Abstract
Recent technical advances have led to the development of a medical device that can reliably activate the carotid baroreflex with an acceptable degree of safety. Because activation of the sympathetic nervous system plays an important role in the pathogenesis of hypertension and heart failure, the unique ability of this device to chronically suppress central sympathetic outflow in a controlled manner suggests potential value in the treatment of these conditions. This notion is supported by both clinical and experimental animal studies, and the major aim of this article is to elucidate the physiological mechanisms that account for the favorable effects of baroreflex activation therapy in patients with resistant hyper-tension and heart failure. Illumination of the neurohormonal, renal, and cardiac actions of baroreflex activation is likely to provide the means for better identification of those patients that are most likely to respond favorably to this device-based therapy.
Keywords: Resistant hypertension, Blood pressure, Sympathetic nervous system, Renin-angiotensin system, Renal nerves, Glomerular filtration rate, Baroreflex, Baroreflex sensitivity, Heart rate, Heart rate variability, Cardiac autonomics, Device-based therapy
Introduction
Due to challenges in developing a safe medical device capable of producing reliable suppression of sympathetic activity by electrical activation of the carotid baroreflex, it has taken several decades for baroreflex activation therapy (BAT) to evolve as a potential treatment for cardiovascular diseases, especially for those in which the pathogenesis is linked to overactivity of the sympathetic nervous system [1]. Recent technological advances by CVRx, Inc. have led to the development of implantable baroreflex activation systems based on a pulse generator and electrodes that allow electric field stimulation of the carotid sinus wall [1]. One of the targets for BAT has been resistant hypertension, defined as blood pressure that remains above goal despite the concurrent use of at least three antihypertensive drugs at maximally tolerated doses, with one being a diuretic [2]. In this review, we will briefly discuss the clinical trials using the prototype Rheos system and the second-generation Barostim neo to deliver BAT in patients with resistant hypertension. A more extensive discussion of these clinical trials has been published by others [3, 4•, 5–7]. The primary focus of this review is on the clinical findings and experimental animal studies that have provided insight into the mechanisms that account for the physiological effects of BAT and the relevance of these mechanisms to hypertension therapy. Indeed, elucidation of these mechanisms may help identify patients with resistant hypertension who stand to benefit the most from BAT. Additionally, we discuss how the mechanistic insight from these studies provides a strong rationale for the potential use of BAT in the treatment of heart failure, a condition in which sympathetic activation is a major determinant of prognosis [8].
Rheos System and Surgical Implantation
The first-generation Rheos system for activation of the carotid baroreflex consists of stimulating electrodes connected to a pulse generator implanted subcutaneously in the infraclavicular region. During surgery, the optimal placement of the electrodes on the carotid sinus is assessed based upon maximal reductions in arterial pressure and heart rate during electrical stimulation of the carotid baroreflex [9]. Once the optimal placement of the electrodes is confirmed, the electrodes are secured around the carotid sinuses. The implanted pulse generator is completely programmable by radiofrequency control using an external programming system. This allows for controlled current delivery throughout the day, including the possibility of providing customized patterns of baroreflex activation. However, to date, only continuous current delivery has been used clinically. Implantation of the second-generation Barostim neo is much less invasive. This system uses a miniaturized electrode for unilateral implantation and therapy, as discussed below (see Barostim neo Trial).
Clinical Trials of BAT in Resistant Hypertension
Device-Based Therapy of Hypertension Trial (DEBuT-HT)
Confirming previous experimental studies [10, 11], a first-in-human proof-of-concept study of the Rheos system indicated that acute electrical activation of the carotid sinus in patients undergoing elective carotid surgery elicits significant and immediate reductions in blood pressure and heart rate [12]. Based on evidence that reductions in blood pressure caused by continuous electrical activation of the baroreflex are sustained over the long term, both in normotensive and hypertensive animal models [10, 11], the phase II open-label nonrandomized feasibility DEBuT-HT study was designed to evaluate the safety and efficacy of the Rheos system over three months of activation in 45 resistant hypertensive patients, with follow-up at yearly intervals for up to two years [3]. No significant changes in antihypertensive medications occurred during the study. The efficacy of electrical activation of the baroreflex with the Rheos system in these high-risk patients was indicated by the sustained lowering of blood pressure after three months of therapy (mean ± SE; 21±4 mmHg systolic, 12±2 mmHg diastolic), a response that was even more pronounced in 17 subjects who completed two years of follow-up (33±8 systolic, 22±6 mmHg diastolic). While the trial indicated an acceptable safety profile, some procedure-related events did occur, likely reflecting the initial lack of procedural experience.
