Abstract
Mitochondrial disorders are the most common inborn errors of metabolism affecting the oxidative phosphorylation system (OXPHOS). Because the poor knowledge of the pathogenic mechanisms, a cure for these disorders is still unavailable and all the treatments currently in use are supportive more than curative. Therefore, in the past decade a great variety of mouse models have been developed to assess the in vivo function of several mitochondrial proteins involved in human diseases. Due to the genetic and physiological similarity to humans, mice represent reliable models to study the pathogenic mechanisms of mitochondrial disorders and are precious to test new therapeutic approaches. Here we summarize the features of several mouse models of mitochondrial diseases directly related to defects in subunits of the OXPHOS complexes or in assembly factors. We discuss how these models recapitulate many human conditions and how they have contributed to the understanding of mitochondrial function in health and disease.
Keywords: Mitochondria, oxidative phosphorylation, OXPHOS, mouse models, mitochondrial diseases
1. Introduction
Mitochondria have a crucial role in energy production in eukaryotic cells, providing the primary energy source in the cells, ATP, by the oxidative phosphorylation system (OXPHOS). This system is composed of five functionally coupled multi-protein complexes (CI-CV) embedded in the inner mitochondrial membrane and of two mobile electron carriers, coenzyme Q (CoQ10) and cytochrome c (Cytc). The OXPHOS system couples the electron flow and proton translocation across the mitochondrial inner membrane generating the electrochemical gradient necessary for ATP synthesis. The OXPHOS complexes are composed by subunits encoded by the nuclear DNA (nDNA) and the mitochondrial DNA (mtDNA). The exception of the dual genetic origin is complex II, which is entirely encoded by the nDNA. Hence, the biosynthesis of the OXPHOS complexes requires coordination on the expression of both nuclear and mitochondrial-encoded proteins, import of those encoded in the nucleus to the mitochondria and the assembly and addition of prosthetic groups. This biosynthetic process requires a series of chaperones better known as assembly factors, which tend to be specific for each complex and do not form part of the final enzyme (Fernandez-Vizarra, 2009). Defects in the OXPHOS system result in a heterogeneous group of pathologies and metabolic syndromes, commonly known as “mitochondrial diseases”. Mitochondrial disorders can rise from mutations either in nDNA or mtDNA resulting in a compromised ATP synthesis and/or chronic oxidative stress. The most affected organs are those with high energy demands such as heart, skeletal muscle and brain but also other tissues may be affected, partially explaining the broad clinical spectrum that includes neurodegeneration and/or muscular weakness, in children and adults (DiMauro and Schon, 2008). To date, hundreds of mutations, either point mutations or large-scale rearrangements, have been found in the mtDNA (www.mitomap.org) and more than 1,000 nuclear genes have been involved in disease-causing mutations (MitoCarta human inventory, Broad Institute). Of these nDNA mutations identified, beside those encoding for subunits of the respiratory complexes, many are related to their respective assembly factors (Diaz, 2011).
Because of the numerous genes involved in the development of the disease; the dual genetic control; the wide variety of the clinical symptoms and the variable onset of the disease, finding a diagnosis for a mitochondrial disease is often challenging.
Effective treatments for mitochondrial disorders still unavailable, mostly due to the poor knowledge of the pathological mechanisms underlying these diseases. For this reason, in the last decade, an effort has been made by the scientific community to develop mouse models to improve our knowledge of the pathophysiology of mitochondrial disorders and to provide a platform for testing therapeutic interventions. Due to the extensive information and number of models created in the last few years, we decided to create a miniseries review comprised of three parts. Part I (this chapter) focuses on those mice models directly related to components of the OXPHOS system, mitochondrial complexes subunits and assembly factors necessary for proper respiratory complexes biogenesis. Part II focuses on the defects caused by mutations in factors that are not part of the OXPHOS system but are required for its function such as those factors involved in mtDNA maintenance, replication, transcription, translation, mitochondrial dynamics and mitochondrial protein quality control. Part III of the review, focuses on the therapeutic interventions that have been tested on several of the mouse models described in parts I and II. We summarized the main features of the animal models and described how they recapitulate human pathogenic phenotypes and how they have advanced our knowledge on the pathological mechanisms of disease. Figure 1 summarizes the animal models described in the text for each respiratory complex or assembly factor.
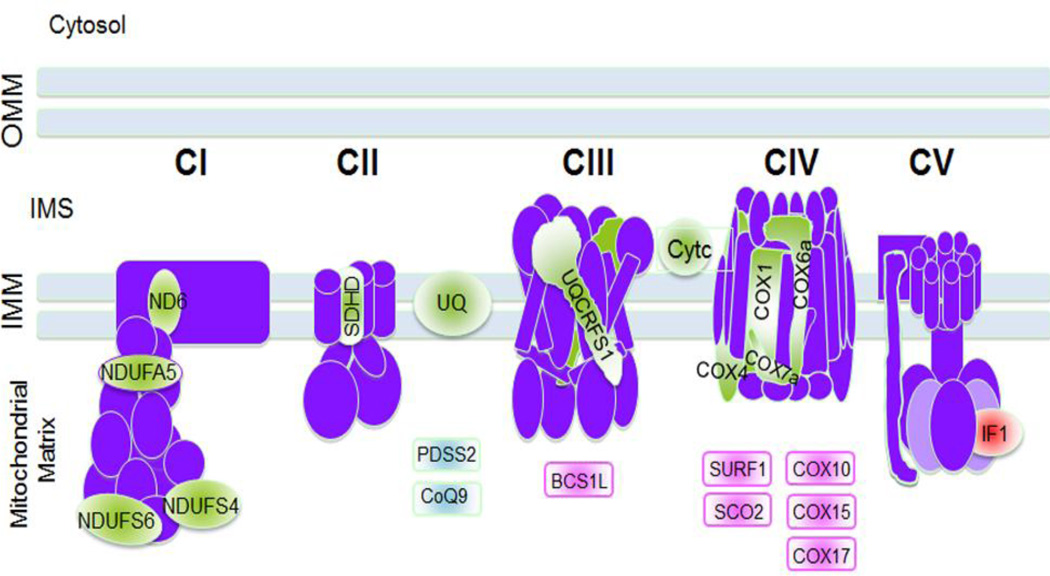
Figure 1. Mouse models of mitochondrial diseases.
Mouse models with defects in OXPHOS complexes subunits, electron carriers and assembly factors are summarized in the figure. In light green are represented the mouse models related to subunits of the electron transport chain (ND6, NDUFA5, NDUFS4, NDUFS6, SDHD, UQCRFS1, COX1, COX4, COX6a and COX7a), the electron carriers ubiquinone (UQ) or CoQ and cytochrome c (Cytc). In light blue are the mouse models of enzymes of the CoQ biosynthetic pathway (PDSS2 and CoQ9). In light pink are represented those mouse models of assembly factors for CIII and CIV (BCS1L, SURF1,SCO2, COX10, COX15, and COX17) and in red the mouse models of IF1, the natural inhibitor of CV.
OMM: outer mitochondrial membrane, IMS: inter membrane space; IMM: inner mitochondrial membrane
2. Mouse models of Complex I deficiency
Mammalian complex I (CI, NADH: ubiquinone oxidoreductase) is the largest enzyme of the OXPHOS system with an estimated size of 1 MDa. It is an L-shaped multi-protein complex and consists of at least 44 subunits, 38 of which are encoded by the nuclear DNA and the rest by the mitochondrial genome (Hirst, 2003). CI deficiency is the most frequent cause of mitochondrial defects. The majority of the mutations affect genes encoding for structural subunits, but lately, a growing number of genes, encoding for assembly factors of the enzyme, have been found mutated in patients with severe neurological diseases (Mckenzie and Ryan, 2010; Pagniez-Mammeri, 2012). Isolated CI defects are associated with a wide variety of clinical phenotypes ranging from Leigh syndrome (LS) or infantile subacute necrotizing encephalomyelopathy, fatal infantile lactic acidosis, leukodystrophy, cardiomyopathy, hepatopathy, tubulopathy and optic neuropathy (Bugiani, 2004; Ngu, 2012; Ogilvie, 2005; Swalwell, 2011). Such diversity of phenotypes makes even more urgent the need for generating mouse models mirroring the broad spectrum of clinical outcomes to study pathogenic mechanisms in detail and to design specific therapies. Table 1 shows a summary of the various mouse models with defects in complex I.
Table I.
Mouse models of Complex I deficiency
| Gene | Mitochondrial function |
Genetic Manipulation | Phenotype | References |
|---|---|---|---|---|
| Ndufs4 | Assembly/stability of CI |
Knockout: -Whole body (Mox2-Cre) |
Leigh-like, blindness, ataxia, retarded growth rate, lethargy |
(Kruse, 2008) |
| Knockout: -Whole body (transposable element) |
Neurological impairment |
(Leong, 2012) | ||
| Conditional knockout: -Neurons and glia (Nestin-Cre and local injection of AAV Cre) |
Fatal progressive encephalopathy, ataxia, glial reactivity and neuronal loss; breathing dysfunction |
(Quintana, 2010; Quintana, 2012) |
||
| -Dopaminergic neurons (Dat-Cre) |
No overt neurodegeneration |
(Sterky, 2012) | ||
| -Heart/skeletal-muscle (Mck-Cre) |
Cardiomyopathy | (Sterky, 2012) | ||
| -Heart (αMhc-Cre) | Cardiomyopathy induced after stress condition |
(Karamanlidis, 2013) | ||
| Knock-in: (Stop codon) |
Embryonic lethality | (Ingraham, 2009) | ||
| Ndufs6 | Involved in the activity of CI |
Knock-down: -whole body (gene trap) |
Cardiomyopathy, systolic dysfunction |
(Ke, 2012) |
| Renal disease, with altered ultrastructure and release of kidney damage biomarkers in urine |
(Forbes, 2013) | |||
| Ndufa5 | Unknown function (accessory subunit that is inserted at a late stage in the assembly process of CI) |
Knockout: -Whole body (gene trap) |
Embryonic lethality | (Peralta, 2013) |
| Conditional knockout: - Neurons (CaMKIIα-Cre) |
Partial defect of CI in neurons that leads to lethargy and loss of motor skills at 10 months. |
(Peralta, 2013) |
2.1. Ndufs4
NDUFS4 encodes a small protein of about 18 kDa, essential for CI assembly and stability. It is inserted at a late stage of CI biogenesis (Antonicka, 2003c; Lazarou, 2007) and mutations in this gene are responsible of LS or Leigh-like disease (Bénit, 2003; Budde, 2000; Petruzzella, 2001; van den Heuvel, 1998). The first Ndufs4 knockout (KO) mouse was created by ablating floxed exon 2 in the germ line using the Mox2-Cre transgenic mice (Kruse, 2008). The Ndufs4−/− mice developed a Leigh-like phenotype characterized by ataxia, blindness, retarded growth rate and lethargy leading to premature death at about 7 weeks of age. CI abundance and activity were variably reduced in several tissues, being almost undetectable in liver and in the central nervous system (CNS). In contrast, CI-dependent oxygen consumption in the KO mouse was reduced to about 50% of control levels in skeletal muscle (Kruse, 2008). This marked variation in CI levels in different tissues suggested that the Ndufs4−/− mice may have died from the loss of neuronal function during development. To test this hypothesis, a conditional Ndufs4 KO in neurons and glia, using a Nestin-Cre recombinase was generated (Quintana, 2010). The NesKO mice developed a phenotype that completely overlapped the one of Ndufs4−/− mice and recapitulated several LS hallmarks. Analysis of brain sections showed vacuolization in some areas of the brainstem and cerebellum and some hemorrhagic foci in the brainstem and midbrain. Reactive glia was also evident as a result of neuronal loss. A striking difference between patients and Ndufs4−/− mice was the absence of alterations in the basal ganglia of the KO mice, likely due to the low expression of Cre in this brain region. Further analysis of the Ndufs4 KO mouse model demonstrated that the neuronal loss in the CNS, particularly in the ventral brain stem, resulted in an aberrant breathing rhythm and contributed to the premature death (Quintana, 2012). Selective ablation of Ndufs4 in the vestibular nucleus of adult mice using adeno-associated virus (AAV) expressing Cre resulted in neuronal loss and severe microglia activation followed by respiratory failure and death. Local injection of AAV-Ndufs4 in the affected brain area partially restored brain functionality by reducing gliosis and lesions. This turned into an improved respiratory capacity and increased lifespan of the injected KO mice (Quintana, 2012).
Conditional deletion of Ndufs4 in heart led to a milder phenotype characterized by cardiomyopathy without heart failure (Sterky, 2012). The resulting mice lived longer than the complete KO and looked healthy up to 1 year of age. Interestingly, there was a severe CI deficiency in disrupted but not in intact mitochondria. The mutant CI, although unstable, was able of form supercomplexes with CIII and CIV. The complex lacking NDUFS4 associated into supercomplexes and displayed dehydrogenase activity (Sterky, 2012). Karamanlidis and colleagues that selectively knocked out Ndufs4 in the heart observed the same mild phenotype. Despite the severe CI impairment in the heart, the KO mice had normal lifespan and cardiac function up to 30 months of age (Karamanlidis, 2013). The only significant pathological trait was a small increase of the left ventricular posterior wall thickness. Nevertheless, when challenged by transverse aortic constriction, Ndufs4 KO mice developed heart failure. The pathomechanism proposed involved the alteration of NAD+/NADH ratio, caused by the CI defect, which in turn inhibited the activity of some deacetylases, such as Sirt3, causing hyperacetylation of target proteins. Increased protein acetylation would induce cell death by increasing the sensitivity of the mitochondrial permeability transition pore (mPTP) (Karamanlidis, 2013). The variable CI deficiency in tissues observed in the systemic inactivation of Ndufs4 was partially clarified by performing biochemical studies on multiple tissues of Ndufs4−/− mice (Calvaruso, 2012). Despite the absence of a fully assembled CI, a residual activity of the enzyme was always present and ranged from 25 to 45% of controls in kidney, brain, skeletal muscle and from 9 to 20% in lung, liver and pancreas. In gel activity assays on Blue Native gels (BN-IGA) revealed in some tissues the presence of subcomplexes of CI. These subcomplexes once stabilized by associating with complex III (CIII), produced a supercomplex that retained the enzymatic activity of CI (Calvaruso, 2012).
CI defects have been also related to Parkinson’s disease (PD), an aging-related neurodegenerative disorder characterized by the loss of the dopaminergic (DA) neurons of the substantia nigra pars compacta in the midbrain (Olanow and Tatton, 1999). Several studies have shown that when animals are exposed to CI inhibitors, like rotenone, they developed PD-like phenotype (Pan-Montojo, 2010). To address whether CI inhibition is sufficient to induce features of PD in the animals, a conditional deletion of Ndufs4 in dopaminergic neurons was achieved using DAT-Cre. The DA neurons of the DAT-KO dysplayed an altered metabolism and release of dopamine and increased toxicity to MPP+ suggesting that CI impairment may contribute to the development of PD (Sterky, 2011). Since the mechanism has not been described yet, the exact role of CI deficiency in the development of PD is still a matter of debate. Indeed, other studies have argued against a direct role of CI defect in PD, as observed in the substantia nigra of patients with mtDNA maintenance disorders (Palin, 2013). On the other hand, a recent study has demonstrated an indirect role for CI dysfunction in PD, proposing that a defective PINK1 activity prevents the phosphorylation of the NDUFA10 subunit, leading to CI loss of function, as observed in Pink1−/− murine cells and in cells derived from PD patients with Pink1 mutations (Morais, 2014).
