Abstract
Functional metagenomic analysis of soil metagenomes is a method for uncovering as-yet unidentified mechanisms for antibiotic resistance. Here we report an unconventional mode by which a response regulator derived from a soil metagenome confers resistance to the β-lactam antibiotic carbenicillin in Escherichia coli. A recombinant clone (βlr16) harboring a 5,169 bp DNA insert was selected from a metagenomic library previously constructed from a remote Alaskan soil. The βlr16 clone conferred specific resistance to carbenicillin, with limited increases in resistance to other tested antibiotics, including other β-lactams (penicillins and cephalosporins), rifampin, ciprofloxacin, erythromycin, chloramphenicol, nalidixic acid, fusidic acid, and gentamicin. Resistance was more pronounced at 24°C than at 37°C. Zone-of-inhibition assays suggested that the mechanism of carbenicillin resistance was not due to antibiotic inactivation. The DNA insert did not encode any genes known to confer antibiotic resistance, but did have two putative open reading frames (ORFs) that were annotated as a metallopeptidase and a two-component response regulator. Transposon mutagenesis and subcloning of the two ORFs followed by phenotypic assays showed that the response regulator gene was necessary and sufficient to confer the resistance phenotype. Quantitative reverse transcriptase PCR showed that the response regulator suppressed expression of the ompF porin gene, independently of the small RNA regulator micF, and enhanced expression of the acrD, mdtA, and mdtB efflux pump genes. This work demonstrates that antibiotic resistance can be achieved by the modulation of gene regulation by heterologous DNA. Functional analyses such as these can be important for making discoveries in antibiotic resistance gene biology and ecology.
Introduction
Culture-independent metagenomic analysis of soils has led to myriad discoveries concerning the genetics, genomics, and ecology of bacteria that inhabit this environment. While most metagenomic studies of soil are based on high-throughput sequencing of total DNA or 16S rRNA gene amplicons, functional screening of metagenomic DNA libraries (functional metagenomics [1]) offers a different method for discovering genes of interest from soil metagenomes [2]. Functional metagenomics is the expression of metagenomic DNA in a host bacterium, and one of the most straightforward functional metagenomic approaches is selection for antibiotic resistance. This strategy has been applied to diverse soils with various potential for selection pressure due to anthropogenic activities [2–8]. These environments include remote soils absent of human intervention as well as soils on which antibiotics and resistant bacteria are applied, such as manure-amended soils. From these and other studies, a picture emerges of soil as a rich reservoir for antibiotic resistance genes [9]—even in so-called “pristine environments”. Furthermore, the genes recovered using functional metagenomic selection often show little homology with canonical antibiotic resistance genes that have been identified from bacteria to date, thereby illustrating the power of this approach for resistance gene discovery [2,4].
The β-lactams consistently make up greater than 50% of the antibiotic market share, with the cephalosporins and broad-spectrum penicillins the two largest classes [10], and the widespread dissemination of β-lactam resistance represents a major medical concern [11,12]. The β-lactam antibiotics inhibit bacterial d,d-transpeptidase enzymes, ultimately disrupting the periplasmic steps of peptidoglycan synthesis [13,14]. By definition, therefore, β-lactams must traverse the outer membrane of Gram negative bacteria to reach the periplasmic space. In E. coli, they do so primarily via passive diffusion through outer membrane porins [17,18], most notably OmpF and OmpC. From the periplasm, β-lactams can be pumped back across the outer membrane using one of a number of TolC-assisted pumps [15]. The AcrAB-TolC, AcrAD-TolC, and MdtABC-TolC pumps of E. coli have been implicated in increased β-lactam tolerance [15]. However, resistance to β-lactams is more commonly mediated by β-lactamase enzymes [16]. The mechanisms by which bacteria are known to develop resistance to the β-lactams therefore include decreased influx, increased efflux, target modification, and acquisition of a β-lactamase [16]. Here we describe a foreign two-component response regulator gene from an Alaskan soil metagenome that confers resistance to the β-lactam carbenicillin on E. coli.
Two-component signal transduction is a common mechanism by which bacteria sense and respond to environmental stimuli [17]. The two components comprise a sensor histidine kinase protein, which typically contains periplasmic receptor and cytoplasmic kinase/phosphatase domains, and a response regulator protein. The sensor kinase senses the stimulus, which results in the transfer of a phosphoryl group from its cytoplasmic domain to an aspartate residue on the response regulator receiver domain. Phosphorylation of the response regulator receiver domain results in activation of its output domain. An active response regulator typically achieves its goal by directly altering bacterial transcription of genes necessary for response to the original stimulus [18]. Two-component regulation is ubiquitous within the prokaryotic domain, with the numbers of two component proteins varying among bacteria from only a few to over 250 proteins (E. coli has and 29 sensor kinases and 32 response regulators) [18]. Our study shows that the introduction of a foreign response regulator gene into E. coli can directly impact gene expression, leading to an antibiotic resistance phenotype.
