Abstract
Objective
Pericardial and intra-thoracic fat are associated with prevalent cardiovascular disease (CVD) and CVD risk factors. However, it is unclear if these fat depots predict incident CVD events and/or all-cause mortality. We examined prospective associations between areas of pericardial and intra-thoracic fat and incident CVD and mortality over a 12-year follow-up in a subset of participants without baseline clinical CVD from the Rancho Bernardo Study (RBS).
Methods
Participants were 343 community-dwelling older adults (mean baseline age=67) who completed a clinic visit in 2001–02, including a computed tomography scan of the chest. Incident CVD and mortality were recorded through January 2013.
Results
Over a 12.6-year median follow-up, there were 60 incident CVD events and 49 deaths. Pericardial fat was associated with all-cause mortality, such that each standard deviation increment predicted a 34% higher chance of death after adjusting for demographics, lifestyle factors, comorbidities, and visceral fat (95% CI=1.01–1.78). When categorized by tertile, those in the middle tertile of pericardial fat showed no increased risk of mortality, while those in the highest tertile had 2.6 times the risk (95% CI=1.10–5.97) compared to the lowest tertile. There was a marginal association between intra-thoracic fat and mortality (p=0.06). Neither pericardial nor intra-thoracic fat was significantly associated with incident CVD. There were no significant interactions by sex.
Conclusions
Higher pericardial, but not intra-thoracic, fat was associated with earlier all-cause mortality in older adults over a 12-year follow-up. This association was primarily driven by a higher mortality rate in those in the highest tertile of pericardial fat.
Keywords: Pericardial fat, intra-thoracic fat, incident CVD, all-cause mortality, fat distribution, body composition
Introduction
There is robust evidence that central body fat distribution is predictive of chronic disease and is often a better predictor than body mass index (BMI)(1, 2) or total body fat(3, 4). Specific fat depots, such as fat in the pericardial, visceral, and intra-thoracic cavities, are associated with a range of cardiovascular and metabolic risk factors and conditions, including diabetes and hypertension (5). These associations remain significant after adjusting for BMI and/or total body fat, suggesting that the location of fat in the body, rather than the overall quantity, is a key factor in determining disease risk.
Not surprisingly, pericardial fat in particular has been associated with cardiovascular outcomes. Pericardial fat is associated with prevalent cardiovascular disease (CVD)(6, 7) and incident coronary heart disease (CHD)(8), suggesting it is not simply a marker of existing disease, but contributes to the development of CHD. Intra-thoracic fat, or fat external to the pericardial sac and within the thoracic cavity, is anatomically close to pericardial fat, and is also associated with cardiovascular(5) and metabolic risk factors.(9, 10) However, there is little research reporting the associations between intra-thoracic fat and incident morbidity. Similarly, little is known about the associations between fat depositions in the chest and all-cause mortality. One study using data from the Framingham Heart Study found no association between pericardial fat and mortality, but this sample was quite young, and associations with intra-thoracic fat were not reported.(11)
Given the robust relationship between pericardial and intra-thoracic fat deposition and CVD risk factors, further examination of the associations between these measures and future morbidity and mortality is warranted. Therefore, the purpose of this paper was to examine the associations between pericardial fat and intra-thoracic fat with incident CVD and all-cause mortality in a subsample of older non-Hispanic white men and women from the Rancho Bernardo Study, and to determine if these associations varied by sex.
Methods
Study Design
The Rancho Bernardo Study (RBS) is a prospective cohort study established between 1972 and 1974 in a suburb of San Diego, California, when 82% of community-dwelling adults in this predominantly older, non-Hispanic White, middle to upper-middle class area agreed to participate in a survey of heart disease risk factors. A full account of inclusion/exclusion criteria and recruitment details has been published (12). Participants have subsequently completed periodic clinic examinations in the Rancho Bernardo research clinic as well as follow-up via phone and mailers. A subsample of the majority of surviving participants who attended a 1997–1999 clinic visit, were free of clinical CVD, and were at least 55 years of age, completed a follow-up clinical examination in 2001–2002 at which time computed tomography (CT) scans of the chest and abdomen were obtained to determine the presence and extent of coronary artery calcium. These scans were also evaluated for region-specific fat deposition. In the current study, we report on 187 women and 156 men who had pericardial and intra-thoracic fat data and complete follow-up for cardiovascular events and mortality through January 2013.