Another Ndufs4 KO mouse model was generated by spontaneous transposable element insertion within the gene that produced a premature stop codon that resulted in an unstable transcript (Leong, 2012). CI activity was reduced in all tissue of the KO mice and the protein was undetectable in those with high-energy demand. Compared to wild type littermates, the KO mice were smaller at birth, lost their fur at two weeks of age, displayed neurological impairment at 5 weeks and died prematurely at about 7 weeks. These mice showed also metabolic abnormalities, such as increased levels of hydroxyacylcarnitines, glycine, phenylalanine and homocitrulline. According to the authors, while increased glycine, phenylalanine and homocytrulline did not impact the overall phenotype of the KO mice, the increased levels of hydroxyacylcarnitines may suggest a block of the fatty acid β-oxidation at the conversion of 3-hydroxyacylcoenzyme A into the corresponding acylcarnitine species (Leong, 2012).
A Ndufs4 knock-in mouse was generated by introducing a mutation in Ndufs4 that produced a premature stop codon in the protein. Homozygous knock-in mice were embryonic lethal and the heterozygous mice showed a partial phenotype (Ingraham, 2009), making them suitable models for studies of mild CI deficiency. Heterozygous mice showed a reduction of CI activity of about 30% in heart, brain and skeletal muscle mitochondria and elevated levels of lactate in brain and heart, as observed in NDUFS4 deficient patients (Anderson, 2008; Budde, 2003; Ingraham, 2009). The reason why the knock-in mouse was embryonic lethal whereas all the Ndufs4 KO mice generated were viable, at least in the first weeks of their life, may be explained by a possible dominant-negative effect created by the point mutation. The mutation in the knock-in mouse creates a stop codon that results in the expression of a truncated Ndufs4 protein of about 14.4 kDa lacking the last 10–15 amino acids. The truncated protein is expressed at low levels and is incorporated into the fully assemble CI in heterozygous KI mouse heart mitochondria resulting in significant complex deficiency (Ingraham, 2009). Further studies are required to determine if the truncated NDUFS4 indeed has a dominant-negative effect.
The effort on generating of such a large number of Ndufs4 mouse models underlines the importance of this subunit in CI function and regulation. NDUFS4 is considered a mutational “hot spot” for LS and Leigh-like disease. Understanding the pathobiological mechanisms will be useful for the development and experimentation of therapeutic and pharmacological treatments for these diseases with fatal outcomes.
2.2. Ndufs6
NDUFS6 is a conserved subunit of CI and is part of the enzymatic core of the complex (Fearnley and Walker, 1992; Hirst, 2003). Mutations in NDUFS6 have been linked to a severe phenotype that affects newborns and leads to death in the first weeks of life (Kirby, 2004; Spiegel, 2009). Common features of patients with mutations in NDUFS6 include severe metabolic acidosis and a consistent reduction of CI activity. Additional signs may include myopathy and encephalopathy.
Ndufs6 has been recently knocked down in mice using the gene-trap (gt) embryonic cell line technique. Expression of Ndufs6 was variably reduced in different tissues, being almost undetectable in the heart, the most compromised tissue (Ke, 2012). Biochemical and structural analysis showed an isolated defect of CI with a reduction in the fully assembled holoenzyme. The homozygous mice for the genetrap Ndufs6gt/gt mice displayed cardiomyopathy, systolic dysfunction and a reduction in functional heart capacity. Males were more affected than females. They worsened earlier, around 4 months after birth, and died shortly after. Interestingly, sex bias has also been observed in patients with certain OXPHOS defects (Yaplito-Lee, 2007), suggesting a hormonal involvement in the progression of the disease. CI dysfunction was evident also in the kidneys of both homozygous and heterozygous mice (Ndufs6gt/gt and Ndufs6gt/+) with different degree of deficiency (32% and 83% residual activity, respectively). Homozygous mice showed also a mild renal impairment, starting during the first days of life and becoming more pronounced during adulthood. An altered glomerular ultrastructure was evident both in Ndufs6gt/gt and in the Ndufs6gt/+, and was paralleled by an increase in the levels of specific markers of glomerular and tubular damage in urine. These data clearly indicate that some organs, such as kidney, may be so dependent of energy supply that they manifest a malfunction even when presenting a mild biochemical defect (Forbes, 2013). Kidney malfunction has also been observed in the Pdss2 mouse (Hallman, 2006) with defective CoQ10 biosynthesis (see section 4.1) and in a mouse with a proviral insertion in the Mpv17 gene leading to mtDNA depletion (Weiher, 1990). However, renal impairment is not a feature observed in patients with mutations in NDUFS6 who die soon after birth of severe metabolic acidosis (Spiegel, 2009). Due to the short life of patients, we cannot exclude that they would have developed renal dysfunction over time. A low percentage of patients with mitochondrial disease have altered kidney function (Emma, 2012; Martin-Hernandez, 2005; Niaudet and Rotig, 1997). Mutations in the mtDNA (Guillausseau, 2001; Iwasaki, 2001; Majander, 1991; Rotig, 1995) , mutations in nuclear genes encoding for OXPHOS assembly factors (de Lonlay, 2001; Valnot, 2000) and CoQ10 biosynthetic pathway genes (Diomedi-Camassei, 2007; Heeringa, 2011; Lopez, 2006), have been associated with kidney malfunction.
2.3. Ndufa5
NDUFA5 is a nuclear encoded structural subunit of CI, and ubiquitously expressed in mammals (Carroll, 2006). To date, no mutations in NDUFA5 have been reported in patients, most likely due to its crucial role (Rak and Rustin, 2014), suggesting not being compatible with life. In mice, when the Ndufa5 gene was inactivated in the full body by the gene trap methodology, the KO mice never developed beyond E9, demonstrating that this protein is required for embryonic development (Peralta, 2013). To study the role of NDUFA5 in adult mice, we created a conditional knockout allele by introducing a rescuing Ndufa5 cDNA transgene flanked by loxP sites in the KO ES cells, which was selectively ablated postnatally in neurons by the CaMKIIα-Cre transgene. The deletion of the rescuing allele in neurons originated a mild phenotype. At 11 months of age, Ndufa5 CNS-KO mice showed lethargy and loss of motor skills. Fully assembled CI levels were greatly reduced in cortex (70–85%), and CI activity was partially reduced (40%). Despite the biochemical phenotype, no oxidative damage, neuronal death or gliosis were detected in the Ndufa5 CNS-KO brain at this age (Peralta, 2013). These results showed that a partial defect in CI in neurons can lead to late-onset motor phenotypes without neuronal loss or oxidative damage. Increased levels of the electron-transferring-flavoprotein subunit A (ETFA) and the ketogenic enzyme ACAT1 (mitochondrial acetyl CoA acetyltransferase) were detected in Ndufa5 CNS-KO cortex homogenates, indicating that two compensatory mechanisms may have attenuated the phenotype: (i) increased electron flow via the electron transfer flavoprotein dehydrogenase to CoQ10, (ii) physiological adaptations to a ketogenic environment.
3. Mouse models of Complex II deficiency
Complex II (CII) or succinate dehydrogenase (SDH) is part of both the Krebs cycle and the electron transport chain. CII is the smallest respiratory complex, being composed by 4 subunits (SDHA-D) encoded by nDNA. It catalyzes the conversion of succinate to fumarate by reducing FAD to FADH2 and transferring electrons through three Fe-S clusters to ubiquinone. CII deficiencies are rare and account for about 2–4% of mitochondrial diseases (Rustin and Rotig, 2002). Mutations have been found in all the structural subunits and in two chaperons SDHAF1 and SDHAF2 reviewed in (Rutter, 2010). The clinical phenotypes include LS, infantile leukoencephalopathy and hereditary paraganglioma-pheochromocytoma. Interestingly, a neurological phenotype has been associated only with mutations in SDHA, SDHAF1 and, in one case, in SDHD. Mutations in SDHB-D subunits and SDHAF2 (assembly factor, SDH5 in yeast) have been associated with tumor formation [reviewed in (Rutter, 2010)]. A summary of mice with CII defects is reported in Table II.
Table II.
Mousel models of Complex II, Complex III and electron carrier deficiencies.
| Gene | Mitochondrial function |
Genetic Manipulation |
Phenotype | References |
|---|---|---|---|---|
| Sdhd | Subunit of CII associated with paraganglioma tumors |
Knockout: -Whole body (replacement of exons 2–4) |
Embryonic lethality | (Piruat, 2004) |
| Knockout: -Whole body (deletion of exon 3) |
Embryonic lethality | (Bayley, 2009) | ||
| Pdss2 | Biosynthesis of Coenzyme Q |
Spontaneous mutation in Pdss2 in homozygosis |
Renal disease, abnormal mitochondria |
(Hallman, 2006) |
| Conditional Knockout: - Kidney (Podocin-Cre) |
Nephropathy and proteinuria |
(Peng, 2008) | ||
| Conditional Knockout: - Liver (Alb-Cre) |
No overt phenotype | |||
| Spontaneous mutation in Pdss2 in homozygosis |
Renal failure, increased oxidative stress, mitochondrial DNA depletion, and reduced citrate synthase activity in kidney and muscle |
(Quinzii, 2013) | ||
| Coq9 | Biosynthesis of Coenzyme Q |
Knock-in: 239R>X |
Encephalomyopathy with astrogliosis and neuronal death |
(Garcia-Corzo, 2013) |
| Bcs1l | CIII assembly factor (last steps of assembly of CIII) |
Knock-in: 232A>G |
Hepatopathy and tubolopathy characteristic of GRACILE syndrome |
(Leveen, 2011) |
| CycS | Cytochrome c somatic isoform |
Knockout: - whole body (replacement of 2 exons) |
Embryonic lethal. Developmental delay at E8.5. No viable embryos after E10.5 |
(Li, 2000) |
| Knock-in: K72A | Abnormal brain development (exencephaly and hydrocephaly), cachexia and lymphopenia |
(Hao, 2005) | ||
| CycT | Cytochrome c testis isoform |
Disruption of exon 3 | Testicular atrophy due to accelerated apoptosis Decreased sperm motility however mice are fertile. |
(Narisawa, 2002) |
| Uqcrfs1 | Rieske iron sulfur protein (RISP), one of the catalytic subunits of CIII |
Conditional Knockout: -Neurons (CaMKII-Cre) |
Fatal encephalopathy with severe oxidative stress in piriform cortex |
(Diaz, 2012) |
| Knock-in: 224P>S | Embryonic lethality in homozygosis. Mild reduction in CIII activity and no apparent phenotype in heterozygous mouse |
(Hughes and Hekimi, 2011) |
3.1. Sdhd
Piruat and colleagues produced an Sdhd KO mice by replacing exons 2–4 with a neomycin cassette, which resulted in embryonic lethality (Piruat, 2004). Sdhd−/− embryos died at about E7.5 during early stages of organogenesis. Sdhd+/− mice survived and by 6 months of age did not display any gross overt phenotype albeit the CII activity in brain, heart, liver and kidney was about 50% lower than control mice. Contrary to the phenotype observed in humans, Sdhd+/− mice did not develop tumors. However, cells in the carotid body had an abnormal increase in their resting activity (Piruat, 2004). Unfortunately, these animals were not followed for their entire lifespan to determine if there was an age related phenotype.
Looking further into the role of SDHD in paraganglioma and pheochromocytoma, Bayley and collaborators created another Sdhd KO mouse by deleting only exon 3 (Bayley, 2009). Ablation of exon 3, which codes for most of the active protein, also resulted in embryonic lethality as described in the first model. The authors in this study followed the heterozygous Sdhd+/− mice for their entire life span in two different genetic backgrounds; however these mice were unable to develop any tumors. Moreover, double KO mouse of Sdhd and H19, a putative modifier gene of SDHD tumorogenesis in humans, also failed to produce tumors or any other genotype related pathology (Bayley, 2009).
Although these two Sdhd−/− mouse models of CII deficiency are not models for mitochondrial disease or hereditary forms of cancer, they clearly demonstrate the pitfalls and risks of creating mouse models to recapitulate human diseases.
4. Mouse models of defect of the OXPHOS electron carriers
4.1. Coenzyme Q deficiency
Coenzyme Q is an essential component of the OXPHOS system, that transfers electron from CI and CII and from electron transferring flavoprotein dehydrogenase (ETF-DH) to complex III (Turunen, 2004). CoQ10 is a lipophilic molecule and is synthesized within the mitochondria through an intricate pathway (Kawamukai, 2009). Several pathogenic mutations in genes encoding proteins involved in the biosynthesis of CoQ10 have been identified. These mutations lead to a multitude of symptoms, including myopathy, encephalopathy, and nephrotic syndrome (Hirano, 2012). Beside its role in the OXPHOS system, CoQ10 is a potent antioxidant molecule and participates in the de novo pyrimidine biosynthesis pathway. Table II shows a summary of OXPHOS soluble electron carrier deficient mice.
4.1.1. Pdss2
Pdss2 encode for the subunit 2 of decaprenyl diphosphate synthase, an enzyme implicated in the biosynthesis of CoQ10. Mutations in this subunit have been reported in an infant male with nephropathy and Leigh syndrome (Lopez, 2006). Mice harboring a spontaneous missense mutation in Pdss2 gene developed renal failure, with ultrastructural mitochondria abnormalities (Hallman, 2006), and decreased CoQ10 levels (Peng, 2008) in kidney. As expected, dietary suplementation of CoQ10 treatment restored the kidney disease in the Pdss2 mouse model (Saiki, 2008). Liver-conditional Pdss2 KO mice had no overt phenotype, although the liver showed impaired respiratory capacity and undetectable levels of CoQ10 precursors (Peng, 2008). These results suggest that the disease manifestation of CoQ10 deficiency relates to tissue-specific respiratory capacity thresholds. Renal tissue displayed the greatest sensitivity to Pdss2 impairment as observed in humans (Lopez, 2006). Recently, Hirano’s group found that the onset/progression of phenotype and the tissue specificity of the defect in CoQ was associated with increased levels of oxidative damage (Quinzii, 2013).
4.1.2. Coq9
The ubiquinone biosynthesis protein COQ9 is one of the 11 proteins required for the biosynthesis of CoQ10 (Johnson, 2005). Duncan et al. reported a patient with CoQ10 deficiency caused by a homozygous stop mutation in the COQ9 gene leading to a truncated protein (Duncan, 2009). The patient showed neonatal lactic acidosis and later developed multisystem disease including intractable seizures, global developmental delay, hypertrophic cardiomyopathy, and renal tubular dysfunction (Duncan, 2009). Recently, a Coq9 knock-in mouse was generated carrying the mutation that is homologous to the one found in the patient (Garcia-Corzo, 2013). The homozygous knock-in (Coq9X/X) mice were normal at birth but between 3–6 months they developed a rapid and progressive paralysis followed by death, suggesting an involvement of the central nervous system in the pathogenic mechanism of the disease. Accordingly, the authors showed that the lack of CoQ10 caused loss of CI and an increase of free CIII, leading to a decrease in mitochondrial respiration and ATP synthesis only in brain of the knock-in mouse (Garcia-Corzo, 2013). Brain tissue showed intense vacuolization, astrogliosis and neuronal death whereas skeletal muscles showed a severe demyelinization (Garcia-Corzo, 2013). Thus, the Coq9X/X mouse is an excellent model of mitochondrial encephalomyopathy associated with CoQ deficiency. Unfortunately, it still unknown why the CNS was more sensitive than other tissues to the lack of CoQ10. Perhaps, this is due to the fact that the biosynthetic pathway of CoQ is shared with the cholesterol pathway which is foundamental for proper brain development in fetuses and newborns.
4.2. Cytochrome c
Cytochrome c (Cytc) plays an important role not only in mitochondrial respiration but also in the initiation of the apoptotic intrinsic pathway. Cytc transfers electrons from CIII to CIV in the electron transport chain in the mitochondria. When Cytc is released into the cytoplasm, it promotes the formation of the apoptosome and activation of caspases that eventually lead to cell death. Two Cytc isoforms are present in mammals, the ubiquitous somatic isoform (CYCS) and the testis-specific isoform (CYCT). Male germ cells and spermatozoids only express the testis isoform whereas other tissues express the somatic isoform. There is no description of Cytc deficiencies per se as mitochondrial diseases, perhaps because they are incompatible with life. To date, only two mutations in the CYCS gene have been described in patients suffering from a rare disease named thrombocytopenia 4 (De Rocco, 2014; Morison, 2008).