Materials and Methods
Bacterial strains, plasmids, and growth conditions
Bacterial strains and plasmids used in this study are in Table 1. The metagenomic libraries AK10, AK14, AK16, and AK18 are in the vector pCF430 [19] and are reported elsewhere [2]. Metagenomic libraries were expressed in TransforMax EPI300 Escherichia coli cells (Epicentre, Madison, WI, USA) for antibiotic resistance screening purposes. Metagenomic libraries were selected on eight β-lactam antibiotics at 24°C and 37°C as described [2]. The recombinant clone designated as βLR16 (Table 1) was recovered only on carbenicillin (50μg ml-1) after growth at 24°C. Subsequent growth of the parent clone and subclones was in Luria-Bertani (LB) medium at 24–28°C. Growth media was amended with tetracycline at 20 μg ml-1 or kanamycin at 20 μg ml-1 for plasmid maintenance where appropriate. The DNA sequence of the βLR16 DNA insert was previously submitted to GenBank (EU408353.1).
Table 1. Strains and plasmids used in this study.
| Strain or plasmid | Genotype or relevant characteristics | Reference or Source |
|---|---|---|
| E. coli strains | ||
| EPI300 | F- mcrA Δ(mrr-hsdRMS-mcrBC)Φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ- rpsL (StrR) nupG trfA dhfr | Epicentre, Madison, WI |
| BL21(DE3) | F- ompT hsdSB(rB–, mB–) gal dcm (DE3) | Novagen, Madison, WI |
| BW25113 | Host strain for Keio knockout collection; F- Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ - rph-1 Δ(rhaD-rhaB)568 hsdR514 | [28] Coli Genetic Stock Center (CGSC), Yale Univ. |
| Δ ompF | ompF gene deletion from Keio collection; BW25113 Δ ompF746::kan; KanR | CGSC, Yale Univ. |
| Δ ompC | ompC gene deletion from Keio collection; BW25113 Δ ompC768::kan; KanR | CGSC, Yale Univ. |
| Plasmids | ||
| pCF430 | Host vector for metagenomic library; TetR | [19] |
| βLR16 | Metagenomic clone in pCF430 conferring CrbR phenotype on E. coli | [2] GenBank accession EU408353.1 |
| pUVRA28b | Vector with native E. coli promoter driving expression from pET28b multicloning site; KanR | This study |
| pCRC01UVRA | Subclone of βLR16 metallopeptidase gene in pUVRA28b; KanR | This study |
| pCRC02UVRA | Subclone of βLR16 metallopeptidase and regulator genes in pUVRA28b; KanR | This study |
| pCRC03UVRA | Subclone of βLR16 regulator gene in pUVRA28b; KanR | This study |
Antibiotic susceptibility testing
The antibiotic susceptibility of βLR16 and subclones was tested in minimum inhibitory concentration assays according to CLSI guidelines except for lower incubation temperatures (24–28°C) [20]. Briefly, serial two-fold dilutions (from 512 μg ml-1 to 0.5 μg ml-1, no antibiotic in the last well) of antibiotics were made in Mueller-Hinton (MH) broth (Becton, Dickinson and Company, Sparks, MD, USA) in 96-well plates. The following antibiotics were tested: ampicillin, rifampin (both from Research Products International Corp., Mt. Prospect, IL, USA), carbenicillin (Fisher Scientific, Fair Lawn, NJ, USA), ciprofloxacin (Wako Chemicals USA, Inc., Richmond, VA), erythromycin (Fluka BioChemika, Buchs, Switzerland), amoxicillin, cefamandole, cefoxitin, ceftazidime, cephalexin, piperacillin, chloramphenicol, nalidixic acid, fusidic acid, and gentamicin (all from Sigma, St. Louis, MO, USA). Each well was inoculated with 10 μl containing 105 colony forming units of each clone being tested. The assay was performed in duplicate at least three times, and E. coli strains EPI300, BL21(DE3), or BW25113 (Table 1) containing empty vector always served as the negative controls. The minimum inhibitory concentration (MIC) corresponded to the antibiotic concentration of the first well in which no growth was visible.
Transposon mutagenesis
Plasmids were isolated by alkaline lysis followed by ethanol precipitation [21]. In vitro transposon mutagenesis of βLR16 was carried out with the GPS-1 Genome Priming System (New England Biolabs, Beverly, MA, USA). Insertion mutants that failed to grow on carbenicillin had presumed insertions in the active gene and were sequenced using the manufacturer’s primers. Additional insertion mutants were randomly chosen for sequencing the entire insert. All sequencing reactions were carried out at the University of Wisconsin-Madison DNA sequencing facility using Big Dye Terminator (v. 3.1, Applied Biosystems, Foster City, CA, USA). The Lasergene software package (DNASTAR, Madison, WI, USA) was used to assemble and annotate the sequence, which accesses the Basic Local Alignment Search Tool (BLAST) [22] through the National Center for Biotechnology Information (NCBI).
