Abstract
Peripheral blood fibrocytes are a newly identified circulating leukocyte subpopulation that migrates into injured tissue where it may display fibroblast-like properties and participate in wound healing and fibrosis of skin and other organs. Previous studies in our lab demonstrated that A2A receptor-deficient and A2A antagonist-treated mice were protected from developing bleomycin-induced dermal fibrosis, thus the aim of this study was to determine whether the adenosine A2A receptor regulates recruitment of fibrocytes to the dermis in this bleomycin-induced model of dermal fibrosis. Sections of skin from normal mice and bleomycin-treated wild type, A2A knockout and A2A antagonist-treated mice were stained for Procollagen α2 Type I and CD34 and the double stained cells, fibrocytes, were counted in the tissue sections. There were more fibrocytes in the dermis of bleomycin-treated mice than normal mice and the increase was abrogated by deletion or blockade of adenosine A2A receptors. Because fibrocytes play a central role in tissue fibrosis these results suggest that diminished adenosine A2A receptor-mediated recruitment of fibrocytes into tissue may play a role in the pathogenesis of fibrosing diseases of the skin. Moreover, these results provide further evidence that adenosine A2A receptors may represent a new target for the treatment of such fibrosing diseases as scleroderma or nephrogenic fibrosing dermopathy.
Keywords: fibrocyte, adenosine A2A receptor, scleroderma
INTRODUCTION
Adenosine and its receptors are important physiological and pharmacologic regulators of many different processes including fibrosis. Adenosine A2A and A2B receptors regulate proliferation, cytokine and collagen production and other functions by dermal, pulmonary and cardiac fibroblasts and hepatic stellate cells [1–10]. First shown to play a role in wound healing in normal and diabetic mice and rats [11], adenosine and its receptors have been shown to play a central role in the pathogenesis of pulmonary and hepatic fibrosis and in a model of Scleroderma, diffuse dermal fibrosis following bleomycin treatment [2, 8, 12–16].
Although stimulation of local fibroblasts may account for fibrosis in some settings recent work suggests that fibrocytes, circulating bone marrow-derived cells, may be recruited to injured or inflamed areas where they play a central role in promoting wound healing and fibrosis (Recently reviewed in [17, 18]). These cells, which are recognizable in the circulation and in tissues as CD34+/procollagen 1 α2+ cells, can differentiate into fibroblasts in the tissue after recruitment by chemokines produced at the inflamed site. Fibrocytes have been reported to play a role in such diseases as scleroderma, a disease of unknown etiology that is characterized by fibrosis of the skin and other organs, and nephrogenic fibrosing dermopathy, a recently described fibrosing syndrome related to gadolinium use [17–19].
As noted above, adenosine and adenosine A2A receptors play a critical role in the development of dermal fibrosis following bleomycin treatment [2] and fibrocytes play a central role in fibrosing disorders [20, 21]. We therefore asked whether fibrocytes are present in the skin of bleomycin-treated mice and whether adenosine A2A receptor deletion or blockade altered the recruitment of these cells to the skin. We report here that there is a marked increase in dermal fibrocyte number in bleomycin-treated mice, even before the onset of significant fibrosis. Moreover, significantly fewer fibrocytes accumulate in the dermis of mice treated with bleomycin when adenosine A2A receptors are deleted or blocked. These results suggest that adenosine A2A receptors regulate fibrocyte recruitment to the skin and may present a novel target for the treatment of scleroderma and other fibrotic disorders.
MATERIALS AND METHODS
Tissue Sections from Bleomycin-treated Mice
Paraffin-embedded, fixed skin sections from wild type or adenosine A2A receptor knockout mice treated with bleomycin, and wild type mice treated with bleomycin plus an adenosine A2A receptor antagonist (ZM241385) were used in these studies. The effects of adenosine receptor blockade and deletion on dermal fibrosis in these mice were previously reported by us [2] and sections from these same blocks, stained for hematoxylin and eosin or picrosirius red were also published previously [2].