Rheos Pivotal Trial
The promising results in DEBuT-HT led to the design of the randomized double-blind placebo-controlled Phase III Rheos Pivotal Trial. The goal of this study was to assess the efficacy and safety of the first-generation Rheos System in 265 patients with resistant hypertension [4•]. All patients were implanted with the Rheos system, and then were randomized one month after implantation in a 2:1 ratio to receive BAT immediately or six months later. In both groups, medication was maintained throughout the trial, but was allowed to change according to physician judgment. The trial was successful in meeting the prespecified sustained efficacy endpoint, with 81 % of the group that received immediate BAT showing a reduction of systolic blood pressure (SBP) of at least 10 mmHg at month 12 as compared to month 0, and of a magnitude at least 50 % of that obtained at month 6. However, the acute efficacy criterion was not met, since the proportion of patients with a reduction in SBP of at least 10 mmHg at six months was only 8 % greater in the group receiving BAT from the beginning as compared to the group with device implantation but without immediate BAT. The failure to meet the prespecified acute efficacy endpoint was apparently primarily due to a larger and more variable reduction than expected in SBP at six months in the group with inactive implants, and likely reflected the less-than-optimal trial design [4•, 7]. Notwithstanding the issues related to trial design, the results of the Rheos Pivotal Trial further underscored the promise of BAT for the treatment of resistant hypertension, as both the immediate and delayed treatment groups had 12-month BAT-induced reductions in SBP of more than 30 mmHg, with 50 % of patients achieving SBP of less than 140 mmHg. Moreover, a long-term single-arm open-label follow-up study in 276 subjects demonstrated prolonged efficacy of the Rheos system for an average of 28 months (maximum 53 months), with a responder rate greater than 75 % and a sustained SBP reduction of more than 30 mmHg in the responders [5].
While the safety of both BAT and the device itself were confirmed in the Rheos Pivotal Trial, issues related to procedural safety, as previously noted in the DEBuT-HT study, emerged once again, and the trial failed to meet the prespecified 82 % event-free performance endpoint. The most prevalent serious adverse events (SAE) were either transient or permanent nerve injury. Unfortunately, the failure to achieve this endpoint may have implied not that procedural complications were too frequent, but that the target set for the procedure was overly optimistic [4•, 7]. Indeed, the SAE profile compares favorably with the results from endarterectomy trials involving intervention in the anatomical region. Nonetheless, these results prompted the development of a second-generation device to deliver BAT, the Barostim neo.
Barostim neo Trial
To address potential safety concerns with surgical manipulation of the carotid arteries for perivascular implantation of electrodes around the carotid sinuses using the Rheos system, a miniaturized electrode was designed to minimize the invasiveness of the implant procedure [6]. This new electrode is sutured to the surface of the carotid sinus and does not require the extensive isolation of the carotid arteries needed for wrapping the Rheos electrodes around the carotid bifurcation. In addition to miniaturization, the new system utilizes a unilateral approach, with obvious procedural benefits, stemming from the finding that 77 % of the patients in the Rheos trial ultimately received unilateral stimulation despite the majority having had bilateral implants [4•]. The primary objective of the open-label Barostim neo Trial was to determine safety and efficacy of the Barostim neo system in 30 patients with resistant hypertension. Reductions in SBP (mean ± SE) with BAT in this trial were 26±3 mmHg and 26±4 mmHg at three and six months of follow-up respectively, values comparable to those in previous trials. Furthermore, 43 % of the resistant hypertensive patients achieved SBP <140 mmHg by the sixth month of therapy. Regarding safety, 90 % of patients were free from system- or procedure-related events within 30 days post-implant, compared with 75 % in the Rheos Pivotal Trial, and the few events that did occur were fully resolved with only modest treatments. Additionally, the benign long-term safety profile was demonstrated by the fact that 97 % of patients remained event-free, compared to 87 % in the Rheos Pivotal Trial. On the heels of these encouraging preliminary findings, a larger, FDA-approved multicenter randomized double-blind pivotal clinical trial has been designed to further evaluate the safety and efficacy of the Barostim neo system. The U.S. Barostim Hypertension Pivotal Trial (NCT01679132) is ongoing and is randomizing patients with resistant hypertension to medical therapy versus BAT, utilizing the smaller-unilateral-lead Barostim neo system, in a safety and efficacy trial.
Mechanisms of Blood Pressure Lowering by BAT
In light of the encouraging results from the clinical studies, as summarized above, understanding the critical mechanisms that account for blood pressure lowering during BAT is of paramount importance in identifying those hypertensive patients most likely to benefit from this device-based therapy.
Suppression of Central Sympathetic Outflow
Natural activation of the baroreceptors by increased pressure leads to suppression of central sympathetic outflow and attendant reductions in blood pressure and heart rate. However, this effect has long been considered to be transient, as the baroreceptors adapt to the prevailing level of arterial pressure [13, 14]. Bypassing mechanotransduction by electrical activation of the carotid sinus provides sustained afferent baroreceptor input into the brain, and consequently may chronically suppress central sympathetic outflow. The acute sympathoinhibitory effects of BAT were confirmed in a subset of hypertensive patients from the DEBuT-HTstudy, where electrical activation of the baroreflex for several minutes elicited immediate and pronounced reductions in both arterial pressure and muscle sympathetic nerve activity (MSNA) [15]. While long-term determinations of MSNA during chronic BAT have not been reported in subjects with resistant hypertension, experimental studies provide compelling evidence that suppression of central sympathetic outflow is a sustained response to chronic activation of the baroreflex. More specifically, chronic blood pressure lowering during three weeks of BAT in normotensive dogs was associated with marked and sustained suppression of whole-body norepinephrine spillover to plasma [16•].
