Abstract
Urotensin II (UII) is an evolutionarily conserved neuropeptide initially isolated from teleost fish on the basis of its smooth muscle-contracting activity. Subsequent studies have demonstrated the occurrence of several UII-related peptides (URPs), such that the UII family is now known to include four paralogue genes called UII, URP, URP1 and URP2. These genes probably arose through the two rounds of whole genome duplication that occurred during early vertebrate evolution. URP has been identified both in tetrapods and teleosts. In contrast, URP1 and URP2 have only been observed in ray-finned and cartilaginous fishes, suggesting that both genes were lost in the tetrapod lineage. In the present study, the distribution of urp1 mRNA compared to urp2 mRNA is reported in the central nervous system of zebrafish. In the spinal cord, urp1 and urp2 mRNAs were mainly colocalized in the same cells. These cells were also shown to be GABAergic and express the gene encoding the polycystic kidney disease 2-like 1 (pkd2l1) channel, indicating that they likely correspond to cerebrospinal fluid-contacting neurons. In the hindbrain, urp1-expressing cells were found in the intermediate reticular formation and the glossopharyngeal-vagal motor nerve nuclei. We also showed that synthetic URP1 and URP2 were able to induce intracellular calcium mobilization in human UII receptor (hUT)-transfected CHO cells with similar potencies (pEC50=7.99 and 7.52, respectively) albeit at slightly lower potencies than human UII and mammalian URP (pEC50=9.44 and 8.61, respectively). The functional redundancy of URP1 and URP2 as well as the colocalization of their mRNAs in the spinal cord suggest the robustness of this peptidic system and its physiological importance in zebrafish.
Introduction
Urotensin II (UII) is a cyclic neuropeptide which was first isolated from the teleost urophysis on the basis of its spasmogenic properties [1]. Subsequent studies have shown that UII occurs in all vertebrate classes [2–6] where it exerts various biological effects including regulation of behaviors, neuroendocrine activities and control of cardiovascular functions [7], [8]. UII-related peptide (URP) is a peptide structurally related to UII which was first identified from the rodent brain [9] then in birds [10], amphibians [11] and teleosts [12]. In mammals, UII and URP have been shown to exert their action through a single receptor called UT, a member of the G protein-coupled receptor superfamily [13–16].
In teleosts, as in chondrichthyans, UII mRNA and/or peptide has been mainly found in Dahlgren cells of the caudal neurosecretory system [5,6,17–20] but its presence has also been reported in several subdivisions of the brain [19]. In contrast, in tetrapods, the UII gene is primarily expressed in motoneurons of the brainstem and spinal cord [21,22]. For its part, URP mRNA is mainly located in motoneurons in both tetrapods [11,23,24] and teleosts (Quan et al., unpublished results). Note that in lampreys, UII was isolated from an extract of the whole brain [6] but its precise localization in the central nervous system is still unknown.
Recently, the occurrence of two additional members of the UII gene family has been reported in teleosts, called URP1 and URP2 [25–28]. It has been proposed that both genes, together with UII and URP arose through the two rounds of whole genome duplication that occurred during early vertebrate evolution [26]. In agreement with this scenario, the existence of URP1 and/or URP2 genes has recently been reported in the spotted gar (a non-teleost actinopterygian) and elephant shark (a chondrichthyan) [27,28]. In contrast, the lack of URP1 and URP2 genes in tetrapods is believed to result from their loss in this lineage specifically [26]. The primary structure of both URP1 and URP2 is exactly the same in all fish species investigated so far.
Up to now, the expression pattern of the URP1 gene has only been studied in one species, the Japanese eel (Anguilla japonica) [25] while the URP2 gene expression has solely been reported in zebrafish (Brachydanio rerio) [26]. RT-PCR revealed that in both species urp1 and urp2 genes are mainly expressed in the brainstem and spinal cord. In zebrafish, it has been shown by in situ hybridization (ISH) that the urp2 mRNA occurs in cells located along the ventral edge of the fourth ventricle and the ependymal canal. It has been suggested that these cells may correspond to cerebrospinal fluid- in cells located along the ventral edge of the fourth ventricle contacting neurons (CSF-cNs) [26].
In the present study, we report the distribution of urp1 mRNA in the central nervous system of zebrafish and compare it to that of urp2 mRNA. We demonstrate that urp1 and urp2 are mainly colocalized in the same cells in the spinal cord but not in the hindbrain. In the spinal cord, we provide evidence that cells containing both urp1 and urp2 mRNA are GABAergic and express the gene encoding the polycystic kidney disease 2-like 1 (pkd2l1) channel, indicating that they likely correspond to CSF-cNs. In the hindbrain, we show that urp1-expressing cells are located in the intermediate reticular formation and the glossopharyngeal-vagal motor nerve nuclei. Finally, we show that synthetic URP1 and URP2 are able to induce intracellular calcium mobilization in human UT-transfected Chinese hamster ovary cells (CHO) cells.
Materials and Methods
Chemicals and reagents
L-Amino acid residues were purchased from Senn Chemicals (Dielsdorf, Switzerland). Preloaded polyethylene glycol-polystyrene resins (Fmoc-Val-PEG-PS and Fmoc-Asn(Trt)-PEG-PS) and O-benzotriazol-1-yl-N,N,N’,N’-tetramethyluronium hexafluorophosphate (HBTU) were from Life Technologies (Saint Aubin, France, France). Acetonitrile, and N-methylpyrrolidinone (NMP) were from Biosolve Chimie (Dieuze, France). Diisopropylethylamine (DIEA), piperidine, trifluoroacetic acid (TFA), thallium (III) trifluoroacetate (Tl(OCOCF3)3) and other reagents were from Sigma-Aldrich (Saint-Quentin Fallavier, France).
Peptide synthesis
Human UII (hUII; H-Glu-Thr-Pro-Asp-Cys-Phe-Trp-Lys-Tyr-Cys-Val-OH), mammalian URP (mURP; H-Ala-Cys-Phe-Trp-Lys-Tyr-Cys-Val-OH), URP1 (H-Ala-Cys-Phe-Trp-Lys-Tyr-Cys-Val-Thr-Asn-OH) and URP2 (H-Val-Cys-Phe-Trp-Lys-Tyr-Cys-Ser-Gln-Asn-OH) were synthesized (0.1 mmol scale) on a Fmoc-Val-PEG-PS or a Fmoc-Asn(Trt)-PEG-PS resin using an Applied Biosystems model 433A automatic peptide synthesizer and the standard procedures, as previously described [29,30]. All Fmoc-amino acids (1 mmol, 10 eq.) were coupled by in situ activation with HBTU (1.25 mmol, 12.5 eq.) and DIEA (2.5 mmol, 25 eq.) in NMP. Reactive side-chains were protected as follows: Cys, acetamidomethyl (Acm) thioether; Asn and Gln, trityl (Trt) amide; Ser, Thr and Tyr, tert-butyl (tBu) ether; Lys and Trp, tert-butyloxycarbonyl (Boc) carbamate and, Asp and Glu, tert-butyl (OtBu) ether. After completion of the chain assembly, cyclization of UII and URPs was performed by Tl(OCOCF3)3 oxidation as previously described [31]. Peptides were deprotected and cleaved from the resin by TFA as previously described [30,32]. Crude peptides were purified by reversed-phase HPLC (RP-HPLC) on a Vydac 218TP1022 C18 column (2.2 x 25 cm; Grace Discovery Sciences Alltech, Templemars, France) using a linear gradient (10–50% over 50 min) of acetonitrile/TFA (99.9:0.1, v/v) at a flow rate of 10 ml/min. Peptides were analyzed by RP-HPLC on a Vydac 218TP54 C18 column (0.46 x 25 cm; Grace Discovery Sciences Alltech) using a linear gradient (10–60% over 25 min) of acetonitrile/TFA (99.9:0.1, v/v) at a flow rate of 1 ml/min. The purity of all peptides was higher than 99.9%. The peptides were characterized by MALDI-TOF mass spectrometry on a Voyager DE-PRO (Applera, Courtaboeuf, France) in the reflector mode with α-cyano-4-hydroxycinnamic acid as a matrix.