Inactivation of the CycS gene in mouse caused embryonic lethality (Li, 2000), whereas the inactivation of the CycT gene did not impaired viability of the mice (Narisawa, 2002). CycS+/− mice did not have any apparent phenotype (Li, 2000) and CycT−/− mice showed testicular atrophy and sperm motility impairment although they were fertile (Narisawa, 2002). Embryonic fibroblasts derived from both KO mice had altered bioenergetics and apoptotic properties.
To study the role of apoptosis in development and tissue homeostasis, Hao and colleagues created a knock-in mouse with a K72A mutation (Hao, 2005). The lysine 72 is required for the apoptotic function of cytochrome c but not for its bioenergetic function. The CycSKA/KA mutant mouse had partial embryonic lethality and those animals that survived died within 3–4 weeks after birth. The knock-in mice had numerous abnormalities including exencephaly, hydrocephaly, spleenomegaly, lymphopenia and cachexia (Hao, 2005), highligthing the importance of the apoptotic pathway in tissue development.
To better understand the complete function of Cytc in bioenergetics, Moraes’ group is working on the characterization of conditional CycS and CycT double KO mouse (Vempati, 2008). Such mice have been produced by crossing CycS+/−, CycT−/− and a trangenic mouse carrying a ubiquitously expressed CycS floxed trangene. The introduction of the floxed CycS transgene rescued the lethality of the ablation of CycS and allowed for the subsequent deletion of the transgene in a tissue specific maner (Vempati, 2008). Phenotypic characterization of the double KO mouse still ongoing but preliminary studies on lung fibroblasts null for both Cytc isoforms showed an impairment on the assembly/stability of CI and CIV. Cytc was found in OXPHOS supercomplex arrangements possibly stabilizing these associations (Vempati, 2009).
5. Mouse models of Complex III deficiency
Mammalian complex III (CIII, ubiquinol cytochrome c reductase or cytochrome bc1 complex), in its monomeric form, is comprised of 3 catalytic and 8 structural subunits. One of the catalytic subunits, cytochrome b, is encoded by the mtDNA while the others are encoded by the nuclear genome. As the other OXPHOS complexes, CIII also requires ancillary proteins for its biogenesis and assembly. CIII deficiencies are rare with a broad spectrum of clinical symptoms and tissue specificity, however there are no clinical features specific for CIII deficiencies (Benit, 2009; Mourmans, 1997). Clinical presentations range from Leber’s hereditary optic neuropathy (LHON), cardiomyopathy and myopathy, exercise intolerance, neurological abnormalities, lactic acidosis, psychomotor retardation, GRACILE and Björnstad syndromes (Diaz, 2011; Haut, 2003; Johns and Neufeld, 1991; Keightley, 2000). Table II shows a summary of complex III deficient mice.
5.1. Bcs1L
BCS1L is one of the ancillary factors required for CIII assembly and participates in the last step of assembly by incorporating the Rieske iron sulfur protein (RISP) and UQCR11 into the complex. Mutations in this gene cause CIII deficiency including Björnstad syndrome, neurological disorders, tubulopathy and liver failure. The most severe mutation found in patients is the homozygous missense variation S78G that induces a severe liver phenotype, causing the GRACILE (growth restriction, aminoaciduria, cholestasis, iron overload, lactic acidosis and early death) syndrome (Visapaa, 2002). Leveen and colleagues created a GRACILE knock-in mouse carrying the Bcs1L 232A>G mutation which creates the S78G amino acid substitution found in humans. Heterozygous Bcs1LA/G mice where indistinguishable from control animals Bcs1LA/A, whereas the homozygous mutant Bcs1LG/G mouse showed growth retardation starting at 3 weeks of age and by P39 (postnatal day 39) they weight about 59–68% of control littermates (Leveen, 2011). These mice had also a short lifespan and the majority of animals died by 6 weeks of age. Histological analysis of tissues from the mutant mouse revealed abnormalities only in liver and kidney starting at P24 with periportal hepatocytes degeneration, microvesicular steatosis (lipid accumulation) and depletion of glycogen. In symptomatic mice (P30 and older) a development of nodular hepatic structures and fibrosis was observed. The kidney pathology was characterized by reduction in size and number of proximal tubuli that contained exudates indicating renal tubolopathy. A significant decrease in the steady-state levels of BCS1L in Bcs1LG/G liver mitochondria was observed and although the levels of RISP were not altered, its incorporation into CIII was dramatically affected at P32 in liver and to a less extent in kidney and heart mitochondria. These resulted in a progressive reduction in CIII activity and the affected mice had about 20% of control levels in liver and 40% in heart and kidney (Leveen, 2011).
The Bcs1LG/G mouse model recapitulated quite well the human phenotype of GRACILE syndrome particularly in its tissue specificity (liver and kidney) with the remarkable exception of the lack of iron accumulation in liver reported in patients. Recent studies on the metabolism of the Bcs1LG/G mouse liver revealed that the mutation led to an energy deficit with starvation-like characteristics where storage supplies were depleted and compensatory mechanisms were not observed (Davoudi, 2014). Interestingly, an increase in oxidative stress markers and a decrease in the mRNA levels of antioxidant enzymes (SOD2 and catalase) was observed only at late stages, whereas cells derived from patients showed consistently higher ROS levels (Hinson, 2007; Moran, 2010).
5.2. Uqcrfs1
Rieske iron sulfur protein (RISP) is one of the catalytic subunits of CIII encoded in the nucleus by the UQCRFS1 gene. To date there are no reports of mutations in this gene in CIII deficiencies, perhaps accounting for incompatibility with life. We have created a conditional KO mouse model by ablation of the Uqcrfs1 gene (exon 2) in neurons using the CaMKIIα Cre (Diaz, 2012). The Risp KO mouse had a short life span. The majority of the KO died by 3 months of age and weighted less than control littermates. Risp KO had no overt phenotypes although they displayed reduced nocturnal ambulatory activity when compared to controls. Biochemical analysis in both cortex and hippocampus showed a progressive CIII deficiency in the KO with 43% of control values at 1 month of age, which declined to 25% by 3 months of age. Consistently, there was a progressive reduction in the steady-state levels of RISP with age in the KO neurons. Interestingly, the CIII deficiency was accompanied by an increase in citrate synthase and CIV activities and by mtDNA/nDNA ratios, suggesting a compensatory mitochondrial biogenesis. Magnetic resonance imaging of the Risp KO brain revealed the presence of lesions in the piriform cortex already evident at 2 months, progressively extending to different areas and posterior regions of the brain with age. These lesions were related to increased oxidative stress. Indeed, we found increased lipid peroxidation protein-adducts, nitrosylated proteins, increased SOD2 levels and oxidized nucleic acids. The increase in oxidative stress with age led to neuronal cell death mainly in the piriform cortex and hippocampus at 3 months (Diaz, 2012).
Mitochondrial dysfunction has been associated to increases in reactive oxygen and nitrogen species (ROS and RNS) and causative of multiple human diseases. Having such a clear oxidative stress phenotype, the Risp KO mice will be an excellent model to test if antioxidant therapeutic agents can mitigate mitochondrial dysfunction.
In Caenorhabditis elegans, mutations in RISP and in other proteins related to the mitochondrial electron transport chain have been associated with increased lifespan. This increase in lifespan has been attributed to ROS signaling events, however the underlying mechanism still unknown (Feng, 2001). To investigate this in mammals, Hughes and Hekimi created a knock-in mouse carrying the point mutation (P224S) corresponding to the one in worms (Hughes and Hekimi, 2011). When homozygous, this point mutation was embryonic lethal, however in heterozygous state it produced a decreased life span in males (10% shorter than wt mice) and an increase in females (9% longer than wt). The mutation caused a mild decrease of CIII enzymatic activity of 15–21% in liver and heart in the Risp+/P224S mice, however the knock-in mice did not showed any apparent phenotype associated with mitochondrial diseases. Unlike what occured in the Risp KO mouse, the point mutation in RISP did not produced increased oxidative stress (Hughes and Hekimi, 2011).
6. Mouse models of Complex IV deficiency
Complex IV (CIV) or cytochrome c oxidase (COX) is the terminal enzyme of the respiratory chain that transfers electrons from reduced cytochrome c to molecular oxygen, a process coupled to the translocation of protons from the matrix into the intermembrane space. Mammalian CIV is a homodimer composed by 14 subunits per monomer of dual genetic origin. Three of the subunits are encoded by the mtDNA (COX1, COX2 and COX3 that form the catalytic core of the enzyme) and the remaining structural subunits are encoded by the nuclear genome. The phenotype exhibited in patients associated with CIV deficiencies comprehends a wide variety of disorders that could affect single or multiple organs (DiMauro, 2012; Pecina, 2004; Shoubridge, 2001). In humans, most of the mutations causing CIV deficiency have been found in all mtDNA encoded subunits (DiMauro, 2012) and in many assembly factors (reviewed in (Diaz, 2010)). The only CIV nuclear encoded structural subunits that have been found mutated in patients are COX6B1 and COX7B (Indrieri, 2012; Massa, 2008). Table III summarizes of mouse models with complex IV deficiencies.
Table III.
Animal Models of Complex IV and Complex V deficiencies
| Gene | Mitochondrial function |
Genetic Manipulation |
Phenotype | References |
|---|---|---|---|---|
| Cox4-2 | Lung specific isoform of the subunit Cox4 of CIV |
Knockout: -Whole body (Homologous recombination) |
Lung pathology with inflammation and Charcot-Leyden crystals. |
(Huttemann, 2012b) |
| Cox6a-2 | Muscle specific subunit of the CIV implicated in the modulation and dimerization of CIV |
Knockout: -Whole body (Homologous recombination) |
Cardiomyopathy | (Radford, 2002) |
| Cox7a-2 | Heart/skeletal muscle specific subunit of CIV. Unknown function, involved in activity regulation |
Knockout: -Whole body (Homologous recombination) |
Cardiomyopathy and limited endurance capacity |
(Huttemann, 2012a; Lee, 2012) |
| Surf1 | Assembly factor of CIV, involved in early assembly. Specific function unknown |
Knockout: -Whole body (Homologous recombination) -replacement exon 5–7 |
Embryonic lethality | (Agostino, 2003) |
| -replacement exon 7 | Viable, prolonged lifespan |
(Dell'agnello, 2007) | ||
| Cox10 | Assembly factor required for biosynthesis of heme a, an essential prosthetic group of CIV |
Conditional Knockout: -Skeletal muscle (Mlc-1f-Cre and Mef2c Cre) |
Progressive myopathy | (Diaz, 2005; Wenz, 2008) |
| -Liver (Alb-Cre) |
Liver steatosis and hepatomegalia |
(Diaz, 2008) | ||
| -Neurons (CaMKII-Cre) |
Cortical neurodegeneration and hypo-and hyperactivity behavioral abnormalities |
(Diaz, 2012) | ||
| -Neurons and glia (Cnp1-Cre) |
Peripheral Nervous system neuropathy with dismyelination and muscular atrophy |
(Funfschilling, 2012) | ||
| Cox15 | Assembly factor required for Heme a biosynthesis, a essential prosthetic group of CIV |
Knockout: -Whole body (Homologous recombination) |
Embryonic lethality | (Viscomi, 2011) |
| -skeletal muscle (Acta1-Cre) |
Severe myopathy | |||
| Cox17 | Copper chaperone involved in CIV assembly |
Knockout: -Whole body (Homologous recombination) |
Embryonic lethality | (Takahashi, 2002) |
| Sco2 | Copper chaperone involved in CIV assembly |
Knockout: -Whole body (Homologous recombination) |
Embryonic lethality | (Yang, 2010) |
| Knock-in: -E140K (Sco2 KI/KI) |
Mild myopathy with impaired motor function at 8 months of age |
(Yang, 2010) | ||
| Knockout/Knock-in: -E140K (Sco2 KO/KI) |
Mild myopathy with impaired motor function at 4 months of age |
(Yang, 2010) | ||
| Atpif1 | Encodes IF1, inhibiting factor of CV. Blocks F1 module to avoid ATP hydrolysis |
Knockout: -Whole body (Homologous recombination) |
No phenotype ATP hydrolysis reduced |
(Nakamura, 2013) |
| Overexpression of inducible TRE-human mutant IF1 H49K and CaMKII-tTA in neurons |
Inhibition of OXPHOS switching neuronal metabolism to glycolysis Mild oxidative stress Refractive to excitotoxicity |
(Formentini, 2014) |
6.1. Cox4-2
In mammals, there are 2 isoforms of the nuclear encoded cytochrome c oxidase subunit 4: the ubiquitously expressed isoform 1, COX4-1, and the lung specific isoform 2, COX4-2. The highest levels of COX4-2 are found in the lining epithelium of the lung and in smooth muscle although both COX4-1 and COX4-2 isoforms are expressed at similar levels in this tissue (Huttemann, 2001). In mammalian cells, the expression of the COX4-1 and COX4-2 subunits is regulated by oxygen (Fukuda, 2007). Under hypoxic conditions, COX4-2 isoform is highly induced and COX4-1 isoform is degraded. COX4-1 plays a key role in the allosteric regulation of CIV activity by binding ATP (allosteric inhibitor) or ADP (allosteric activator), thereby adjusting energy production to energy demands (Acin-Perez, 2011). To ablate the Cox4-2 gene, exons 2 and 3 were replaced by homologous recombination with a neomycin cassette (Huttemann, 2012b). In the Cox4-2 KO mice, CIV activity in lung was reduced to half of control values and ATP levels were significantly reduced to 29% of control. Cox4-2 KO animals developed lung pathology, starting at 18 weeks and deteriorated with age. In humans, only one mutation in Cox4-2 gene has been reported to be associated to congenital exocrine pancreatic insufficiency (Shteyer, 2009). However, lung function was never examined in these patients. In the Cox4-2 KO mice, the pancreas histology showed no sign of dysfunction (Huttemann, 2012b). Moreover, the authors were unable to detect expression of the lung isoform in pancreas of control mice. It remains to be determined if the Cox4-2 isoform is expressed in the murine pancreas, and if the additional factors contribute to the phenotype observed patients.
6.2. Cox6a-2
Mammalian COX6A is represented by two different isoforms: COX6A-1 or COX6A-L (liver isoform) and COX6A-2 or COX6A-H (heart isoform). The COX6A-1 subunit is ubiquitously expressed, whereas COX6A-2 subunit is specific of heart and skeletal muscle (Parsons, 1996). COX6A-2 is implicated in the modulation of CIV activity (by binding ADP/ATP) and in the dimerization of the monomers. Cox6a-2 gene was ablated in the mouse by inserting a neomycin cassette in exon 2. The resulting Cox6a-2 KO mouse showed CIV deficiency (reduced to 23% of controls) in cardiac tissue and the levels of the fully assembled CIV were significantly decreased (Radford, 2002). Accordingly, the mice exhibited a cardiac phenotype, as a consequence of a diastolic dysfunction, without impact on their lifespan. Further characterization of this model revealed that CIV activity was also moderate but significantly reduced in soleus, gastrocnemius, and diaphragm muscles (Quintens, 2013). The partial loss of CIV in the KO mice was accompanied by increased ROS production in the skeletal muscle. Metabolic characterization indicated that the Cox6a-2 KO mice had decreased body mass, consumed more oxygen and generated more heat when compared to control animals. However, no differences in the ratio of carbohydrate over fat oxidation were observed. At the molecular level, Cox6a-2 KO mice showed constitutive activation of AMP-activated protein kinase (AMPK) in skeletal muscle and enhanced expression of uncoupling proteins in skeletal muscle and adipose tissue. The expression of markers of oxidative fibers was enhanced in both gastrocnemius and diaphragm in the Cox6a-2 KO mice indicating a fiber type switch. Increased mitochondrial size and PGC-1α levels were also detected in the skeletal muscles of the KO mice. Moreover, the Cox6a-2 KO mice were protected against high-fat diet-induced obesity, insulin resistance, and glucose intolerance. This phenotype appears to be the result of elevated energy expenditure and muscle fiber type switch towards more oxidative fibers. The results obtained with this mouse model suggested that COX6A-2 subunit is an important regulator of energy homeostasis, thermogenesis and response to the fasted state. The authors proposed that the loss of COX6A2 subunit enhances ROS production, which in turn could activate AMPK, PGC-1α and upregulates uncoupling protein expression in muscle. This subsequently results in increased energy expenditure, non-shivering thermogenesis, as well as muscle fiber type switch and enhanced insulin sensitivity (Quintens, 2013).
