Abstract
There is increasing evidence that many human cancers, including breast cancer, are driven and maintained by cancer stem cells (CSCs) which mediate tumor metastasis and contribute to treatment resistance and relapse. Our group was the first to describe “breast cancer stem cells” (BCSCs) characterized by expression of the cell surface markers ESA and CD44 and the absence of expression of the marker CD24. More recently, we have demonstrated that breast cancer cells contain subpopulations with stem cell properties that can be isolated by virtue of their expression of Aldehyde dehydrogenase (ALDH) as assessed by the Aldefluor assay. Interestingly, these markers identify overlapping, but not identical cell populations. Recent studies have suggested similarities between cancer stem cells and the epithelial mesenchymal transition (EMT) state. Our studies suggest that both normal and malignant breast stem cells exist in distinct, inter-convertible states (EMT and MET), the inter-conversion of which is regulated by micro-RNAs. EMT-like CSCs have a mesenchymal morphology, are largely quiescent, invasive and characterized by expression of the CSC markers CD24−CD44+ and are EpCAM−CD49f+. In contrast, the MET (mesenchymal epithelial transition) state of CSCs is characterized by active self-renewal and expression of the CSC markers ALDH and EpCAM+CD49f+. A subpopulation of cells expressing both CD24−CD44+ and ALDH may represent cells in transition between these states. This transition is regulated by signals originating in the microenvironment which in turn modulate microRNA networks in the CSC populations. The existence of multiple stem cell states suggests the necessity of developing therapeutic strategies capable of effectively targeting CSCs in all of these states. In addition, since CSC states are regulated by miRNAs, these small non-coding RNAs may be useful therapeutic agents to target CSCs.
Keywords: microRNA, Breast cancer stem cell, EMT, MET
Breast Cancer Stem Cells
With over 200,000 new cases yearly, breast cancer is the most common malignancy of women in the United States (US) [1]. The past 20 years have seen significant reductions in mortality from breast cancer in the United States and elsewhere [2]. This reduction has been largely due to improvement in early detection and the development of more effective adjuvant therapies [2]. Despite the fact that there have been significant advances in the treatment of breast cancer, the fact remains that once metastatic, the disease remains incurable. Recent studies in our laboratory, and others, have provided strong support for the cancer stem cell hypothesis which suggests that breast cancers are driven by a subpopulation of cells which display stem cell properties. These properties include self-renewal which generates other cancer stem cells and differentiation which generates populations of cells forming the bulk of the tumor. There is increasing evidence that cancer stem cells are resistant to chemotherapy and radiation therapy and, thus, contribute to treatment resistance and relapse.
The development of biomarkers to identify CSCs, as well as validation of in vitro and mouse models, has facilitated the isolation and characterization of these cells from both murine and human tumors. Our group was the first to describe a subpopulation in BC that displayed stem cell properties and was characterized by expression of the cell surface markers ESA and CD44 in the absence of expression of the marker CD24 [3]. These cells have been termed “breast cancer stem cells” (BCSCs). As few as 200 ESA-positive CD44+/CD24−Lin− cells were able to generate tumors in immunocompromised NOD/SCID mice, whereas 100-fold more cells without these markers isolated from the same tumors were non-tumorigenic [3]. Furthermore, the tumor-initiating populations regenerated tumors that recapitulated the heterogeneity of the initial tumor [3]. We also developed an in vitro “mammosphere” assay as a means of quantitating normal and malignant stem cells [4]. More recently, we have described the expression of aldehyde dehydrogenase (ALDH) as assessed by the Aldefluor assay (StemCell Technologies, Canada) or the isoform ALDH1 by immunohistochemistry (IHC) as a means of further identifying and enriching for tumor initiating CSC populations in human BCs [5]. Interestingly, we reported that these markers identify overlapping, but not identical cell populations [5]. Furthermore, we and others have found that these markers can be utilized to isolate CSC populations from established breast cancer cell lines, as well as primary tumor xenografts [8]. The development and validation of breast cancer stem cell (BCSC) biomarkers, in vitro mammosphere formation assays, and xenograft models by our laboratory and others [3, 6–8] has permitted assessment of chemotherapy and radiation resistance of BCSCs. These studies [9–11] have demonstrated the relative resistance of BCSC to chemotherapy and radiation therapy. Furthermore, it has recently been demonstrated that the percent of BCSC as assessed either by CD44+/CD24low, mammosphere assays [12] or by ALDH expression [13] increases following neoadjuvant chemotherapy providing direct clinical evidence for the therapeutic resistance of BCSC. Together, these studies suggest that significant improvement in patient outcome will require the successful targeting of BCSCs.