Animals
All zebrafish (B. rerio) lines were maintained and raised under standard conditions of 10/14 hours light cycle and water was regulated at 28.5°C, 500 μS and pH = 7.4. Wild-type animals (AB and Tüpfel) were obtained from local suppliers. The nacre mutants (microphthalmia-a, mitfa) which were selected for the embryo transparency were provided by Dr Jean-Pierre Levraud (Institut Pasteur, Paris, France). All procedures were approved by the Institutional Ethics Committee Cuvier at the Museum National d’Histoire Naturelle (protocol # 68–020; approval period from December 21, 2012 through December 19, 2017). In accordance to these guidelines, all efforts were made to minimize the number of animals used and their suffering.
Reverse transcription-polymerase chain reaction (RT-PCR)
The expression profiles of urp1 and urp2 genes in adult zebrafish were examined by RT-PCR using gene-specific primers of each transcript (see S1 Table for the primer sequences). Total RNA was extracted from various tissues, including brain, spinal cord, eyes, skin, muscle, heart, spleen, gill, gas bladder, intestine, liver, kidney, ovary, and testis, following the protocol provided with RNAble reagent (Eurobio, Les Ulis, France) and further purified by using RNAeasy Plus Mini kit (Qiagen, Courtabœuf, France). For each tissue, 242 ng of total RNA was reverse transcribed by using ImProm-II Reverse Transcription System (Promega, Charbonnières, France). PCR was carried out using a MyCycler thermal cycler (Bio-Rad, Marne la Coquette, France) for 25 cycles (denaturation: 95°C, 2 min; annealing: 56°C, 30 sec; extension: 72°C, 30 sec). The zebrafish β-actin gene was amplified as an internal control to verify the integrity of all cDNA samples (primer sequences are given in S1 Table). Negative controls were performed without cDNA template. All PCR products were electrophoresed in 1% agarose gel stained with SybrSafe (Invitrogen, Pontoise, France) then detected under UV light with the Gel Doc XR+ System (Bio-Rad).
Synthesis of the riboprobes for in situ hybridization
To generate the urp1 and urp2 ISH probes, two PCR fragments of 476 and 356 base pairs, respectively, were amplified from zebrafish brain and spinal cord RACE-ready cDNA (see S1 Table for the primer sequences) then subcloned into pGEM-T easy (Promega). Digoxigenin- and fluorescein-labeled probes were synthesized from the pre-linearized plasmid using SP6 or T7 RNA polymerases with the RNA Labeling Kit (Roche Diagnostics, Mannheim, Germany), according to the manufacturer’s instructions. Other probes, namely islet-1 (isl1), somatostatin 1 (ss1) and gad 67, were synthesized as previously described [33,34].
Sample preparation for in situ hybridization
Zebrafish embryos were fixed with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer at 4°C overnight and rinsed in 0.1 M phosphate-buffered saline, 0.1% Tween-20 (PBST). Adult zebrafish were deeply anesthetized in 0.02% MS-222 (Sigma-Aldrich) and killed by decapitation. Dissected brains and spinal cords were fixed with PFA as described above for embryos. For whole-mount ISH, fixed embryos or adult tissues were stored in 100% methanol at -20°C until use. For ISH of sections, fixed tissues were cryoprotected in 15% then 30% sucrose/PBS and embedded in Tissue-Tek (Sakura, Netherlands). Frontal or para-sagittal sections of brains (10–20 μm) and spinal cords (8–16 μm) were cut at -20°C using a cryostat (CM 3050, Leica, Nanterre, France), collected on Superfrost Plus slides (O. Kindler, Freiburg, Germany), dried at room temperature for 24 h and stored at -80°C until use. Free-floating sections (40 μM) were sometimes used for spinal cord observations.
In situ hybridization procedures
Only ISH using fluorescent probes is described here, whole-mount ISH and ISH being exhaustively described elsewhere [26]. Fixed samples (whole-mount embryos and adult brain and spinal cords) were treated with 2% H2O2 in 100% methanol for 20 min, rehydrated through a series of solutions with descending ethanol percentage (75, 50, 25% ethanol all in PBST, for 10 min each) and permeabilized by treatment with 10 μg/ml of proteinase K in PBST for 1–20 min, depending on the developmental stage. They were then post-fixed in 4% PFA for 20 min, washed four times in PBST for 5 min, incubated in 2mg/ml glycine for 30 min and washed again in PBST. Samples were prehybridized in hybridation buffer (50% formamide, 5X saline-sodium citrate (SSC), 50 μg/ml heparin, 0.5 mg/ml yeast RNA (Sigma-Aldrich), 0.1% Tween-20) for 1 h at 65°C. Hybridization was performed overnight at 65°C in the same buffer containing the heat-denaturated digoxigenin-labeled riboprobe. Embryos were washed in a descending washing buffer (50% formamide, 5X SSC, 0.1% Tween-20) series (75, 50, 25 and 0% washing buffer all in 2X SSC for 15 min each) at 65°C. They were rinsed twice in 0.05X SSC for 30 min at the same temperature, once in 50% 0.05X SSC/50% PBST for 5 min, then twice in PBST. Following the final wash, brain and spinal cord were included in 3% agarose and sectioned at 50 μm using a vibratome (VT1000S Leica).
Embryos or adult tissue sections were then pre-incubated in blocking buffer (1% blocking reagent (Roche Diagnostics) in 100 mM maleic acid pH 7.5, 150 mM NaCl 0.1% Tween-20) for 1 h. Probe detection was carried out as follows: 1) incubation with specific anti-digoxigenin- and/or anti-fluorescein- antibodies conjugated to peroxidase (Roche Diagnostics; dilution: 1:300), 2) incubation in 0.0015% H2O2 for 30 min with the suitable fluorochrome- conjugated tyramide, and 3) peroxidase inactivation, by 2% H2O2. Labeled probes were revealed as previously described [35] by a home-made FITC- (Fischer scientific, Illkirch, France), TAMRA- (Life Technologies) or Cy5- (Perkin Elmer, Courtaboeuf, France) conjugated tyramide (protocol available on Xenbase: http://www.xenbase.org/other/static/methods/FISH.jsp). After extensive washes in PBST (at least five times for 20 min each), samples were postmounted on Superfrost Plus slides (O. Kindler) in Mowiol (Sigma Aldrich). For double fluorescent ISH, the fluorescein-labeled probe was revealed first. The specificity of each probe was verified using the sense probe as negative control (data not shown).
Combined fluorescent ISH and immunofluorescence
ISH was performed before IHC as described above and revealed using FITC-, TAMRA- and/or Cy5-conjugated tyramide. After several washes in 0.1% Triton/0.1 M PBS, tissues were pre-incubated in 4% BSA, 0.1% Triton, 0.1 M PBS for 1 h. Primary antibodies (rabbit anti-GAD65/67 (Abcam, Cambridge, UK) 1:100 or goat anti-choline acetyltransferase (ChAT, Millipore), 1:100) were incubated in the same solution overnight at 4°C. Secondary antibodies (donkey anti-rabbit IgG Alexa Fluo 488/596 (Invitrogen) 1:500, or donkey anti-goat IgG Alexa Fluor 488 (Invitrogen) 1:500) were incubated after three washes in 0.1% Triton/0.1 M PBS for 2 hours, then washed twice in PBS. The specificity of the immunostaining was verified by omitting the primary antibodies (data not shown).