While these studies support the contention that chronic BAT has sustained effects to inhibit central sympathetic out-flow and lower blood pressure, they also raise the question of whether similar efficacy can be obtained by complete pharmacologic blockade of postjunctional adrenergic receptors with established roles in mediating the long-term effects of the sympathetic nervous system on blood pressure. This question was addressed by chronic administration of α-1/β-1,2 antagonists alone and subsequently during simultaneous carotid baroreflex activation [17]. Complete adrenergic blockade led to a sustained reduction in blood pressure of approximately 20 mmHg in association with an approximate threefold increase in plasma NE concentration. Most importantly, when BAT was administered for one week during complete adrenergic blockade, blood pressure decreased an additional 10 mmHg in parallel with suppression of plasma NE levels to control values. These data indicate that BAT has sustained effects to inhibit drug-induced increases in central sympathetic outflow and to lower blood pressure by actions beyond those mediated by well-established adrenergic mechanisms.
Renal Sympathetic Nerve Activity and Renin Secretion
It is well established that the changes in autonomic activity to the heart and peripheral vasculature account for immediate reductions in arterial pressure during acute activation of the baro-reflex. However, these actions of baroreflex activation are not sufficient to maintain reduced arterial pressure during long-term suppression of sympathetic outflow to non-renal territories because the kidneys would respond to the fall in pressure by retaining salt and water until blood volume increased sufficiently to restore arterial pressure to control levels [13, 18]. Therefore, an increase in renal excretory function at a lower prevailing level of arterial pressure is an absolute requirement for the maintenance of sodium balance during BAT [13, 18].
Increases in renal sympathetic nerve activity (RSNA) may reduce sodium excretion by promoting sodium reabsorption and by decreasing GFR as a result of increasing preglomerular renal vascular resistance [19]. Modest increases in RSNA promote sodium reabsorption directly by stimulation of tubular α-adrenergic receptors and indirectly by generation of angiotensin during stimulation of renin secretion through activation of β-adrenergic receptors located on the juxtaglomerular cells. Reductions in GFR and renal blood flow occur at higher levels of RSNA that stimulate renal vascular α-adrenergic receptors. Accordingly, reduced RSNA may provide the critical link between suppression of central sympathetic outflow and the obligate increase in renal excretory function required to maintain reduced arterial pressure during BAT [13 18]. Although RSNA has not been directly measured during BAT, experimental studies in chronically instrumented animals have demonstrated that the natural activation of the baroreflex in hypertension has sustained effects to suppress RSNA and promote sodium excretion [13, 18].
Activation of the renin-angiotensin system plays a critical role in the restoration of arterial pressure when the kidneys are exposed to reduced renal perfusion pressure. As the renal nerves tonically stimulate renin release and enhance renin responses to reductions in renal perfusion pressure [19], suppression of RSNA and attendant inhibition of renin secretion may be an important factor in the lowering of arterial pressure during BAT. Indeed, in normotensive dogs subjected to three weeks of baroreflex activation, renin secretion did not increase during suppression of central sympathetic outflow [16•] despite sustained reductions in arterial pressure (approximately 20 mmHg) that would normally increase renin release [20, 21]. Furthermore, in the sympathetically mediated model of obesity-induced hypertension in dogs, chronic baroreflex activation abolished the hypertension in association with parallel suppression of sympathetic activity and plasma renin activity [22•]. These studies suggest that the lowering of arterial pressure during BAT is mediated, at least in part, by sympathoinhibition and concomitant suppression of renin secretion, most likely mediated by reductions in RSNA.
If inhibition of pressure-dependent renin release is, in fact, an important contributing factor to the chronic hypotensive effects of BAT, then increases in plasma levels of angiotensin II or aldosterone would be expected to minimize blood pressure lowering during baroreflex activation. This contention is supported by responses to baroreflex activation in canine models of hypertension produced by exogenous infusion of angiotensin II and aldosterone [23, 24]. In both models, clamping these hormones at higher than normal plasma levels by constant infusion produced non-sympathetically mediated forms of hypertension. More to the point, after angiotensin and aldosterone hypertension were established, acute reductions in blood pressure during baroreflex activation were substantial and comparable to those measured before induction of hypertension. Thus, the effects of sympathetic inhibition on the peripheral vasculature were comparable under these conditions. However, in contrast to the impressive sustained lowering of blood pressure in normotensive dogs and in dogs with obesity-induced hypertension in which activation of the renin-angiotensin-aldosterone system during BAT was inhibited, the long-term antihypertensive effects of baroreflex activation in the angiotensin and aldosterone models of hypertension were relatively insignificant. Following initial reductions in blood pressure in the angiotensin and aldosterone models of hypertension, blood pressure slowly returned to initial hypertensive levels during BAT. These observations suggest that, in the presence of high circulating levels of these potent sodium-retaining hormones, inhibition of α-adrenergic receptor-mediated tubular reabsorption of sodium during suppression of RSNA has only modest effects to promote sodium excretion and lower arterial pressure. Therefore, because GFR decreases modestly during BAT, as is discussed below, maximal blood pressure lowering is dependent upon inhibition of both the direct and indirect (angiotensin-mediated) effects of the renal nerves on sodium reabsorption. Taken together, these studies suggest that BAT may provide the greatest clinical benefit in hypertensive patients whose high blood pressure is associated with sympathetic activation and a renin-angiotensin-aldosterone system that is responsive to neural modulation.