6.3. Cox7a-1
COX7A-1 or COX7A-H is the heart/skeletal muscle isoform of COX7A and it seems to be involved in adapting CIV activity to tissue-specific energy demands, although the mechanism is still not elucidated. The other COX7A isoform is the COX7A-2, which is predominantly expressed in liver and it has been detected in low proportion in hearts of mice and humans (Van Kuilenburg, 1992). To generate the mouse model the complete Cox7a-1 gene (composed by 3 exons) was replaced by homologous recombination with a neomycin cassette. The Cox7a-1 KO mice developed dilated cardiomyopathy and reduced systolic and diastolic function at 6 weeks of age. However, this cardiac defect tended to improve with age and remained stable, resulting in a mild dilated cardiomyopathy (Huttemann, 2012a). Interestingly, when COX7A-1 was absent, an upregulation of the COX7A-2 isoform in hearts was observed (Huttemann, 2012a). This compensatory upregulation of COX7A-2 could explain the partial recovery and the mild cardiomyopathy showed in the Cox7a-1 KO mouse. CIV activity in heart homogenates of KO and heterozygous mice was reduced to 53% and 26%, respectively. Surprisingly, ATP levels were significantly higher in Cox7a-1 KO hearts and intermediate in heterozygotes when compared to controls suggesting the presence of adaptive responses to maintain energetic demands.
Subsequent work showed a severe and limited endurance capacity of the Cox7a-1 KO mouse. The lack of COX7A-1 isoform in the hind limb muscles (quadriceps and soleus) resulted in a 62–65% decrease in CIV activity and in a 60% decrease in ATP levels (Lee, 2012). The deletion of COX7A-1 resulted in impaired muscle function. Hence, it is reasonable to hypothesize that ablation of the Cox7a-1 gene results in distinct adaptive responses in heart and skeletal muscle. Unfortunately, the authors did not address if the levels of the COX7A-2 subunit were increased in the hind limb muscles to compensate the defect, as shown in the heart tissue. This would be a key point to determine tissue specific adaptive responses.
6.4. Surf1
The SURF1 protein is known to participate in the early assembly steps of CIV, however its precise function is still not well understood (Tiranti, 1999; Williams, 2001). Mutations in SURF1 gene cause severe neurological diseases, such as LS, and represent the primary cause of CIV defects of nuclear origin. Zeviani’s group has generated two Surf1 KO mouse models. The first model was obtained by disrupting the gene by targeted insertion of a neomycin cassette and replacement of exons 5 to 7. This genetic disruption caused high embryonic lethality with only few KO animals surviving. The authors attributed the lethality to the neomycin insertion rather than directly to the ablation of the Surf1 gene (Agostino, 2003). The surviving Surf1 KO mice showed an isolated CIV deficiency, that was higher in skeletal muscle and liver, but mild in heart and brain tissues. Surprisingly, the Surf1 KO mice did not show any apparent neurological abnormality. Brain regions frequently affected in LS, such as cortex, hippocampus and thalamus, appeared normal in the KO mice (Agostino, 2003). In the second constitutive Surf1 KO animal model, the neomycin cassette was targeted to the exon 7, disrupting only the last portion of the Surf1 gene (Dell'agnello, 2007). After the excision of the neomycin cassette, the resulting Surf1 KO mice were viable. This result confirmed the hypothesis that the neomycin cassette in the previous model induced the lethality. The Surf1 KO mice showed a mild CIV deficiency in brain, skeletal muscle, heart and liver (30% – 40% of control values), the latter being the most affected tissue. This new model also failed to show any overt neurodegenerative phenotype. Unexpectedly, the Surf1 KO showed a prolonged lifespan, about 5 months longer than control mice. The reasons for increased longevity remain unexplained although the same phenotype was observed in CNS specific Surf1 KO in Drosophila melanogaster (Zordan, 2006). To produce a neurological phenotype and test the sensitivity of CIV deficient neurons to toxic stimulus, the Surf1 KO mice were treated with kainic acid (glutamate agonists that induces Ca++ excitotoxicity), but no difference was found in terms of severity of seizures and mortality rate between control and Surf1 KO. However, extensive markers of apoptosis found in controls were absent in the KO mice, suggesting a possible role of Surf1 in other pathways responsible for the neuroprotective mechanism (Dell'agnello, 2007). Recently, a detailed study of the whole-body metabolism in Surf1−/− mice has showed increased mitochondrial biogenesis in the white adipose tissue, increased fat utilization and improved insulin sensitivity (Deepa, 2013). The pathways responsible for the mitochondrial biogenesis due to a CIV deficiency are still unknown. The improved insulin sensitivity due to CIV deficiency detected in the Surf1−/− mice is a novel finding that may contribute for the prolonged life span exhibited by this model.
6.5. Cox10
The product of the COX10 gene is a farnesyl transferase that catalyzes the first step of the biosynthesis of heme a, an essential prosthetic group for the function of CIV (Antonicka, 2003a). In humans, mutations in COX10 have been associated to leukodystrophy and tubolopathy, hypotonia, lactic acidemia, anemia, LS, sensorineural deafness and fatal infantile hypertrophic cardiomyopathy (Coenen, 2004; Valnot, 2000). Conditional ablation of Cox10 in mouse by the Cre-loxP system (Cox10flox/flox) allowed us to create tissue-specific models of CIV deficiency in liver, brain and skeletal muscle (Diaz, 2008; Diaz, 2012; Diaz, 2005). More recently a specific KO mouse of Cox10 in oligodendrocytes and Schwann cells has been generated (Funfschilling, 2012). To ablate this gene, loxP sites were introduced flanking exon 6, which encodes part of the active site of the enzyme. Disruption of the floxed gene using the Cre recombinase driven by the myosin light chain 1f promoter (Mlc-1f) allowed the creation of a myopathy mouse model (Diaz, 2005). The myopathy mouse had a severe progressive CIV deficiency in the skeletal muscle (13% of residual activity) already at 1 month of age, but the myopathic signs were not evident until approximately 3 months of age. The CIV deficiency progressed with age, showing a mosaic pattern resembling the pathology observed in patients with mitochondrial myopathies and ended with an early death at about 6 months. The Cox10-Mlc-1f KO females were more affected than the males, having shorter life span and pronounced exercise intolerance (Diaz, 2005).
We generated a second myopathy mouse model by expressing the Cre recombinase from the myocyte enhancer factor 2c (Mef2c) promoter. The Cox10-Mef2c KO mouse model showed a milder myopathy compared to the Cox10-Mlc-1f KO one. The difference in severity correlated with the rate of deletion of the floxed Cox10 gene (Wenz, 2008). At the age of 3 months the Cox10-Mef2c KO mice showed reduced CIV activity in skeletal muscle (40% of control levels) and an impaired treadmill performance. Similar to the Cox10-Mlc-1f KO model, the Cox10-Mef2c KO females were more affected and the myopathy progressed with age, dying earlier than the control animals.
Crossing the floxed Cox10 mouse with transgenic mice expressing a liver specific Cre recombinase allowed us to create a mitochondrial hepatopathy mouse model, the Cox10-Alb-KO (Diaz, 2008). These mice presented at 2 months of age a severe CIV deficiency and liver pathology (increased transaminases, lipid accumulation and decreased glycogen storage). The CIV defect triggered a massive compensatory mitochondrial proliferation in the hepatocytes, however, it was not sufficient to maintain the hepatocytes viable for extended periods. Interestingly, at older ages, no differences were found between control and Cox10 KO mice, due to the fact that hepatocytes that had escaped the Cox10 deletion proliferated and repopulated the defective liver, subsequently restoring CIV activity and hepatic function. In contrast, those animals that were homozygous for the Alb-Cre transgene, presented a more severe hepatic phenotype including steatosis and hepatomegaly, due to the higher efficiency of recombination. These later mice had shorter lifespan and died at about 2 months of age. The Cox10 liver KO model recapitulates several clinical features of mitochondrial diseases with liver involvement often including microvesicular steatosis, increased mitochondrial density, elevated transaminases and moderated hepatomegaly.
A Cox10 encephalopathy mouse model was also developed by crossing the Cox10flox/flox mouse with a transgenic mouse expressing Cre under the CaMKIIα promoter leading to ablation of the gene in forebrain structures (Diaz, 2012). The Cox10-CaMKIIα KO mouse developed a progressive CIV deficiency that resulted in behavioral abnormalities (alternated cycles of hypo and hyperactivity) and severe cortical atrophy long after the onset of the mitochondrial defect, that resulted in premature death between 10–12 months of age. CIV deficiency was already detected at 2 months of age in cortex and hippocampus homogenates. The onset of the behavioral abnormalities was at 4 months of age and coincided with the first signs of neuronal death. Neurons in the cingulated cortex, hippocampus and, to a less extent, piriform cortex were particularly affected by the mitochondrial defect. Gliosis and increased oxidative stress were observed late in the pathology of this mouse model.
Ablation of the Cox10 gene in oligodendrocytes and Schwann cells under the Cnp1 promoter caused severe neuropathy in the peripheral nervous system characterized by demyelination, leading to muscular atrophy and paralysis by 9 months of age (Funfschilling, 2012). This mouse model recapitulates the leukodystrophy (dysmyelination) observed in some COX10 patients. Interestingly, CIV deficiency did not cause cell death of post-myelination oligodendrocytes. However, there are studies indicating that myelinating cells are sensitive to mitochondrial defects. In fact, demyelination has been reported in patients with MELAS (Ohara, 1988; Rusanen, 1995), Leber’s Hereditary Optic Neuropathy (Kovacs, 2005), Friedreich’s Ataxia (Carelli, 2002) and Dominant Optic Atrophy (Johnston, 1979). In addition, cultured rat or human oligodrendrocytes increase mitochondrial function during differentiation and the differentiated cells become more sensitive to rotenone (Schoenfeld, 2010). These results in vitro are in agreement with the demyelination observed in patients with certain mitochondrial defects.
6.6. Cox15
Cox15 encodes a protein involved in the conversion of heme o into heme a (Khalimonchuk and Rodel, 2005) and mutations in this gene cause fatal encephalocardiopathy with early or late onset LS (Antonicka, 2003b; Bugiani, 2005; Oquendo, 2004). Constitutive Cox15 KO mice showed a consistent embryonic lethality at E7.5 (Viscomi, 2011). A knock-in mouse with the Cox15 exons 1–2 flanked by the loxP sites was crossed with a transgenic mouse expressing the Cre recombinase under the control of the human skeletal muscle-specific alpha-actin (ACTA1) promoter to create the ACTA-Cox15−/− mouse. Albeit born at the expected Mendelian ratio, the ACTA-Cox15−/− animals were smaller than control littermates at 1 month and had reduced motor performance due to a sever myopathy. Histochemical analysis of skeletal muscle confirmed a severe reduction of CIV and a compensatory proliferation of aberrant mitochondria (Viscomi, 2011).
6.7. Cox17
CIV requires copper for enzymatic activity and in humans there are at least 8 chaperones involved in this process (Barrientos, 2009). The COX17 gene encodes one of this copper chaperones that transfers the metal to two CIV assembly factors, COX11 and SCO1. A Cox17 KO mouse model was created by targeting the insertion of a GFP-neomycin cassette to replace half of exon 1 and the complete exon 2. Deletion of Cox17 in mouse showed that this protein is indispensable not only for CIV activity but also for embryonic development since Cox17 KO mice died between E8.5 and E10 (Takahashi, 2002). These results indicate that loss of function of Cox17 is incompatible with life and to date mutations in this gene have not been found in humans.
6.8. Sco2
SCO2 encodes for a chaperone implicated in copper metabolism that is required for the proper assembly and function of CIV. The specific function of SCO2 is still not completely clear (Leary, 2009). In humans, mutations in SCO2 cause hypertrophic cardiomyopathy, encephalopathy and myopathy soon after birth (Papadopoulou, 1999). The most common mutation found in patients is the n.1541G>A missense mutation which induces an amino acid change E140K in SCO2 close to the copper-binding domain. Patients homozygous for the n.1541G>A mutation had a delayed onset of symptoms and a longer survival compared to compound heterozygotes (E140K on one allele and a null mutation on the other allele) who display more severe clinical phenotype and dye in the first months or in the first years of life (Jaksch, 2000; Knuf, 2007; Vesela, 2008). Recently, a mouse model harboring a Sco2 KO allele (Sco2KO) and a Sco2 E129K knock-in allele (Sco2KI), equivalent to E140K mutation in humans was created (Yang, 2010). The Sco2KO/KO mouse resulted in embryonic lethality prior to E8.5, implying that Sco2 plays a crucial role during early development. On the other hand, homozygous mice for the knock-in allele, Sco2KI/KI and the compound heterozygous Sco2KO/KI were viable. Both genotypes showed CIV deficiency in heart, muscle, brain and liver and accumulation of CIV assembly intermediates in brain and liver (Yang, 2010). Similar to what happens in patients, the phenotype of the Sco2KI/KI was less severe than the compound heterozygous Sco2KO/KI mice. Both mice had impaired motor function with a later onset in the Sco2KI/KI than in the Sco2KO/KI mouse (8 and 4 months respectively). Accordingly, the CIV defect was higher in the Sco2KO/KI than in the Sco2KI/KI mouse. However, whereas most SCO2 deficient patients die in infancy of a combined cardiopathy and myopathy, the Sco2KI/KI and Sco2KO/KI mice do not show any evidence of cardiomyopathy or reduction in lifespan (Yang, 2010). Two major differences between mice and humans could explain this milder phenotype. First, in patients the most affected tissue is the skeletal muscle whereas in mice is the liver. Second, it has been proposed a further role of Sco2 independent of CIV function/assembly. An additional regulatory role of SCO2 on copper homeostasis would explain the reduction in total cellular cooper found in patient cells and tissues (Leary, 2007; Stiburek, 2009). However, Sco2 mutant animals did not show any reduction in the total amount of copper in the examined tissues (brain, heart, liver and muscle), but interestingly, only a decline in copper levels in the mitochondrial fraction was observed (Yang, 2010).
7. Mouse models of Complex V deficiency
Mitochondrial complex V or ATP synthase is the key enzyme of mitochondrial ATP production. ATP synthase is composed by two functional modules connected by two stalks: the hydrophilic F1 portion involved in the synthesis and hydrolysis of ATP and the hydrophobic Fo module that translocates protons from the intermembrane space back to the matrix (Stock, 1999; Walker, 1995). Defects of the ATP synthase are mostly linked to mutations of the mtDNA. In particular, neuropathy, ataxia and retinitis pigmentosa (NARP syndrome) and maternally inherited LS are associated to ATP6 mutations (Holt, 1990), whereas hypertrophic cardiomyopathy is usually caused by a mutation found in ATP8 (Jonckheere, 2008). A distinct group of inherited inborn defects of ATP synthase is due to mutations in nuclear genes that give rise to a typical phenotype characterized by 3-methylglutaconic aciduria, hypertrophic cardiomyopathy, neonatal-onset hypotonia and lactic acidosis. Only one nuclear encoded structural subunit (ATP5E) has been found mutated in patients (Mayr, 2010), while the most of nuclear defects affect assembly factors of complex V as ATP12 (De Meirleir, 2004) and TMEM70 (Cizkova, 2008; Torraco, 2012). In particular, TMEM70 is so far considered the most common cause of mendelian-inherited isolated ATP synthase deficiency (Spiegel, 2011).