Image acquisition
Samples stained by BM Purple were imaged using a Leica DM 5500 B microscope connected to LAS V4.1 software. Samples stained by tyramide system amplification were imaged by the Olympus FV1000 or Zeiss LSM700 confocal microscopes using 405, 488, 543 and 633 nm laser lines. Images were processed using the ZEN (Zeiss, Marly Le Roi, France), Fiji [36] and Adobe Illustrator (Adobe Systems, Mountain View, CA, USA) softwares.
Intracellular calcium mobilization assays
CHO cells stably transfected with the human UII receptor (hUT-CHO) (Chatenet et al. 2004) were plated at a density of 4 x 104 cells/well in flat clear bottom black 96-well plates. Measures of intracellular calcium mobilization were performed as previously described (Dubessy et al. 2008) with slight modifications. Briefly, after 24 h in culture, cells were incubated for 1 h in a humidified incubator (37°C, 5% CO2) with 2 μM Fluo-4 acetoxymethyl ester (AM) calcium dye (Life Technologies) in Hank’s Buffer Saline Solution (HBSS; Life Technologies) buffered with 5 mM HEPES and supplemented with 2.5 mM probenecid (Sigma-Aldrich, Saint-Quentin Fallavier, France). Cells were washed twice with HBSS/HEPES/probenecid to remove Fluo-4 AM from the incubation medium and incubated in 150 μl of the same medium at 37°C for 15 min. Fluorescence was recorded using a Flexstation 3 fluorescence plate reader system (Molecular Devices, Saint-Grégoire, France) during 180 sec with an excitation wavelength of 485 nm, an emission wavelength of 525 nm and a cutoff filter of 515 nm. After 18 sec recording in basal conditions, 50 μl of graded concentration (10-12 to 10-6 M) of different peptides (4-fold final concentration) was added to the incubation medium with the built-in 8 channel pipettor at a rate of 47 μl/sec to assess their agonistic activity. After subtraction of mean fluorescence background from control wells without Fluo-4 AM, baseline was normalized to 100% and fluorescence peak values were determined for each concentration of peptide. Potency (EC50) and efficacy (Emax) were calculated with the Prism 5.0 software (GraphPad Software In., La Jolla, CA, USA) using a four-parameter logistic equation to fit peak fluorescence data.
Statistical analysis
For intracellular calcium mobilization assays, results from 7–13 independent experiments were expressed as mean ± SEM of Log(EC50) and plotted with box and whiskers. The normality of each data set was verified with the Shapiro-Wilk’s test and a one-way ANOVA test followed by a Tukey’s multiple comparison test to compare Log(EC50) between each peptide. Differences were considered significant where P<0.05.
Results
Both urp1 and urp2 genes are expressed in the hindbrain and spinal cord in embryo and adult zebrafish
The expression of the urp1 gene in zebrafish embryos was investigated by ISH at stages 18, 20, 22, 28, 30, 36 and 48 hours post fertilization (hpf) stages. The first urp1-expressing (urp1 +) cells were detected at 22 hpf in the rostral half of the spinal cord (Fig. 1A). From 22 to 36 hpf, the urp1 staining expanded more caudally within the spinal cord (Fig. 1B1, 2). At any stage analyzed, urp1 + cells were exclusively located at the base of the neural tube, ventral to the central canal (Fig. 1B3,4). These cells, which were distributed in two rows along the midline, belong to the lateral floor plate (Fig. 1B4,5). At 48 hpf, the urp1 mRNA was primarily detected in a tight bilateral cluster of cells in the hindbrain, while it was hardly detectable in the spinal cord (Fig. 1C).
Fig 1. urp1 mRNA is restricted to the ventral spinal cord and hindbrain at early stages of development in zebrafish.
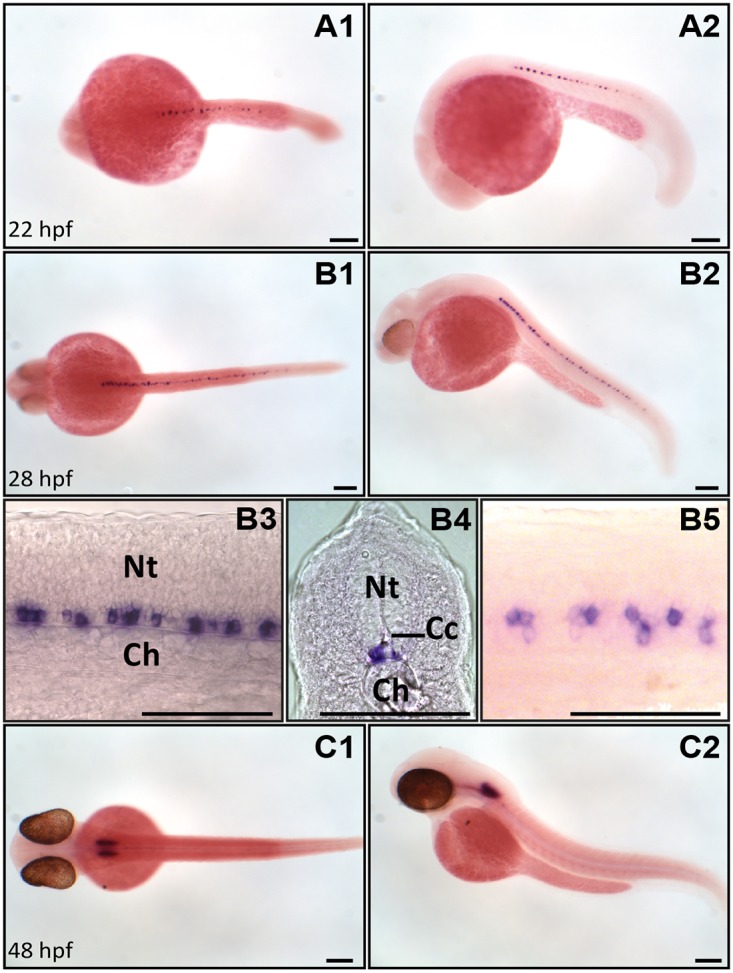
Expression of urp1 revealed by ISH (BM purple, violet) on nacre embryos at 22 (A), 28 (B) and 48 hpf (C). At 22 hpf and 28 hpf, urp1 + cells occur only in the spinal cord at the level of the lateral floor plate (A, B), while from 48 hpf, they are mainly visible in the hindbrain (C). A1, B1, B5 and C1, dorsal views; A2, B2, B3 and C2, lateral views with dorsal up; B4, coronal section with dorsal up; all embryos oriented anterior left; B3 and B5 are details at higher magnifications of B2 and B1, respectively. Ch, chord; Cc, central canal; Nt, neural tube; Scale bar: 100 μm.
RT-PCR was used to determine the distribution of urp1 and urp2 mRNAs in different organs of adult zebrafish. Both mRNAs were detected in the hindbrain and the spinal cord (Fig. 2). The urp2 mRNA was also found in the middle part of the brain, as previously reported [26]. In all the others tissues tested, the expression of urp1 and urp2 genes was undetectable (Fig. 2).