β-lactam hydrolysis assay
Thick MH agar (Becton, Dickinson and Company) plates were swabbed with an overnight culture of EPI300 E. coli across each plate three times, turning the plate 60° between swabs. Then, using the wide end of a sterile P1000 pipetman tip, an agar plug was aseptically removed from the center of each plate. E. coli strain EPI300 containing pCF430, βLR16, βLR2, or no plasmid was inoculated into four separate tubes containing 5 ml LB plus 100 μg ml-1 carbenicillin. Experimental controls were βLR2, a metagenomic clone containing a metallo-β-lactamase [2], and EPI300 E. coli containing pCF430. Cultures were shaken at room temperature for 48 hr. At 30 minutes, 6 hr, 24 hr, and 48 hr post-inoculation, 500 μl was removed and centrifuged. 400 μl of each supernatant was pipetted into a well in the MH agar. Plates were incubated at room temperature for 24 hr, at which time the diameter of the zone of growth inhibition was measured.
Subcloning the genes encoding the metallopeptidase and response regulator
The full-length metallopeptidase gene alone (pCRC01), the full-length regulator gene alone (pCRC03) and the combination of both the metallopeptidase and the regulator gene (pCRC02) were subcloned in a pET28b (Novagen, Madison, WI) background using primers (Table 2) targeting XbaI and BamHI restriction enzyme sites (pCRC01, pCRC02), or NcoI and BamHI restriction enzyme sites (pCRC03) present in the pET28b multicloning region. A Thr to Ala mutation was introduced at Thr2 in the regulator gene coding sequence in pCRC03 to facilitate cloning using the NcoI restriction enzyme site.
Table 2. Primers (5′-3′) used for cloning and qPCR/RT-PCR.
| Clone | Forward primer | Reverse primer |
|---|---|---|
| Subcloning the active gene a | ||
| pCRC01 | cccctctaga aggagaacggcATGCTTCTAGGCATGCTGATGGG | cgtcggatccTTATGTAGAGCTGATGCATGACGCCTGTTCTCCG |
| pCRC02 | cccctctaga aggagaacggcATGCTTCTAGGCATGCTGATGGG | cgtcggatccTTAGTTGTTAGACACCTCAAATCGATAGCCAATTCC |
| pCRC03 | cagtccATGGCGAATAATGATGTGCATATTCTGGTAGTTG | cgtcggatccTTAGTTGTTAGACACCTCAAATCGATAGCCAATTCC |
| uvrA promoter | ||
| pCRC01UVRA, pCRC02UVRA, and pCRC03UVRA | GATCTGATATCTTTACACTTTATGCTT | CTAGATTATACGAGCCGGAAGCATAA |
| qPCR/RT-PCR | ||
| acrA b | CTTAGCCCTAACAGGATGTG | TTGAAATTACGCTTCAGGAT |
| acrB b | CGTACACAGAAAGTGCTCAA | CGCTTCAACTTTGTTTTCTT |
| acrD b | GATTATCTTAGCCGCTTCAA | CAATGGAGGCTTTAACAAAC |
| mdtA | TAACGCGCGCATGTTAGT | GCTATTCAGCACCCAGACAA |
| mdtB | AACTGCGTCGTCCGTTAG | GCGGTCGAACAGCAAATAAA |
| ompF | GGCGCAACCTACTACTTCAA | GAACCTACGCCCAGTTTGT |
| ompC | GTCCACCTACGTTGACTACAAA | GACCCAGAGCTACGATGTTATC |
| micF b | TCATCATTAACTTTATTTATTACCG | GCATCCGGTTGAAATAGG |
| GAPDH b | ACTTACGAGCAGATCAAAGC | AGTTTCACGAAGTTGTCGTT |
aCapital letters indicate coding regions, underline indicates restriction enzyme sites, and italics indicate an engineered E. coli ribosomal binding site.
bPrimers are from Viveiros et al. 2007 [23].
To construct a system in which the genes present on these constructs would be expressed in an E. coli strain in the absence of the λDE3 lysogen, a native E. coli promoter (uvrA) was cloned into each of these constructs using preexisting BglII and XbaI restriction enzyme sites from their respective pET28b multicloning regions. The cloned promoter DNA was engineered with these restriction enzyme sites, and was constructed from two synthetic oligonucleotides that were annealed and digested accordingly (Table 2). This strategy directly added the native E. coli promoter, displacing the existing T7 promoter region. The newly constructed vectors were named pCRC01UVRA, pCRC02UVRA, and pCRC03UVRA, according to the notation introduced in the above paragraph. Verification of expression was performed by SDS-PAGE analysis of the protein from EPI300 E. coli containing pCRC03UVRA as well as phenotypic assays.