MicroRNAs
Until recently, the function of non-coding regions of the genome was unknown. However, it is now clear that many of these regions code for microRNAs. Each microRNA is capable of regulating the expression of multiple proteins and as a result, can have very potent effects on cellular functions. The miRNA gene is first transcribed by RNA polymerase II into a primary transcript (pri-miRNA) in the nucleus, where the hairpin stem-loop structure is processed into precursor miRNA (pre-miRNA) by a microprocessing complex, which includes Drosha and DGCR8 [14]. The 60–70 nt-long pre-miRNAs is exported from the nucleus [14]. Within the cytoplasm, the RNAse III enzyme Dicer processes the pre-miRNA to yield the 18–25 nt mature miRNAs which mediate gene silencing through imperfect hybridization to 3′ untranslated regions (3′ UTR) in target mRNAs [15] and modulate a variety of cellular processes including regulating m-RNA stability and proliferation, differentiation translation, microRNAs and apoptosis [16].
MicroRNAs Regulate Cancer Stem Cells (CSCs)
Recent studies have demonstrated a link between dysregulated expression of miRNAs and carcinogenesis. A number of miRNAs have been shown to function as oncogenes or tumor suppressors during carcinogenesis [17, 18]. In addition, emerging evidence suggests that miRNAs also play essential roles in stem cell self-renewal and differentiation by negatively regulating the expression of key stem cell regulating genes [19]. Furthermore, abnormal miRNA expression may result in dysregulation of self-renewal in cancer stem cells during cancer progression [20–22]. Silber et al. reported that mir124 and mir137 induce differentiation of neural and glioblastoma stem cells and induce cell cycle arrest [23]. These results suggest that targeted delivery of mir124 and mir137 to glioblastoma cells may be therapeutically efficacious for the glioblastoma treatment. miRNA181 and miRNA17-92 clusters were shown to be up-regulated in hepatocellular carcinoma (HCC) CSCs [24]. More recently, Tang’s group showed that prostate cancer stem and/or progenitor cell populations have lower levels of miR-34a and let-7b compared to bulk tumor cells [25]. In addition, they reported that miR34a targets CD44, resulting in impaired tumor growth and decreased metastases in mouse models of prostate cancer. The increased survival of mice treated with systemically delivered miR34a suggests a novel strategy to target prostate CSCs, thereby inhibiting tumor growth and metastasis [25].
MicroRNAs and Breast Cancer Stem Cells (BCSCs)
There have been a number of studies describing a role of microRNA in the regulation of normal and malignant breast stem cells. Hannon’s group showed that both mir-205 and mir-22 are highly expressed in mouse mammary stem/progenitor cells whereas mir-93 and Let7 are depleted in this population [26]. Rosen’s group reported that miR-205 overexpression in mouse mammary cells led to an expansion of the progenitor cell population, decreased cell size and increased cellular proliferation [27]. More recent studies have shown that overexpression of mir-200c reduced the clonogenic and tumor-initiation activities of BCSCs and suppressed mammary duct formation by normal mammary stem cells. This occurred through the down-regulation of the polycomb gene Bmi-1, a target of mir-200c. This work demonstrated a molecular link between normal breast stem cells and BCSCs [28]. Yu et al. showed that Let7 is decreased in BCSCs and that overexpression of Let7 inhibits the cell proliferation, mammosphere formation, BCSC self-renewal and differentiation, and tumor formation and metastasis in NOD/SCID mice [22]. These effects were shown to be mediated through down-regulation of the Let7 targets H-Ras and HMGA2 [22]. This group also demonstrated that expression of miR-30 markedly reduced BCSCs by targeting ubiquitin-conjugating enzyme 9 (UBC9) and integrin b3 (ITGB3). More complete inhibition of self-renewal and mammosphere formation of BCSCs was observed when both Let7 and miR-30 were simultaneously introduced compared to each microRNA individually [29]. The ability of these microRNAs to target BCSCs suggests that they may have significant therapeutic potential.