Fig 2. urp1 and urp2 mRNAs are exclusively detected in the brain and spinal cord in adult zebrafish.
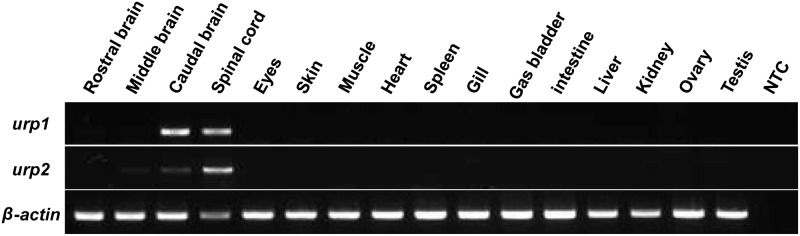
Tissue distribution of urp1 and urp2 mRNAs assessed by RT-PCR. Parallel amplification of zebrafish β-actin mRNA served as internal control. NTC, non-template control.
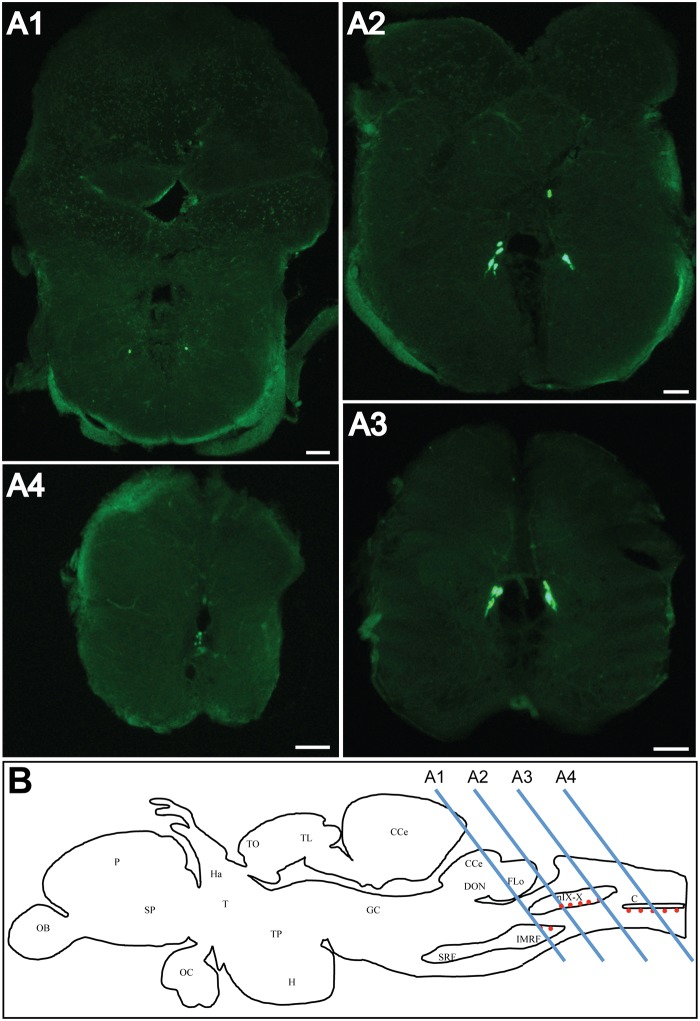
In line with PCR results, ISH analysis showed the presence of urp1 + cells in the medulla oblongata (Fig. 3A, B) at the level of the intermediate reticular formation (Fig. 3A1) and the roots of the glossopharyngeal and vagal nerves (Fig. 3A2,3) [37,38]. Most caudal, a group of urp1 + cells was found at the junction between the rhombencephalon and spinal cord, at the ventral edge of the central canal (Fig. 3A4).
Fig 3. urp1 mRNA is found in the caudal part of the hindbrain in adult zebrafish.
Expression of urp1 revealed by fluorescent ISH (FITC, green) on coronal sections of adult brain (A). urp1 mRNA is visible in neurons located in the intermediate reticular formation (A1) and the region of the glossopharyngeal-vagal motor nerve nuclei (A2–A3). More caudally, at the level of the junction between hindbrain and spinal cord, urp1 mRNA occurs at the ventral edge of the central canal (A4). Schematic sagittal view of an adult zebrafish brain depicting the distribution of urp1 mRNA (red dots). Levels of sections shown in A are indicated. The anatomical structures are designated according to [38] (B). CC, cerebellar crest; C, central canal; CCe, corpus cerebelli; DON, dorsal octavolateralis nucleus; EW, Edinger-Westphal nucleus; FLo, facial lobe; Ha, habenula; H, hypothalamus; IMRF, intermediate reticular formation; MO, medulla oblongata; NC, commissural nucleus of Cajal; nIX-X, glossopharyngeal-vagal motor nerve nuclei; OB, olfactive bulbs; OC, optic chiasma; P, pallium; PN, preopic nucleus; RV, rhombencephalic ventricle; SCsm, spinal cord somatomotor neurons; SP, subpallium; T, thalamus; TO, tectum opticum; TL, torus longitudinalis; TP, posterior tuberculum; TS, torus semicircularis; VLo, vagal lobe. Scale bars: 100 μm.
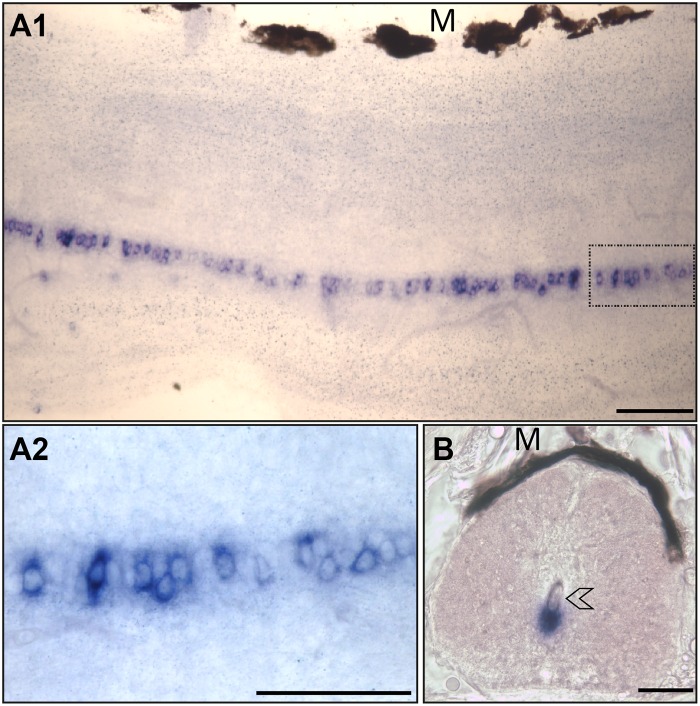
In the spinal cord, urp1 + cells formed a quasi-continuous column in the ventral margin along the central canal (Fig. 4A1,2) with exception to its more caudal part (data not shown). In this regard, it is noteworthy that no labeling was observed in Dahlgren cells nor in the urophysis. As shown in Fig. 4B, urp1 staining occurred in close contact to the lumen of the central canal. The distribution of the urp2 mRNA has been previously reported both in the midbrain and the spinal cord [20]. S1 Fig. highlights the occurrence of urp2 + cells at the ventral edge of the fourth ventricle.
Fig 4. urp1 mRNA occurs in cells located along the ventral edge of the central canal of spinal cord in adult zebrafish.