Renal Sympathetic Nerve Activity and Renal Hemodynamics
In addition to promoting tubular reabsorption of sodium, increases in RSNA may decrease sodium excretion by increasing preglomerular vascular constriction and concomitantly reducing GFR, although this is most likely only at the elevated levels of nerve activation that are present under pathophysio-logical conditions such as heart failure and possibly resistant hypertension [19]. However, the possibility that neurally mediated increases in GFR increase renal excretory function during suppression of sympathetic activity during BAT is not supported by empirical data in patients with resistant hypertension and in normotensive and hypertensive canines.
The chronic effects of baroreflex activation on GFR have been evaluated in both clinical and experimental studies. In patients with resistant hypertension enrolled in the Rheos Pivotal Trial, eGFR was modestly reduced (approximately 10 %) after six months of BAT [25•]. Further changes in eGFR did not occur when additional measurements were assessed after 12 months of therapy, and there were no changes in urinary albumin excretion during the 12-month period of BAT. Stratification of the Rheos patients based on baseline eGFR indicated that the greatest reduction in GFR occurred in patients in the highest eGFR category (>90 mL/min/1.73 m2), whereas those in the lowest initial range (<60 mL/min/ 1.73 m2) were devoid of changes in GFR during BAT. In experimental studies in normotensive dogs and in dogs with obesity hypertension and glomerular hyperfiltration (GFRs 35–40 % above control levels), reductions in GFR of approximately 10 % occurred along with the lowering of arterial pressure during chronic BAT [22•, 26]. In both of these canine studies, GFR returned to control levels after BAT was terminated. As glomerular hyperfiltration may lead to progressive glomerular injury, and add to the impairment of renal excretory function and exacerbation of hypertension, this effect of producing a modest non-progressive physiological reduction in GFR may be especially relevant to the long-term beneficial effects of BAT in patients with resistant hypertension, many of whom are obese [3, 4•, 6].
While the mechanisms that account for the reduction in GFR during BAT are unresolved, there are several relevant considerations. One possibility is that decreased GFR is simply the result of a drop in renal perfusion pressure. However, this would hold true only if pre- and post-glomerular arteriolar tone is unchanged, which is not the normal response to a reduction in renal perfusion pressure. Under normal conditions, a chronic reduction in perfusion pressure yielding a lower GFR would decrease sodium chloride delivery to the macula densa, and concomitantly activate two mechanisms geared towards reestablishment of glomerular capillary pressure: 1) afferent arteriolar vasodilation via the tubuloglomerular feedback (TGF) mechanism, and 2) efferent arteriolar constriction as a result of increased angiotensin II generation during stimulation of renin secretion. However, both of these compensatory responses are likely altered by BAT. First, as RSNA promotes sodium reabsorption in the proximal tubule and in the loop of Henle, suppression of RSNA by BAT would increase sodium chlo-ride delivery to the macula densa and increase the TGF signal to constrict the afferent arteriole. This effect of BAT may be especially important in counteracting the hyperfiltration of obesity. According to the tubular hypothesis for glomerular filtration, in obesity, increased neurally mediated sodium re-absorption prior to the macula densa may account for TGF-dependent dilation of the afferent arteriole [27]. Second, because BAT inhibits renin secretion, this would likely diminish the compensatory increase in postglomerular resistance that normally contributes to the maintenance of glomerular pressure during reduced renal perfusion pressure [28]. An analogous fall in GFR occurs during antihypertensive therapy with inhibitors to the renin-angiotensin system [29]. Because blockade of the renin-angiotensin system lowers intraglomerular pressure during hypertension therapy, moderate acute decreases in GFR do occur, but they are commonly associated with long-term preservation of renal function [29]. The non-progressive moderate fall in GFR during 12 months of BAT in patients with resistant hypertension is consistent with these observations [25•].
BAT and Cardiac Autonomic Activity
Buffering of beat-to-beat fluctuations in arterial blood pressure is achieved by dynamic alterations in central input from arterial baroreceptors and compensatory changes in sympathetic and parasympathetic activity. In contrast to pulse-synchronous baroreceptor discharge, the Rheos and Barostim neo systems deliver continuous nonpulsatile electrical impulses to carotid sinus afferent fibers, which leads to the conceptual concern that this device-based therapy may disrupt normal physiological baroreflex function. Patients subjected to BAT, however, do not exhibit signs of orthostatic hypotension or syncope [3]. Furthermore, clinical studies in subsets of patients from the DEBut-HT study further dispute the notion that BAT may disrupt natural baroreflex function. In one study, 24-hour ECG recordings were made before and after three months of BAT [30]. In 21 subjects, BAT not only decreased arterial pressure appreciably but reduced heart rate as well. Of greater relevance to this issue, heart rate variability actually increased during BAT. Furthermore, baroreflex-mediated measures of heart rate variability suggested an increase in cardiac baroreflex sensitivity. Another study found no worsening of baroreflex regulation of heart rate or sympathetic activity even in the face of impressive reductions in MSNA during acute baroreflex activation [15].
