Abstract
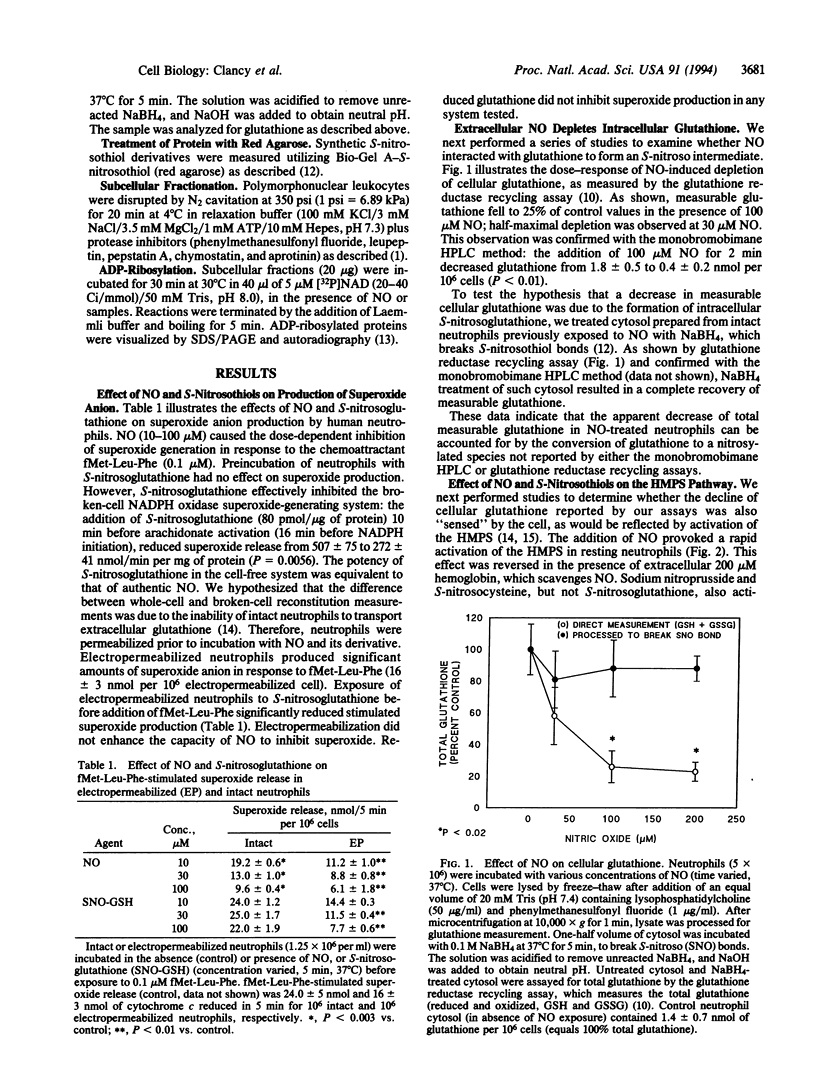
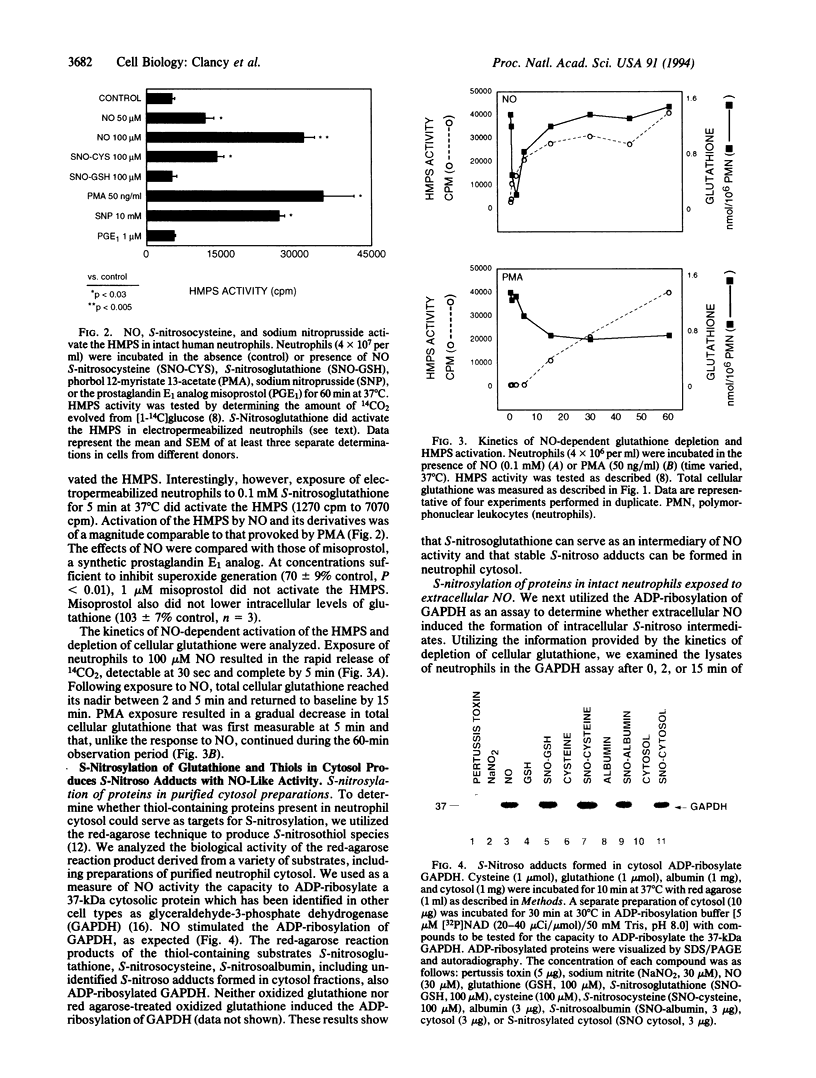
We performed experiments to determine whether nitric oxide promoted the formation of intracellular S-nitrosothiol adducts in human neutrophils. At concentrations sufficient to inhibit chemoattractant-induced superoxide anion production, nitric oxide caused a depletion of measurable intracellular glutathione as determined by both the monobromobimane HPLC method and the glutathione reductase recycling assay. The depletion of glutathione could be shown to be due to the formation of intracellular S-nitrosoglutathione as indicated by the ability of sodium borohydride treatment of cytosol to result in the complete recovery of measurable glutathione. The formation of intracellular S-nitrosylated compounds was confirmed by the capacity of cytosol derived from nitric oxide-treated cells to ADP-ribosylate glyceraldehyde-3-phosphate dehydrogenase. Depletion of intracellular glutathione was accompanied by a rapid and concomitant activation of the hexose monophosphate shunt (HMPS) following exposure to nitric oxide. Kinetic studies demonstrated that nitric oxide-dependent activation of the HMPS was reversible and paralleled nitric oxide-induced glutathione depletion. Synthetic preparations of S-nitrosoglutathione shared with nitric oxide the capacity to inhibit superoxide anion production and activate the HMPS. These data suggest that nitric oxide may regulate cellular functions via the formation of intracellular S-nitrosothiol adducts and the activation of the HMPS.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Mastrofrancesco B. Modulation of glucose metabolism in macrophages by products of nitric oxide synthase. Am J Physiol. 1993 Jun;264(6 Pt 1):C1594–C1599. doi: 10.1152/ajpcell.1993.264.6.C1594. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Abramson S. B. Novel synthesis of S-nitrosoglutathione and degradation by human neutrophils. Anal Biochem. 1992 Aug 1;204(2):365–371. doi: 10.1016/0003-2697(92)90253-4. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Leszczynska-Piziak J., Abramson S. B. Nitric oxide stimulates the ADP-ribosylation of actin in human neutrophils. Biochem Biophys Res Commun. 1993 Mar 31;191(3):847–852. doi: 10.1006/bbrc.1993.1294. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Leszczynska-Piziak J., Abramson S. B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992 Sep;90(3):1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy R. M., Miyazaki Y., Cannon P. J. Use of thionitrobenzoic acid to characterize the stability of nitric oxide in aqueous solutions and in porcine aortic endothelial cell suspensions. Anal Biochem. 1990 Nov 15;191(1):138–143. doi: 10.1016/0003-2697(90)90400-4. [DOI] [PubMed] [Google Scholar]