The RBS was approved by the Institutional Review Board of the University of California, San Diego, and all participants gave written informed consent.
Measurement of Covariates
Information on demographics, lifestyle factors, and cardiovascular risk factors ascertained at the 1997–1999 clinic visits included standard self-administered or interviewer-administered questionnaires were used to record age, sex, physical activity, and smoking status. Medication use was assessed via a medication inventory. Height and weight were measured with participants in light clothing and no shoes, and used to compute body mass index (BMI; kg/m2). Blood pressure was recorded as the average of two measurements taken with participants resting in a seated position after rest for five minutes. Hypertension was defined as ≥140 mmHg systolic and/or ≥90 mmHg diastolic, and/or use of anti-hypertension medication.
Fasting plasma glucose, cholesterol, and triglycerides were measured in morning fasting blood samples obtained after an 8 to 12-hour fast. Diabetes was defined as use of diabetes medication, or fasting plasma glucose of ≥126 mg/dL. Low-density cholesterol (LDL) was calculated using the Friedewald equation(13), and high-density cholesterol (HDL) was determined using precipitation analysis.
Adipocytokines were measured using EDTA fasting plasma samples stored at −70C. Adiponectin was measured via radioimmunoassay (Millipore Linco Research, St Charles, MO) with intra- and inter-assay coefficients of variation (CVs) ranging from 1.97% – 7.32%. Interleukin (IL)-6 was measured via ELISA (R&D Systems, Minneapolis, MN) with intra- and inter-assay CVs for IL-6 of 4.9% – 10.5%,
Measurement of Regional Adiposity
During the 2001–02 exam, areas of pericardial and intra-thoracic fat were measured from the computed tomography (CT) scans of the chest. Full details of regional fat measurement and quantification, including intra-thoracic and pericardial fat with accompanying example CT images, have been published elsewhere (9). In brief, 15 CT slices of 3mm thickness were selected originating from the right coronary artery (slice 1), 4 slices above the right coronary artery and 10 slices below. The anterior border of the total thoracic fat volume was defined by the interior margin of the chest wall and the posterior border by the aorta and bronchus. The pulmonary vessels were excluded. The anterior border of the pericardial area was defined by the line of the fibrous pericardium and the posterior border was shared with the thoracic area. Intra-thoracic fat area was defined as the total thoracic fat area minus the pericardial fat area. Using a 6mm cross sectional CT slice taken at the umbilicus, visceral fat was measured at the same visit. The chest and abdominal CT scans were read by three experienced CT analysts using body composition segmentation software (MIPAV 4.1.2, National Institutes of Health) on networked computers. CT reader intra- and inter-rater reliability in the lab ranged from 0.85–0.99. Tissues with a voxel count between -190 and -30 Hounsfield units were counted as adipose tissue, and reported in cm2. With the non-contrast CT scans and the current MIPAV software, epicardial fat cannot be adequately or precisely quantified; thus, it was not measured in this study.
Incident Cardiovascular Disease and Total Mortality Outcomes
Follow-up for mortality and cardiovascular events was obtained through January 2013 for all 343 RBS participants who completed regular clinic visits, annual mailings, and/or telephone surveys. Incident CVD included fatal and non-fatal myocardial infarction (MI), stroke, and transient ischemic attack (TIA), coronary artery bypass, angioplasty, and/or carotid endarterectomy. Information on non-fatal CVD events was obtained via self-report of physician diagnosis at clinic exams, or on annual mailed questionnaires or telephone surveys. Additionally, evidence of a previous MI on a 12-lead electrocardiogram was taken into account, including Minnesota codes 1.1 to 1.2 (major Q-wave) and 7.1.1 (left branch bundle block). As indicated elsewhere(14), validation of self-reported physician diagnosis has been previously performed in a sample of 30% of cases in the RBS, and was validated in 85% of those cases.
