Abstract
The coinhibitory receptor CTLA-4 is a master regulator of T cell responses and its function is critical in models of transplant tolerance. The CD28/CTLA-4 pathway is also an important therapeutic target, as the costimulation blocker belatacept was recently approved for use following renal transplantation. While the traditional model of CTLA-4 coinhibition focuses on its ability to directly counteract CD28 costimulation, recently this paradigm has significantly broadened. Recent work has uncovered the ability of CTLA-4 to act as a cell-extrinsic coinhibitory molecule on CD4+ T cell effectors. While it has been appreciated that CTLA-4 is required FoxP3+ Treg suppression, current studies have elucidated important differences in the function of CTLA-4 on Tregs compared to effectors. CTLA-4 expression patterns also differ by T cell subsets, with Th17 cells expressing significantly higher levels of CTLA-4. Thus, in contrast to the traditional model of CTLA-4 as a negative receptor to counter CD28 costimulation, recent work has begun to define CTLA-4 as a global regulator of T cell responses with subset-specific functions. Future studies must continue to uncover the molecular mechanisms that govern CTLA-4 function. These novel findings have implications for novel strategies to maximize the regulatory potential of CTLA-4 during allogeneic T cell responses.
Keywords: Costimulation, coinhibition
Introduction
The CD28/CTLA-4 pathway is the prototypic cosignaling pathway in T cells. T cells that receive inadequate CD28 costimulation via suboptimal ligation of CD80 and CD86 (B7.1 and B7.2, respectively) become anergized or apoptotic (1). CTLA-4 has been characterized as a counter-signal to CD28 costimulation, as CTLA-4 also binds CD80/CD86 (2). In contrast to CD28, which is constitutively expressed in most populations of T cells at rest and following activation, CTLA-4 is not expressed on the surface of resting T cells, but is maintained in intracellular Golgi-associated vesicles beneath the plasma membrane. Upon antigen stimulation, CTLA-4 surface expression is induced through both de novo gene expression and cycling of existing CTLA-4 protein to the cell surface.
CTLA-4 expression is induced not only by antigen stimulation, but also by CD28 signaling (3). Indeed, multiple studies have shown that CD28 triggering is required for maximal CTLA-4 expression and coinhibition coinhibition (3, 4). The pathways that control de novo CTLA-4 expression are not completely understood (5). Seminal work demonstrated that the mTOR inhibitor rapamycin or the NFAT pathway inhibitor cyclosporine function to reduce CTLA-4 expression (6). Consistent with a role for the Akt/mTOR signaling pathway to control CTLA-4 expression, the FOXO family of transcription factors was recently shown to bind to the upstream regulatory region of CTLA-4 and induce expression (7, 8).
CTLA-4 in Alloreactive T Cell Responses
CTLA-4 has been established as a critical molecule for controlling antigen specific T cell responses (9, 10) and in models of autoimmune disease, pathogen-responses, and cancer (1, 2, 11). In transplantation, the function of CD28 as a critical regulator of T cell activation led to many studies investigating its potential as a target to induce long term graft survival (1). Indeed, many early studies investigating mechanisms of tolerance found enhanced graft survival using the CD80/CD86-binding molecule CTLA-4 Ig. While it was largely presumed that this was due to the inhibition of CD28 signals, work from several groups demonstrates that the inhibitory effects of blocking CD28 require CTLA-4 signals. In a cardiac allograft model of tolerance induction with CTLA-4 Ig and DST, Judge et al. showed that CTLA-4 signals early following transplantation were required for long term graft survival. Interestingly, donor CD80, but not CD86, signals were critical for the effect of CTLA-4 in this model (12). Similarly, cardiac allografts transplanted into CD28−/− recipients displayed accelerated rejection kinetics when CTLA-4 signals were blocked (13).
More recently, CTLA-4 has been demonstrated to be a critical regulator of alloreactive T cell responses (Table 1). In a model of islet allograft tolerance with anti-CD45RB, CTLA-4 was selectively upregulated and CTLA-4 signals were required for allograft survival (14, 15). Two recent studies utilizing selective CD28 blocking reagents have demonstrated long-term survival of skin and cardiac allografts (16, 17). In both models, the efficacy of CD28 blockade was dependent on CTLA-4 signals, as concurrent CD28 and CTLA-4 blockade abrogated the enhanced graft survival. Together, these studies establish the importance of CTLA-4 as a regulator of alloreactive T cell responses, and demonstrate that CTLA-4 coinhibitory signals are critical for multiple strategies that enhance allograft survival.