EMT and MET of BCSCs
Epithelial to mesenchymal transition (EMT) is involved in many biological processes including embryonic development, wound healing and cancer progression [30]. During EMT, epithelial cells lose cell-cell contacts and undergo cytoskeletal remodeling and polarity changes, resulting in acquisition of a mesenchymal morphology as well as enhanced migratory ability. Importantly, EMT is reversible and these cells can undergo a mesenchymal to epithelial transition (MET), so that polarized epithelium can be generated at a new site. Both EMT and MET play central roles in embryogenesis [30]. During development, the process of EMT is required for tissue and organ formation [31]. The EMT state has been associated with loss of epithelial characteristics including apical basal polarity and expression of E-Cadherin and acquisition of mesenchymal characteristics including loss of polarity and increased expression of the transcription factors slug, snail and twist and mesenchymal proteins including vimentin and fibronectin [32]. During early embryonic development, the mesoderm generated by EMTs develops into multiple tissue types, and later in development, mesodermal cells generate epithelial organs (e. g., kidney and ovary) by METs [33]. In adult tissues, TGF-β can induce EMT characterized by downregulation of epithelial markers such as E-cadherin and upregulation of EMT-inducing factors, such as Twist and Snail [34, 35]. It has been proposed that EMT plays an important role in tumorigenesis and progression. Furthermore, a number of developmental pathways such as the Wnt and HGF-cMet pathways which are frequently deregulated in cancers are also regulators of EMT [36]. Both the inflammatory immune response [37] and the hypoxic tumor environment [38] induce EMT in cancers. It is increasingly recognized that EMT plays an important role in the metastasis of breast cancer [39] and other types of carcinoma [40, 41]. EMT has also been implicated in therapeutic resistance and tumor recurrence [42–44]. Since EMT is a key developmental program that is often activated during cancer invasion and metastasis, and CSCs that maintain and initiate tumors have also been implicated in invasion and metastasis, the relationship between EMT and CSCs is an important question. Recently, a number of studies have linked the EMT state to cancer stem cells. Mani and Weinberg demonstrated that acquisition of EMT is associated with expression of CSC markers such as CD24−CD44+ and generation of tumorispheres in breast cancer models [45]. A defining characteristic of CSCs is their ability to self-renew, a property that endows these cells with the ability to initiate and sustain tumor growth. However, although the EMT state has been linked to tumor invasion and metastasis, EMT cells are largely quiescent [46]. The differences in invasive and proliferative characteristics of CSCs and EMT cells has led to the proposition that contrary to Mani, et al., 2008, CSCs and EMTs are mutually exclusive [46].