To create a mouse model of CV deficiency, Cuezva’s group produced a transgenic mouse that overexpresses a mutant human IF1 (hIF1, inhibitory factor 1) in neurons (Formentini, 2014) (Table III). Although not a structural subunit of CV, IF1 is an endogenous inhibitor exerting regulatory function. IF1 prevents the futile conversion of ATP into ADP when substrates for the OXPHOS system become limiting and the cell must utilize glycolysis to generate ATP. IF1 binds to the ATPase between the alpha and beta subunits of the F1 module and blocks ATP hydrolysis (Pullman and Monroy, 1963). The transgenic mouse overexpressing the hIF1 carries a mutation in this gene (H49K) that conferred increased affinity of hIF1 to the beta subunit and inhibited ATPase hydrolysis activity. Inhibition of the ATP synthase in neurons caused a mild oxidative stress phenotype associated with increased protein carbonyls and increased levels of catalase, SOD1 and SOD2. Besides a decreased respiration and lower ATP levels, the hIF1 transgenic mouse had a significant reduction on CIV activity and its association into supercomplexes whereas activities of CI and CII+CIII were not affected. Interestingly, the hIF1 trangenic mouse was refractive to excitotoxicity caused by quinolinic acid injection into the striatum indicating an important role of this factor in apoptosis and cell survival pathways. The authors postulate that the mild ROS caused by the ATPase inhibition produced a preconditioning effect perhaps mediated by an activation of AKT and a metabolic reprograming (Formentini, 2014).
An IF1 KO mouse was created by replacing exons 1, 2 and part of 3 with a neomycin cassette in the Atpif1 locus (Table III). IF1 KO did not show any phenotype for at least the first year of life when compared to the control mice. Unexpectedly, no abnormalities in tissues histology, or mitochondria and cristae morphology were observed. At the biochemical level, CV was expressed in similar levels in both IF1 KO and controls in the tissues examined. The ATP synthesis driven by succinate was similar too and only the ATP hydrolysis activity was small but significantly higher in the KO. These results revealed a non essential role for the IF1 in normal conditions however it may become essential under stress (Nakamura, 2013). This remains to be tested.
IF1 KO mouse did not represent a suitable model for CV deficiencies therefore would be of importance to develop a model to resemble the human phenotype. A good candidate would be the TMEM70 gene since a growing number of patients with ATP synthase deficiencies have mutations mostly in this gene (Torraco, 2012). A CV deficient mouse will be indispensable to characterize the mechanism of pathogenesis and test compounds that have already given promising results in both yeast and cybrid cell lines (Couplan, 2011).
8. Mouse models of mtDNA mutations in OXPHOS subunits and transmitochondrial mouse models
The scientific community has put lots of effort in generating mouse models of mtDNA defects, but the first attempts gave rise to models carrying mutations whose pathogenicity is quite controversial (Jenuth, 1997; Levy, 1999; Marchington, 1999; Sligh, 2000; Watanabe, 1978). The main difficulty in generating mice carrying mtDNA deletions is to stably introduce mutagenized mtDNA in mitochondria of mammalian cells. Nevertheless, selecting mouse cell lines with naturally occurring mtDNA mutations circumvented this problem. A summary of the mouse models harboring mtDNA mutations is shown in table IV.
Table IV.
Mouse models carrying mutations in the mtDNA
| Gene | Mitochondrial Function |
Genetic Manipulation | Phenotype | References |
|---|---|---|---|---|
| “Common deletion” |
Encompasses tRNAs and 7 structural genes of CI, CIV, CV |
Electrofusion of pronucleus- stage embryos with several enucleated cytoplasts of the ΔmtDNA cybrids. Positive clones were transferred to the oviduct of pseudo pregnant females |
Mosaic pattern of COX negative and positive fibers both in heart and muscle; peaks of lactic acid in peripheral blood; kidney impairment |
(Inoue, 2000) |
| Cox1 | Catalytic subunit of CIV |
Fusion of cytoplasts carrying the homoplasmic T6589C missense mutation with ES cells deprived of mtDNA. Positive clones were introduced into 8-cell-stage embryos |
Growth retardation and CIV deficiency in brain, heart, liver and skeletal muscles. No motor or neurological phenotype |
(Kasahara, 2006) |
| Cox1/Nd6 | Catalytic subunits of CIV and CI |
Fusion of cytoplasts carrying both the homoplasmic T6589C missense mutation and a Nd6 frameshift mutation with ES cells devoid of mitochondria (ρ0) |
Mitochondrial myopathy and cardiomyopathy; decrease CIV activity in brain, liver, heart and skeletal muscle; RRF and abnormal mitochondria in muscle. No motor or neurological phenotype |
(Fan, 2008) |
| Nd6 | Core subunit of CI |
Cells containing the homoplasmic G13997A missense mutation were fused with ρo ES cells. Positive clones were fused with a 8- cell-stage embryos and implanted into pseudo pregnant female |
CI+III in different tissues. Age associated disorders at longer observation |
(Hashizume , 2012) |
| Enucleated LMTK cell lines carrying homoplasmic G13997A mutation were fused with female mouse ES cells deprived of mitochondria. Positive clones were injected into blastocyst to produce chimera females |
Phenotype resembling the clinical features of LHON patients, characterized by decreased retinal response and swollen axons in the optic tract; CI deficiency in liver and brain; high levels of ROS in the brain. |
(Lin, 2012) | ||
| tRNALys | Mitochondrial translational component |
The somatic G7731A mutation was introduced in female ES cells lacking mitochondria. Mice with different loads of G7731A mtDNA were obtained in subsequent generations. |
Transmitochondrial mice carrying 85% tRNALys G8344A displayed short body length, muscle weakness and RRF. Respiratory chain defect and ROS production was particularly evident in skeletal muscle. |
(Shimizu, 2014) |
8.1. ΔmtDNA
Inoue and colleagues generated transmitochondrial mouse carrying a 4.7 Kb deletion resembling the “common deletion” found in humans (Inoue, 2000). They modeled the disease by electrofusing human enucleated cybrid cells, carrying the 4.7 Kb mtDNA deletion, with mouse pronucleus of early stage embryos. Surprisingly, the ΔmtDNA was successfully transmitted to the progeny, from F1 to F3, but the mutational load was not higher than 90%, probably due to embryonic lethality. Histochemical analysis of both skeletal muscle and cardiac tissue showed a mosaic pattern of COX positive and negative fibers, according to the mutant load. Lactic acid accumulation in peripheral blood was proportional to the amount of mutated mtDNA. The gross phenotype observed in ΔmtDNA mice was characterized by systemic ischemia and enlarged kidney. Due to the high concentration of urea, nitrogen and creatinine, the authors suggested that death may occur for a kidney failure subsequent to progressive respiratory defect (Inoue, 2000). However, this model do not resemble the major features typically found in patients such as diabetes, hearing loss, exercise intolerance and pancreatic dysfunction underlining a diverse species segregation of ΔmtDNA among different tissues. Moreover, the maternal inheritance of deleted mtDNA molecules is not common in humans. The authors explain that the transmission of ΔmtDNA in their mice may be due either to the transmission of duplicated molecules that, subsequently, recombine and give rise to deleted molecules or to the transmission of deleted/duplicated molecules in those tissues that are resistant to respiratory defects.
8.2. Cox1
The first transmitochondrial mouse (mito-mouse) carrying a point mutation in the mtDNA was generated by Kasahara and colleagues (Kasahara, 2006). They introduced the missense mutation T6589C in the Cox1 gene that lowered CIV activity up to 50%. The T6589C mouse model was created by fusing the cytoplast of mouse cells carrying the homoplasmic mutation with mitochondria-devoid ES cells. Positive clones for the mutation were selected, injected into the embryos and subsequently transferred to a pseudo pregnant female. The resulted mice had 100% T6589C mutation in all tissues analyzed (Kasahara, 2006). To confirm that the observed phenotype was due exclusively to the Cox1 T6598C mutation and not to additional contribution of nuclear background, female mito-mice were backcrossed with B6 males and F6 progeny was analyzed. The F6 mito-mice showed growth retardation until 18 weeks and at the age of 6 months they showed a marked decrease of CIV activity in the brain, heart, liver and skeletal muscle (50% – 70% of the control levels). Increased blood lactate levels were detected in these mice but no signs of epilepsy were found, which has been reported in patients (Kasahara, 2006).
8.3. Nd6
By using the cytoplast fusion technique, that consists in the fusion of an enucleated mouse cell line (LA9) with mtDNA deprived (ρ0) mouse cell line (LMEB4), the group of Wallace created a second transmitochondrial mouse model carrying two mutations, the T6589C mutation in Cox1 mentioned above and the 13885insC frameshift mutation in the Nd6 gene, able to totally inactivate CI function (Fan, 2008). Interestingly, it was observed that the amount of heteroplasmy of the Nd6 mutation decreased in the female germ line. Total loss of the mutation occurred in 4 generations, whereas the Cox1 mutation persisted at homoplasmic levels over time, causing mitochondrial myopathy and cardiomyopathy (Fan, 2008). This study is the first strong evidence indicating the existence of a mechanism in the female germ line that recognizes and eliminates the more pathogenic mutation from the mtDNA.
Another Mito-mouse model harbors the missense G13997A mutation in Nd6 (P25L) found in a lung carcinoma cell line that was associated with pro-metastatic activity and enhanced ROS production (Ishikawa, 2008). Interestingly, this mutation in murine mtDNA corresponds to the G14600A mutation in the human MT-ND6 gene found in homoplasmy in a patient with a progressive encephalomyopathy with MRI features consistent with LS. Interestingly, in heteroplasmy the same mutation caused optic atrophy and cerebellar ataxia (Malfatti, 2007). Mito-mice Nd6 were homoplasmic for the mutation and displayed a mild reduction of CI+III activity in multiple tissues (about 20–30%). Young mice looked healthy and showed only a moderate increase of lactate in blood. A long-term observation of Mito-mice Nd6 led to the conclusion that the G13997A mutation was responsible for age-associated disorders, such as increased glucose level in the blood and development of B-lymphoma, but no neurological or ophthalmological symptoms were displayed (Hashizume, 2012).
Surprisingly, the same G13997A mutation placed into a different mouse strain led to the generation of the first real mtDNA mouse model resembling human pathology (Lin, 2012). The heteroplasmic Nd6 mouse was obtained by mutagenizing LMTK- cell lines and selecting the respiratory defective clones using glucose or galactose containing media. Homoplasmic G13997A clones displayed isolated CI deficiency (23% residual activity), 65% reduction in ATP synthesis and increased ROS production (Lin, 2012). Enucleated clones were fused with female mouse ES cells deprived of mitochondria and the resulted fused cells containing the mutation were injected into blastocyst. Despite the systemic reduction of CI activity, the phenotype was restricted to optic nerve and mirrored some typical LHON (Leber hereditary optic neuropathy) pathological features. In fact, 14-months old mice showed decreased retinal response, preferential loss of small retinal fibers and swollen axons of retinal ganglion cells, due to the accumulation of morphologically abnormal mitochondria. ROS levels were consistently high in mitochondria and synaptosomes isolated from the brain. Surprisingly, ATP production was not compromised in synaptosomes, indicating that the primary cause of the retinal impairment found in LHON patients is the chronic oxidative damage rather than the energetic failure (Lin, 2012). This mouse represents a valuable model to test antioxidant therapies in vivo that may be effective in preventing the development of optic disease due to mtDNA mutations.
8.4. tRNALys
The first transmitochondrial mouse carrying a point mutation in tRNALys gene was generated very recently by selecting a tRNA mutation by PCR screening of somatic variants occurring in mouse lung carcinoma P29 cells (Shimizu, 2014). The G7731A mutation was highly conserved among the species and had a counterpart in human mtDNA from patients affected by mitochondrial disease. Subcloning of the initial 48% mutational load clones allowed to select higher percentage of mutation, up to more than 90%. The resulted clones had defective respiration and increased ROS production when compared to parental P29 cells (Shimizu, 2014). To avoid interference of the genetic background, the cytoplast of G7731A clones were fused with ρ0 B82 cells. The resulting cybrids carried 70 and 90% mutational load, maintained reduced oxygen consumption and increased ROS production. Transmitochondrial mice were created by fusing female ES cells devoid of mitochondria with the cybrid clone with 70% heteroplasmy of the tRNALys G7731A. Analysis of F1 “Mito-mice-tRNALys7731” confirmed the transmission of the G7731A. Quantification of the mutation in the subsequent generation did not showed percentages higher than 85% heteroplasmy due to the loss of oocytes containing higher mutational load. Phenotypically, mice with 85% G7731A mtDNA displayed some features of human mtDNA disease, such as short body length, muscle weakness and ragged red-fibers (RRF), due to the respiratory deficiency of skeletal muscle mitochondria. However, mito-mice-tRNALys7731 did not reproduced the severe phenotype of patients with tRNALys G8344A associated with MERRF (myoclonic epilepsy with ragged red fibers) because of the lower percentage of mtDNA mutation transmitted. However, it cannot be excluded a late onset of the disease due to accumulation of the mutation in aged mice. A striking difference between Mito-mice-tRNALys7731 and humans carrying mutations in the tRNALys gene is represented by the loss of oocyte with high mutational load in mice. This aspect underlines ones more the difficulties in creating transmitochondrial mice models resembling human defects and, probably, species specific differences in mtDNA selection during germ line transmission and segregation in tissues. Nevertheless, in our opinion, this mouse model is suitable for studying specific forms of human mitochondrial myopathies and eventually test either antioxidant compounds or engineered mitonucleases (Bacman, 2013) able to recognize specifically the mutant mtDNA molecules and shift the heteroplasmic levels towards more wild type mtDNA.
9. Concluding remarks
The aim of this review was to comprehensively summarize the mouse models of mitochondrial diseases generated in the last decade and highlight their importance in the recapitulation of human clinical presentations. In particular we focused on those models related to subunits and assembly factors of the OXPHOS system. While a great advance in this field has been made by the creation of a wide variety of mouse models carrying nuclear gene defects, the generation of models with mutations in the mtDNA is still scarce, mostly due to the difficulties in manipulating the mitochondrial genome.
Important studies have clarified the molecular and pathogenic mechanisms of some genetic defects and have paved the way for pioneering therapeutic approaches (discussed in the third part of this review miniseries). Despite the variety of mouse models already available, we still have a gap of knowledge on the pathogenic mechanisms of many mitochondrial diseases. With the creation of new models, we will be a step closer to design therapies that would be beneficial for more than one particular disease. We foresee that in the next few years this will be a very fruitful field in medical research.
Highlights.
Mitochondrial diseases are characterized by defects in the OXPHOS system
Mitochondrial diseases can be caused by mutations in either mitochondrial or nuclear DNA genes encoding for subunits of the respiratory complexes or assembly factors.
Most of the mouse models recapitulate phenotypic features of the human mitochondrial disorders.
Mouse models of mitochondrial disorders contribute to understand the pathogenic mechanisms of disease.
Acknowledgements
We are thankful to Jose Oca for critical comments of this manuscript. FD research has been funded by the James and Esther King Research Program Florida Department of Health (grant number 08KN-01), by the National Institute of Health (grant number GM101225) and by the United Mitochondrial Disease Foundation (grant number 14050R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: Authors have nothing to declare.
Contributor Information
Alessandra Torraco, Email: alessandra.torraco@opbg.net.
Susana Peralta, Email: SPeralta@med.miami.edu.
Luisa Iommarini, Email: luisa.iommarini2@unibo.it.
Francisca Diaz, Email: FDiaz1@med.miami.edu.