Gene expression analysis
Quantitative PCR following reverse transcription of purified E. coli RNA was used to assess expression of the acrA, acrB, acrD, mdtA, mdtB, ompC, and ompF genes, as well as the small RNA micF. EPI300 harboring pCRC03UVRA or pUVRA28b was grown in LB supplemented with kanamycin to an OD600 value of 0.5 and the cells were harvested by centrifugation. Total RNA was purified using the RNeasy Mini kit according to the manufacturer’s specifications (Qiagen). Purified RNA was quantified using a Take3 micro-volume plate with UV spectrophotometry (BioTek) and supplemented with the SUPERase•In RNase inhibitor (Life Technologies). Purified RNA was subjected to RNase-free DNase (Promega) treatment to eliminate any contaminating DNA. 500ng DNase-treated RNA was used for cDNA synthesis using SuperScript VILO mastermix (Invitrogen) in a 20μl reaction at 42°C for 2 h. Quantitative PCR was performed on 1μl of a 1:8 dilution of the resulting cDNA in a 10μl reaction that contained 300 nM PCR primers (Table 2) and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). The PCRs amplified a ~70–200 base region of DNA in each case (acrA 189 bp, acrB 183 bp, acrD 187 bp, mdtA 103 bp, mdtB 101 bp, ompF 92 bp, ompC 95 bp, micF 70 bp). Quantitative PCR was performed on a CFX384 real-time PCR detection system (Bio-Rad) using the following cycling parameters: one cycle at 95°C for 10 min as hot start, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 47°C (acrA/B/D) or 52°C (mdtA/B) or 55°C (ompC/F) or 45°C (micF) for 30 s, and extension at 72°C for 30 s. Control PCRs were also included using a reverse transcriptase-minus (RT-minus) template to verify that genomic DNA did not contribute to the observed results. Expression levels under each condition were normalized to the GAPDH housekeeping gene [23]. Melting curve analysis (65–99°C with a heating rate of 1°C per second and a continuous fluorescence measurement) was done to verify the identity of the PCR products. The 2-ΔΔCt method was used to calculate fold change in gene expression [24].
Results
Identification of βLR16
Four metagenomic libraries from Alaskan soil DNA were selected on various β-lactam antibiotics, yielding 14 resistant clones [2]. Thirteen of the clones encoded β-lactamases, but one did not. This clone, designated βLR16, grew in the presence of carbenicillin (50 μg ml-1) at 24°C but not at 37°C. When resuspending cell pellets of βLR16, it was noted that the pellet was stickier and harder to resuspend than E. coli containing empty vector.
Phenotypic characterization of βLR16
To assess the range of resistance conferred by the βLR16 clone, resistance to 15 antibiotics was tested in minimum inhibitory concentration (MIC) assays. βLR16 conferred elevated and specific resistance to carbenicillin on EPI300 E. coli (Table 3). When tested against additional β-lactams, the βLR16 clone was consistently more resistant than EPI300 E. coli containing empty vector, but the difference was never more than 2-fold.
Table 3. Minimum inhibitory concentration (MIC; μg ml-1) of β-lactams on recombinant E. coli cells harboring constructs from this work.
| Construct a | Antibiotic b | |||||||
|---|---|---|---|---|---|---|---|---|
| Amo | Amp | Cfm | Cft | Cfx | Cph | Crb | Pip | |
| βLR16 in EPI300 | 4 | 4 | <0.5 | 1 | 4 | 4 | 64 | 4 |
| pCF430 in EPI300 | 2 | 2 | <0.5 | <0.5 | 2 | 2 | 8 | 2 |
| pCRC03UVRA in EPI300 | 4 | 4 | 1 | 1 | 8 | 4 | 128 | 4 |
| pUVRA28b in EPI300 | 2 | 4 | <0.5 | <0.5 | 2 | 4 | 8 | 4 |
| pCRC03UVRA in BL21(DE3) | 8 | |||||||
| pUVRA28b in BL21(DE3) | 2 | |||||||
| βLR16 in BW25113 | 32 | |||||||
| pCF430 in BW25113 | 8 | |||||||
| βLR16 in ΔompF | 32 | |||||||
| pCF430 in ΔompF | 16 | |||||||
| βLR16 in ΔompC | 16 | |||||||
| pCF430 in ΔompC | 2 | |||||||
a pCRC01UVRA showed no difference in levels of Crb resistance compared to vector alone (pUVRA28b), while pCRC02UVRA showed the same Crb resistance level as pCRC03UVRA. ΔompF and ΔompC are in the BW25113 E. coli background and are from the Keio collection of gene knockouts (see Table 1).
b Antibiotic abbreviations are as follows: Amo, Amoxicillin; Amp, ampicillin; Cfm, cefamandole; Cft, ceftazidime; Cfx, cefoxitin; Cph, cephalexin; Crb, carbenicillin; Pip, piperacillin.