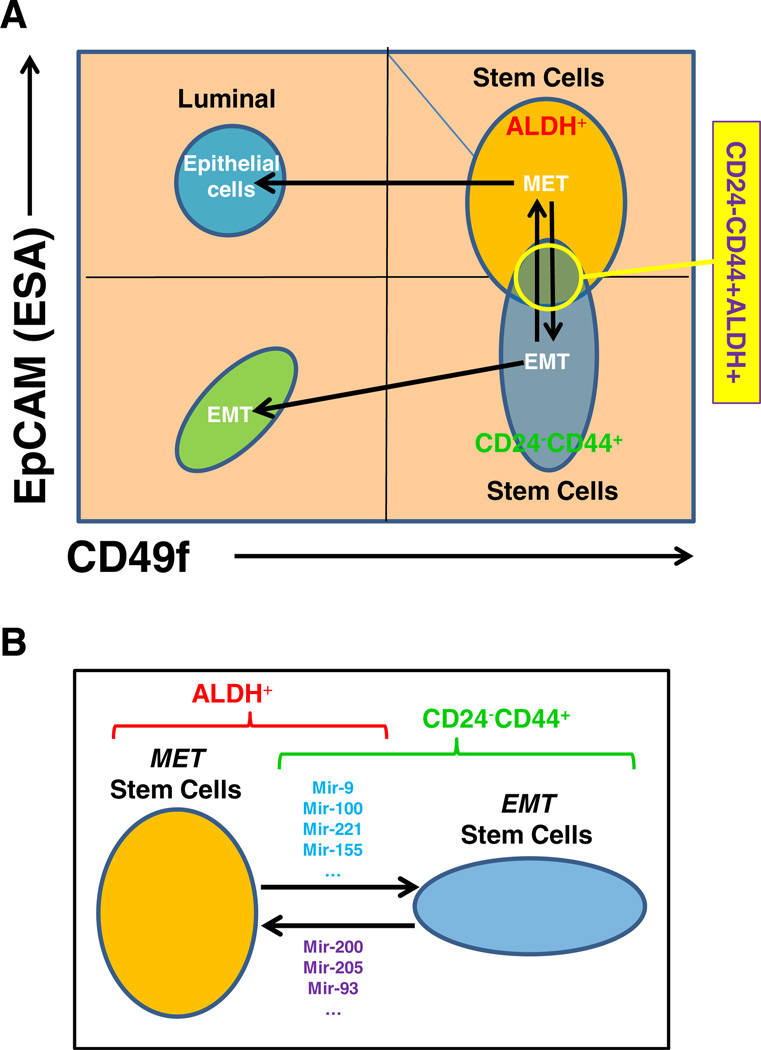
Recently it has been suggested that expression of the cell surface markers EpCAM and CD49f can be used to define functional populations of normal mouse and human mammary cells [47]. Based on in vitro and mouse fat pad re-implantation studies it has been suggested that EpCAM−CD49f+ cells represent mammary stem cells, EpCAM+CD49+ (double-positive cells): luminal progenitors; EpCAM+CD49f−: epithelial cells; and EpCAM−CD49f −: stromal cells [47]. However, double-positive (EpCAM+CD49f+) so-called luminal progenitor cells, have been found to give rise to basal as well as luminal cells when cultured in vitro [48]. Furthermore, it has recently been reported that both the EpCAM−CD49f+ and EpCAM+CD49f+ fractions of normal human mammary cells have the ability to form complete mammary trees consisting of basal as well as luminal cells when transplanted into the fatpads of immunosuppressed mice, which have been “humanized” with normal mammary fibroblasts [48]. These results suggest that in addition to luminal progenitors, the EpCAM+CD49f+ population may also contain a sub-population with stem cell characteristics. As described previously by our lab, CD24−CD44+ and ALDH identify overlapping, but not identical cell populations. We have generated preliminary data suggesting that EpCAM+CD49f+ cells (MET-like) contain an ALDH enriched population, whereas CD24−CD44+ cells are mainly contained in EpCAM−CD49f+ population (EMT-like). These results suggest a model in which breast cancer stem cells can exist in two alternative states: one, an EMT-like state which is EpCAM−CD49f+ expresses the stem cell markers CD24−CD44+, and an MET-like population which is EpCAM+CD49f+ (double-positive) and expresses the CSC marker ALDH (Fig. 1a). The existence of multiple states of cancer stem cells has also been suggested by recent studies in squamous carcinoma [49].
Figure 1.
Two stem cell states. a Breast cancer stem cells (BCSCs) can exist in two alternative states: one, an EMT-like state which is EpCAM-CD49f+ expresses the stem cell markers CD24−CD44+, and an MET-like population which is EpCAM+CD49f+ (double-positive) and expresses the CSC marker ALDH. b The two stem cell states are interconvertable, which is regulated by the microRNA networks. Such as: mir-9, mir-100, mir-221 and mir-155 can induce EMT stem cells; mir-200, mir-205 and mir-93 can induce MET stem cells
microRNA Regulation of EMT-MET States of BCSCs
There is substantial evidence linking BCSCs and EMT. BCSCs isolated from primary breast tumors and metastatic pleural effusions express EMT markers [45, 50, 51]. However, there is less evidence linking BCSCs and MET. Consistent with the expression of EMT markers by BCSCs, these cells also express EMT related microRNAs. miRNA expression profiling of BCSCs isolated from human breast tumors compared to the remaining breast cancer cells revealed high levels of expression of EMT-inducing miR-155 [28]. Furthermore, mir-200 which is downregulated in BCSCs is associated with MET [28, 52].