Immunohistochemistry
Double immunohistochemistry was carried out on the Formalin-fixed, paraffin-embedded tissue sections. Antigen Retrieval was performed using Tris–EDTA buffer/pH 9.0 at 95–100° C for 20 min. Sections were blocked by incubation in normal rabbit serum—species same as secondary antibody and endogenous peroxidase was inactivated with H2O2. Tissue sections were first incubated with a rat anti-mouse CD34 mAb (30 min at room temperature) and secondly with a rabbit anti-Rat Biotinylated secondary antibody (Vector lab. inc. Burlingame, CA) in PBS for 30 min at room temperature. For detection, slides were incubated with ABC-Peroxidase Solution for 30 min at room temperature and developed in DAB peroxidase substrate (brown chromagen; all from Vector Laboratories, Burlingame, CA, USA). Then, sections were pre-incubated with donkey serum, incubated with Goat anti-mouse type I procollagen (Vector) followed by incubation with alkaline phosphatase-conjugated Donkey anti-goat antibody (Vector). The color reaction was performed using the Fast Red substrate system (Dako). Slides were counterstained with Mayer’s hematoxylin and mounted in glycerol gelatin. Controls included omission of the primary and/or secondary antibodies and substitution of the primary antibodies with irrelevant antibodies of the same species. Quantification of procollagen-positive and CD34-positive cells was based on the average cell number of ten high-power fields per slide using an ×10 eyepiece and ×40 objective lens and the results from three slides from three different mice in each group were counted.
Statistical Analysis
Statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL, USA) statistical software. Experimental data are reported as means SD of triplicate independent samples.
RESULTS
Fibrocytes are Present in Normal Skin and the Skin of Mice Treated with Bleomycin
Some fibrocytes (CD34+/procollagen 1+ cells) were found in the skin of PBS treated-wild type mice although there was a marked increase in the skin of bleomycin-treated mice, as compared to PBS-treated mice (Figs. 1, 2, 3). The fibrocytes were found primarily in groups in the peri-vascular space and individually just under the epidermis (Fig. 1) and their presence preceded dramatic fibrosis of the skin since similar numbers and tissue localization of dermal fibrocytes were seen in mice treated with bleomycin for 1 or 3 weeks (Fig. 2).
Fig. 1.
Double immunohistochemical staining for CD34 and procollagen 1 expression in mouse skin. Shown is a representative section of dermis from a bleomycin-treated mouse stained for CD34 and procollagen 1, originally described in [2], and counterstained with Mayer’s hematoxylin, as described in “Materials and Methods.” In this section several CD34+ (brown stain), procollagen 1+ (red stain) cells are seen surrounding a vessel (arrow). Original magnification ×400.
Fig. 2.
Double immunohistochemical staining for CD34 and procollagen 1 expression in murine skin. Representative sections of skin from mice treated as described in [2], stained for CD34 and procollagen 1 were prepared as described in “Materials and Methods.” A Skin from a normal PBS-treated mouse. B Skin from a bleomycin-treated wild type mouse treated with bleomycin (1 mg kg−1 week−1 for 3 weeks). C Skin from a bleomycin-treated A2A receptor knockout mouse. D Skin from a bleomycin- and ZM241385-treated mouse. Original magnification ×200.
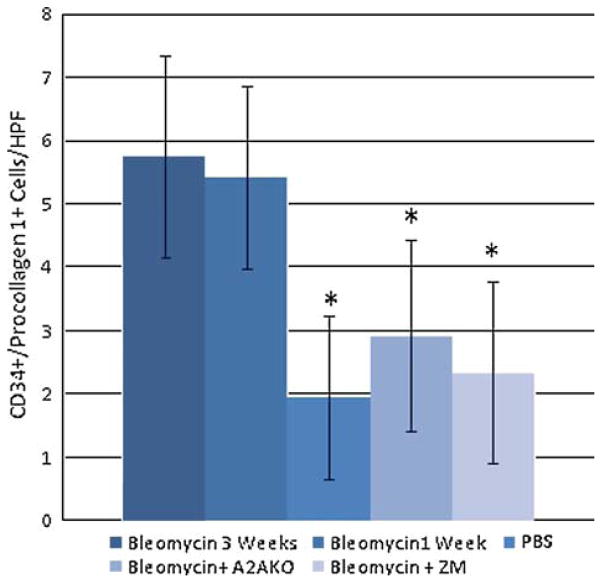
Fig. 3.