Expression of urp1 revealed by ISH (BM purple, violet) on free-floating sections of adult spinal cord. urp1 + cells form a quasi-continuous line at the ventral edge of the central canal (A). urp1 + cells are in close contact to the lumen of the central canal (arrowhead) (B). A1 and A2, lateral sections with dorsal up; B, coronal section with dorsal up. urp1 + cells boxed in A1 are shown in A2 at higher magnification. M, melanocytes. Scale bars: 50 μm.
urp1 and urp2 mRNAs are mainly localized in the same cells of the spinal cord
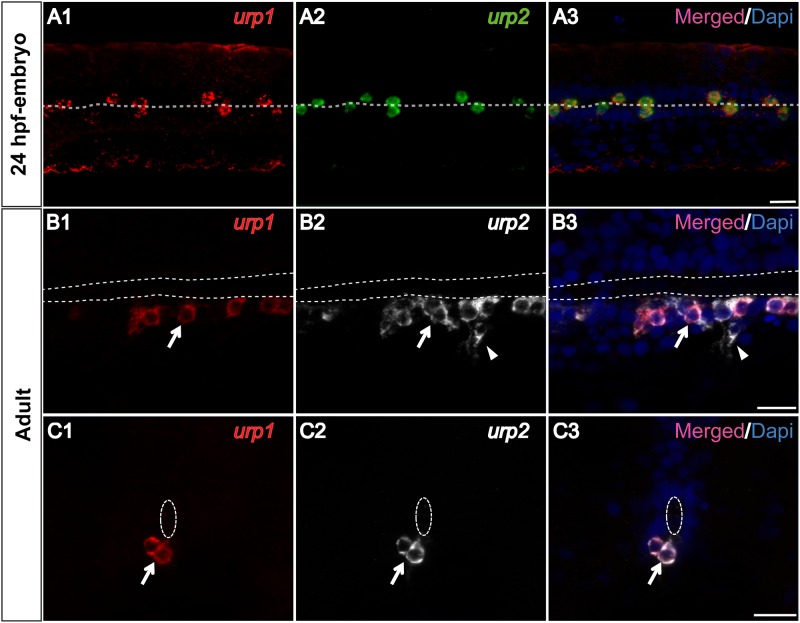
The expression pattern of the urp1 gene in the spinal cord appeared very similar to that of the urp2 gene [26]. To assess whether the two genes are expressed in the same cells, we performed double fluorescent ISH using urp1 and urp2 antisense probes, both in embryos and adults. In 24 hpf-embryos, we observed that all stained cells contained both urp1 and urp2 mRNAs (Fig. 5A). In adults, all urp1 + cells were stained by the urp2 probe (Fig. 5B,C), while about 20% of the urp2 + cells did not contain the urp1 mRNA (Fig. 5B).
Fig 5. urp1 and urp2 mRNAs are mainly coexpressed in the same spinal cord cells in zebrafish.
Simultaneous expression of urp1 and urp2 revealed by double fluorescent ISH (TAMRA, red for urp1, FITC, green for urp2 and DAPI in blue) on 24 hpf-embryo (A) and adult spinal cord sections (B, C). In embryo, all the stained cells contain both urp1 and urp2 mRNAs (A). In adult, although most of the stained cells are doubly-positive for urp1 and urp2 (arrow), some of the urp2 + cells are devoid of any urp1 mRNA (arrowhead). The white dash line indicates the central canal. A, dorsal view; B, sagittal section with dorsal up; C, coronal section with dorsal up. Scale bars: 15 μm.
Cells containing both urp1 and urp2 mRNA in the spinal cord also express markers of the CSF-contacting neurons
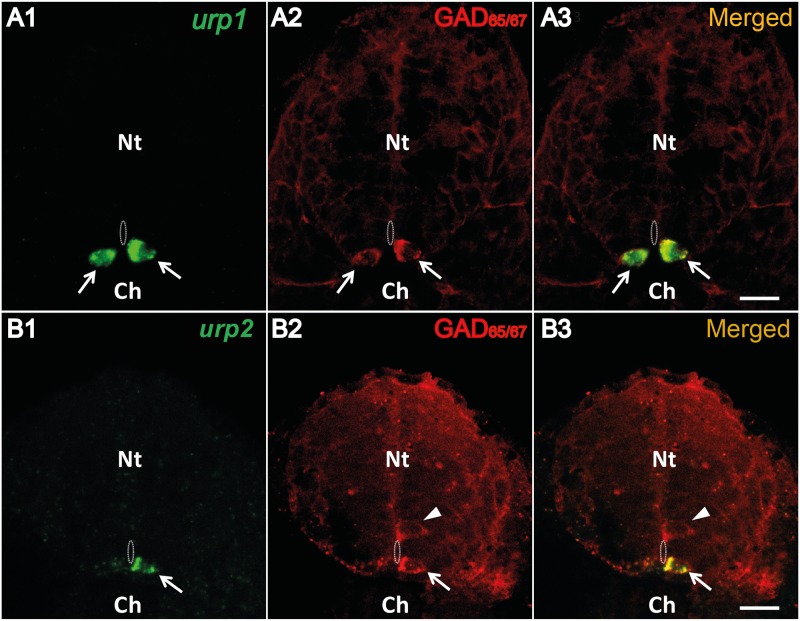
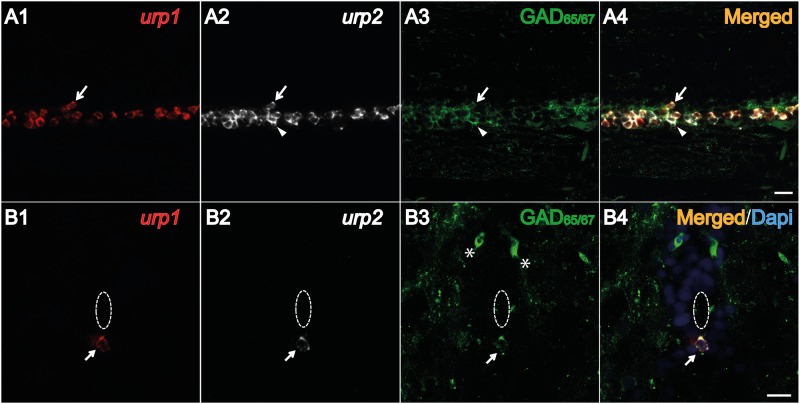
urp1 + cells, as urp2 + cells, were located in close contact to the central canal, indicating that they may both correspond to CSF-cNs. In zebrafish embryos, it has been shown that spinal CSF-cNs are GABAergic [39,40]. Therefore, we asked whether urp1 + and urp2 +cells may express GABAergic markers. In 24 hpf-embryos, single fluorescent ISH using either the urp1 or urp2 probe followed by IHC for GAD65/67 showed that all urp1 + cells as well as all urp2 + cells were GAD65/67 immunoreactive (Fig. 6A, B, arrows).
Fig 6. Both urp1 + and urp2 + cells are GABAergic neurons in the zebrafish embryo.
urp1 (A) and urp2 (B) expression revealed by fluorescent ISH (FITC, green) on 24 hpf-embryo, together with a fluorescent immunostaining for GAD65/67 (Alexa Fluor 546, red). Both urp1 + and urp2 + cells are GAD+ (arrows). Note that only ventral KA (KA”) cells are doubly stained. In contrast, dorsal KA (KA’) cells are GAD+ but do not express urp1 (arrowhead). The white dash line indicates the central canal. A and B, coronal sections with dorsal up. Scale bars: 15 μm.