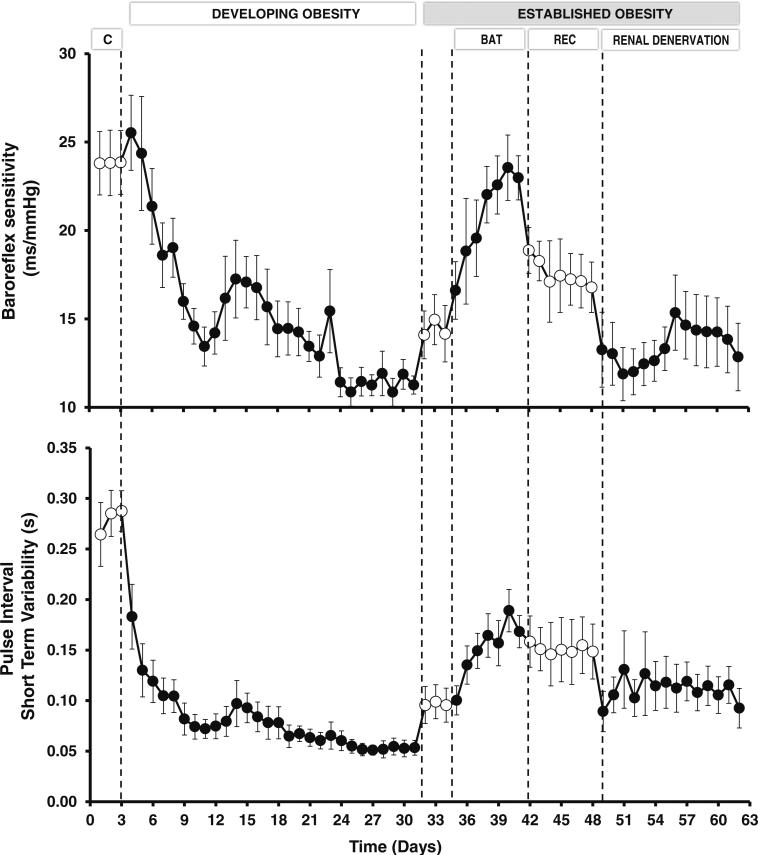
Experimental studies in normotensive and hypertensive dogs have provided further insight into modulation of cardiac autonomic function during baroreflex activation. In normotensive dogs, levels of carotid sinus stimulation producing sustained reductions in MAP and heart rate of 20 mmHg and 15 bpm, respectively, were actually associated with significant increases in heart rate variability and in the sensitivity of the natural cardiac baroreflex responses to spontaneous fluctuations in arterial pressure throughout the three weeks of baroreflex activation [16•]. Decreases in heart rate variability and cardiac baroreflex sensitivity have been identified as predictors and risk factors for cardiac arrhythmias and mortality in cardiovascular and renal disease [31–34], and are common findings in obese individuals [35, 36], along with their propensity for cardiac arrhythmias [37–39]. Therefore, from 24-hour recordings of spontaneous fluctuations in arterial pressure and heart rate, we evaluated whether BAT restored impaired spontaneous baroreflex control and heart rate variability in dogs with obesity-induced hypertension [40•]. As illustrated in Fig. 1, the development of obesity by fat-feeding led to dramatic reductions in baroreflex sensitivity and heart rate variability, while arterial pressure and heart rate progressively increased (not shown). Remarkably, baroreflex activation abolished the hypertension and markedly reduced the tachycardia in the established phase of obesity when the increase in body weight was stable. More specifically, BAT completely restored cardiac baroreflex sensitivity and considerably improved heart rate variability. In addition, daily power spectral analysis of heart rate throughout the duration of the study showed that reduced parasympathetic activity played a key role in mediating impairment of cardiac baroreflex sensitivity and heart rate variability and, most importantly, their restoration by BAT.
Fig. 1.
Changes in baroreflex sensitivity and heart rate variability during developing obesity and responses to baroreflex activation therapy and bilateral renal denervation during established obesity. Control (C), recovery (Rec). During days 4–31, obesity hypertension was induced by feeding dogs a high-fat diet. During this time, mean arterial pressure and heart rate increased from 100±2 to 117±3 mm Hg and from 86±3 to 130±4 bpm, respectively, along with an increase in body weight of approximately 50 %. After day 31, dietary fat was minimal, and there were no further change in body weight throughout the remainder of the study
Parenthetically, bilateral renal denervation, while also abolishing the hypertension, had no effect on the tachycardia and did not improve the impairment in baroreflex sensitivity and heart rate variability of obesity (Fig. 1), discounting the importance of renal afferent nerves in this pathophysiology. Therefore, a potential benefit of BAT in patients with resistant hypertension, who are commonly obese, may be the restoration of cardiac rhythmicity by improving spontaneous baroreflex sensitivity and shifting cardiac autonomic balance in favor of parasympathetic activity. As arrhythmias are known to contribute significantly to mortality in heart failure, the actions of BAT to decrease sympathetic and increase parasympathetic activity may be particularly important in this patient population.
