOI] [PubMed] [Google Scholar]
- Sacker A, Worts D, McDonough P.Social influences on trajectories of self–rated health: evidence from Britain, Germany, Denmark and the USA. J Epidemiol Community Health. 2011;65(2):130–136. [DOI] [PubMed] [Google Scholar]
- Lucke JC, Brown W, Tooth L, et al. Health across generations: findings from the Australian Longitudinal Study on Women's Health. Biol Res Nurs. 2010;12(2):162–170. [DOI] [PubMed] [Google Scholar]
- Health Canada. Strong foundation, renewed focus: an overview of Canada's federal tobacco control strategy 2012–2017. 2012. http://www.hc-sc.gc.ca/hc-ps/pubs/tobac-tabac/ns-sn/index-eng.php#exec. Accessed June 6, 2014.
- Buckley J.Baby boomers, obesity, and social change. Obes Res Clin Pract. 2008;2(2):73–82. [DOI] [PubMed] [Google Scholar]
- Powell LM, Han E, Chaloupka FJ.Economic contextual factors, food consumption, and obesity among U.S. adolescents. J Nutr. 2010;140(6):1175–1180. [DOI] [PubMed] [Google Scholar]
- Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E.2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ. 2007;176(8):S1–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada and the Canadian Institute for Health Information. Obesity in Canada 2011. http://www.phac-aspc.gc.ca/hp-ps/hl-mvs/oic-oac/index-eng.php. Accessed June 6, 2014.
- Statistics Canada. Life expectancy. Statistics Canada; 2010. http://www.statcan.gc.ca/pub/82-229-x/2009001/demo/lif-eng.htm. Accessed June 6, 2014.
- Jemal A, Ward E, Hao Y, Thun M.Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294(10):1255–1259. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Vos T, Lozano R, et al. Disability–adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Life expectancy at birth. http://gamapserver.who.int/gho/interactive_charts/mbd/life_expectancy/atlas.html. Accessed September 11, 2014.
- Christensen K, Doblhammer G, Rau R, Vaupel JW.Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Beltran–Sanchez H.Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66 B(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine JM, Saito Y, Jagger C.The relationship between longevity and healthy life expectancy. Quality in Ageing. 2009;10(2):5–14. [Google Scholar]
- Vaupel JW.Biodemography of human ageing. Nature. 2010;464(7288):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF.Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303(3):130–135. [DOI] [PubMed] [Google Scholar]
- Fries JF, Bruce B, Chakravarty E.Compression of morbidity 1980–2011: a focused review of paradigms and progress. J Aging Res. 2011;(2011):Article ID 261702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg EM.The failures of success. Milbank Q. 1977;55(1):3–24. [PubMed] [Google Scholar]
- Olshansky SJ, Rudberg MA, Carnes BA, Cassel CK, Brody JA.Trading off longer life for worsening health: the expansion of morbidity hypothesis. J Aging Health. 1991;3(2):194–216. [Google Scholar]
- Manton KG.Changing concepts of morbidity and mortality in the elderly population. Milbank Q. 1982;60(2):183–244. [PubMed] [Google Scholar]
- Robine JM, Cambois E.Healthy life expectancy in Europe. Popul Soc. 2013;(499):1–4. [Google Scholar]
- Ford ES, Capewell S.Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu. Rev. Public Health 2011;32:5–22. [DOI] [PubMed] [Google Scholar]
- Hardoon SL, Whincup PH, Lennon LT, Wannamethee SG, Capewell S, Morris RW.How much of the recent decline in the incidence of myocardial infarction in British men can be explained by changes in cardiovascular risk factors? Evidence from a prospective population–based study. Circulation. 2008;117(5):598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M, Townsend N, Scarborough P, Rayner M.Trends in age–specific coronary heart disease mortality in the European Union over three decades: 1980–2009. Eur Heart J. 2013;34(39):3017–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos AF, McCarty DJ, Zimmet P.The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetes Med. 1997;14 (Suppl. 5):S1–S85. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Asthma Facts—CDC's National Asthma Control Program Grantees. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]