All-cause mortality was confirmed by death certificates in approximately 95% of all decedents, with underlying cause of death coded by a certified nosologist using the ninth revision of the International Classification of Diseases (ICD-9), Adapted. CVD death codes included 401–414, 426–438, and 440–448, all causes codes were 0–999.
Statistical Analysis
Univariate associations were assessed using ANOVA, chi-square or t-tests as appropriate. Unadjusted event rates were calculated per 1000 person-years. To examine the multivariate associations of intra-thoracic and pericardial fat with all-cause mortality and incident CVD events, staged Cox proportional hazards regression models were used. The first model stage included age and sex. Then, for model 2 we added physical activity, ever smoking, height, visceral fat, prevalent hypertension, prevalent diabetes, and lipid-lowering medication to the first model. The final staged model additionally included LDL cholesterol, HDL cholesterol, triglycerides, fasting plasma glucose, adiponectin and IL-6. In these models, height was used rather than BMI, because height was less correlated than BMI with the other adiposity measures, yet still provides adjustment for overall body size. This was important given the small sample size, the number of adjustment variables, and already high correlations of pericardial and intra-thoracic fat with visceral fat (0.60, 0.70, respectively). The proportional hazards assumption was checked for all models via plots of Schoenfeld residuals, and global chi-square tests.
Interactions of pericardial fat and intra-thoracic fat with sex were assessed on a multiplicative scale in models that included age, as well as sex, physical activity, ever smoking, height, visceral fat, prevalent hypertension, prevalent diabetes, and lipid-lowering medication use. To ensure the highest statistical power, pericardial and intra-thoracic fat as continuous variables were used to examine interactions.
Results
The overall mean (+/−SD) baseline age was 67 (+/− 7). Half (50%) of participants had hypertension, and 7.8% had prevalent diabetes. Approximately half (52%) reported ever smoking, while 76% reported exercising at least three times per week. Table 1 shows the unadjusted baseline participant characteristics by tertiles of pericardial fat area. With the exception of prevalent diabetes (p=0.10), smoking (p=0.32), and regular exercise (p=0.07), all other characteristics differed significantly across tertiles (p<0.05). All characteristics (including diabetes, exercise, and smoking) varied across tertiles, such that the risk factors for CVD were progressively higher in those with more pericardial fat. Visceral fat was moderately correlated with pericardial fat (r=0.60) and intra-thoracic fat (r=0.70).