BAT for Treatment of Chronic Heart Failure
Heart failure (HF) is associated with autonomic imbalance and neurohormonal activation [8, 31, 41]. Increased sympathetic activity, withdrawal of vagal activity, and stimulation of the renin-angiotensin system all contribute to the pathogenesis of HF. Accordingly, sympathetic inhibition with β-adrenergic blockers and pharmacological inhibition of the reninangiotensin system have clear therapeutic benefit [42, 43]. However, despite experimental and clinical studies indicating that reduced baroreflex sensitivity and heart rate variability (markers of vagal activity) and increased heart rate are associated with heart failure and are risk factors for cardiac arrhythmias and mortality [31, 44], less attention has been given to increasing vagal activity to correct the autonomic imbalance of HF. More recently, experimental studies have demonstrated that electrical cervical vagal nerve stimulation improves long-term survival and left ventricular function in experimental models of chronic heart failure [45–47], suggesting that vagal withdrawal has a detrimental role in the pathogenesis of heart failure.
Table 1 summarizes the physiological effects of BAT reported in normotensive and hypertensive dogs and in patients with resistant hypertension. Because these findings indicate that BAT suppresses sympathetic activity, augments parasym-pathetic activity, suppresses neurohormonal activation, increases renal excretory function, and reduces cardiac hyper-trophy, it is reasonable to hypothesize that BAT may provide a beneficial therapeutic approach for the treatment of heart failure. This theory is supported by canine studies conducted in two different experimental models of heart failure.
Table 1.
Responses to BAT in Resistant Hypertension and in Normotensive and Hypertensive Canines
| • Sustained suppression of central sympathetic outflow |
| • Reduced arterial pressure |
| • Reduced left ventricular hypertrophy |
| • Improved renal excretory function |
| • Suppression of renin secretion |
| • Reduced heart rate and increased heart rate variability |
| • Increased cardiac baroreflex sensitivity |
Experimental Studies
Zucker et al. evaluated the effects of chronic BAT in the rapid ventricular pacing model of heart failure [48]. After two weeks of pacing, dogs were randomly assigned to either BAT or a control group without BAT. Subsequently, dogs were paced until they either died or were moribund. A most impressive response reported in this study was that BAT increased survival to 68 days, compared to 37 days in controls. In the BAT-treated group, there was substantially less activation of the sympathetic nervous and the renin-angiotensin systems, suggesting a plausible relationship between suppression of neurohormonal activation and increased survival. The effects of BAT were also evaluated in the coronary microembolization-induced model of heart failure [49•]. After coronary microembolization to produce an initial reduction in ejection fraction to 25 %, dogs were randomized into BAT and non-BAT control groups. Measures of left ventricular function, remodeling, and ventricular arrhythmogenesis were evaluated after 3–6 weeks of heart failure. In dogs with chronic heart failure, BAT increased left ventricular ejection fraction, stroke volume, and cardiac output, and decreased left ventricular end-systolic volume, indicating improvement in left ventricular systolic function. These measures were either unchanged or deteriorated further in control dogs with heart failure. Cellular and structural markers indicated partial reversal of left ventricular remodeling in the BAT-treated group. In addition, BAT therapy abolished the tachycardia associated with heart failure, and markedly increased the threshold for lethal ventricular arrhythmias.
In summary, experimental models of heart failure in dogs indicate that BAT improves autonomic imbalance and left ventricular function, increases the threshold for lethal ventricular arrhythmias, and prolongs survival. These findings, along with those reported in normotensive and hypertensive dogs and in patients with resistant hypertension (Table 1), including a report indicating substantial regression of left ventricular hypertrophy in 21 patients after 12 months of BAT [50], provide a strong rationale for conducting a small single-arm open-label study in patients with heart failure.
Clinical Studies
Eleven subjects with heart failure and reduced ejection fraction (NYHA class III) were enrolled in an open-label feasibility trial to determine whether BAT improved clinical outcome. Temporal changes during up to six months of follow-up showed sustained reductions in MNSA along with improvement in NYHA class and 6-minute walk distance [51]. There were no changes in either MAP or heart rate during BAT, suggesting improved cardiac function associated with increased stroke volume. The results of this preliminary open-label trial are encouraging and are consistent with the experimental and clinical studies discussed above. A larger-scale trial in 140 subjects, randomized 1:1 to BAT plus optimal medical management versus optimal medical management alone, is ongoing to confirm these clinical improvements and to determine whether this device-based therapy truly leads to desirable clinical outcomes (NCT01471860 and NCT 1720160).
Conclusions
Technical advances have led to the development of a medical device – the Barostim neo – that can reliably activate the carotid baroreflex with an acceptable degree of safety. Because activation of the sympathetic nervous system plays a key role in the pathogenesis of hypertension and heart failure, the unique ability of this medical device to chronically suppress central sympathetic outflow in a controlled manner, with favorable physiological effects on the heart and kidneys, underscores its potential value in the treatment of these conditions. Along with suppression of sympathetic activity, sustained baroreflex-mediated vagal modulation may add to the clinical benefit of BAT in heart failure by improving cardiac autonomic dysfunction, which contributes to the poor prognosis in these patients. Randomized controlled trials are ongoing, and results will influence whether BAT assumes a role in the treatment of resistant hypertension and heart failure.