Resistance to carbenicillin was examined in a zone-of-inhibition assay to assess whether the mechanism of resistance conferred by βLR16 resulted from antibiotic inactivation. After 48 hours, the zone diameter resulting from the supernatant of a βLR16 culture was the same as the zone diameter from the supernatant of carbenicillin-containing media inoculated with E. coli containing empty vector (Table 4). This was in contrast to the diameter of the zone resulting from the supernatant of the 48-hour βLR2 culture (Table 4). βLR2 contains a gene encoding a metallo-β-lactamase homologue, and over time this clone inactivated the carbenicillin in the media and reduced the size of the zone of inhibition of E. coli. The zone observed with the βLR16 supernatant was not different from the negative control, suggesting that the resistance does not result from inactivation of carbenicillin.
Table 4. Zones of inhibition of EPI300 E. coli growth resulting from 400 μl supernatant following incubation with 100 μg ml-1 carbenicillin.
aAll plasmids in EPI300 E. coli. pCF430 is empty vector, and βLR2 contains a putative metallo-β-lactamase.
b10 mm-diameter well.
cSamples taken at 30 minutes (t0), 6 hours (t1), 24 hours (t2), and 48 hours (t3) post-inoculation.
Sequence analysis
The DNA insert from the βLR16 metagenomic clone was 5,169 base pairs. Sequence analysis further supported the phenotypic results, showing that the metagenomic DNA insert from βLR16 did not encode a predicted β-lactamase gene (Fig. 1). In silico open reading frame (ORF) analysis suggested two ORFs that share homology to known genes, and loss-of-phenotype transposon mutants mapped to these two putative genes (Fig. 1). The location of the loss-of-phenotype transposon insertions suggested that either both genes were required for resistance, or that mutations in the upstream metallopeptidase gene were polar on the downstream response regulator gene (Fig. 1).
Fig 1. Open reading frame (ORF) map of the insert of metagenomic clone βLR16.
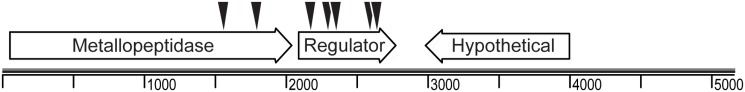
Gray triangles designate the locations of transposon insertions that eliminated the resistance phenotype.
A blastP query using the predicted amino acid sequence of the βLR16 upstream gene product showed the highest identity with a hypothetical protein from the unclassified Zetaproteobacterium SCGC AB-137-C09 (WP_018282215.1), at 39% identity over 632 amino acids. This βLR16 protein showed evidence of an N-terminal urea transport membrane domain, and a peptidase family M23 domain (zinc metallopeptidase). A blastP query using the predicted amino acid sequence of the βLR16 downstream gene product showed a putative transcriptional regulator from the Betaproteobacteria Pseudogulbenkiania ferrooxidans (WP_008955746.1) to have the highest identity, with 56% identity over 228 amino acids. This βLR16 protein is predicted to encode an N-terminal response regulator receiver domain (Conserved Domain cd00156) and a C-terminal effector domain (Conserved Domain cd00383) [25], and can be assigned to the OmpR/PhoB family of response regulators [26]. The function of either of the proteins described above in their host organism is not known.
Considering that the response regulator gene may impact gene expression in the E. coli host, a blastP query of E. coli K-12 (substr. MG1655) was conducted to determine whether the βLR16 response regulator shows homology with a known E. coli response regulator. This search revealed that the βLR16 regulator protein sequence showed the highest identity with the BaeR response regulator protein (NP_416583.1), with 51% identity over 227 amino acids. Alignment of the βLR16 regulator protein with BaeR (Fig. 2) revealed conservation of key amino acids, including the aspartate residue (Asp 56 in βLR16) that is phosphorylated in the receiver domain, and the “switch residues” (Thr 83 and Tyr 102 in βLR16) that undergo distinct rotameric shifts upon domain activation. Further, nine of the ten amino acids involved in domain swap dimerization interactions necessary for activation in the BaeR protein are conserved in the βLR16 protein [27] (Fig. 2).
Fig 2. Amino acid alignment of the BaeR response regulator of E. coli and the response regulator from βLR16.
Alignment was performed with MegAlign from the Lasergene software package from DNASTAR (Madison, WI) using the Lipman-Pearson alignment tool (Ktuple:2; Gap Penalty: 4; Gap Length Penalty: 12). Levels of similarity between the sequences are indicated as lines (identical) or dots (similar). The conserved aspartate residue that is phosphorylated is indicated in blue (Asp 56 in βLR16). Switch residues are indicated in gray (Thr 83 and Tyr 102 in βLR16), and amino acids from BaeR involved in domain swap interactions, and their βLR16 counterparts, are shown in red.