Table 1.
Baseline Characteristics by Tertiles of Pericardial Fat
| Tertile 1 < 126.5 cm2 n=113 |
Tertile 2 126.5 – 194.2 cm2 n=116 |
Tertile 3 ≥ 194.3 cm2 n=114 |
p-value* | |
|---|---|---|---|---|
| Age, yrs | 65 ± 7 | 67 ± 6 | 68 ± 7 | 0.02 |
| Female sex, n(%) | 73 (65%) | 71 (61%) | 43 (38%) | <0.001 |
| Ever Smoker, n(%) | 55 (49%) | 58 (50%) | 66 (58%) | 0.32 |
| BMI, kg/m2 | 24 ± 3 | 27 ± 4 | 28 ± 5 | <0.001 |
| Height, m | 1.66 ± 0.09 | 1.67 ± 0.10 | 1.71 ± 0.09 | 0.001 |
| Visceral Fat, cm2 | 80 ± 37 | 125 ± 53 | 173 ± 66 | <0.001 |
| Exercise≥3×/week, n(%) | 95 (84%) | 86 (74%) | 82 (72%) | 0.07 |
| Prevalent Hypertension, n(%) | 44 (39%) | 55 (47%) | 72 (63%) | 0.001 |
| Systolic Blood Pressure, mmHg | 125 ± 19 | 131 ± 20 | 134 ± 18 | 0.001 |
| Diastolic Blood Pressure, mmHg | 75 ± 8 | 77 ± 9 | 78 ± 8 | 0.004 |
| Prevalent Diabetes, n(%) | 6 (5%) | 7 (6%) | 14 (12%) | 0.10 |
| Fasting Plasma Glucose, mg/dL | 98 ± 18 | 103 ± 29 | 108 ± 23 | 0.009 |
| LDL cholesterol, mg/dL | 116 ± 32 | 123 ± 25 | 131 ± 31 | 0.001 |
| HDL cholesterol, mg/dL | 64 ± 18 | 60 ± 17 | 53 ± 13 | <0.001 |
| Triglycerides, mg/dL† | 98 (73, 134) | 109 (75, 152) | 128 (95, 186) | <0.001 |
| Lipid-lowering medication, n(%) | 8 (7%) | 20 (17%) | 25 (22%) | 0.007 |
| Interleukin (IL)-6, pg/mL† | 1.19 (0.92, 1.72) | 1.52 (1.17, 2.11) | 1.68 (1.13, 2.60) | <0.001 |
| Adiponectin, ug/mL† | 12.5 (9.0, 16.5) | 11.2 (7.2, 15.3) | 9.2 (6.8, 14.4) | 0.01 |
p-value by ANOVA, chi-square or Kruskal-Wallis as appropriate
Median (Quartile 1, Quartile 3)
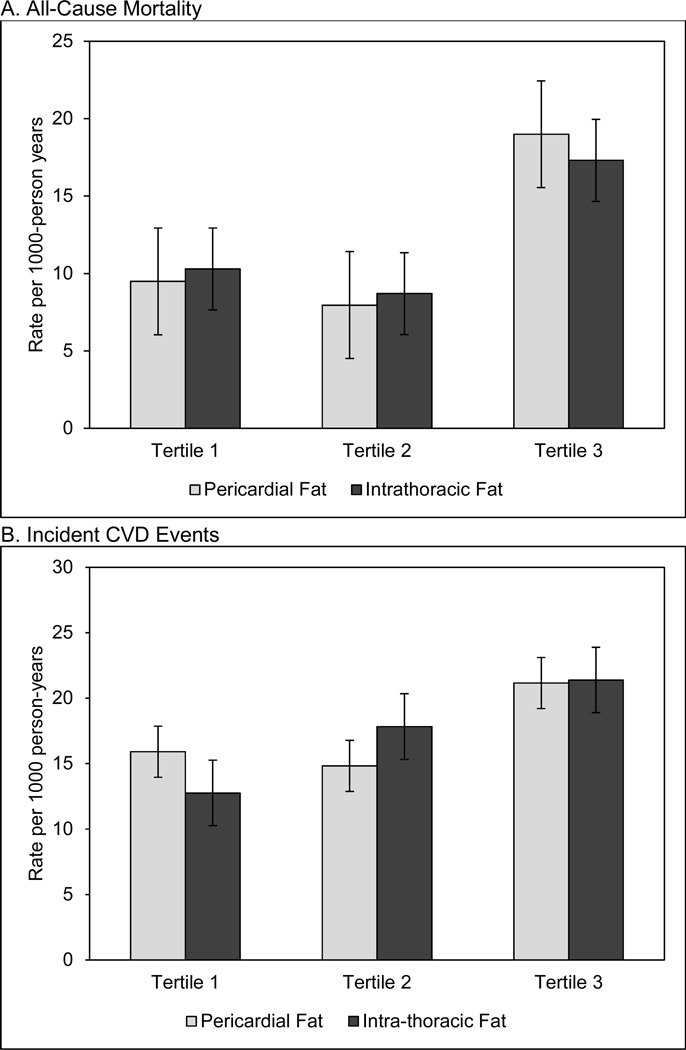
Unadjusted rates of incident CVD events and all-cause mortality across tertiles of pericardial and intra-thoracic fat are shown in Figures 1a and 1b. Generally, higher levels of incident CVD and mortality were seen only in the highest fat tertiles. Specifically, there were 19.0 deaths per 1,000 person years among those in the highest tertile of pericardial fat, compared to 9.5 in the lowest tertile (Figure 1a). Similarly, 17.3 deaths were recorded per 1,000 person years in the highest tertile of intra-thoracic fat, compared to 10.3 in the lowest tertile (Figure 1a). Those in the middle tertiles showed no elevated risk. A similar pattern was observed for incident CVD (Figure 1b).