The number of CD34/procollagen 1 double-stained cells/hpf in mice. Mice were treated as previously described in [2], and the number of fibrocytes/high powered field were counted, as described in “Materials and Methods.” Asterisk, P<0.05 compared to 3 weeks bleomycin treatment.
Adenosine Receptor Deletion or Blockade Diminishes Dermal Fibrocyte Numbers After Bleomycin Treatment
In the skin sections studied here we had previously demonstrated that adenosine A2A receptor blockade or deletion suppressed the development of fibrosis [2], thus we counted the number of fibrocytes in the skin of bleomycin-treated mice in which adenosine A2A receptors were either deleted or blocked. We found that adenosine A2A receptor blockade or deletion led to a significant reduction in the number of fibrocytes in the skin of bleomycin-treated mice associated with the previously reported reduction in fibrosis (Fig. 3).
DISCUSSION
We report here that fibrocytes accumulate in the skin of bleomycin-treated mice and fewer fibrocytes are recruited to the skin of bleomycin-treated mice when their adenosine A2A receptors are deleted or blocked. These results are consistent with the hypothesis that fibrocytes play a role in dermal fibrosis in this model of scleroderma and that adenosine A2A receptors are critical regulators of fibrocyte accumulation.
In contrast to our results Boban and colleagues [22] found no evidence of an increase in fibrocyte trafficking to the skin following bleomycin treatment. In our experiments mice were treated every other day with bleomycin for only 3 weeks whereas Boban and colleagues treated their mice daily for 4 weeks and it is possible that either the high doses of the drug diminished production of these bone marrow-derived cells or that maximal fibrosis/fibrocyte recruitment occurs at earlier time points than that used by Boban et al. [22].
Prior studies have demonstrated that topical application of adenosine A2A receptor agonists to wounds promotes wound healing by increasing both angiogenesis and matrix production [11, 23–25] and, conversely, deletion of adenosine A2A receptors diminishes granulation tissue formation in wounds [23]. Although adenosine and its A2A receptor stimulate mature dermal fibroblasts to produce increased matrix [2] and mature microvascular endothelial cells to form microtubules and produce angiogenic factors [26, 27] results of other studies indicate that adenosine A2A receptors are required for optimal recruitment of circulating progenitor cells to wounds. Thus, Montesinos and colleagues observed that adenosine A2A receptor agonists increase the recruitment of endothelial progenitor cells, bone marrow-derived mesenchymal cells, from peripheral blood to healing wounds [25] contributing to micro-vessel formation in healing wounds.
Bucala and colleagues first demonstrated the presence of fibrocytes in connective tissues scars in 1994 [28] and subsequent studies demonstrated that these cells traffic to skin wounds [29] and to the pulmonary interstitium in a model of pulmonary fibrosis induced by bleomycin [30]. Chemokines, primarily CCL21 and CXCL12 (SDF-1), and their receptors, CCR and CXCR4, respectively, have been shown to play an important role in recruiting fibrocytes to injured tissue and in models of pulmonary and renal fibrosis (reviewed in [17]). Consistent with this hypothesis is the recent report that adenosine, acting at A2A and A2B receptors, stimulates increased expression of CXCR4 and increased migration to CXCL12 by human carcinoma cells [31]. Fibrocytes may also mature from a population of CD14+ circulating monocytes that are recruited by the same chemokines to inflamed or injured sites (reviewed in [17]) and adenosine A2A receptors have previously been shown to promote the differentiation of monocytes to an alternatively stimulated, pro-angiogenic phenotype in vitro [32]. Although the results of the studies reported here do not reveal whether adenosine and its A2A receptor promote recruitment of fibrocytes or their monocyte precursors directly or stimulate differentiation of monocyte precursors to fibrocytes in the tissue it is possible that both mechanisms are operative at sites of tissue injury or fibrosis.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR41911, AA13336, GM56268), the Scleroderma Foundation, King Pharmaceuticals, the General Clinical Research Center (M01RR00096) and by the Kaplan Cancer Center of New York University School of Medicine.