References
- Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostino A, Invernizzi F, Tiveron C, Fagiolari G, Prelle A, Lamantea E, Giavazzi A, Battaglia G, Tatangelo L, Tiranti V, Zeviani M. Constitutive knockout of Surf1 is associated with high embryonic lethality, mitochondrial disease and cytochrome c oxidase deficiency in mice. Hum Mol Genet. 2003;12:399–413. doi: 10.1093/hmg/ddg038. [DOI] [PubMed] [Google Scholar]
- Anderson SL, Chung WK, Frezzo J, Papp JC, Ekstein J, DiMauro S, Rubin BY. A novel mutation in NDUFS4 causes Leigh syndrome in an Ashkenazi Jewish family. J Inherit Metab Dis. 2008;31(Suppl 2):S461–S467. doi: 10.1007/s10545-008-1049-9. [DOI] [PubMed] [Google Scholar]
- Antonicka H, Leary SC, Guercin GH, Agar JN, Horvath R, Kennaway NG, Harding CO, Jaksch M, Shoubridge EA. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum Mol Genet. 2003a;12:2693–2702. doi: 10.1093/hmg/ddg284. [DOI] [PubMed] [Google Scholar]
- Antonicka H, Mattman A, Carlson CG, Glerum DM, Hoffbuhr KC, Leary SC, Kennaway NG, Shoubridge EA. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am J Hum Genet. 2003b;72:101–114. doi: 10.1086/345489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonicka H, Ogilvie I, Taivassalo T, Anitori RP, Haller RG, Vissing J, Kennaway NG, Shoubridge EA. Identification and characterization of a common set of complex I assembly intermediates in mitochondria from patients with complex I deficiency. J Biol Chem. 2003c;278:43081–43088. doi: 10.1074/jbc.M304998200. [DOI] [PubMed] [Google Scholar]
- Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med. 2013;19:1111–1113. doi: 10.1038/nm.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A, Gouget K, Horn D, Soto IC, Fontanesi F. Suppression mechanisms of COX assembly defects in yeast and human: insights into the COX assembly process. Biochim Biophys Acta. 2009;1793:97–107. doi: 10.1016/j.bbamcr.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley JP, van Minderhout I, Hogendoorn PC, Cornelisse CJ, van der Wal A, Prins FA, Teppema L, Dahan A, Devilee P, Taschner PE. Sdhd and SDHD/H19 knockout mice do not develop paraganglioma or pheochromocytoma. PLoS One. 2009;4:e7987. doi: 10.1371/journal.pone.0007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benit P, Lebon S, Rustin P. Respiratory-chain diseases related to complex III deficiency. Biochim Biophys Acta. 2009;1793:181–185. doi: 10.1016/j.bbamcr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Bénit P, Steffann J, Lebon S, Chretien D, Kadhom N, de Lonlay P, Goldenberg A, Dumez Y, Dommergues M, Rustin P, Munnich A, Rötig A. Genotyping microsatellite DNA markers at putative disease loci in inbred/multiplex families with respiratory chain complex I deficiency allows rapid identification of a novel nonsense mutation (IVS1nt −1) in the NDUFS4 gene in Leigh syndrome. Hum Genet. 2003;112:563–566. doi: 10.1007/s00439-002-0884-2. [DOI] [PubMed] [Google Scholar]
- Budde SM, van den Heuvel LP, Janssen AJ, Smeets RJ, Buskens CA, DeMeirleir L, Van Coster R, Baethmann M, Voit T, Trijbels JM, Smeitink JA. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochemical and biophysical research communications. 2000;275:63–68. doi: 10.1006/bbrc.2000.3257. [DOI] [PubMed] [Google Scholar]
- Budde SM, van den Heuvel LP, Smeets RJ, Skladal D, Mayr JA, Boelen C, Petruzzella V, Papa S, Smeitink JA. Clinical heterogeneity in patients with mutations in the NDUFS4 gene of mitochondrial complex I. J Inherit Metab Dis. 2003;26:813–815. doi: 10.1023/b:boli.0000010003.14113.af. [DOI] [PubMed] [Google Scholar]
- Bugiani M, Invernizzi F, Alberio S, Briem E, Lamantea E, Carrara F, Moroni I, Farina L, Spada M, Donati MA, Uziel G, Zeviani M. Clinical and molecular findings in children with complex I deficiency. Biochim Biophys Acta. 2004;1659:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bugiani M, Tiranti V, Farina L, Uziel G, Zeviani M. Novel mutations in COX15 in a long surviving Leigh syndrome patient with cytochrome c oxidase deficiency. J Med Genet. 2005;42:e28. doi: 10.1136/jmg.2004.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvaruso MA, Willems P, van den Brand M, Valsecchi F, Kruse S, Palmiter R, Smeitink J, Nijtmans L. Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum Mol Genet. 2012;21:115–120. doi: 10.1093/hmg/ddr446. [DOI] [PubMed] [Google Scholar]
- Carelli V, Ross-Cisneros FN, Sadun AA. Optic nerve degeneration and mitochondrial dysfunction: genetic and acquired optic neuropathies. Neurochemistry international. 2002;40:573–584. doi: 10.1016/s0197-0186(01)00129-2. [DOI] [PubMed] [Google Scholar]
- Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE. Bovine complex I is a complex of 45 different subunits. J Biol Chem. 2006;281:32724–32727. doi: 10.1074/jbc.M607135200. [DOI] [PubMed] [Google Scholar]
- Cizkova A, Stranecky V, Mayr JA, Tesarova M, Havlickova V, Paul J, Ivanek R, Kuss AW, Hansikova H, Kaplanova V, Vrbacky M, Hartmannova H, Noskova L, Honzik T, Drahota Z, Magner M, Hejzlarova K, Sperl W, Zeman J, Houstek J, Kmoch S. TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat Genet. 2008;40:1288–1290. doi: 10.1038/ng.246. [DOI] [PubMed] [Google Scholar]
- Coenen MJ, van den Heuvel LP, Ugalde C, Ten Brinke M, Nijtmans LG, Trijbels FJ, Beblo S, Maier EM, Muntau AC, Smeitink JA. Cytochrome c oxidase biogenesis in a patient with a mutation in COX10 gene. Ann Neurol. 2004;56:560–564. doi: 10.1002/ana.20229. [DOI] [PubMed] [Google Scholar]
- Couplan E, Aiyar RS, Kucharczyk R, Kabala A, Ezkurdia N, Gagneur J, St Onge RP, Salin B, Soubigou F, Le Cann M, Steinmetz LM, di Rago JP, Blondel M. A yeast-based assay identifies drugs active against human mitochondrial disorders. Proc Natl Acad Sci U S A. 2011;108:11989–11994. doi: 10.1073/pnas.1101478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoudi M, Kotarsky H, Hansson E, Fellman V. Complex I Function and Supercomplex Formation Are Preserved in Liver Mitochondria Despite Progressive Complex III Deficiency. PLoS One. 2014;9:e86767. doi: 10.1371/journal.pone.0086767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lonlay P, Valnot I, Barrientos A, Gorbatyuk M, Tzagoloff A, Taanman JW, Benayoun E, Chretien D, Kadhom N, Lombes A, de Baulny HO, Niaudet P, Munnich A, Rustin P, Rotig A. A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat Genet. 2001;29:57–60. doi: 10.1038/ng706. [DOI] [PubMed] [Google Scholar]
- De Meirleir L, Seneca S, Lissens W, De Clercq I, Eyskens F, Gerlo E, Smet J, Van Coster R. Respiratory chain complex V deficiency due to a mutation in the assembly gene ATP12. J Med Genet. 2004;41:120–124. doi: 10.1136/jmg.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rocco D, Cerqua C, Goffrini P, Russo G, Pastore A, Meloni F, Nicchia E, Moraes CT, Pecci A, Salviati L, Savoia A. Mutations of cytochrome c identified in patients with thrombocytopenia THC4 affect both apoptosis and cellular bioenergetics. Biochim Biophys Acta. 2014;1842:269–274. doi: 10.1016/j.bbadis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Pulliam D, Hill S, Shi Y, Walsh ME, Salmon A, Sloane L, Zhang N, Zeviani M, Viscomi C, Musi N, Van Remmen H. Improved insulin sensitivity associated with reduced mitochondrial complex IV assembly and activity. FASEB J. 2013;27:1371–1380. doi: 10.1096/fj.12-221879. [DOI] [PubMed] [Google Scholar]
- Dell'agnello C, Leo S, Agostino A, Szabadkai G, Tiveron C, Zulian A, Prelle A, Roubertoux P, Rizzuto R, Zeviani M. Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet. 2007;16:431–444. doi: 10.1093/hmg/ddl477. [DOI] [PubMed] [Google Scholar]
- Diaz F. Cytochrome c oxidase deficiency: patients and animal models. Biochim Biophys Acta. 2010;1802:100–110. doi: 10.1016/j.bbadis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Diaz F, Garcia S, Hernandez D, Regev A, Rebelo A, Oca-Cossio J, Moraes CT. Pathophysiology and fate of hepatocytes in a mouse model of mitochondrial hepatopathies. Gut. 2008;57:232–242. doi: 10.1136/gut.2006.119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Garcia S, Padgett KR, Moraes CT. A defect in the mitochondrial complex III, but not complex IV, triggers early ROS-dependent damage in defined brain regions. Hum Mol Genet. 2012;21:5066–5077. doi: 10.1093/hmg/dds350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Kotarsky H, Fellman V, Moraes CT. Mitochondrial disorders caused by mutations in respiratory chain assembly factors. Semin Fetal Neonatal Med. 2011;16:197–204. doi: 10.1016/j.siny.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Thomas CK, Garcia S, Hernandez D, Moraes CT. Mice lacking COX10 in skeletal muscle recapitulate the phenotype of progressive mitochondrial myopathies associated with cytochrome c oxidase deficiency. Hum Mol Genet. 2005;14:2737–2748. doi: 10.1093/hmg/ddi307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annual review of neuroscience. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Tanji K, Schon EA. The many clinical faces of cytochrome c oxidase deficiency. Adv Exp Med Biol. 2012;748:341–357. doi: 10.1007/978-1-4614-3573-0_14. [DOI] [PubMed] [Google Scholar]
- Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A, Muda AO, Valente ML, Bertini E, Emma F. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. Journal of the American Society of Nephrology : JASN. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- Duncan AJ, Bitner-Glindzicz M, Meunier B, Costello H, Hargreaves IP, Lopez LC, Hirano M, Quinzii CM, Sadowski MI, Hardy J, Singleton A, Clayton PT, Rahman S. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emma F, Bertini E, Salviati L, Montini G. Renal involvement in mitochondrial cytopathies. Pediatric nephrology. 2012;27:539–550. doi: 10.1007/s00467-011-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Waymire KG, Narula N, Li P, Rocher C, Coskun PE, Vannan MA, Narula J, Macgregor GR, Wallace DC. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science. 2008;319:958–962. doi: 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley IM, Walker JE. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim Biophys Acta. 1992;1140:105–134. doi: 10.1016/0005-2728(92)90001-i. [DOI] [PubMed] [Google Scholar]
- Feng J, Bussière F, Hekimi S. Mitochondrial Electron Transport Is a Key Determinant of Life Span in Caenorhabditis elegans. Developmental Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vizarra E, Tiranti V, Zeviani M. Assembly of the oxidative phosphorylation system in humans: what we have learned by studying its defects. Biochim Biophys Acta. 2009;1793:200–211. doi: 10.1016/j.bbamcr.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Ke BX, Nguyen TV, Henstridge DC, Penfold SA, Laskowski A, Sourris KC, Groschner LN, Cooper ME, Thorburn DR, Coughlan MT. Deficiency in mitochondrial complex I activity due to Ndufs6 gene trap insertion induces renal disease. Antioxid Redox Signal. 2013;19:331–343. doi: 10.1089/ars.2012.4719. [DOI] [PubMed] [Google Scholar]
- Formentini L, Pereira MP, Sanchez-Cenizo L, Santacatterina F, Lucas JJ, Navarro C, Martinez-Serrano A, Cuezva JM. In vivo inhibition of the mitochondrial H+-ATP synthase in neurons promotes metabolic preconditioning. The EMBO journal. 2014;33:762–778. doi: 10.1002/embj.201386392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Corzo L, Luna-Sanchez M, Doerrier C, Garcia JA, Guaras A, Acin-Perez R, Bullejos-Peregrin J, Lopez A, Escames G, Enriquez JA, Acuna-Castroviejo D, Lopez LC. Dysfunctional Coq9 protein causes predominant encephalomyopathy associated with CoQ deficiency. Hum Mol Genet. 2013;22:1233–1248. doi: 10.1093/hmg/dds530. [DOI] [PubMed] [Google Scholar]
- Guillausseau PJ, Massin P, Dubois-LaForgue D, Timsit J, Virally M, Gin H, Bertin E, Blickle JF, Bouhanick B, Cahen J, Caillat-Zucman S, Charpentier G, Chedin P, Derrien C, Ducluzeau PH, Grimaldi A, Guerci B, Kaloustian E, Murat A, Olivier F, Paques M, Paquis-Flucklinger V, Porokhov B, Samuel-Lajeunesse J, Vialettes B. Maternally inherited diabetes and deafness: a multicenter study. Annals of internal medicine. 2001;134:721–728. doi: 10.7326/0003-4819-134-9_part_1-200105010-00008. [DOI] [PubMed] [Google Scholar]
- Hallman TM, Peng M, Meade R, Hancock WW, Madaio MP, Gasser DL. The mitochondrial and kidney disease phenotypes of kd/kd mice under germfree conditions. J Autoimmun. 2006;26:1–6. doi: 10.1016/j.jaut.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, Yeh WC, Ohashi P, Wang X, Mak TW. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Hashizume O, Shimizu A, Yokota M, Sugiyama A, Nakada K, Miyoshi H, Itami M, Ohira M, Nagase H, Takenaga K, Hayashi J. Specific mitochondrial DNA mutation in mice regulates diabetes and lymphoma development. Proc Natl Acad Sci U S A. 2012;109:10528–10533. doi: 10.1073/pnas.1202367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut S, Brivet M, Touati G, Rustin P, Lebon S, Garcia-Cazorla A, Saudubray JM, Boutron A, Legrand A, Slama A. A deletion in the human QP-C gene causes a complex III deficiency resulting in hypoglycaemia and lactic acidosis. Hum Genet. 2003;113:118–122. doi: 10.1007/s00439-003-0946-0. [DOI] [PubMed] [Google Scholar]
- Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, Xie LX, Salviati L, Hurd TW, Vega-Warner V, Killen PD, Raphael Y, Ashraf S, Ovunc B, Schoeb DS, McLaughlin HM, Airik R, Vlangos CN, Gbadegesin R, Hinkes B, Saisawat P, Trevisson E, Doimo M, Casarin A, Pertegato V, Giorgi G, Prokisch H, Rotig A, Nurnberg G, Becker C, Wang S, Ozaltin F, Topaloglu R, Bakkaloglu A, Bakkaloglu SA, Muller D, Beissert A, Mir S, Berdeli A, Varpizen S, Zenker M, Matejas V, Santos-Ocana C, Navas P, Kusakabe T, Kispert A, Akman S, Soliman NA, Krick S, Mundel P, Reiser J, Nurnberg P, Clarke CF, Wiggins RC, Faul C, Hildebrandt F. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. The Journal of clinical investigation. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JT, Fantin VR, Schonberger J, Breivik N, Siem G, McDonough B, Sharma P, Keogh I, Godinho R, Santos F, Esparza A, Nicolau Y, Selvaag E, Cohen BH, Hoppel CL, Tranebjaerg L, Eavey RD, Seidman JG, Seidman CE. Missense mutations in the BCS1L gene as a cause of the Bjornstad syndrome. The New England journal of medicine. 2007;356:809–819. doi: 10.1056/NEJMoa055262. [DOI] [PubMed] [Google Scholar]