Analysis of βLR16 subclones
To assess the potential roles of the putative metallopeptidase and response regulator genes in resistance, subclones were generated and carbenicillin resistance was assayed in an E. coli EPI300 background. Analysis of subclones harboring the metallopeptidase gene alone (pCRC01UVRA), the two genes together (pCRC02UVRA), and the response regulator gene alone (pCRC03UVRA) revealed that the response regulator gene was necessary and sufficient to confer carbenicillin resistance on the E. coli host (Table 3). No resistance was seen with the metallopeptidase alone, while the combination of the two genes yielded a resistance profile that was the same as the regulator gene alone. Based on these results, we hypothesized that the two-component regulator gene may be altering gene expression in E. coli in a way that results in elevated, specific resistance to carbenicillin.
MIC analysis in different E. coli strains
Antibiotic susceptibility was analyzed for pUVRA28b (empty vector) and pCRC03UVRA (response regulator subclone) in E. coli strains EPI300 and in BL21(DE3) (Table 3). A four-fold increase in MIC with carbenicillin can be seen with empty vector between BL21(DE3) and EPI300, presumably due to genomic differences between the K-12-derived (EPI300) and B-derived (BL21(DE3)) strains. The increase in carbenicillin resistance conferred by the response regulator subclone is significantly greater in the EPI300 background when compared to the same vector in the BL21(DE3) background; nonetheless, some increase in resistance is noted in BL21(DE3). Expression of the regulator gene from pCRC03UVRA in EPI300 did not increase resistance to certain outer membrane-permeating antibiotics such as erythromycin, fusidic acid, and gentamicin (data not shown). Owing to the differences in porin content and regulation between the two strains, we reasoned that variations in expression of the OmpC or OmpF porin could result in the observed carbenicillin resistance phenotype. We therefore employed single gene, in-frame deletions of the ompF and ompC genes (Table 1) for two purposes. Assessing the relative carbenicillin resistances of the deletion strains compared to the wild type strain would allow us to determine whether regulation of these genes may be involved in carbenicillin resistance, and to determine whether the βLR16 regulator gene impacts resistance in the absence of either of the porin genes. The kanamycin resistance cassette insertions in the ΔompC and ΔompF strains (Table 1) precluded use of the pCRC03UVRA subclone in these strains, owing to the kanamycin resistance selectable marker in the vector. Comparing the empty vector (pCF430) MIC values between the K-12-derived strain (BW25113, the progenitor for the Keio knockout collection [28]) and the two porin knockout strains, the data suggested that elimination of the OmpF porin increases carbenicillin resistance while elimination of the OmpC porin renders E. coli more sensitive to carbenicillin. The βLR16 clone enhances carbenicillin resistance in each of the strains, with the greatest difference between empty vector and βLR16 resistance in the ΔompC strain. This is consistent with downregulation of ompF expression as a means of carbenicillin resistance, but the enhanced resistance conferred by βLR16 in the ΔompF strain suggests the involvement of additional mechanisms.
Gene expression analysis
The expression of genes involved in pumping β-lactams (acrA, acrB, acrD, mdtA, mdtB) as well as genes involved in β-lactam outer membrane permeability (ompC, and ompF porin genes, and the micF small RNA) was analyzed in EPI300 grown with the regulator subclone (pCRC03UVRA) as compared to EPI300 grown with the empty vector (pUVRA28B) (Table 5). The acrA and acrB genes showed less than twofold change in expression under these two conditions, while the acrD gene was upregulated over 10 fold in the presence of the response regulator. The mdtA and mdtB genes both exhibited >10-fold increases in expression in the presence of the response regulator gene. Each of the three “permeability” genes (ompC, ompF, micF) was downregulated somewhat in the presence of the regulator subclone, but the impact was most noteworthy in the case of the ompF gene, where a >20-fold decrease in expression was seen. The ompC gene was downregulated ~2-fold, while the micF small RNA exhibited less than 2-fold change in expression.
Table 5. Fold change in gene expression of select E. coli genes in the presence of the regulator-containing vector (pCRC03UVRA) versus empty vector (pUVRA28b).