The same results were obtained in adults by triple staining experiments using both urp1 and urp2 probes combined with an anti-GAD65/67 antibody. All urp1 + and urp2 +cells were GAD65/67 immunoreactive (Fig. 7A, B, arrows). As mentioned above, some of the urp2 + cells did not contained the urp1 mRNA (Fig. 7A4, arrowhead).
Fig 7. Both urp1 + and urp2 + cells in the spinal cord are GABAergic neurons in adult zebrafish.
Simultaneous expression of urp1 and urp2 revealed by double fluorescent ISH (TAMRA, red for urp1 and Cy5, white for urp2) in adult spinal cord, coupled to a fluorescent immunostaining for GAD65/67 (Alexa Fluor 488, green) (A, B). Both urp1 + and urp2 + cells are GAD+ (A, B). Arrows designate triple-stained cells (A, B). Note the occurrence of some doubly-positive cells (urp2/GAD) that do not contain any urp1 (arrowhead) (A). Asterisks designate GABAergic interneurons located at the dorsal part of the spinal cord. The white dash line indicates the central canal. A, sagittal section with dorsal up; B, coronal section with dorsal up. Scale bars: 15 μm.
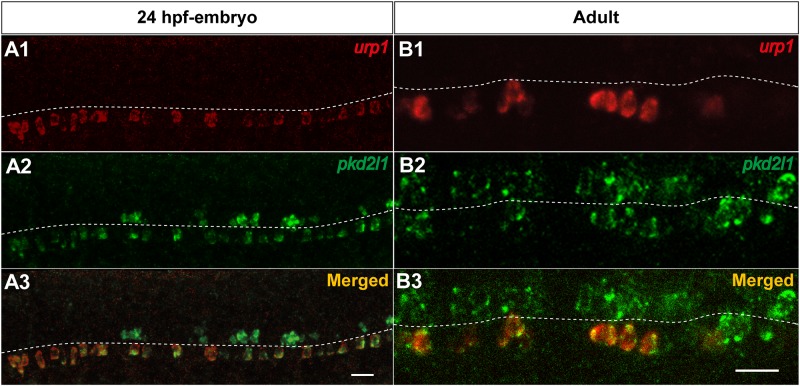
The gene encoding for the calcium-permeable PKD2L1 channel has been recently reported as a specific marker of CSF-cNs in various vertebrate species including zebrafish [41–43]. To determine whether urp1 + cells express pkd2l1, we performed double fluorescent ISH using urp1 and pkd2l1 probes, both in embryos and adults. As depicted in Fig. 8A, in 24 hpf-embryos, all urp1 + cells contained pkd2l1 mRNA, but their localization was restricted to the ventral subpopulation of pkd2l1 +cells. The same results were observed in adults, since urp1 + cells represented a fraction of pkd2l1 +cells located at the ventral edge of the central canal (Fig. 8B). Likewise, all urp2 + cells were identified as pkd2l1 + both in embryo and adult (data not shown).
Fig 8. urp1 + cells express pkd2l1, a specific marker of spinal cerebrospinal fluid- contacting neurons in zebrafish.
Simultaneous expression of urp1 and pkd2l1 revealed by double fluorescent ISH (TAMRA, red for urp1 and FITC, green for pkd2l1) on 24 hpf-embryo (A) and adult spinal cord sections (B). pkd2l1 mRNA is distributed in two rows of cells along the rostro-caudal axis of the spinal cord both in embryo and adult (A2, B2). All the urp1 + cells are pkd2l1 + (A1,3, B1,3) but only the ventral pkd2l1 + cells are urp1 +. The white dash line indicates the central canal. A, lateral views; B, sagittal sections with dorsal up. Scale bars: 20μm.
Hindbrain cells containing urp1 mRNA also express motoneuron markers
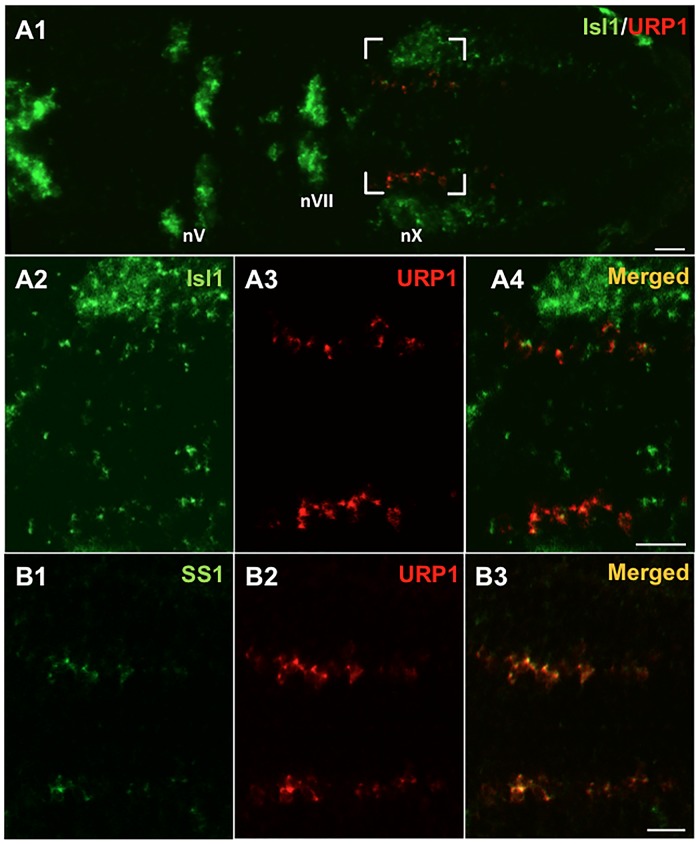
To better characterize the urp1 gene expression pattern in the hindbrain, we tested the colocalization of urp1 mRNA with different markers, namely isl1, ss1, ChAT and GAD. isl1 is a member of the LIM/homeobox gene family which is expressed in all postmitotic motoneurons at early stages of development [44]. Its expression pattern is particularly suitable to discriminate the different types of cranial motor nuclei in the hindbrain. In the 48 hpf-embryo, double fluorescent ISH using urp1 and isl1 probes revealed that all urp1 mRNA was exclusively present in isl1 +cells (Fig. 9A) at the level of the medial motor nucleus of the vagus [45]. Double fluorescent ISH using the urp1 and ss1 probes was also carried out, since ss1 was previously shown to be expressed in neurons of the vagal motor nucleus [33]. As depicted in Fig. 9B, all urp1 mRNA colocalized with ss1 mRNA. It is noteworthy that urp1 + cells were detected only in the most ventral part of the ss1-positive area (data not shown).
Fig 9. urp1 + cells express isl1 and ss1, two markers of the vagus motor nucleus in zebrafish embryo.
Simultaneous expression of urp1 and isl1 (A) or ss1 (B) revealed by double fluorescent ISH (TAMRA, red for urp1 and FITC, green for isl1 or ss1) on 48 hpf-embryo. urp1 + cells are located at the level of the medial motor nucleus of the vagus. Most of them appear to be both isl1 + and ss1 +. All pictures are dorsal views with anterior left. The boxed region in A1 is shown at higher magnification in A2–A4 and the same region is shown in B. nV, trigeminal nerve motor nuclei; nVII, facial nerve motor nuclei; nX, vagal nerve motor nuclei. Scale bars: 20 μm.