Acknowledgements
The authors’ studies cited in this report were funded by National Heart, Lung, and Blood Institute Grant HL-51971.
Footnotes
This article is part of the Topical Collection on Device-Based Approaches for Hypertension
Compliance with Ethics Guidelines
Conflict of Interest Radu Iliescu and Ionut Tudorancea declare that they have no conflict of interest.
Thomas E. Lohmeier has received consulting fees from CVRx, Inc., and research support from National Institutes of Health.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Radu Iliescu, Department of Physiology and Biophysics, University of Mississippi Medical Center, 2500 North State St., Jackson, MS 39216-4505, USA; Department of Physiology, University of Medicine and Pharmacy, “Gr. T. Popa,” Iasi, 16, University St., Iasi, Romania.
Ionut Tudorancea, Department of Physiology, University of Medicine and Pharmacy, “Gr. T. Popa,” Iasi, 16, University St., Iasi, Romania.
Thomas E. Lohmeier, Department of Physiology and Biophysics, University of Mississippi Medical Center, 2500 North State St., Jackson, MS 39216-4505, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation. Mechanisms and potential for hyper-tension therapy. Hypertension. 2011;57:880–6. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. Hypertension. 2008;51:1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 3.Scheffers IJM, Kroon AA, Schmidli J, et al. Novel baroreflex activation therapy in resistant hypertension. J Am Coll Cardiol. 2010;56:1254–8. doi: 10.1016/j.jacc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 4•.Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal trial. J Am Coll Cardiol. 2011;58:765–73. doi: 10.1016/j.jacc.2011.06.008. [The first randomized controlled trial investigating safety and efficacy of baroreflex activation therapy as treatment for resistant hypertension.] [DOI] [PubMed] [Google Scholar]
- 5.Bakris GL, Nadim MK, Haller H, et al. Baroreflex activation therapy provides durable benefit in patients with resistant hyper-tension: results of long-term follow-up in the Rheos Pivotal trial. J Am Soc Hypertens. 2012;6:152–8. doi: 10.1016/j.jash.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe UC, Brandt M-C, Wachter R, et al. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens. 2012;6:270–6. doi: 10.1016/j.jash.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Gasser JP, Bisognano JD. Baroreflex activation therapy in hyper-tension. J Hum Hypertens. 2014:1–6. doi: 10.1038/jhh.2013.139. [DOI] [PubMed] [Google Scholar]
- 8.Cohn JN, Levine TB, Olivari MT. Plasma norepinephrine as a guide to prognosis in patients with chronic heart failure. N Engl J Med. 1984;311:819–23. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 9.Tordoir JHM, Scheffers I, Schmidli J, Savolainen H, Liebeskind U, Hansky B, et al. An implantable carotid sinus baroreflex activating system: surgical technique and short-term outcome from a multi-center feasibility trial for treatment of resistant hypertension. Eur J Vasc Endovasc Surg. 2007;33:414–21. doi: 10.1016/j.ejvs.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Lohmeier TE, Irwin ED, Rossing MA, et al. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–11. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 11.Lohmeier TE, Dwyer TM, Irwin ED, et al. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007;49:1307–14. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 12.Schmidli J, Savolainen H, Eckstein F, et al. Acute device-based blood pressure reduction: electrical activation of the carotid barore-flex in patients undergoing elective carotid surgery. Vascular. 2007;15:63–9. doi: 10.2310/6670.2007.00024. [DOI] [PubMed] [Google Scholar]
- 13.Lohmeier TE, Drummond HA. The baroreflex in the pathogenesis of hypertension. In: Lip GYH, Hall JE, editors. Comprehensive hypertension. Elsevier; New York: 2007. pp. 265–79. [Google Scholar]
- 14.Malpas SC. Sympathetic nervous system activity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–57. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 15.Heusser K, Tank J, Engeli S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–26. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 16•.Lohmeier TE, Iliescu R, Dwyer TM, et al. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol. 2010;299:H402–9. doi: 10.1152/ajpheart.00372.2010. [Shows that chronic baroreflex activation has sustained effects to suppress central sympathetic outflow and lower arterial pressure without activating the renin-angiotensin system.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohmeier TE, Hildebrandt DA, Dwyer TM, et al. Prolonged activation of the baroreflex decreases arterial pressure even during chronic adrenergic blockade. Hypertension. 2009;53:833–8. doi: 10.1161/HYPERTENSIONAHA.109.128884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohmeier TE, Iliescu R. Lowering of blood pressure by chronic suppression of central sympathetic outflow: insight from prolonged baroreflex activation. J Appl Physiol. 2012;113:1652–8. doi: 10.1152/japplphysiol.00552.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Ehmke H, Persson PB, Fischer S, et al. Resetting of pressure-dependent renin release by intrarenal α1-adrenoceptors in conscious dogs. Pflugers Arch. 1989;413:261–6. doi: 10.1007/BF00583539. [DOI] [PubMed] [Google Scholar]
- 21.Yang HM, Lohmeier TE, Kivlighn SD, et al. Sustained increases in plasma epinephrine concentration do not modulate renin release. Am J Physiol. 1989;257:E57–64. doi: 10.1152/ajpendo.1989.257.1.E57. [DOI] [PubMed] [Google Scholar]