| Gene | Fold change ± standard deviation |
|---|---|
| acrA | 0.67 ± 0.09 |
| acrB | 0.51 ± 0.11 |
| acrD | 11.51 ± 3.25 |
| mdtA | 11.93 ± 2.03 |
| mdtB | 13.30 ± 1.26 |
| micF | 0.69 ± 0.16 |
| ompC | 0.45 ± 0.07 |
| ompF | 0.05 ± 0.01 |
Discussion
The structural and mechanistic similarities between metallopeptidase enzymes and metallo-β-lactamase enzymes led us to first postulate that the putative metallopeptidase gene from βLR16 was responsible for resistance. However, the phenotypic analyses suggested that carbenicillin resistance conferred by βLR16 does not result in inactivation of the β-lactam antibiotic. Additionally, a subclone of the metallopeptidase did not increase carbenicillin resistance in E. coli EPI300, while a subclone of the response regulator gene increased resistance markedly, leading us to conclude that the response regulator was necessary and sufficient for β-lactam resistance. Considering the nature of the resistance gene, we hypothesized that the response regulator altered expression of one or more native E. coli genes that would allow for increased, specific tolerance to the β-lactam carbenicillin
Previous work has established that spontaneous mutants showing a phenotype similar to what we observed (temperature-dependent carbenicillin resistance) map to the ompF gene in E. coli and other Gram negative organisms [29,30]. While porin specificity is dictated by the size, charge, and hydrophobicity of the substrate [31], OmpF is thought to preferentially allow influx of anionic compounds, while OmpC is more cation selective [31]. It is noteworthy that carbenicillin is a dianionic molecule, while the other β-lactams tested here are monoanionic or zwitterionic. This may explain the apparent selectivity of the OmpF porin for carbenicillin, as opposed to structurally related monoanionic or zwitterionic β-lactams. Nonetheless, both porins are known to be involved in antibiotic influx [30].
The resistance phenotype analyses showed that the E. coli BW25113 ompF knockout (ΔompF) displayed slightly elevated resistance to carbenicillin compared to the wild type strain. In contrast, the ompC knockout (ΔompC) was more susceptible. This is consistent with previous work showing that the OmpF porin is the primary porin through which carbenicillin enters the E. coli periplasm [29]. Considering that either porin is likely overrepresented in the absence of the other, a proportionally larger quantity of OmpF (in the ΔompC strain) would mean a higher influx of carbenicillin. The qRT-PCR data were consistent with a role for the response regulator in decreasing expression of the ompF gene—in a micF-independent manner—in E. coli.
The 2-fold increase in resistance in the ΔompF strain suggested that the regulator impacts more than just expression of the ompF gene, so genes encoding efflux pumps were investigated. Previous work indicates that the AcrAB-TolC pump system of E. coli has very broad substrate specificity and can enhance resistance to a wide variety of antibiotics, detergents, and solvents [32]. This pump is also proposed to be the primary means by which β-lactam antibiotics are pumped from the cytoplasm or periplasm into the extracellular space [33]. Indeed, both deletion and overexpression studies of the acrAB genes in E. coli show that these genes confer “across the board” resistance to β-lactams [34]. In contrast, overexpression of the mdtABC genes and acrD gene show enhanced, yet specific, carbenicillin resistance [32]. These observations are consistent with our gene expression analysis, which showed negligible changes in expression to the acrA and acrB genes in the presence of the βLR16 response regulator gene, yet increases in expression of both the acrD gene as well as the mdtA and mdtB genes. The βLR16 response regulator gene, therefore, is responsible for both repressing porin expression and activating multidrug efflux pump expression.
Regulation of the mdtABC genes and the acrD gene by the same response regulator is not without precedent. The BaeSR two-component system of E. coli is shown to activate expression of both the mdtABC genes and the acrD gene [35,36]. Overall similarities (Fig. 2) between the βLR16 response regulator and the BaeR response regulator suggest that they may act in a similar fashion to enhance antibiotic resistance. Conversely, the βLR16 response regulator may act as a repressor in a manner that indirectly impacts gene expression to give the observed results.
This study introduces a number of concepts that should be further explored, including alternative mechanisms by which gene regulation can impact antibiotic tolerance, co-regulation of antibiotic influx and efflux genes, and the genetics and biochemistry of modular response regulators in alternative host organisms. We also propose that further explorations of the soil metagenome using functional screening and selection strategies will be an outstanding method for advancing discovery in the broader biology of microorganisms.