Recently, we demonstrated that the double-positive EpCAM+CD49f+ population is characterized by the highest expression of mir-93 and that forced overexpression of mir-93 increases the proportion of EpCAM+CD49f+ and ALDH+ cells in non-transformed MCF-10A cells as well as primary normal human mammary cells isolated from reduction mammoplasty specimens (manuscript submitted). Furthermore, we demonstrated that expression levels of mir-221 and mir-100 are significant higher in EpCAM−CD49f+ and EpCAM−CD49f− populations than in EpCAM+CD49f+ and EpCAM+CD49f− populations. Furthermore, forced overexpression of mir-100 or mir-221 increased the proportion of EpCAM−CD49f+ cells in non-transformed MCF-10A cells as well as primary normal human mammary cells isolated from reduction mammoplasty specimens. This resulted in an increase in the proportion of CD24−/CD44+ CSC cells with a concomitant decrease in the ALDH+ CSC population (manuscript in preparation). These results suggest that mir-93, mir-100 and mir-221 may be important regulators of the transition between the EMT and MET stem cell states. We demonstrated that induction of mir-93 in EMT-like SUM159 cells induces an MET in the ALDH-positive CSC population characterized by increased expression of E-Cadherin and Claudin, and downreguation of mesenchymal genes, such as vimentin, N-Cadherin and Twist. We have found that mir-93 also inhibits TGFβ signaling by targeting TGFβR2, an effect seen within 12 h of mir-93 induction. This was followed by an EMT/MET transition in the Aldefluor-positive CSC population. Since TGFβ is a major regulator of EMT, abrogation of this signaling pathway may facilitate MET. Of interest, it has been recently reported that the mir106b-25 cluster including mir-93 is induced in the early stages of nuclear reprogramming of fibroblasts into IPS cells [53]. This is accompanied by an EMT to MET conversion in these cells which is obligatory for reprogramming to occur. This suggests that this miRNA cluster may regulate EMT to MET in multiple biological contexts. Furthermore, expression of mir-100 or mir-221 in MCF10A cells and several cancer cell lines resulted in a decrease of the ALDH-positive CSC population with a concomitant increase in the CD24−CD44+ population accompanied by induction of EMT. We demonstrated mir- 100 effects are mediated by targeting BMPR2, SMARCA5 and SMARCD1, all of which may contribute to induction of EMT.
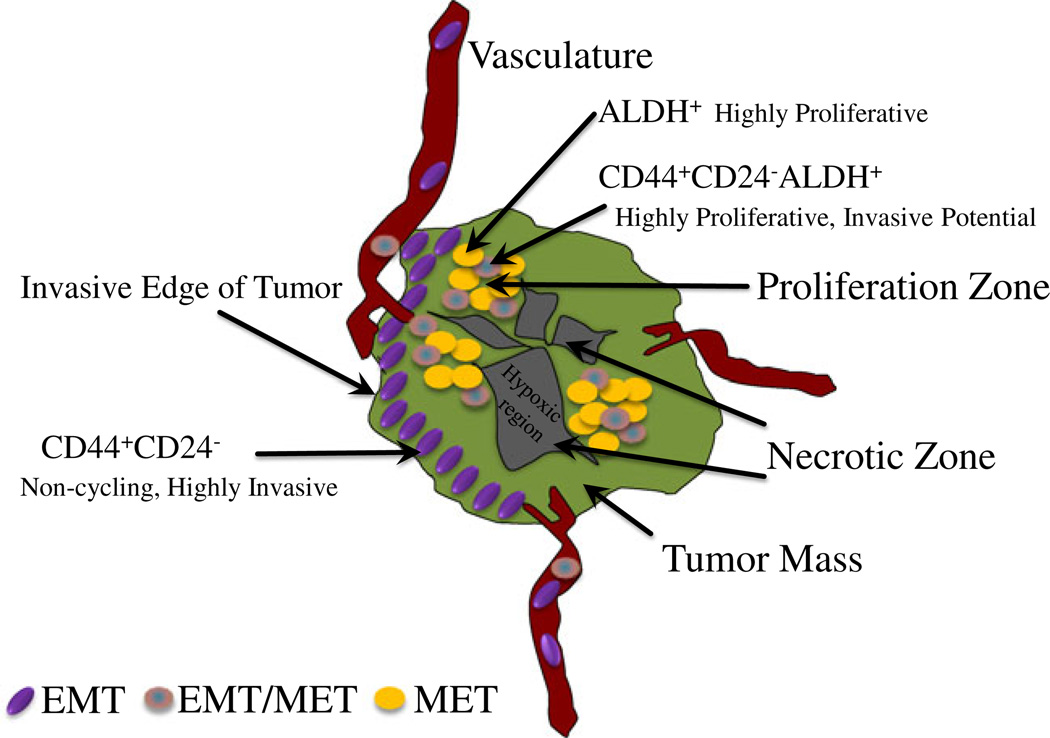
The existence of reversible alternative states of CSCs provides an explanation for the seemingly disparate hypothesis concerning the relationship between CSCs and EMT. We propose that CSCs may exist in either EMT or MET states, the inter-conversion of which is regulated by the microenvironment which in turn regulates CSC microRNA networks as illustrated in Fig. 1b. The existence of alternative CSC states provides an explanation for how these cells promote tumor invasion as well as growth at metastatic sites. For example, it has been shown that bladder cancer cells selected for bone metastatic competence are overtly epithelial as compared to their parental cells [54]. Similarly, human breast cancer, liver, lung and brain metastasis often express more E-cadherin than their corresponding primary tumors [55, 56]. Previous studies together with our current work suggests a model (Fig. 2) in which CSCs located inside the tumor mass exist predominantly in the MET state in which they are highly proliferative and express ALDH, in contrast at the tumor invasive front. Factors such as TGF-β in the microenvironment induce EMT in the CSC populations. The population which is characterized as CD24−CD44+ is highly invasive and mediates tumor