Table 1.
Murine transplant models in which CTLA-4 signals prevent graft rejection.
| Study | Allograft | Recipient | Treatment* |
|---|---|---|---|
| Lin et al, 1998 (13) | BALB/c hearts | C57BL/6 or CD28−/− | CTLA-4 Ig, anti-B7 |
| Judge et al, 1999 (12) | BALB/c hearts | C57BL/6 | CTLA-4 Ig/DST, anti-B7-1/anti-B7-2 |
| Fecteau et al, 2001 (15) | BALB/c islets | Stz treated C57Bl/6 | Anti-CD45RB |
| Ariyan et al, 2003 (14) | BALB/c islets | Stz treated C57Bl/6, B7-1−/−/B7-2−/, and B7-1−/−/B7-2−/−/CTLA-4−/− | Anti-CD45RB |
| Zhang et al, 2011 (17) | C57BL/6 hearts | BALB/c | Anti-CD28 scFc |
| Liu et al, 2014 (16) | BALB/c or mOVA skin | C57BL/6 or C57BL/6 OT-I chimera | Anti-CD28 dAb |
Includes only treatments that were shown to depend on CTLA-4 signals
Abbreviations: Stz, streptozotocin
Mechanisms of CTLA-4 Coinhibition
Traditional model of CTLA-4 coinhibition
CTLA-4 has long been understood to function as a coinhibitor that restrains T cell responses, owing to early studies using monoclonal antibodies to block CTLA-4 that augmented murine and human T cell proliferation (5), and the profound auto-proliferative phenotype of CTLA-4 knockout mice, which develop severe polyclonal T cell infiltration in multiple tissues (18). However, a precise understanding of the mechanism of CTLA-4 coinhibition has been elusive, as a number of proposed mechanisms of coinhibition have been proposed.
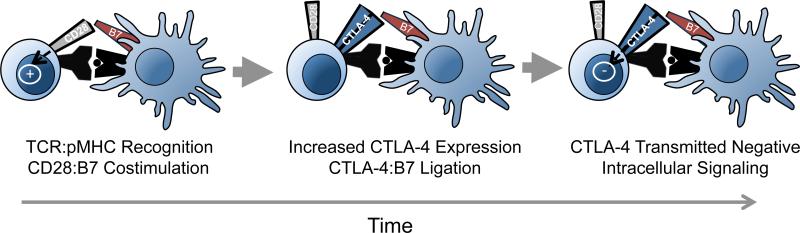
Here we define the traditional model of CTLA-4 cell-intrinsic coinhibition that is based on two phenomena (Figure 1): 1) the higher binding affinity of CTLA-4 vs. CD28 for the shared ligands CD80/CD86, and 2) the transmission of negative intracellular signals through the CTLA-4 cytoplasmic tail (for a detailed review, see Reference 4). While CD28 and CTLA-4 share the same ligand binding motifs, CD28 binds its ligands monovalently, while CTLA-4 binds bivalently. Multiple groups have demonstrated that CTLA-4 expression can prevent CD28 ligand binding, possibly through its recruitment to the immunological synapse (5). Once CTLA-4 engages its ligands, inhibitory intracellular signaling cascades are initiated that involve the ITIM motif of CTLA-4 and SHP-2, PP2A, and/or cbl-b (5). This model has established that the localization, expression kinetics, and biochemistry of CTLA-4 inhibit T cell responses. However, recent studies have uncovered additional roles for CTLA-4 that significantly broaden our understanding of CTLA-4 as a global regulator of T cell responses.
Figure 1. The traditional model of CTLA-4 coinhibition.
Successful recognition of a T cell receptor with cognate peptide:MHC induces CD28:B7 costimulation. Following a brief period of activation, CTLA-4 is expressed on the T cell suface and extinguishes CD28:B7 binding. CTLA-4 also transmits intracellular signals that limit T cell activation.