Acknowledgments
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U S Department of Agriculture. USDA is an equal opportunity provider and employer.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded in part by grants from United States Department of Agriculture National Institute of Food and Agriculture (2011-67020-30195) and the National Research Initiative of the United States Department of Agriculture Cooperative State Research, Education and Extension Service (WIS01312) to LAM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Handelsman J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev. 2004;68: 669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen HK, Moe LA, Rodbumrer J, Gaarder A, Handelsman J. Functional metagenomics reveals diverse β-lactamases in a remote Alaskan soil. ISME J. 2009;3: 243–251. 10.1038/ismej.2008.86 [DOI] [PubMed] [Google Scholar]
- 3. Donato JJ, Moe LA, Converse BJ, Smart KD, Berklein FC, McManus PS, et al. Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl Environ Microbiol. 2010;76: 4396–4401. 10.1128/AEM.01763-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wichmann F, Udikovic-Kolic N, Andrew S, Handelsman J. Diverse antibiotic resistance genes in dairy cow manure. mBio. 2014;5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lang KS, Anderson JM, Schwarz S, Williamson L, Handelsman J, Singer RS. Novel florfenicol and chloramphenicol resistance gene discovered in Alaskan soil by using functional metagenomics. Appl Environ Microbiol. 2010;76: 5321–5326. 10.1128/AEM.00323-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riesenfeld CS, Goodman RM, Handelsman J. Uncultured soil bacteria are a reservoir of new antibiotic resistance genes. Environ Microbiol. 2004;6: 981–989. [DOI] [PubMed] [Google Scholar]
- 7. McGarvey KM, Queitsch K, Fields S. Wide variation in antibiotic resistance proteins identified by functional metagenomic screening of a soil DNA library. Appl Environ Microbiol. 2012;78: 1708–1714. 10.1128/AEM.06759-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres-Cortés G, Millán V, Ramírez-Saad HC, Nisa-Martínez R, Toro N, Martínez-Abarca F. Characterization of novel antibiotic resistance genes identified by functional metagenomics on soil samples. Environ Microbiol. 2011;13: 1101–1114. 10.1111/j.1462-2920.2010.02422.x [DOI] [PubMed] [Google Scholar]
- 9. Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: Antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8: 251–259. 10.1038/nrmicro2312 [DOI] [PubMed] [Google Scholar]
- 10. Hamad B. The antibiotics market. Nat Rev Drug Discov. 2010;9: 675–676. 10.1038/nrd3267 [DOI] [PubMed] [Google Scholar]
- 11. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74: 417–433. 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antibiotic resistance threats in the United States, 2013. Center for Disease Control and Prevention.
- 13. Waxman DJ, Strominger JL. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu Rev Biochem. 1983;52: 825–869. [DOI] [PubMed] [Google Scholar]
- 14. Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to β-lactam antibiotics: Compelling opportunism, compelling opportunity. Chem Rev. 2005;105: 395–424. [DOI] [PubMed] [Google Scholar]
- 15. Nikaido H, Pagès J-M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev. 2011;36: 340–363. 10.1111/j.1574-6976.2011.00290.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poole K. Resistance to β-lactam antibiotics. Cell Mol Life Sci. 2004;61: 2200–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69: 183–215. [DOI] [PubMed] [Google Scholar]
- 18. Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Annu Rev Microbiol. 2012;66: 325–347. 10.1146/annurev-micro-092611-150039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman JR, Fuqua C. Broad-host-range expression vectors that carry the L-arabinose-inducible Escherichia coli AraBAD promoter and the AraC regulator. Gene. 1999;227: 197–203. [DOI] [PubMed] [Google Scholar]
- 20. NCCLS. Performance standards for antimicrobial susceptibility testing; fourteenth informational supplement. Wayne, PA: The National Committee for Clinical Laboratory Standards; 2004. pp. 96–130. 15099651 [Google Scholar]
- 21. Sambrook JF, Russell DW. Molecular cloning: A laboratory manual; CSHL Press; 2001. [Google Scholar]
- 22. Altschul SF, Gish W, Miller W, Meyers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215: 403–410. [DOI] [PubMed] [Google Scholar]
- 23. Viveiros M, Dupont M, Rodrigues L, Couto I, Davin-Regli A, Martins M, et al. Antibiotic stress, genetic response and altered permeability of Escherichia coli . PLoS ONE. 2007;2: e365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods. 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- 25. Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, et al. CDD: Conserved domains and protein three-dimensional structure. Nucleic Acids Res. 2013;41: D348–D352. 10.1093/nar/gks1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galperin MY. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 2010;13: 150–159. 10.1016/j.mib.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choudhury HG, Beis K. The dimeric form of the unphosphorylated response regulator BaeR. Protein Sci. 2013;22: 1287–1293. 10.1002/pro.2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harder KJ, Nikaido H, Matsuhashi M. Mutants of Escherichia coli that are resistant to certain β-lactam compounds lack the OmpF porin. Antimicrob Agents Chemother. 1981;20: 549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pages J-M, James CE, Winterhalter M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 2008;6: 893–903. 10.1038/nrmicro1994 [DOI] [PubMed] [Google Scholar]
- 31. Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67: 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishino K, Nikaido E, Yamaguchi A. Regulation and physiological function of multidrug efflux pumps in Escherichia coli and Salmonella . Biochim Biophys Acta. 2009;1794: 834–843. 10.1016/j.bbapap.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 33. Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (mar) mutants. J Bacteriol. 1996;178: 306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazzariol A, Cornaglia G, Nikaido H. Contributions of the AmpC β-lactamase and the AcrAB multidrug efflux system in intrinsic resistance of Escherichia coli K-12 to β-lactams. Antimicrob Agents Chemother. 2000;44: 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baranova N, Nikaido H. The BaeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J Bacteriol. 2002;184: 4168–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leblanc SKD, Oates CW, Raivio TL. Characterization of the induction and cellular role of the BaeSR two-component envelope stress response of Escherichia coli . J Bacteriol. 2011;193: 3367–3375. 10.1128/JB.01534-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.