Figure 1. Rates of all-cause mortality (panel A) and incident CVD (panel B) by tertiles of pericardial and intra-thoracic fat*.
*standard error bars are shown
Unadjusted rates of mortality (panel A) and incident CVD events (panel B) by teriles of pericardial fat (light grey) and intra-thoracic fat (dark grey). Event rates per 1000 person-years are on the y-axis while tertiles are on the x-axis for each plot.
For all-cause mortality, the median follow-up from the 2001–02 examination was 12.6 years (interquartile range 11.6–13.0). There were 49 all-cause deaths (14.3%), eight of which (16.3%) were attributed to CVD, including one CHD death. For pericardial fat, each standard deviation higher was associated with a 34% increased risk of all-cause mortality in unadjusted models (HR=1.34, p<0.01; Table 2). This association did not change after adjustment for demographics, lifestyle factors, comorbidities, visceral fat, and height. Further adjustment for lipids, glucose, and adipocytokines did not materially change the results. In unadjusted analyses, intra-thoracic fat was associated with a 40% increased risk of all-cause mortality (p<0.01). This was attenuated to marginal significance (HR = 1.40, p=0.06) after adjusting for additional cardiovascular risk factors, including lipids, glucose, and adipocytokines, as well as visceral fat (Table 2). There were no significant interactions of pericardial fat or intra-thoracic fat by sex for all-cause mortality (data not shown).
Table 2.
Associations of pericardial and intra-thoracic fat with all-cause mortality and incident CVD*
| All-Cause Mortality HR (95% CI) |
p-value | Incident CVD HR (95% CI) |
p-value | |
|---|---|---|---|---|
| Pericardial Fat | ||||
| Unadjusted | 1.34 (1.10,1.63) | 0.004 | 1.07 (0.89,1.29) | 0.46 |
| +Age, sex | 1.25 (1.00,1.57) | 0.05 | 1.00 (0.82, 1.21) | 0.99 |
| +Lifestyle/Comorbidities/Body Size† | 1.46 (1.03,1.78) | 0.03 | 0.93 (0.71, 1.21) | 0.57 |
| +Lipids, Glucose, Adipocytokines‡ | 1.34 (1.01,1.78) | 0.04 | 0.80 (0.60,1.07) | 0.13 |
| Intra-thoracic Fat | ||||
| Unadjusted | 1.40 (1.11, 1.77) | 0.004 | 1.14 (0.92,1.41) | 0.23 |
| +Age, sex | 1.22 (0.95, 1.56) | 0.13 | 0.99 (0.78, 1.26) | 0.96 |
| +Lifestyle/Comorbidities/Body Size† | 1.39 (0.99, 1.96) | 0.06 | 0.94 (0.67, 1.31) | 0.71 |
| +Lipids, Glucose, Adipocytokines‡ | 1.40 (0.98, 1.99) | 0.06 | 0.95 (0.67, 1.36) | 0.79 |
Per standard deviation of intra-thoracic (75 cm2) or pericardial fat (79 cm2)
Physical activity, ever smoker, height, visceral fat, prevalent hypertension, prevalent diabetes, lipid-lowering medication use
LDL cholesterol, HDL cholesterol, triglycerides, fasting plasma glucose, interleukin-6, adiponectin
For incident CVD events, the median follow-up time was 10.7 years (IQ range 10.2–11.0). There were 60 total incident CVD events, with 19 incident CHD events (diagnosed myocardial infarction). For both pericardial and intra-thoracic fat, there were no significant associations with incident CVD across any models (Table 2). There were also no significant interactions of pericardial fat or intra-thoracic fat by sex for incident CVD (data not shown). When modeled as tertiles, there were no significant associations of pericardial or intra-thoracic fat with incident CVD (data not shown).