Cell extrinsic CTLA-4 function
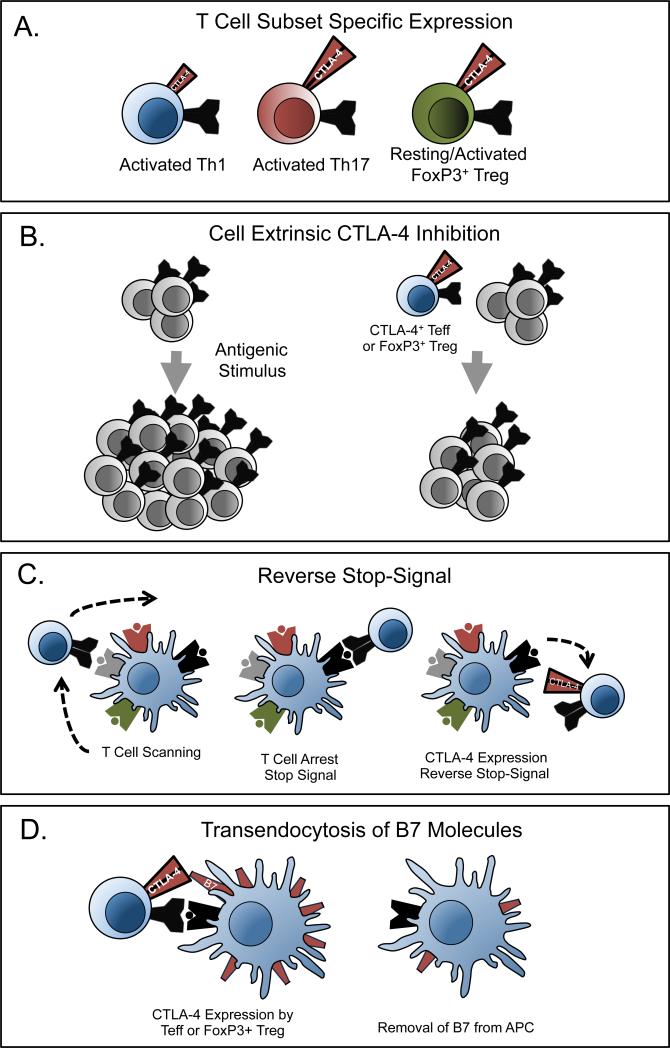
While early studies employing selective CTLA-4 blockade or genetic ablation demonstrated that CTLA-4 functions to dampen T cell proliferative responses, considerable evidence demonstrates that CTLA-4 also functions in a non-cell autonomous, or cell extrinsic, mechanism. Although CTLA-4 KO T cells have an autoreactive phenotype, two groups reported that in bone marrow chimeras of CTLA-4 KO and sufficient cells, CTLA-4 KO cells do not hyper-proliferate (19, 20). This finding demonstrates that the expression of CTLA-4 on T cells is sufficient to provide coinhibition to CTLA-4 deficient T cells. A cell extrinsic function for CTLA-4 found on activated T cells has also been demonstrated in vitro (21).
Recently the importance of the cell extrinsic function of CTLA-4 in vivo has been further investigated. Using a co-adoptive transfer approach of antigen-specific CTLA-4 deficient and WT CD4+ T cells, Corse et al. showed that the presence of CTLA-4 on WT CD4+ T cells limited the hyper-proliferation of CTLA-4 KO cells to WT levels (22). Interestingly, gene expression profiling on CD4+ CTLA-4 KO and WT cells demonstrates that the absence of CTLA-4 induces a signature of cell cycle progression during in vivo immune responses. Similarly, Wang et al. showed in dual adoptive transfer experiments that the presence of CTLA-4 on CD4+ Teffs limited the proliferative response of CTLA-4 KO Teffs (23). However, only CTLA-4+/+ Tregs, but not CTLA-4+/+ Teffs, were sufficient to suppress disease in a RIP-OVA model of diabetes.