- Corradin S. B., Mauël J. Phagocytosis of Leishmania enhances macrophage activation by IFN-gamma and lipopolysaccharide. J Immunol. 1991 Jan 1;146(1):279–285. [PubMed] [Google Scholar]
- Drapier J. C., Pellat C., Henry Y. Generation of EPR-detectable nitrosyl-iron complexes in tumor target cells cocultured with activated macrophages. J Biol Chem. 1991 Jun 5;266(16):10162–10167. [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZE C. E., LANGDON R. G. Hepatic glutathione reductase. I. Purification and general kinetic properties. J Biol Chem. 1962 May;237:1589–1595. [PubMed] [Google Scholar]
- Malawista S. E., Montgomery R. R., van Blaricom G. Evidence for reactive nitrogen intermediates in killing of staphylococci by human neutrophil cytoplasts. A new microbicidal pathway for polymorphonuclear leukocytes. J Clin Invest. 1992 Aug;90(2):631–636. doi: 10.1172/JCI115903. [DOI] [PMC free article] [PubMed] [Google Scholar]
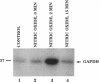
- McDonald L. J., Moss J. Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6238–6241. doi: 10.1073/pnas.90.13.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Merryman P. F., Clancy R. M., He X. Y., Abramson S. B. Modulation of human T cell responses by nitric oxide and its derivative, S-nitrosoglutathione. Arthritis Rheum. 1993 Oct;36(10):1414–1422. doi: 10.1002/art.1780361014. [DOI] [PubMed] [Google Scholar]
- Molina y Vedia L., McDonald B., Reep B., Brüne B., Di Silvio M., Billiar T. R., Lapetina E. G. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992 Dec 15;267(35):24929–24932. [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Newton G. L., Dorian R., Fahey R. C. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Sandborg R. R. Ca2(+)-induced secretion by electropermeabilized human neutrophils. The roles of Ca2+, nucleotides and protein kinase C. Biochim Biophys Acta. 1990 Apr 9;1052(1):133–142. doi: 10.1016/0167-4889(90)90068-o. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Jaraki O., Osborne J. A., Francis S., Mullins M., Singel D., Loscalzo J. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8087–8091. doi: 10.1073/pnas.89.17.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Stadler J., Evans C. H. Nitric oxide and arthritis. Arthritis Rheum. 1993 Aug;36(8):1036–1044. doi: 10.1002/art.1780360803. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]