Table 3 shows hazard ratios of all-cause mortality across tertiles of pericardial and intra-thoracic fat. Compared to those in the lowest tertile, those in the middle tertile of pericardial fat showed no elevated risk of mortality across any models of adjustment. However, those in the highest tertile had more than double the risk of mortality in unadjusted models (HR=2.66, p=0.008), which decreased modestly after adjusting for demographics, lifestyle factors, comorbidities, visceral fat, and height (HR=2.56, p=0.03). Additional adjustment for lipids, glucose, and inflammation did not materially change the results. Those in the second and third tertiles of intra-thoracic fat area did not show significantly greater risk of mortality compared to the lowest tertile in any of the models (Table 3).
Table 3.
Association of tertiles of pericardial and intra-thoracic fat with all-cause mortality
| All-Cause Mortality HR (95% CI);p |
||||||
|---|---|---|---|---|---|---|
| Pericardial Fat, cm2 | Intra-thoracic Fat, cm2 | |||||
| Tertile 1 <126.5 |
Tertile 2 126.5–194.2 |
Tertile 3 ≥194.3 |
Tertile 1 <45.9 |
Tertile 2 45.9–104.7 |
Tertile 3 ≥104.8 |
|
| Unadjusted | ref | 1.01 (0.44,2.30); 0.98 | 2.66 (1.29, 5.45); 0.008 | ref | 0.96 (0.44.2.11); 0.92 | 1.87 (0.95,3.71); 0.07 |
| +Age, sex | ref | 0.95 (0.42, 2.18); 0.91 | 2.00 (0.96, 4.18); 0.06 | ref | 0.74 (0.33,1.64); 0.45 | 1.15 (0.55,2.41); 0.71 |
| +Lifestyle/Comorbidities/Body Size* | ref | 1.00 (0.42, 2.39); 0.98 | 2.56 (1.10, 5.97); 0.03 | ref | 0.64 (0.27,1.55); 0.32 | 1.14 (0.45, 2.89): 0.79 |
| +Lipids, Glucose, Adipocytokines† | ref | 1.09 (0.44, 2.73); 0.85 | 2.63 (1.06, 6.55); 0.04 | ref | 0.49 (0.19,1.25); 0.13 | 0.96 (0.36, 2.55); 0.93 |
Physical activity, ever smoker, height, visceral fat, prevalent hypertension, prevalent diabetes, lipid-lowering medication use
LDL cholesterol, HDL cholesterol, triglycerides, fasting plasma glucose, interleukin-6, adiponectin
Discussion
In the current study, pericardial fat was significantly associated with all-cause mortality. This association remained significant after adjusting for potential confounders, such that each standard deviation increment in pericardial fat was associated with 34% increased risk of mortality in fully adjusted models. Associations between pericardial fat and mortality were primarily driven by elevated risk in the highest tertile of pericardial fat, while those in the middle tertile showed no elevated risk compared to those in the lowest tertile. In unadjusted models, the highest tertile of pericardial fat was associated with approximately 19 deaths per 1,000 person years, compared to half that rate (9.5) in the lowest quartile. The risk of death was even higher (HR=2.56) after adjusting for other cardiovascular risk factors. While mortality and incident CVD were also higher in the highest tertile of intra-thoracic fat, this was not statistically significant after adjusting for potential confounders.
These findings are inconsistent with previous data in 3,086 participants followed for 5 years from the Framingham Heart Study (FHS) (11) that showed no association between pericardial fat and all-cause mortality (HR=1.17, 95% CI = 0.95–1.44). Notably, participants in our study were significantly older (mean age 67 vs. 50 years) and had a longer follow-up time (median follow-up 12.6 vs. 5.0 years). Consequently, the mortality rate in the RBS was much higher than in the FHS (14.6% vs. 2.3%). In addition, the FHS did not consider the possibility of non-linear association with increased risk only at the highest levels.