In a seminal study, Qureshi et al. provided a compelling mechanistic explanation for cell extrinsic CTLA-4 coinhibition by demonstrating that CTLA-4 facilitates the transendocytosis of CD80/CD86 from antigen presenting cells (24). The authors demonstrated that in both human and murine T cells, CTLA-4 selectively removes its ligands from the surface of neighboring cells and targets it for degradation in the endocytic vesicles of the recipient cell. This mechanism is sufficient for suppressor function of CD4+ Tregs and Teffs, as blockade of CTLA-4 with monoclonal antibodies preserved the expression of CD80/CD86 on APCs and enhanced costimulatory function of T cells.
Together, these studies demonstrated that CTLA-4 expressed by Teff acts during primary CD4+ clonal expansion in a cell extrinsic suppressive manner. One potential cell extrinsic CTLA-4 mechanism of coinhibition is the induction of IDO expression in APCs mediated through B7 signals, which inhibits T cell responses by limiting tryptophan availability (25). However, outcomes beyond inhibiting proliferation – including limiting effector functions – remain to be shown. As well, CD8+ effectors and memory cells upregulate CTLA-4 following TCR engagement, and it remains to be seen whether CTLA-4 on CD4+ or CD8+ cells limits expansion of CD8+ T cells. While CTLA-4 is recognized as a critical attenuator of alloreactive T cell responses, strategies to maximize cell extrinsic CTLA-4 function in settings of transplantation have not been fully explored. For example, strategies aimed at transiently inducing cellular expression of CTLA-4 at the time of transplantation could effectively inhibit alloreactive T cell responses.
CTLA-4 as T Cell:APC Conjugation Inhibitor
Recently an entirely novel mechanism of CTLA-4 inhibition of T cell activation has been elucidated for CD4+ T effector (Teff) cells involving the control T cell:APC conjugation. T cell activation requires a sustained stable conjugation between the T cell and cell presenting its cognate antigen. In the absence of cognate antigen, T cells rapidly scan antigen-presenting cells in search of cognate antigen. Recognition of a TCR and its cognate pMHC induces expression of molecules that enable the T cell to arrest its motion and facilitate sustained conjugation. Seminal work from Schneider and colleagues demonstrated that CTLA-4 plays an important role in reversing the TCR-mediated stop signals that occurs when a T cell encounters peptide:MHC complex (26). Using two-photon microscopy, this groups showed that CTLA-4 negative cells increased their contact times upon contacting antigen while CTLA-4 positive cells were unable to increase APC dwell times. These observations corroborate earlier observations that CTLA-4 is able to associate with the immunological synapse, and provide another distinct cell intrinsic mechanism of action for CTLA-4.
CTLA-4 on Tregs
The CD4+ T cell compartment is comprised of pro-inflammatory effector as well as anti-inflammatory suppressor cells, the best characterized of which are CD4+CD25+FoxP3+ regulatory T cells. FoxP3+ Tregs play a critical role in maintaining self-tolerance in models of autoimmunity and transplantation (27). Prior to the discovery of FoxP3 as the master regulator of CD4+CD25+ suppressor function, CTLA-4 was shown to be constitutively expressed on Tregs (28-30). The identification of FoxP3 as a master factor of Treg function revealed that CTLA-4 is in fact a transcriptional target, leading to the investigation of CTLA-4 in FoxP3+ Tregs (31).
While experiments using CTLA-4 blockade in vivo and in vitro produced conflicting results, more recent work has established that CTLA-4 is required for Treg-mediated suppression of immune responses (31). In a FoxP3+ conditional knockout mouse model of CTLA-4 deletion, Wing et al. definitively demonstrated that CTLA-4 deficiency in FoxP3+ Treg cells is sufficient to allow the development of spontaneous and fatal autoimmunity, reminiscent of CTLA-4 KO mice (32). Conditional deletion of CTLA-4 on Tregs also leads to enhanced tumor immunity and an enhanced allogeneic proliferative response (32). Recently, several groups have shown that reduced expression of CTLA-4 on Tregs leads to dysfunctional Treg function in vitro and in vivo (7, 8, 33). Similarly, suppression by human T cells requires CTLA-4 but not FoxP3 (34). Recently, Zhang et al showed that CD28-deficient Tregs were unable to maintain self-tolerance (33). CD28-deficient Tregs had diminished CTLA-4 expression and fail to survive following a skin graft challenge in the presence of CD28 sufficient Tregs.