- Hirano M, Garone C, Quinzii CM. CoQ(10) deficiencies and MNGIE: two treatable mitochondrial disorders. Biochim Biophys Acta. 2012;1820:625–631. doi: 10.1016/j.bbagen.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Carroll J, Fearnley IM, Shannon RJ, Walker JE. The nuclear encoded subunits of complex I from bovine heart mitochondria. Biochim Biophys Acta. 2003;1604:135–150. doi: 10.1016/s0005-2728(03)00059-8. [DOI] [PubMed] [Google Scholar]
- Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990;46:428–433. [PMC free article] [PubMed] [Google Scholar]
- Hughes BG, Hekimi S. A Mild Impairment of Mitochondrial Electron Transport Has Sex-Specific Effects on Lifespan and Aging in Mice. PLoS ONE. 2011;6:e26116. doi: 10.1371/journal.pone.0026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttemann M, Kadenbach B, Grossman LI. Mammalian subunit IV isoforms of cytochrome c oxidase. Gene. 2001;267:111–123. doi: 10.1016/s0378-1119(01)00385-7. [DOI] [PubMed] [Google Scholar]
- Huttemann M, Klewer S, Lee I, Pecinova A, Pecina P, Liu J, Lee M, Doan JW, Larson D, Slack E, Maghsoodi B, Erickson RP, Grossman LI. Mice deleted for heart-type cytochrome c oxidase subunit 7a1 develop dilated cardiomyopathy. Mitochondrion. 2012a;12:294–304. doi: 10.1016/j.mito.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttemann M, Lee I, Gao X, Pecina P, Pecinova A, Liu J, Aras S, Sommer N, Sanderson TH, Tost M, Neff F, Aguilar-Pimentel JA, Becker L, Naton B, Rathkolb B, Rozman J, Favor J, Hans W, Prehn C, Puk O, Schrewe A, Sun M, Hofler H, Adamski J, Bekeredjian R, Graw J, Adler T, Busch DH, Klingenspor M, Klopstock T, Ollert M, Wolf E, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Weissmann N, Doan JW, Bassett DJ, Grossman LI. Cytochrome coxidase subunit 4 isoform 2-knockout mice show reduced enzyme activity, airway hyporeactivity, and lung pathology. FASEB J. 2012b;26:3916–3930. doi: 10.1096/fj.11-203273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrieri A, van Rahden VA, Tiranti V, Morleo M, Iaconis D, Tammaro R, D'Amato I, Conte I, Maystadt I, Demuth S, Zvulunov A, Kutsche K, Zeviani M, Franco B. Mutations in COX7B cause microphthalmia with linear skin lesions, an unconventional mitochondrial disease. Am J Hum Genet. 2012;91:942–949. doi: 10.1016/j.ajhg.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham CA, Burwell LS, Skalska J, Brookes PS, Howell RL, Sheu SS, Pinkert CA. NDUFS4: creation of a mouse model mimicking a Complex I disorder. Mitochondrion. 2009;9:204–210. doi: 10.1016/j.mito.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Nakada K, Ogura A, Isobe K, Goto Y, Nonaka I, Hayashi JI. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat Genet. 2000;26:176–181. doi: 10.1038/82826. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Hashizume O, Koshikawa N, Fukuda S, Nakada K, Takenaga K, Hayashi J. Enhanced glycolysis induced by mtDNA mutations does not regulate metastasis. FEBS letters. 2008;582:3525–3530. doi: 10.1016/j.febslet.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Iwasaki N, Babazono T, Tsuchiya K, Tomonaga O, Suzuki A, Togashi M, Ujihara N, Sakka Y, Yokokawa H, Ogata M, Nihei H, Iwamoto Y. Prevalence of A-to-G mutation at nucleotide 3243 of the mitochondrial tRNA(Leu(UUR)) gene in Japanese patients with diabetes mellitus and end stage renal disease. Journal of human genetics. 2001;46:330–334. doi: 10.1007/s100380170068. [DOI] [PubMed] [Google Scholar]
- Jaksch M, Ogilvie I, Yao J, Kortenhaus G, Bresser HG, Gerbitz KD, Shoubridge EA. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum Mol Genet. 2000;9:795–801. doi: 10.1093/hmg/9.5.795. [DOI] [PubMed] [Google Scholar]
- Jenuth JP, Peterson AC, Shoubridge EA. Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nat Genet. 1997;16:93–95. doi: 10.1038/ng0597-93. [DOI] [PubMed] [Google Scholar]
- Johns DR, Neufeld MJ. Cytochrome b mutations in Leber hereditary optic neuropathy. Biochemical and biophysical research communications. 1991;181:1358–1364. doi: 10.1016/0006-291x(91)92088-2. [DOI] [PubMed] [Google Scholar]
- Johnson A, Gin P, Marbois BN, Hsieh EJ, Wu M, Barros MH, Clarke CF, Tzagoloff A. COQ9, a new gene required for the biosynthesis of coenzyme Q in Saccharomyces cerevisiae. J Biol Chem. 2005;280:31397–31404. doi: 10.1074/jbc.M503277200. [DOI] [PubMed] [Google Scholar]
- Johnston PB, Gaster RN, Smith VC, Tripathi RC. A clinicopathologic study of autosomal dominant optic atrophy. American journal of ophthalmology. 1979;88:868–875. doi: 10.1016/0002-9394(79)90565-8. [DOI] [PubMed] [Google Scholar]
- Jonckheere AI, Hogeveen M, Nijtmans LG, van den Brand MA, Janssen AJ, Diepstra JH, van den Brandt FC, van den Heuvel LP, Hol FA, Hofste TG, Kapusta L, Dillmann U, Shamdeen MG, Smeitink JA, Rodenburg RJ. A novel mitochondrial ATP8 gene mutation in a patient with apical hypertrophic cardiomyopathy and neuropathy. J Med Genet. 2008;45:129–133. doi: 10.1136/jmg.2007.052084. [DOI] [PubMed] [Google Scholar]
- Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr., Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Ishikawa K, Yamaoka M, Ito M, Watanabe N, Akimoto M, Sato A, Nakada K, Endo H, Suda Y, Aizawa S, Hayashi J. Generation of trans-mitochondrial mice carrying homoplasmic mtDNAs with a missense mutation in a structural gene using ES cells. Hum Mol Genet. 2006;15:871–881. doi: 10.1093/hmg/ddl005. [DOI] [PubMed] [Google Scholar]
- Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem. 2009;53:217–226. doi: 10.1042/BA20090035. [DOI] [PubMed] [Google Scholar]
- Ke BX, Pepe S, Grubb DR, Komen JC, Laskowski A, Rodda FA, Hardman BM, Pitt JJ, Ryan MT, Lazarou M, Koleff J, Cheung MM, Smolich JJ, Thorburn DR. Tissue-specific splicing of an Ndufs6 gene-trap insertion generates a mitochondrial complex I deficiency-specific cardiomyopathy. Proc Natl Acad Sci U S A. 2012;109:6165–6170. doi: 10.1073/pnas.1113987109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley JA, Anitori R, Burton MD, Quan F, Buist NR, Kennaway NG. Mitochondrial encephalomyopathy and complex III deficiency associated with a stop-codon mutation in the cytochrome b gene. Am J Hum Genet. 2000;67:1400–1410. doi: 10.1086/316900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalimonchuk O, Rodel G. Biogenesis of cytochrome c oxidase. Mitochondrion. 2005;5:363–388. doi: 10.1016/j.mito.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kirby DM, Salemi R, Sugiana C, Ohtake A, Parry L, Bell KM, Kirk EP, Boneh A, Taylor RW, Dahl HH, Ryan MT, Thorburn DR. NDUFS6 mutations are a novel cause of lethal neonatal mitochondrial complex I deficiency. The Journal of clinical investigation. 2004;114:837–845. doi: 10.1172/JCI20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuf M, Faber J, Huth RG, Freisinger P, Zepp F, Kampmann C. Identification of a novel compound heterozygote SCO2 mutation in cytochrome c oxidase deficient fatal infantile cardioencephalomyopathy. Acta Paediatr. 2007;96:130–132. doi: 10.1111/j.1651-2227.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Hoftberger R, Majtenyi K, Horvath R, Barsi P, Komoly S, Lassmann H, Budka H, Jakab G. Neuropathology of white matter disease in Leber's hereditary optic neuropathy. Brain. 2005;128:35–41. doi: 10.1093/brain/awh310. [DOI] [PubMed] [Google Scholar]
- Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 2008;7:312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, McKenzie M, Ohtake A, Thorburn DR, Ryan MT. Analysis of the assembly profiles for mitochondrial- and nuclear-DNA-encoded subunits into complex I. Mol Cell Biol. 2007;27:4228–4237. doi: 10.1128/MCB.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary SC, Cobine PA, Kaufman BA, Guercin GH, Mattman A, Palaty J, Lockitch G, Winge DR, Rustin P, Horvath R, Shoubridge EA. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5:9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Leary SC, Sasarman F, Nishimura T, Shoubridge EA. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum Mol Genet. 2009;18:2230–2240. doi: 10.1093/hmg/ddp158. [DOI] [PubMed] [Google Scholar]
- Lee I, Huttemann M, Liu J, Grossman LI, Malek MH. Deletion of heart-type cytochrome c oxidase subunit 7a1 impairs skeletal muscle angiogenesis and oxidative phosphorylation. J Physiol. 2012;590:5231–5243. doi: 10.1113/jphysiol.2012.239707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong DW, Komen JC, Hewitt CA, Arnaud E, McKenzie M, Phipson B, Bahlo M, Laskowski A, Kinkel SA, Davey GM, Heath WR, Voss AK, Zahedi RP, Pitt JJ, Chrast R, Sickmann A, Ryan MT, Smyth GK, Thorburn DR, Scott HS. Proteomic and metabolomic analyses of mitochondrial complex I-deficient mouse model generated by spontaneous B2 short interspersed nuclear element (SINE) insertion into NADH dehydrogenase (ubiquinone) Fe-S protein 4 (Ndufs4) gene. J Biol Chem. 2012;287:20652–20663. doi: 10.1074/jbc.M111.327601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveen P, Kotarsky H, Morgelin M, Karikoski R, Elmer E, Fellman V. The GRACILE mutation introduced into Bcs1l causes postnatal complex III deficiency: a viable mouse model for mitochondrial hepatopathy. Hepatology. 2011;53:437–447. doi: 10.1002/hep.24031. [DOI] [PubMed] [Google Scholar]
- Levy SE, Waymire KG, Kim YL, MacGregor GR, Wallace DC. Transfer of chloramphenicol-resistant mitochondrial DNA into the chimeric mouse. Transgenic Res. 1999;8:137–145. doi: 10.1023/a:1008967412955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Williams RS. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- Lin CS, Sharpley MS, Fan W, Waymire KG, Sadun AA, Carelli V, Ross-Cisneros FN, Baciu P, Sung E, McManus MJ, Pan BX, Gil DW, Macgregor GR, Wallace DC. Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc Natl Acad Sci U S A. 2012;109:20065–20070. doi: 10.1073/pnas.1217113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, Dimauro S, Hirano M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majander A, Suomalainen A, Vettenranta K, Sariola H, Perkkio M, Holmberg C, Pihko H. Congenital hypoplastic anemia, diabetes, and severe renal tubular dysfunction associated with a mitochondrial DNA deletion. Pediatric research. 1991;30:327–330. doi: 10.1203/00006450-199110000-00007. [DOI] [PubMed] [Google Scholar]
- Malfatti E, Bugiani M, Invernizzi F, de Souza CF, Farina L, Carrara F, Lamantea E, Antozzi C, Confalonieri P, Sanseverino MT, Giugliani R, Uziel G, Zeviani M. Novel mutations of ND genes in complex I deficiency associated with mitochondrial encephalopathy. Brain. 2007;130:1894–1904. doi: 10.1093/brain/awm114. [DOI] [PubMed] [Google Scholar]
- Marchington DR, Barlow D, Poulton J. Transmitochondrial mice carrying resistance to chloramphenicol on mitochondrial DNA: developing the first mouse model of mitochondrial DNA disease. Nat Med. 1999;5:957–960. doi: 10.1038/11403. [DOI] [PubMed] [Google Scholar]
- Martin-Hernandez E, Garcia-Silva MT, Vara J, Campos Y, Cabello A, Muley R, Del Hoyo P, Martin MA, Arenas J. Renal pathology in children with mitochondrial diseases. Pediatric nephrology. 2005;20:1299–1305. doi: 10.1007/s00467-005-1948-z. [DOI] [PubMed] [Google Scholar]
- Massa V, Fernandez-Vizarra E, Alshahwan S, Bakhsh E, Goffrini P, Ferrero I, Mereghetti P, D'Adamo P, Gasparini P, Zeviani M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am J Hum Genet. 2008;82:1281–1289. doi: 10.1016/j.ajhg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr JA, Havlickova V, Zimmermann F, Magler I, Kaplanova V, Jesina P, Pecinova A, Nuskova H, Koch J, Sperl W, Houstek J. Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 epsilon subunit. Hum Mol Genet. 2010;19:3430–3439. doi: 10.1093/hmg/ddq254. [DOI] [PubMed] [Google Scholar]
- Mckenzie M, Ryan MT. Assembly factors of human mitochondrial complex I and their defects in disease. IUBMB Life. 2010;62:497–502. doi: 10.1002/iub.335. [DOI] [PubMed] [Google Scholar]
- Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S, Aerts L, Overbergh L, Grunewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344:203–207. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- Moran M, Marin-Buera L, Gil-Borlado MC, Rivera H, Blazquez A, Seneca S, Vazquez-Lopez M, Arenas J, Martin MA, Ugalde C. Cellular pathophysiological consequences of BCS1L mutations in mitochondrial complex III enzyme deficiency. Human mutation. 2010;31:930–941. doi: 10.1002/humu.21294. [DOI] [PubMed] [Google Scholar]
- Morison IM, Cramer Borde EM, Cheesman EJ, Cheong PL, Holyoake AJ, Fichelson S, Weeks RJ, Lo A, Davies SM, Wilbanks SM, Fagerlund RD, Ludgate MW, da Silva Tatley FM, Coker MS, Bockett NA, Hughes G, Pippig DA, Smith MP, Capron C, Ledgerwood EC. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat Genet. 2008;40:387–389. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
- Mourmans J, Wendel U, Bentlage HACM, Trijbels JMF, Smeitink JAM, de Coo IFM, Gabreëls FJM, Sengers RCA, Ruitenbeek W. Clinical heterogeneity in respiratory chain complex III deficiency in childhood. Journal of the neurological sciences. 1997;149:111–117. doi: 10.1016/s0022-510x(97)05379-3. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Fujikawa M, Yoshida M. IF1, a natural inhibitor of mitochondrial ATP synthase, is not essential for the normal growth and breeding of mice. Biosci Rep. 2013:33. doi: 10.1042/BSR20130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa S, Hecht NB, Goldberg E, Boatright KM, Reed JC, Millan JL. Testis-specific cytochrome c-null mice produce functional sperm but undergo early testicular atrophy. Mol Cell Biol. 2002;22:5554–5562. doi: 10.1128/MCB.22.15.5554-5562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngu LH, Nijtmans LG, Distelmaier F, Venselaar H, van Emst-de Vries SE, van den Brand MA, Stoltenborg BJ, Wintjes LT, Willems PH, van den Heuvel LP, Smeitink JA, Rodenburg RJ. A catalytic defect in mitochondrial respiratory chain complex I due to a mutation in NDUFS2 in a patient with Leigh syndrome. Biochim Biophys Acta. 2012;1822:168–175. doi: 10.1016/j.bbadis.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Niaudet P, Rotig A. The kidney in mitochondrial cytopathies. Kidney international. 1997;51:1000–1007. doi: 10.1038/ki.1997.140. [DOI] [PubMed] [Google Scholar]