Conversely, and similar to the findings in FHS (11), we found no association between pericardial fat (or intra-thoracic fat) and incident CVD. Ding et al., however, did find a significant association between pericardial fat and incident CHD in 998 participants from the Multi-Ethnic Study of Atherosclerosis (MESA), followed for approximately five years (HR=1.33, 95% CI= 1.15–1.54)(8), while Mahabadi et al. found an association between epicardial fat and coronary events over an eight-year follow-up in 4093 participants (HR=1.54, 95% CI=1.09–2.19)(15). This could suggest that the effects of pericardial fat are localized rather than general; that is, pericardial fat may be specifically associated with disease in the heart, where the fat is located, rather than more general diffuse disease in vascular beds throughout the body. This is supported by other previous studies showing that pericardial and intra-thoracic fat are associated with presence and severity of coronary artery calcium (9, 16), and calcified coronary plaques (17), associations not found with BMI or waist-hip ratio. Also, participants in the MESA study were ethnically diverse while participants in the Rancho Bernardo Study were predominantly non-Hispanic White, which could also contribute to differences in incident events. The more likely difference, however, lies in the nature of the CVD events in the current study, approximately two-thirds of which were non-CHD events. Because there were only 19 incident CHD cases in our study, obtaining valid parameter estimates for the association with incident CHD specifically was not feasible.
Additionally, after adjustment for lipids, glucose, and adipocytokines, we observed a slight inverse, yet non-significant, trend in the association between pericardial fat and incident CVD. This could be the result of several factors, including glucose and adipocytokines mediating rather than confounding this association, or a spurious finding (type I error) as a result of adjustment for an increasing number of variables with a relatively small sample size.
There are a number of mechanisms that could potentially explain the association between pericardial fat and mortality. Pericardial fat has been associated with production of adipocytokines, such as IL-6 and TNF- α (18, 19), and therefore is associated with localized and systemic inflammation. However, adjusting for adipocytokines in the current study did not appreciably attenuate any results. The fact that mortality (n=49) was rare and was attributed to a range of causes, with only eight attributed to CVD, may suggest that pericardial fat is a marker of more general declining health or compromised immune function. This is supported by our findings that pericardial fat was strongly associated with many CVD risk factors at baseline. Indeed, only three CVD risk factors, diabetes, exercise, and smoking, did not differ significantly across tertiles of pericardial fat. In general, increasing pericardial fat was associated with a worse CVD risk factor profile in unadjusted analyses. Higher levels of pericardial fat have also been associated with more sedentary behavior (20), which is another potential pathway linking greater pericardial fat to earlier death.
The observation that associations between intra-thoracic fat and mortality were somewhat different from those with pericardial fat may be somewhat surprising. This is consistent, however, with previous studies finding discordant results for pericardial and intra-thoracic fat (6, 10, 21). While proximally closer to pericardial fat, intra-thoracic fat has been found to more highly correlate with visceral fat, and, like visceral fat, has been associated with metabolic outcomes (10). In our study, visceral fat was indeed more highly correlated with intra-thoracic fat than pericardial fat. As this is one of the only studies reporting associations of intra-thoracic fat and incident events and mortality, clearly more research is needed to elucidate the role of intra-thoracic fat in the development of disease.
This study has a number of noteworthy strengths and limitations. Fat depositions were measured by CT and quantified by trained technicians using semi-automated software. Despite the small numbers of events, we do have prospective outcomes over a relatively long follow-up period (median of 12.6 years), and thorough measures of a number of physiologic and behavioral cardiovascular risk factors. Our study is limited, however, in that the majority of the sample were non-Hispanic white, thus the findings may not generalize to other racial/ethnic groups. (Though, on the other hand, the homogenous nature of the RBS participants provides fewer opportunities for confounding.) Also, if regional fat depots changed appreciably over time in this sample, the advanced age of the sample combined with the long follow-up time could have led to misclassification. Finally, it is also possible that selection bias could have influenced our results, as all participants were free of known CVD at baseline despite a relatively advanced age, and thus were in particularly good cardiovascular health. This may explain why the event rates were too low to allow for meaningful examination of more specific types or subsets of CVD or causes of mortality. However, use of this healthy population also adds to the novelty of these findings, as the data show an association between pericardial fat and mortality even in a population with particularly good cardiovascular health.