While these studies have established that CTLA-4 is required for Treg mediated immunosuppression, it remains to be seen whether the suppressive effect of Tregs is mediated by solely by high CTLA-4 expression or whether CTLA-4 has a unique biochemical properties when expressed on Tregs. Many studies have provided evidence of the former scenario, that CTLA-4 expression on non-FoxP3+ Tregs is sufficient to induce a suppressor function. For example, Tai et al. demonstrated that activated Teffs had in vitro suppressive capacity similar to Tregs, and both cell types required the extracellular, but not the intracellular, CTLA-4 domains (21). Studies examining cell extrinsic suppressive function of CTLA-4 have thus far not identified a unique role for CTLA-4 on Tregs (22, 23). This is further supported by the finding that Teffs and Tregs demonstrate a similar ability to trans-endocytose CD80/CD86 molecules via CTLA-4 (24). Together, these findings suggest that suppression is a function of high CTLA-4 expression, not a unique property of CTLA-4 when expressed on Tregs, as evident by the fact that high CTLA-4 expression can induce suppressive function on otherwise effector T cells.
Interestingly, recent work from Rudd and colleagues has demonstrated a divergent function of CTLA-4 on Tregs and Teffs that is independent of expression level (35). Using two-photon microscopy in an antigen-specific system to monitor T cell migration, the investigators were able to sensitively detect the conjugation patterns and kinetics of T cell:APC conjugates. While the presence of CTLA-4 limited the conjugation time with APCs for CD4+ Teffs, Tregs were resistant to CTLA-4-mediated reverse stop signals. This study supports a model in which APC conjugation of Teffs is limited by CTLA-4, while longer conjugation times of Tregs are better able to occupy APC binding sites. While this striking finding is consistent with the function of Tregs as occupying antigen binding sites on APCs and out-competing Teffs for costimulatory receptors, it indicates that the reverse stop potential of a cell does not rely solely on the CTLA-4 expression level. This work is the most thorough demonstration that CTLA-4 might have a different functional role on Tregs compared to non-Tregs, and future studies will examine the mechanistic basis for this result, whether due to a biochemical property of CTLA-4 expressed by Tregs and Teffs, or the presence of additional molecules that can override CTLA-4 function.
CTLA-4 on Th17 cells
Th17 cells are a pro-inflammatory CD4+ lineage that provides immunity to fungi, including Candida albicans, and extracellular bacteria. Th17 cells can be potent mediators of pathogenic T cell responses in autoimmune disease, and have been shown to participate in graft rejection in several animal models and in human studies (36, 37).
Recently it has been demonstrated that Th17 cells are resistant to CD28/CTLA-4 blockade with CTLA-4 Ig in vitro and in vivo (4, 38, 39). Human and murine Th17 cells express high levels of CTLA-4 compared to primary and memory Th1 cells (4, 39). In an antigen-specific pathogen immunization model, murine Th17 cells also expressed more CTLA-4 than Th1 cells and correlated with CTLA-4 Ig resistant skin graft rejection (39). Interestingly, the expression level of CTLA-4 correlated to the level of augmentation of proliferation in the presence of CTLA-4 blockade with antibodies, suggesting that CTLA-4 acts in a cell intrinsic manner on Th1 and Th17 cells. Consistent with the finding that Th17 cells are reliant on CTLA-4 signals, Ying et al found that CD28−/− Th17 cells were augmented by CTLA-4 blockade with CTLA-4 Ig (40).
Interestingly, costimulation blockade with the CTLA-4 Ig derivative belatacept has demonstrated mixed efficacy in Th17 mediated disease (1). Renal transplant recipients who experience rejection while being treated with belatacept, but not calcineurin inhibitors, had an elevated frequency of Th17 memory cells in their blood, suggesting that Th17 memory cells participate in graft rejection in the setting of CD28/CTLA-4 blockade therapy (4). Together, these studies suggest that CTLA-4 Ig, which was designed to induce deletion and anergy through blockade of CD28 signals, might have unintended consequences on specific T cell populations through blockade of endogenous coinhibitory signals.