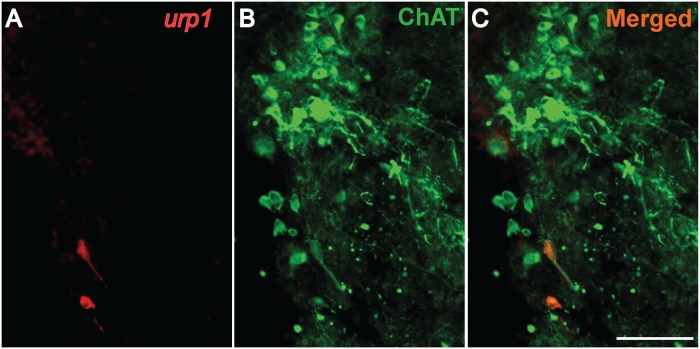
To characterize the urp1 + cells in the adult hindbrain, we performed single fluorescent ISH using the urp1 probe followed by IHC with ChAT, a marker of cholinergic neurons [38,46]. As shown in Fig. 10, urp1 + cells located in the glossopharyngeal-vagal motor nerve nuclei were weakly stained by the anti-ChAT antibody. Double fluorescent ISH using urp1 and gad 67 probes did not reveal any double-labeled cells (S2 Fig.), in contrast to what was observed in the spinal cord. Note that in the intermediate reticular formation, urp1 + cells were both ChAT- and gad 67-negative (data not shown).
Fig 10. urp1 + cells in the hindbrain are cholinergic neurons expressing ChAT in adult zebrafish.
urp1 expression revealed by fluorescent ISH (TAMRA, red) on coronal sections of adult brain, together with a fluorescent immunostaining for ChAT (Alexa 488, green). urp1 + cells express ChAT. Scale bars: 100 μm.
URP1 and URP2 are equipotent to activate the hUT
As a step to elucidate the physiological actions of URP1 and URP2, we tested the in vitro activities of both peptides using transfected CHO cells expressing the human UT using a calcium mobilization assay. Synthetic URP1 and URP2 induced a robust increase in intracellular calcium in hUT-CHO cells with similar efficacies (around 200–250%) than those evoked by hUII and mURP (Fig. 11A). Of note, URP1 and URP2 were equipotent to activate the hUT (pEC50 = 7.99 ± 0.15 and 7.52 ± 0.11 respectively). Nevertheless, they were respectively 28 and 83 times less potent than hUII (pEC50 = 9.44 ± 0.12) to mobilize intracellular calcium. mURP, which is also the natural ligand of hUT, exhibited an intermediate potency (pEC50 = 8.61 ± 0.16) statistically distinct from the others tested peptides (Fig. 11B).
Fig 11. URP1 and URP2 are equipotent to induce intracellular calcium mobilization in a hUT-transfected CHO cell line.
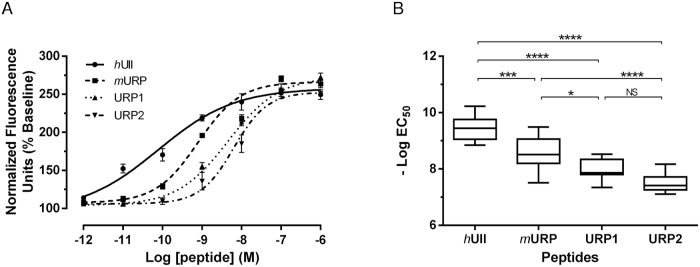
Representative dose-response curves of hUII (●), mURP (■), URP1 (▲) and URP2 (▼) on the intracellular calcium mobilization (A). The values are expressed as percentages of the baseline and each point is the mean (± S.E.M.) of 3 replicates. Experimental data were fitted using a four-parameter logistic equation. The potencies of 7–13 independent experiments for each peptides were plotted as—Log(EC50) with box and whiskers (B). Values were considered as statistically different as assessed by analysis of variance followed by Tukey’s post-test, n.s., not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
The aim of the present work was to compare the distributions and in vitro activities of the urotensin II-related peptides URP1 and URP2 in zebrafish. Previous studies had shown that urp2 gene expression occurs in cells contacting the central canal in the spinal cord [26], while urp1 mRNA was confined in the caudal neurosecretory system [25]. Our data do not confirm the presence of urp1 in the caudal spinal cord reported in the eel [25]. Instead urp1 + cells were found, as urp2 + cells, close to the central canal of the spinal cord. However, in contrast to urp2 mRNA which exhibits alternate zones of strong and weak staining [26], urp2 mRNA was present approximately at the same intensity along the entire length of the spinal cord. Worth mentioning, all urp1 + cells were urp2 +, while the opposite was not necessarily true, at least in adults.
On the basis of a former study reporting the occurrence of UII-like immunoreactive material in CSF-cNs [47], we suggested that urp2 +cells might be CSF-cNs [26]. CSF-cNs are neurons, described in all vertebrate groups [48–50], located on the edge of the neural tube lumen, so-called because they are in direct contact with the CSF via their dendritic pole. In Xenopus as in zebrafish, CSF-cNs have been shown to project an ascending axon ventrally in the spinal cord [39,51–53]. Moreover, analysis of the UII-like immunoreactive-containing system from various fish species showed that CSF-cNs send fibers to several brain regions, including medulla oblongata, thalamus, hypothalamus and telencephalon, but also in the spinal cord ventrolateral surface [47,54].
Although their functions remain largely unknown, CSF-cNs are classically considered as sensory neurons, but the exact nature of their stimuli is not clearly established. Recent studies have shown that they are GABAergic (see [42] for review). They also express PKD2L1, a transient receptor potential channel that could play a role as a sensory receptor [42,43], in good agreement with studies describing these neurons as chemoreceptors or mechanoreceptors sensing the CSF chemical composition and/or movements [42,50]. In addition, using the powerful optogenetic approach, Wyart et al. [53] have shown that CSF-cNs might be involved in motor control as part of the neuronal network that controls spontaneous swimming movement of zebrafish larvae. Thus, our data demonstrate that urp1 + and urp2 + cells both express GABA and pkd2l1 indicating that they actually are CSF-cNs and that URP1 and URP2 might have a role in the neuronal network that controls spontaneous swimming in zebrafish, at least in larvae.
It is likely that the presence of URP1 and/or URP2 in CSF-cNs is an ancestral feature of gnathostomes since the occurrence of CSF-cNs containing UII-like immunoreactivity has also been reported in a chondrichthyan species, Hydolagus collieri [54]. Beside URP1 and URP2, several other neuropeptides have been detected in CSF-cNs, such as vasoactive intestinal peptide (VIP) and SS [53,55–57]. In this respect, it is noteworthy that in coho salmon Oncorhynchus kisutch, SS- and UII-immunoreactive material do not localize in the same CSF-cNs [58] suggesting the occurrence of distinct CSF-cN types. Indeed, in zebrafish embryos, in which GABAergic CSF-cNs were named Kolmer-Agduhr (KA) cells to distinguish them from ciliated ependymal cells [51], KA cells have been divided into two subpopulations, namely KA’ and KA” cells, on the basis of their developmental origin and location. Whereas the dorsal KA’ cells are derived from olig2 + motoneuron precursors, the more ventral KA” cells develop from the lateral floor plate [34,42,59]. Our results showing that urp1 and urp2 gene expression was restricted to the KA” ventral subpopulation, reinforce the idea of the CSF-cNs diversity and reveal urp1 and urp2 as new markers for KA” cells in zebrafish. In adults, the fact that urp1 + and urp2 +cells also occur in a ventral position indicates that KA” cells conserve their location relative to the central canal during development. Whether all these different neuropeptides could help define additional types of CSF-cNs remains to be determined.