Conclusions
In an older population of mostly non-Hispanic white men and women, pericardial fat is associated with all-cause mortality, but not incident CVD, over a 12-year follow-up period. This risk was driven by an especially high mortality rate in those in the highest tertile of pericardial fat. More research is needed to determine the mechanisms linking pericardial fat and mortality, and whether pericardial fat predicts risk of specific cardiovascular conditions, such as CHD.
Highlights.
We used chest CT scans to quantify pericardial and intra-thoracic fat
Participants were followed for incident CVD and mortality over 12.6 years
Pericardial fat area predicted all-cause mortality in older adults
Risk was elevated only in the highest tertile of pericardial fat
Neither pericardial fat nor intra-thoracic fat predicted incident CVD
Acknowledgments
This work was supported by R21HL089622 from the National Heart Lung and Blood Institute to CLW. The Rancho Bernardo Study was supported by National Institute of Aging Grant NIA 5R01 AG07181 and the National Institute of Diabetes and Digestive and Kidney Diseases Grant NIDDK 5R01 DKK31801. GAL was supported by an American Heart Association award. BAL was supported by K01DK101650 from the National Institutes for Diabetes, Digestive, and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts to disclose.
References
- 1.Folsom AR, Kaye SA, Sellers TA, Hong CP, Cerhan JR, Potter JD, et al. Body fat distribution and 5-year risk of death in older women. Journal of the American Medical Association. 1993;269(4):483–487. [PubMed] [Google Scholar]
- 2.Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116(25):2933–2943. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 3.Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, et al. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110(11):867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 4.Myint PK, Kwok CS, Luben RN, Wareham NJ, Khaw KT. Body fat percentage, body mass index and waist-to-hip ratio as predictors of mortality and cardiovascular disease. Heart. 2014 doi: 10.1136/heartjnl-2014-305816. [DOI] [PubMed] [Google Scholar]
- 5.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial, fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 6.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. European heart journal. 2009;30(7):850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157(1):203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) The American journal of clinical nutrition. 2009;90(3):499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wassel CL, Laughlin GA, Araneta MRG, Kang E, Morgan CM, Barrett-Connor E, et al. Associations of pericardial and intra-thoracic fat with coronary calcium presence and progression in a multi-ethnic study. Obesity. 2012 doi: 10.1002/oby.20111. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O'Donnell CJ, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circulation Cardiovascular imaging. 2010;3(5):559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. American Journal of Epidemiology. 1980;111:704–712. doi: 10.1093/oxfordjournals.aje.a112948. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald W, Levy R, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Daniels LB, Laughlin GA, Sarno MJ, Bettencourt R, Wolfert RL, Barrett-Connor E. Lipoprotein-associated phospholipase A2 is an independent predictor of incident coronary heart disease in an apparently healthy older population: the Rancho Bernardo Study. J Am Coll Cardiol. 2008;51(9):913–919. doi: 10.1016/j.jacc.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61(13):1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 16.Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis. 2010;209(1):136–141. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16(8):1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 19.Yun CH, Lin TY, Wu YJ, Liu CC, Kuo JY, Yeh HI, et al. Pericardial and thoracic peri-aortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. European journal of radiology. 2012;81(4):749–756. doi: 10.1016/j.ejrad.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Larsen BA, Allison MA, Kang E, Saad S, Laughlin GA, Araneta MR, et al. Associations of physical activity and sedentary behavior with regional fat deposition. Med Sci Sports Exerc. 2014;46(3):520–528. doi: 10.1249/MSS.0b013e3182a77220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18(5):1039–1045. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]