Future Directions and Challenges
CTLA-4 has been established as a critical regulator of peripheral tolerance, and offers tremendous therapeutic potential as a target that can be harnessed to limit alloreactive T cell responses. In addition to the traditional model of CTLA-4 function, recent studies outlined here have broadened our understanding of the mechanisms of action of CTLA-4 coinhibition. An updated “new” model of CTLA-4 function can be segregated into three components: 1.) CTLA-4 can inhibit cell autonomous T cell activation, 2.) CTLA-4 expressed on FoxP3+ Tregs and Teffs can act in a cell extrinsic manner to suppress T cell responses 3.) CTLA-4 has distinct expression patterns on T cell subsets with functional consequences. Perhaps the greatest challenge facing our complete understanding of CTLA-4 biology is that none of these modes of action are mutually exclusive. As such, more work is needed to elucidate the contexts under which each of these mechanisms do or do not occur.
For investigators, the most salient conceptual dichotomy is how to reconcile cell-intrinsic and cell-extrinsic CTLA-4 functions. As these studies were largely temporally separated – with earlier studies focused on traditional biochemical cell autonomous functions of CTLA-4 and more recent studies elucidating cell extrinsic functions -- more careful investigation of the potential interplay of these two phenomena is needed. For example, do cell-intrinsic and -extrinsic functions occur simultaneously? Do they occur preferentially in particular T cell subsets? Are there synergistic or antagonistic effects of these mechanisms on T cell activation?
Additionally, regarding the cell extrinsic role of CTLA-4, further work needs to elucidate the differences between Teffs and FoxP3+ Tregs. While it is well established that Tregs require CTLA-4 for suppression, it remains unclear whether the function of CTLA-4 is critically different on Tregs than Teffs. Several groups have now shown that the expression of CTLA-4 is sufficient for any CD4+ T cell to have a suppressive function, while work from Lu et al. provides the most compelling subset-specific functional difference to date. The goal of costimulation-blockade based biologic therapies includes limiting effector T cell populations while retaining functional Tregs, greater understanding of distinctions of CTLA-4 function on these populations is critical.
In conclusion, our understanding of “the original” T cell coinhibitory receptor has deepened substantially in recent years. The updated model of CTLA-4 demonstrates the importance of continued mechanistic investigations of established therapeutic pathways, as new understanding can lead to further advances in clinical applications. In the context of the higher-than-anticipated acute rejection rates observed in belatacept-based regimens for renal transplant patients, these new insights suggest that perhaps perturbations in the cell-extrinsic functions of CTLA-4 or Th17 cells might be playing a role. While many challenges remain to unify the model of CTLA-4 coinhibition, it remains a therapeutic target with tremendous promise to effectively limit alloreactive T cells.
Figure 2. Novel mechanisms of CTLA-4 function.
(A) CTLA-4 expression levels are different on Th1, Th17, and FoxP3+ Treg cells. (B) CTLA-4 expressed on T cells functions in a non-cell autonomous manner to limit the proliferation of other T cells. (C) T cells continuously scan antigen presenting cells for cognate peptide:MHC complexes. Upon binding peptide:MHC binding, T cells arrest this scanning motion, termed a stop signal. The presence of CTLA-4 can block this arrest, termed the reverse stop-signal. (D) CTLA-4 can eliminate the expression of B7 molecules on antigen presenting cells through trans-endocytosis.
Acknowledgements
The authors are supported by The Roche Organ Transplant Research Foundation (M.L.F.), R56 AI081789-01 (M.L.F.), R01 AI073707 (M.L.F.), R01 AI104699 (M.L.F.), T32 AI007610-11 (S.M.K.), T32 GM08169-23 (S.M.K.), T32A1070081 (S.M.K.), and F30DK098928-01 (S.M.K.).