invastion and metastasis. The association of EMT and invasion is supported by our studies demonstrating that downregulation of mir-93 using a mirZIP vector or upregulation of mir-100 increases the invasiveness of cancer cells. Highly invasive EMT/CSCs enter the circulation and travel to distant organs where they form micrometastasis [8]. This scenario is supported by studies showing that in women with breast cancer, bone micrometastasis express CSC markers such as CD24−CD44+ as well as EMT markers such as vimentin [57]. These micrometastasis are largely quiescent as indicated by their lack of expression of markers of cellular proliferation such as Ki67 [46]. In order to enter a proliferative state, EMT/CSC cells must undergo an MET transition in which they lose their invasive characteristics and acquire self-renewal capacity. Self-renewing MET/CSCs in turn drive tumor growth at metastatic sites. In this model, the balance of EMT/MET states of CSCs regulated by miRNAs plays an important role in mediating tumor invasion and metastasis, as well as maintaining tumor dormancy or promoting tumor growth at metastatic sites.
Figure 2.
Tumor Heterogeneity. CSCs located inside the tumor mass exist predominantly in the MET state in which they are highly proliferative and express ALDH (ALDH+), in contrast at the tumor invasive front are located with EMT CSCs which are characterized as CD24−CD44+ and are highly invasive, and mediates tumor invastion and metastasis. The intermediate CSCs (CD24−CD44+ALDH+) also reside inside the tumor mass, and they are highly proliferative with invasive potential
Conclusions
The ability of CSCs to exist in alternative EMT and MET states, the transition of which is regulated by the microenvironment and mediated by miRNAs has important implications for understanding the role of the cells carcinogenesis, invasion and metastasis. In addition, the existence of alternative CSC states, associated with expression of different protein markers has important implications for understanding the plasticity of CSCs. For example, it has been claimed that CSCs may be generated from non-CSC tumor populations through induction of EMT [45]. However, the existence of alternative CSC states suggests that the acquisition of stem cell markers may reflect transition of CSC states rather than generation of CSCs from non-CSC populations. Future experiments will need to determine the spectrum of cells capable of acquiring an EMT/CSC phenotype. In addition, the existence of multiple stem cell states suggests the necessity of developing therapeutic strategies capable of effectively targeting CSCs in all of these states. Dysregulation of microRNAs has been implicated in tumor development and microRNAs plays important roles in regulating cancer stem cells. Since CSCs have been shown to be involved in tumor initiation, tumor maintenance, metastasis, and therapeutic resistance, the regulation of microRNA networks in CSCs may provide novel therapeutic targets. However, the use of miRNAs as therapeutic agents poses a number of technical challenges, such as how to achieve efficient systemic delivery. Because, in theory, each CSC has the potential to generate a tumor, prevention of tumor recurrence would require the successful targeting of all CSCs. The existence of multiple CSC states also has important therapeutic implications, since CSCs in these states may respond differently to therapeutic agents. Nevertheless, the complexity of regulatory pathways in CSCs, as well as the heterogeneity of these cell populations, suggests that it may be necessary to combine multiple CSC-targeting agents to eliminate all CSC populations and thus improve the outcome for cancer patients.