- Ogilvie I, Kennaway NG, Shoubridge EA. A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy. The Journal of clinical investigation. 2005;115:2784–2792. doi: 10.1172/JCI26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Ohama E, Takahashi H, Ikuta F, Nishizawa M, Tanaka K, Miyatake T. Alterations of oligodendrocytes and demyelination in the spinal cord of patients with mitochondrial encephalomyopathy. Journal of the neurological sciences. 1988;86:19–29. doi: 10.1016/0022-510x(88)90004-4. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annual review of neuroscience. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Oquendo CE, Antonicka H, Shoubridge EA, Reardon W, Brown GK. Functional and genetic studies demonstrate that mutation in the COX15 gene can cause Leigh syndrome. J Med Genet. 2004;41:540–544. doi: 10.1136/jmg.2003.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagniez-Mammeri H, Rak M, Legrand A, Benit P, Rustin P, Slama A. Mitochondrial complex I deficiency of nuclear origin II. Non-structural genes. Mol Genet Metab. 2012;105:173–179. doi: 10.1016/j.ymgme.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Palin EJ, Paetau A, Suomalainen A. Mesencephalic complex I deficiency does not correlate with parkinsonism in mitochondrial DNA maintenance disorders. Brain. 2013;136:2379–2392. doi: 10.1093/brain/awt160. [DOI] [PubMed] [Google Scholar]
- Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5:e8762. doi: 10.1371/journal.pone.0008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou LC, Sue CM, Davidson MM, Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J, Glerum DM, Coster RV, Lyon G, Scalais E, Lebel R, Kaplan P, Shanske S, De Vivo DC, Bonilla E, Hirano M, DiMauro S, Schon EA. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- Parsons WJ, Williams RS, Shelton JM, Luo Y, Kessler DJ, Richardson JA. Developmental regulation of cytochrome oxidase subunit VIa isoforms in cardiac and skeletal muscle. Am J Physiol. 1996;270:H567–H574. doi: 10.1152/ajpheart.1996.270.2.H567. [DOI] [PubMed] [Google Scholar]
- Pecina P, Houstkova H, Hansikova H, Zeman J, Houstek J. Genetic defects of cytochrome c oxidase assembly. Physiol Res. 2004;53(Suppl 1):S213–S223. [PubMed] [Google Scholar]
- Peng M, Falk MJ, Haase VH, King R, Polyak E, Selak M, Yudkoff M, Hancock WW, Meade R, Saiki R, Lunceford AL, Clarke CF, Gasser DL. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4:e1000061. doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta S, Torraco A, Wenz T, Garcia S, Diaz F, Moraes CT. Partial complex I deficiency due to the CNS conditional ablation of Ndufa5 results in a mild chronic encephalopathy but no increase in oxidative damage. Hum. Mol. Genet. 2013;23:1399–1412. doi: 10.1093/hmg/ddt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzella V, Vergari R, Puzziferri I, Boffoli D, Lamantea E, Zeviani M, Papa S. A nonsense mutation in the NDUFS4 gene encoding the 18 kDa (AQDQ) subunit of complex I abolishes assembly and activity of the complex in a patient with Leigh-like syndrome. Hum Mol Genet. 2001;10:529–535. doi: 10.1093/hmg/10.5.529. [DOI] [PubMed] [Google Scholar]
- Piruat JI, Pintado CO, Ortega-Saenz P, Roche M, Lopez-Barneo J. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol Cell Biol. 2004;24:10933–10940. doi: 10.1128/MCB.24.24.10933-10940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman ME, Monroy GC. A Naturally Occurring Inhibitor of Mitochondrial Adenosine Triphosphatase. J Biol Chem. 1963;238:3762–3769. [PubMed] [Google Scholar]
- Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci U S A. 2010;107:10996–11001. doi: 10.1073/pnas.1006214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD. Fatal breathing dysfunction in a mouse model of Leigh syndrome. The Journal of clinical investigation. 2012;122:2359–2368. doi: 10.1172/JCI62923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintens R, Singh S, Lemaire K, De Bock K, Granvik M, Schraenen A, Vroegrijk IO, Costa V, Van Noten P, Lambrechts D, Lehnert S, Van Lommel L, Thorrez L, De Faudeur G, Romijn JA, Shelton JM, Scorrano L, Lijnen HR, Voshol PJ, Carmeliet P, Mammen PP, Schuit F. Mice deficient in the respiratory chain gene Cox6a2 are protected against high-fat diet-induced obesity and insulin resistance. PLoS One. 2013;8:e56719. doi: 10.1371/journal.pone.0056719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, Garone C, Emmanuele V, Tadesse S, Krishna S, Dorado B, Hirano M. Tissue-specific oxidative stress and loss of mitochondria in CoQ-deficient Pdss2 mutant mice. FASEB J. 2013;27:612–621. doi: 10.1096/fj.12-209361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford NB, Wan B, Richman A, Szczepaniak LS, Li JL, Li K, Pfeiffer K, Schagger H, Garry DJ, Moreadith RW. Cardiac dysfunction in mice lacking cytochrome-c oxidase subunit VIaH. Am J Physiol Heart Circ Physiol. 2002;282:H726–H733. doi: 10.1152/ajpheart.00308.2001. [DOI] [PubMed] [Google Scholar]
- Rak M, Rustin P. Supernumerary subunits NDUFA3, NDUFA5 and NDUFA12 are required for the formation of the extramembrane arm of human mitochondrial complex I. FEBS letters. 2014;588:1832–1838. doi: 10.1016/j.febslet.2014.03.046. [DOI] [PubMed] [Google Scholar]
- Rotig A, Goutieres F, Niaudet P, Rustin P, Chretien D, Guest G, Mikol J, Gubler MC, Munnich A. Deletion of mitochondrial DNA in patient with chronic tubulointerstitial nephritis. The Journal of pediatrics. 1995;126:597–601. doi: 10.1016/s0022-3476(95)70359-4. [DOI] [PubMed] [Google Scholar]
- Rusanen H, Majamaa K, Tolonen U, Remes AM, Myllyla R, Hassinen IE. Demyelinating polyneuropathy in a patient with the tRNA(Leu)(UUR) mutation at base pair 3243 of the mitochondrial DNA. Neurology. 1995;45:1188–1192. doi: 10.1212/wnl.45.6.1188. [DOI] [PubMed] [Google Scholar]
- Rustin P, Rotig A. Inborn errors of complex II--unusual human mitochondrial diseases. Biochim Biophys Acta. 2002;1553:117–122. doi: 10.1016/s0005-2728(01)00228-6. [DOI] [PubMed] [Google Scholar]
- Rutter J, Winge DR, Schiffman JD. Succinate dehydrogenase - Assembly, regulation and role in human disease. Mitochondrion. 2010;10:393–401. doi: 10.1016/j.mito.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R, Lunceford AL, Shi Y, Marbois B, King R, Pachuski J, Kawamukai M, Gasser DL, Clarke CF. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol. 2008;295:F1535–F1544. doi: 10.1152/ajprenal.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld R, Wong A, Silva J, Li M, Itoh A, Horiuchi M, Itoh T, Pleasure D, Cortopassi G. Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion. 2010;10:143–150. doi: 10.1016/j.mito.2009.12.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A, Mito T, Hayashi C, Ogasawara E, Koba R, Negishi I, Takenaga K, Nakada K, Hayashi J. Transmitochondrial mice as models for primary prevention of diseases caused by mutation in the tRNALys gene. Proc Natl Acad Sci U S A. 2014;111:3104–3109. doi: 10.1073/pnas.1318109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge EA. Cytochrome c oxidase deficiency. Am J Med Genet. 2001;106:46–52. doi: 10.1002/ajmg.1378. [DOI] [PubMed] [Google Scholar]
- Shteyer E, Saada A, Shaag A, Al-Hijawi FA, Kidess R, Revel-Vilk S, Elpeleg O. Exocrine pancreatic insufficiency, dyserythropoeitic anemia, and calvarial hyperostosis are caused by a mutation in the COX4I2 gene. Am J Hum Genet. 2009;84:412–417. doi: 10.1016/j.ajhg.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligh JE, Levy SE, Waymire KG, Allard P, Dillehay DL, Nusinowitz S, Heckenlively JR, MacGregor GR, Wallace DC. Maternal germ-line transmission of mutant mtDNAs from embryonic stem cell-derived chimeric mice. Proc Natl Acad Sci U S A. 2000;97:14461–14466. doi: 10.1073/pnas.250491597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel R, Khayat M, Shalev SA, Horovitz Y, Mandel H, Hershkovitz E, Barghuti F, Shaag A, Saada A, Korman SH, Elpeleg O, Yatsiv I. TMEM70 mutations are a common cause of nuclear encoded ATP synthase assembly defect: further delineation of a new syndrome. J Med Genet. 2011;48:177–182. doi: 10.1136/jmg.2010.084608. [DOI] [PubMed] [Google Scholar]
- Spiegel R, Shaag A, Mandel H, Reich D, Penyakov M, Hujeirat Y, Saada A, Elpeleg O, Shalev SA. Mutated NDUFS6 is the cause of fatal neonatal lactic acidemia in Caucasus Jews. Eur J Hum Genet. 2009;17:1200–1203. doi: 10.1038/ejhg.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Hoffman AF, Milenkovic D, Bao B, Paganelli A, Edgar D, Wibom R, Lupica CR, Olson L, Larsson NG. Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum Mol Genet. 2012;21:1078–1089. doi: 10.1093/hmg/ddr537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci U S A. 2011;108:12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiburek L, Vesela K, Hansikova H, Hulkova H, Zeman J. Loss of function of Sco1 and its interaction with cytochrome c oxidase. Am J Physiol Cell Physiol. 2009;296:C1218–C1226. doi: 10.1152/ajpcell.00564.2008. [DOI] [PubMed] [Google Scholar]
- Stock D, Leslie AG, Walker JE. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- Swalwell H, Kirby DM, Blakely EL, Mitchell A, Salemi R, Sugiana C, Compton AG, Tucker EJ, Ke BX, Lamont PJ, Turnbull DM, McFarland R, Taylor RW, Thorburn DR. Respiratory chain complex I deficiency caused by mitochondrial DNA mutations. Eur J Hum Genet. 2011;19:769–775. doi: 10.1038/ejhg.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Kako K, Kashiwabara S, Takehara A, Inada Y, Arai H, Nakada K, Kodama H, Hayashi J, Baba T, Munekata E. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome C oxidase and embryonic development. Mol Cell Biol. 2002;22:7614–7621. doi: 10.1128/MCB.22.21.7614-7621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiranti V, Jaksch M, Hofmann S, Galimberti C, Hoertnagel K, Lulli L, Freisinger P, Bindoff L, Gerbitz KD, Comi GP, Uziel G, Zeviani M, Meitinger T. Loss-of-function mutations of SURF-1 are specifically associated with Leigh syndrome with cytochrome c oxidase deficiency. Ann Neurol. 1999;46:161–166. doi: 10.1002/1531-8249(199908)46:2<161::aid-ana4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Torraco A, Verrigni D, Rizza T, Meschini MC, Vazquez-Memije ME, Martinelli D, Bianchi M, Piemonte F, Dionisi-Vici C, Santorelli FM, Bertini E, Carrozzo R. TMEM70: a mutational hot spot in nuclear ATP synthase deficiency with a pivotal role in complex V biogenesis. Neurogenetics. 2012;13:375–386. doi: 10.1007/s10048-012-0343-8. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Valnot I, von Kleist-Retzow JC, Barrientos A, Gorbatyuk M, Taanman JW, Mehaye B, Rustin P, Tzagoloff A, Munnich A, Rotig A. A mutation in the human heme A:farnesyltransferase gene (COX10 ) causes cytochrome c oxidase deficiency. Hum Mol Genet. 2000;9:1245–1249. doi: 10.1093/hmg/9.8.1245. [DOI] [PubMed] [Google Scholar]
- van den Heuvel L, Ruitenbeek W, Smeets R, Gelman-Kohan Z, Elpeleg O, Loeffen J, Trijbels F, Mariman E, de Bruijn D, Smeitink J. Demonstration of a new pathogenic mutation in human complex I deficiency: a 5-bp duplication in the nuclear gene encoding the 18-kD (AQDQ) subunit. Am J Hum Genet. 1998;62:262–268. doi: 10.1086/301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kuilenburg AB, Van Beeumen JJ, Van der Meer NM, Muijsers AO. Subunits VIIa,b,c of human cytochrome c oxidase. Identification of both 'heart-type' and 'liver-type' isoforms of subunit VIIa in human heart. Eur J Biochem. 1992;203:193–199. doi: 10.1111/j.1432-1033.1992.tb19847.x. [DOI] [PubMed] [Google Scholar]
- Vempati UD, Han X, Moraes CT. Lack of cytochrome c in mouse fibroblasts disrupts assembly/stability of respiratory complexes I and IV. J Biol Chem. 2009;284:4383–4391. doi: 10.1074/jbc.M805972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vempati UD, Torraco A, Moraes CT. Mouse models of oxidative phosphorylation dysfunction and disease. Methods. 2008;46:241–247. doi: 10.1016/j.ymeth.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesela K, Hulkova H, Hansikova H, Zeman J, Elleder M. Structural analysis of tissues affected by cytochrome C oxidase deficiency due to mutations in the SCO2 gene. APMIS. 2008;116:41–49. doi: 10.1111/j.1600-0463.2008.00772.x. [DOI] [PubMed] [Google Scholar]
- Visapaa I, Fellman V, Vesa J, Dasvarma A, Hutton JL, Kumar V, Payne GS, Makarow M, Van Coster R, Taylor RW, Turnbull DM, Suomalainen A, Peltonen L. GRACILE syndrome, a lethal metabolic disorder with iron overload, is caused by a point mutation in BCS1L. Am J Hum Genet. 2002;71:863–876. doi: 10.1086/342773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, Schon EA, Lamperti C, Zeviani M. In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell Metab. 2011;14:80–90. doi: 10.1016/j.cmet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Collinson IR, Van Raaij MJ, Runswick MJ. Structural analysis of ATP synthase from bovine heart mitochondria. Methods Enzymol. 1995;260:163–190. doi: 10.1016/0076-6879(95)60136-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Dewey MJ, Mintz B. Teratocarcinoma cells as vehicles for introducing specific mutant mitochondrial genes into mice. Proc Natl Acad Sci U S A. 1978;75:5113–5117. doi: 10.1073/pnas.75.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990;62:425–434. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Williams SL, Taanman JW, Hansikova H, Houst'kova H, Chowdhury S, Zeman J, Houstek J. A novel mutation in SURF1 causes skipping of exon 8 in a patient with cytochrome c oxidase-deficient leigh syndrome and hypertrichosis. Mol Genet Metab. 2001;73:340–343. doi: 10.1006/mgme.2001.3206. [DOI] [PubMed] [Google Scholar]
- Yang H, Brosel S, Acin-Perez R, Slavkovich V, Nishino I, Khan R, Goldberg IJ, Graziano J, Manfredi G, Schon EA. Analysis of mouse models of cytochrome c oxidase deficiency owing to mutations in Sco2. Hum Mol Genet. 2010;19:170–180. doi: 10.1093/hmg/ddp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaplito-Lee J, Weintraub R, Jamsen K, Chow CW, Thorburn DR, Boneh A. Cardiac manifestations in oxidative phosphorylation disorders of childhood. The Journal of pediatrics. 2007;150:407–411. doi: 10.1016/j.jpeds.2006.12.047. [DOI] [PubMed] [Google Scholar]
- Zordan MA, Cisotto P, Benna C, Agostino A, Rizzo G, Piccin A, Pegoraro M, Sandrelli F, Perini G, Tognon G, De Caro R, Peron S, Kronnie TT, Megighian A, Reggiani C, Zeviani M, Costa R. Post-transcriptional silencing and functional characterization of the Drosophila melanogaster homolog of human Surf1. Genetics. 2006;172:229–241. doi: 10.1534/genetics.105.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]