Abbreviations
- A2AKO
adenosine A2A receptor knockout
References
- 1.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension. 1998;31:943–948. doi: 10.1161/01.hyp.31.4.943. [DOI] [PubMed] [Google Scholar]
- 2.Chan ES, Fernandez P, Merchant AA, Montesinos MC, Trzaska S, Desai A, et al. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–2642. doi: 10.1002/art.21974. [DOI] [PubMed] [Google Scholar]
- 3.Zhong H, Belardinelli L, Maa T, Zeng D. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am J Respir Cell Mol Biol. 2005;32:2–8. doi: 10.1165/rcmb.2004-0103OC. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, et al. Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2004;287:H2478–H2486. doi: 10.1152/ajpheart.00217.2004. [DOI] [PubMed] [Google Scholar]
- 5.Murakami S, Hashikawa T, Saho T, Takedachi M, Nozaki T, Shimabukuro Y, et al. Adenosine regulates the IL-1 beta-induced cellular functions of human gingival fibroblasts. Int Immunol. 2001;13:1533–1540. doi: 10.1093/intimm/13.12.1533. [DOI] [PubMed] [Google Scholar]
- 6.Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK. A(2b) receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension. 2001;37:716–721. doi: 10.1161/01.hyp.37.2.716. [DOI] [PubMed] [Google Scholar]
- 7.Murakami S, Terakura M, Kamatani T, Hashikawa T, Saho T, Shimabukuro Y, et al. Adenosine regulates the production of interleukin-6 by human gingival fibroblasts via cyclic AMP/protein kinase A pathway. J Periodontal Res. 2000;35:93–101. doi: 10.1034/j.1600-0765.2000.035002093.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Che J, Chan ES, Cronstein BN. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol. 2007;72:1626–1636. doi: 10.1124/mol.107.03876. [DOI] [PubMed] [Google Scholar]
- 10.Hashmi AZ, Hakim W, Kruglov EA, Watanabe A, Watkins W, Dranoff JA, et al. Adenosine inhibits cytosolic calcium signals and chemotaxis in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G395–G401. doi: 10.1152/ajpgi.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, et al. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, et al. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, et al. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J Clin Invest. 2006;116:1274–1283. doi: 10.1172/JCI26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chunn JL, Mohsenin A, Young HW, Lee CG, Elias JA, Kellems RE, et al. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am J Physiol Lung Cell Mol Physiol. 2006;290:L579–L587. doi: 10.1152/ajplung.00258.2005. [DOI] [PubMed] [Google Scholar]
- 16.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 17.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 18.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8:145–150. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 19.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 2007;170:843–854. doi: 10.2353/ajpath.2007.051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boban I, Barisic-Dujmovic T, Clark SH. Parabiosis and transplantation models show no evidence of circulating dermal fibroblast progenitors in bleomycin-induced skin fibrosis. J Cell Physiol. 2008;214:230–237. doi: 10.1002/jcp.21182. [DOI] [PubMed] [Google Scholar]
- 23.Victor-Vega C, Desai A, Montesinos M, Cronstein B. Adenosine A2A agonists promote more rapid wound healing than recombinant human platelet derived growth factor (PDGF) Inflammation. 2002;26:19–24. doi: 10.1023/A:1014417728325. [DOI] [PubMed] [Google Scholar]
- 24.Montesinos M, Chen JF, Desai A, Yee H, Jacobson M, Schwarzschild M, et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A2A receptors. Am J Pathol. 2002;160:2000–2009. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the anti-angiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 27.Khoa ND, Montesinos CM, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signalling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 28.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 29.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 30.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard CL, Tan EY, Blay J. Adenosine upregulates CXCR4 and enhances the proliferative and migratory responses of human carcinoma cells to CXCL12/SDF-1alpha. Int J Cancer. 2006;119:2044–2053. doi: 10.1002/ijc.22084. [DOI] [PubMed] [Google Scholar]
- 32.Leibovich S, Chen JF, Belem P, Elson G, Rosania A, Ramanathan M, et al. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in murine macrophages by adenosine A2A receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]