- 22•.Lohmeier TE, Iliescu R, Liu B, et al. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–8. doi: 10.1161/HYPERTENSIONAHA.111.185074. [Provides insight into the mechanisms that account for abolition of hypertension and suppression of renin secretion and GFR during chronic activation of the baroreflex in dogs with obesity-induced hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohmeier TE, Dwyer TM, Hildebrandt DA, et al. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–200. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- 24.Lohmeier TE, Liu B, Georgakopoulos D, et al. Cardiovascular responses to chronic baroreflex activation in aldosterone hypertension. Hypertension. 2013;62:A354. [Google Scholar]
- 25•.Alnima T, de Leeuw PW, Tan ES, et al. Renal responses to long-term carotid baroreflex activation therapy in patients with drug-resistant hypertension. Hypertension. 2013;61:1334–9. doi: 10.1161/HYPERTENSIONAHA.113.01159. [This study reports an initial modest non-progressive decrease in eGFR in association with the antihypertensive response to 12 months of baroreflex activation therapy in patients with resistant hypertension. There were no changes in eGFR during baroreflex activation in patients in the lowest baseline eGFR category (<60 mL/min).] [DOI] [PubMed] [Google Scholar]
- 26.Iliescu R, Irwin ED, Georgakopoulos D, Irwin ED, et al. Renal responses to chronic suppression of central sympathetic outflow. Hypertension. 2012;60:749–56. doi: 10.1161/HYPERTENSIONAHA.112.193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular hyperfiltration. Am J Physiol Renal Physiol. 2004;286:F8–15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 28.Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol. 1999;10:S258–65. [PubMed] [Google Scholar]
- 29.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–93. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 30.Wustmann K, Kucera JP, Scheffers I, et al. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54:530–6. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev. 2011;16:101–7. doi: 10.1007/s10741-010-9179-1. [DOI] [PubMed] [Google Scholar]
- 32.Hildreth CM. Prognostic indicators of cardiovascular risk in renal disease. Front Physiol. 2012;2:1–6. doi: 10.3389/fphys.2011.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuji H, Venditti FJ, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–83. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 34.Reed MJ, Robertson CE, Addison PS. Heart rate variability measurements and the prediction of ventricular arrhythmias. Quart J Med. 2005;98:87–95. doi: 10.1093/qjmed/hci018. [DOI] [PubMed] [Google Scholar]
- 35.Beske S, Alvarez GE, Ballard TP, et al. Reduced cardiovagal gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2002;282:H630–5. doi: 10.1152/ajpheart.00642.2001. [DOI] [PubMed] [Google Scholar]
- 36.Grassi G, Seravalle G, Dell'Oro R, et al. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–42. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 37.Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol. 2007;18:87–95. doi: 10.1111/j.1540-8167.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 38.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of meta-analysis. Am Heart J. 2008;155:310–5. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Wang TJ, Parise H, Levy D, D'Agostino Sr RB, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. J Am Med Assoc. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 40•.Iliescu R, Tudorancea I, Irwin ED. Chronic baroreflex activation restores spontaneous baroreflex control and variability of heart rate in obesity-induced hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1080–8. doi: 10.1152/ajpheart.00464.2013. [Based on continuous 24-hour recordings of spontaneous fluctuations in arterial pressure and heart rate, this study shows that chronic baroreflex activation restores impaired cardiac baroreflex sensitivity and heart rate variability in obesity hypertension.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of left ventricular dysfunction (SOLVD). Circulation. 1990;82:1724–9. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 42.The SOLVD. investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 43.Cibis II. Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBUS II). Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 44.Lechat P. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation. 2001;103:1428–33. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- 45.Sabbah HN, Ilsar I, Zaretsky A, et al. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 2011;16:171–8. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Popovic ZB, Bibevski S, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–9. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 47.Li M, Zheng C, Sato T, et al. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 48.Zucker IH, Hackley JF, Cornish KG, et al. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–10. doi: 10.1161/HYPERTENSIONAHA.107.095216. [DOI] [PubMed] [Google Scholar]
- 49•.Sabbah HN, Gupta RC, Imai M, et al. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011;4:65–70. doi: 10.1161/CIRCHEARTFAILURE.110.955013. [Shows that chronic baroreflex activation improves left ventricular function and partially reverses left ventricular remodeling both globally and at the cellular and molecular levels, and increases the threshold for lethal ventricular arrhythmias in dogs with advanced heart failure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bisognano JD, Kaufmann CL, Bach DS. Improved cardiac structure and function with chronic treatment using an inflatable device in resistant hypertension. J Am Col Cardiol. 2011;57:1787–8. doi: 10.1016/j.jacc.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 51.Gronda E, Seravalle G, Brambilla G. Chronic baroreflex activation reduces sympathetic tone and improves clinical outcomes in reduced-ejection fraction heart failure. Circulation. 2013;128:A16137. [Google Scholar]