Abbreviations
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- B7
CD80 and CD86
- APC
antigen presenting cell
- OVA
ovalbumin
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009 May;229(1):307–21. doi: 10.1111/j.1600-065X.2009.00780.x. PubMed PMID: 19426230. [DOI] [PubMed] [Google Scholar]
- 2.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011 May;241(1):180–205. doi: 10.1111/j.1600-065X.2011.01011.x. PubMed PMID: 21488898. Pubmed Central PMCID: 3077803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996 Jun 1;183(6):2541–50. doi: 10.1084/jem.183.6.2541. PubMed PMID: 8676075. Pubmed Central PMCID: 2192609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummey SM, Cheeseman JA, Conger JA, Jang PS, Mehta AK, Kirk AD, et al. High CTLA-4 expression on Th17 cells results in increased sensitivity to CTLA-4 coinhibition and resistance to belatacept. Am J Transplant. 2014 Mar;14(3):607–14. doi: 10.1111/ajt.12600. PubMed PMID: 24730049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. PubMed PMID: 16551244. [DOI] [PubMed] [Google Scholar]
- 6.Finn PW, He H, Wang Y, Wang Z, Guan G, Listman J, et al. Synergistic induction of CTLA-4 expression by costimulation with TCR plus CD28 signals mediated by increased transcription and messenger ribonucleic acid stability. J Immunol. 1997 May 1;158(9):4074–81. PubMed PMID: 9126965. [PubMed] [Google Scholar]
- 7.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010 Jul;11(7):618–27. doi: 10.1038/ni.1884. PubMed PMID: 20467422. [DOI] [PubMed] [Google Scholar]
- 8.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009 Feb;10(2):176–84. doi: 10.1038/ni.1689. PubMed PMID: 19136962. Pubmed Central PMCID: 2856471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walunas TL, Bluestone JA. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J Immunol. 1998 Apr 15;160(8):3855–60. PubMed PMID: 9558090. [PubMed] [Google Scholar]
- 10.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997 Apr;6(4):411–7. doi: 10.1016/s1074-7613(00)80284-8. PubMed PMID: 9133420. [DOI] [PubMed] [Google Scholar]
- 11.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. PubMed PMID: 11244047. [DOI] [PubMed] [Google Scholar]
- 12.Judge TA, Wu Z, Zheng XG, Sharpe AH, Sayegh MH, Turka LA. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol. 1999 Feb 15;162(4):1947–51. PubMed PMID: 9973463. [PubMed] [Google Scholar]
- 13.Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998;188(1):199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariyan C, Salvalaggio P, Fecteau S, Deng S, Rogozinski L, Mandelbrot D, et al. Cutting edge: transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003 Dec 1;171(11):5673–7. doi: 10.4049/jimmunol.171.11.5673. PubMed PMID: 14634073. [DOI] [PubMed] [Google Scholar]
- 15.Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat Immunol. 2001 Jan;2(1):58–63. doi: 10.1038/83175. PubMed PMID: 11135579. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Krummey SM, Badell IR, Wagener M, Schneeweis LA, Stetsko DK, et al. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft- specific CD8+ T cell responses. J Exp Med. 2014 Feb 10;211(2):297–311. doi: 10.1084/jem.20130902. PubMed PMID: 24493803. Pubmed Central PMCID: 3920565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Fresnay S, Welty E, Sangrampurkar N, Rybak E, Zhou H, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011 Aug;11(8):1599–609. doi: 10.1111/j.1600-6143.2011.03624.x. PubMed PMID: 21749640. Pubmed Central PMCID: PMC3158027. Epub 2011/07/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270(5238):985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann MF, Waterhouse P, Speiser DE, McKall-Faienza K, Mak TW, Ohashi PS. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J Immunol. 1998 Jan 1;160(1):95–100. PubMed PMID: 9551960. [PubMed] [Google Scholar]
- 20.Sevilla N, Homann D, von Herrath M, Rodriguez F, Harkins S, Whitton JL, et al. Virus-induced diabetes in a transgenic model: role of cross-reacting viruses and quantitation of effector T cells needed to cause disease. J Virol. 2000 Apr;74(7):3284–92. doi: 10.1128/jvi.74.7.3284-3292.2000. PubMed PMID: 10708445. Pubmed Central PMCID: 111829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012 May 31;119(22):5155–63. doi: 10.1182/blood-2011-11-388918. PubMed PMID: 22403258. Pubmed Central PMCID: 3369608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corse E, Allison JP. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J Immunol. 2012 Aug 1;189(3):1123–7. doi: 10.4049/jimmunol.1200695. PubMed PMID: 22753941. [DOI] [PubMed] [Google Scholar]