Abbreviation
- CSC
cancer stem cell
- BCSC
breast cancer stem cell
- ALDH
Aldehyde dehydrogenase
- EMT
epithelial mesenchymal transition
- MET
mesenchymal epithelial transition
- ESA
epithelial specific antigen
- IHC
immunohistochemistry
- miRNA
microRNA
- mir
microRNA
- UTR
untranslated regions
- HCC
hepatocellular carcinoma
Footnotes
Disclosure M Wicha holds equity in and is an advisor for OncoMed Pharmaceuticals.
References
- 1.SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. (eds). http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dontu G, Abdallah W, Foley J, Jackson K, Clarke M, Kawamura M, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginestier C, Hur M, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant breast stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7(6) doi: 10.1371/journal.pbio.1000121. e1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 11.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68(9):3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 13.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15(12):4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 14.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303(5654):95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 15.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Kato M, Slack FJ. microRNAs: small molecules with big roles—C. elegans to human cancer. Biol Cell. 2008;100(2):71–81. doi: 10.1042/BC20070078. [DOI] [PubMed] [Google Scholar]
- 17.Lowery AJ, Miller N, McNeill RE, Kerin MJ. MicroRNAs as prognostic indicators and therapeutic targets: potential effect on breast cancer management. Clin Cancer Res. 2008;14(2):360–365. doi: 10.1158/1078-0432.CCR-07-0992. [DOI] [PubMed] [Google Scholar]
- 18.Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer. 2007;43(10):1529–1544. doi: 10.1016/j.ejca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Hatfield S, Ruohola-Baker H. microRNA and stem cell function. Cell Tissue Res. 2008;331(1):57–66. doi: 10.1007/s00441-007-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 21.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 22.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self-renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50(2):472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21(24):3238–3243. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene SB, Gunaratne PH, Hammond SM, Rosen JM. A putative role for microRNA-205 in mammary epithelial cell progenitors. J Cell Sci. 2010;123(Pt 4):606–618. doi: 10.1242/jcs.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29(29):4194–4204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 30.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119(6):1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies JA. Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat (Basel) 1996;156(3):187–201. doi: 10.1159/000147846. [DOI] [PubMed] [Google Scholar]
- 34.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Shin SY, Rath O, Zebisch A, Choo SM, Kolch W, Cho KH. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res. 2010;70(17):6715–6724. doi: 10.1158/0008-5472.CAN-10-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15(5):416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 39.Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107(3):1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15(3):195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 41.Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65(14):5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. [DOI] [PubMed] [Google Scholar]
- 42.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skvortsova I, Skvortsov S, Raju U, Stasyk T, Riesterer O, Schottdorf EM, et al. Epithelial-to-mesenchymal transition and c-myc expression are the determinants of cetuximab-induced enhancement of squamous cell carcinoma radioresponse. Radiother Oncol. 2010;96(1):108–115. doi: 10.1016/j.radonc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Bandyopadhyay A, Wang L, Agyin J, Tang Y, Lin S, Yeh IT, et al. Doxorubicin in combination with a small TGFbeta inhibitor: a potential novel therapy for metastatic breast cancer in mouse models. PLoS One. 2010;5(4):e10365. doi: 10.1371/journal.pone.0010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68(24):10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15(8):907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 48.Keller PJ, Arendt LM, Skibinski A, Logvinenko T, Klebba I, Dong S, Smith AE, Prat A, Perou CM, Gilmore H, et al. Defining the cellular precursors to human breast cancer. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1017626108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, et al. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011;71(15):5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]
- 50.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Wang L, Matyunina LV, Hill CG, McDonald JF. Overexpression of miR-429 induces mesenchymal-to-epithelial transition (MET) in metastatic ovarian cancer cells. Gynecol Oncol. 2011;121(1):200–205. doi: 10.1016/j.ygyno.2010.12.339. [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30(5):823–834. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66(23):11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 55.Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yates CC, Shepard CR, Stolz DB, Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer. 2007;96(8):1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8(5):R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]