- 23.Wang CJ, Kenefeck R, Wardzinski L, Attridge K, Manzotti C, Schmidt EM, et al. Cutting edge: cell-extrinsic immune regulation by CTLA-4 expressed on conventional T cells. J Immunol. 2012 Aug 1;189(3):1118–22. doi: 10.4049/jimmunol.1200972. PubMed PMID: 22753931. Pubmed Central PMCID: 3442233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011 Apr 29;332(6029):600–3. doi: 10.1126/science.1202947. PubMed PMID: 21474713. Pubmed Central PMCID: 3198051. Epub 2011/04/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hainz U, Jurgens B, Heitger A. The role of indoleamine 2,3-dioxygenase in transplantation. Transpl Int. 2007 Feb;20(2):118–27. doi: 10.1111/j.1432-2277.2006.00370.x. PubMed PMID: 17239019. [DOI] [PubMed] [Google Scholar]
- 26.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006 Sep 29;313(5795):1972–5. doi: 10.1126/science.1131078. PubMed PMID: 16931720. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009 Mar 20;30(3):458–69. doi: 10.1016/j.immuni.2008.12.022. PubMed PMID: 19303390. Pubmed Central PMCID: 2737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000 Apr;12(4):431–40. doi: 10.1016/s1074-7613(00)80195-8. PubMed PMID: 10795741. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000 Jul 17;192(2):303–10. doi: 10.1084/jem.192.2.303. PubMed PMID: 10899917. Pubmed Central PMCID: 2193248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000 Jul 17;192(2):295–302. doi: 10.1084/jem.192.2.295. PubMed PMID: 10899916 Pubmed Central PMCID: 2193261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011 Dec;11(12):852–63. doi: 10.1038/nri3108. PubMed PMID: 22116087. [DOI] [PubMed] [Google Scholar]
- 32.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008 Oct 10;322(5899):271–5. doi: 10.1126/science.1160062. PubMed PMID: 18845758. Epub 2008/10/11. eng. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013 Feb 1;123(2):580–93. doi: 10.1172/JCI65013. PubMed PMID: 23281398. Pubmed Central PMCID: 3561819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Manzotti CN, Burke F, Dussably L, Qureshi O, Walker LS, et al. Acquisition of suppressive function by activated human CD4+ CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol. 2008 Aug 1;181(3):1683–91. doi: 10.4049/jimmunol.181.3.1683. PubMed PMID: 18641304. Pubmed Central PMCID: 2758479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Schneider H, Rudd CE. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood. 2012 Nov 29;120(23):4560–70. doi: 10.1182/blood-2012-04-421420. PubMed PMID: 23047820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riella LV, Sayegh MH. T-cell co-stimulatory blockade in transplantation: two steps forward one step back! Expert Opin Biol Ther. 2013 Nov;13(11):1557–68. doi: 10.1517/14712598.2013.845661. PubMed PMID: 24083381. [DOI] [PubMed] [Google Scholar]
- 37.Burrell BE, Bishop DK. Th17 cells and transplant acceptance. Transplantation. 2010 Nov 15;90(9):945–8. doi: 10.1097/TP.0b013e3181f5c3de. PubMed PMID: 20838278. Pubmed Central PMCID: 3191917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouguermouh S, Fortin G, Baba N, Rubio M, Sarfati M. CD28 co-stimulation down regulates Th17 development. PLoS One. 2009;4(3):e5087. doi: 10.1371/journal.pone.0005087. PubMed PMID: 19333372 Pubmed Central PMCID: PMC2658739. Epub 2009/04/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krummey SM, Floyd TL, Liu D, Wagener ME, Song M, Ford ML. Candida-elicited murine Th17 cells express high Ctla-4 compared with Th1 cells and are resistant to costimulation blockade. J Immunol. 2014 Mar 1;192(5):2495–504. doi: 10.4049/jimmunol.1301332. PubMed PMID: 24493820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, et al. Cutting edge: CTLA-4--B7 interaction suppresses Th17 cell differentiation. Journal of immunology. 2010 Aug 1;185(3):1375–8. doi: 10.4049/jimmunol.0903369. PubMed PMID: 20601598. Pubmed Central PMCID: 2915549. Epub 2010/07/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]