At the brain level, urp1 + cells were restricted to the rhombencephalon. In embryos, they were observed from 48 hpf, i.e. more than 24 h later than those detected in the spinal cord, in a small bilateral area also expressing isl1 and ss1 genes. In agreement with previous studies [33–44], these cells likely correspond to motoneurons in the vagal motor nucleus. Supporting this idea, most of the urp1 + cells in adults were identified as cholinergic neurons located in the same nucleus [38]. These data indicate that urp1 gene expression in the vagal motor nucleus persists throughout the entire development period until adulthood. It is noteworthy that UII and URP are known to be expressed mainly in motoneurons in tetrapods [11,21–24,60]. While urp1 and urp2 gene expression largely overlap in the spinal cord, their respective patterns differ completely in the brain since urp2 + cells appear to form an extension of the spinal urp2 CSF-cNs system below the fourth ventricle [26].
In trout (O. mykiss) and eel (A. japonica), central injection of UII evokes an increase in arterial blood pressure and heart rate [25,62–65]. Similar effects have been reported with URP1 [25]. It has been suggested that UII and URP1 may act directly at the central level, even though their site of action are still uncertain [25]. The present data show that the glossopharyngeal-vagal motor nerve nuclei are a putative source of URP1 indicating that URP1, rather than UII absent here [17,19,20,25], may act as a central regulator of the cardiovascular activity. Likewise, central pharmacological effects of UII on motor activity [61] could be achieved by URP1 expressed in the intermediate reticular formation, one type of zebrafish hindbrain nuclei projecting to the spinal cord [65].
To date, the precise mechanism of the URP1 action is unkown. Calcium mobilization assay showed here that synthetic URP1 and URP2 are active peptides toward human urotensin II receptor. This is consistent with the presence of the cyclic hexapeptide (CFWKYC) which is the minimal core involved for the activity of these peptides [31] and which is also present in UII and URP [27,28]. Previous docking [66,67] and photolabelling studies [68] showed that the side chains of Phe6 and Lys9 of hUII interact respectively with the Met185 residue located in the fourth transmembrane domain (TMD) and the Asn130 residue in the third TMD of the hUII receptor. The lower potencies of URP1 and URP2 toward hUT compared to hUII and mURP, the natural cognate peptides of this receptor, might be attributed to their longer C-terminus end. Indeed, steric hindrance and/or chemical nature of these three residues (VTN and SQN for URP1 and URP2 respectively) could interfere with the optimal positioning of the peptides within their putative hydrophobic binding pocket or their interaction with the second and third extracellular loop of the human receptor as shown for UII and URP [69].
UT has long been considered to be the only high affinity receptor for the UII family peptides, at least in mammals [7]. However, in a recent study, Tostivint et al. [28] provided evidence that the vertebrate ancestor likely possessed five distinct UT subtype genes, called UTS2R1–5 and that most of them have been preserved in teleosts. In zebrafish for example, four UT-like sequences have been identified that correspond to Uts2r1, the UT homologue, Uts2r2, uts2r3 and Uts2r4, while in stickleback, Uts2r2 seems to have been lost but is replaced by Uts2r5 [28]. In contrast, only the UTS2R1 subtype is still present in mammals. Considering the large number of UT receptor subtypes in teleosts, it is highly probable that URP1 and URP2 are able to bind to more than one receptor subtype. In support with this view, it is noteworthy that the Met185 and Asn130 residues mentioned above are conserved in the four putative UII receptors identified in zebrafish (data not shown). It is now evident that further studies will be needed to determine which receptor subtype the different UII-related peptides can preferentially bind to. In this regard, it will be interesting to determine whether the Uts2r1 subtype, the hUT counterpart studied in the present work, is the most efficient target of URP1 and URP2 or not.
In conclusion, we show here that, in the spinal cord, urp1 and urp2 are colocalized mainly in CSF-cNs, while, in the hindbrain, urp1 but not urp2 is confined to motoneurons in the glossopharyngeal-vagal motor nerve nuclei. We also demonstrate that URP1 and URP2 are active peptides toward human urotensin II receptor with similar potencies. Taken together, the functional redundancy of URP1 and URP2 as well as the colocalization of their mRNAs in the spinal cord suggest the robustness of this peptidic system and its physiological importance in zebrafish. These results provide the basis for further studies to improve our understanding of the physiological functions of URP1 and URP2 by using zebrafish as an experimental model.
Supporting Information
(TIF)
(TIF)
(DOCX)
Acknowledgments
We thank Jean-Paul Chaumeil and Philippe Durand for zebrafish care, Dr Caroline Parmentier (Université Pierre et Marie Curie, Paris, France) for helpful discussions and advice and Lindsey Marshall (MNHN) for English proofreading of the manuscript. We thank the team of the “Plateforme d’Imagerie Cellulaire Pitié Salpêtrière” for their support. Peptides and calcium data were obtained respectively on the Peptide Synthesis service and on the Bioactive Compounds Screening service of PRIMACEN (http://primacen.crihan.fr), the Cell Imaging Platform of Normandy.
We also thank the following colleagues for their generous gift of plasmids: Drs Francesco Argenton (Universita di Padova, Italy) for isl1 and ss1 and Dr Uwe Strähle (Karlsruhe Institute of Technology, Germany) for gad 67. H.T. and F.B.Q. are especially grateful to Dr. Laure Bally-Cuif (CNRS, Gif-sur-Yvette) for her kind invitation to learn fluorescent in situ hybridization techniques in her lab.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by funding obtained from the Centre National de la Recherche Scientifique and the Muséum National d’Histoire Naturelle to HT, and from the Institut de la Santé et de la Recherche Médicale, the University of Rouen and the Conseil Régional de Haute-Normandie to CD and IL. CW received financial support from the network Ecole des Neurosciences de Paris (ENP), the Fondation Bettencourt Schueller (FBS), Mr Pierre Belle, the City of Paris Emergence program, the Atip/Avenir junior program from INSERM and CNRS, the Fyssen foundation, the International Reintegration Grant from Marie Curie Actions Framework Program 6, and the European Research Council (ERC) starter grant “OptoLoco”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pearson D, Shively JE, Clark BR, Gerchwind II, Barkley M, Nishioka RS, et al. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980; 77: 5021–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conlon JM, O'Harte F, Smith DD, Balment RJ, Hazon N. Purification and characterization of urotensin II and parvalbumin from an elasmobranch fish, Scyliorhinus canicula (common dogfish). Neuroendocrinology. 1992; 55: 230–235. [DOI] [PubMed] [Google Scholar]
- 3. Conlon JM, O'Harte F, Smith DD, Tonon MC, Vaudry H. Isolation and primary structure of urotensin II from the brain of a tetrapod, the frog Rana ridibunda . Biochem Biophys Res Commun. 1992; 188: 578–583. [DOI] [PubMed] [Google Scholar]
- 4. McMaster D, Belenky MA, Polenov AL, Lederis K. Isolation and amino acid sequence of urotensin II from the sturgeon Acipenser ruthenus . Gen Comp Endocrinol. 1992; 87: 275–285. [DOI] [PubMed] [Google Scholar]
- 5. Waugh D, Conlon JM. Purification and characterization of urotensin II from the brain of a teleost (trout, Oncorhynchus mykiss) and an elasmobranch (skate, Raja rhina). Gen Comp Endocrinol. 1993; 92: 419–427. [DOI] [PubMed] [Google Scholar]
- 6. Waugh D, Youson J, Mims SD, Sower S, Conlon JM. Urotensin II from the river lamprey (Lampetra fluviatilis), the sea lamprey (Petromyzon marinus), and the paddlefish (Polyodon spathula). Gen Comp Endocrinol. 1995; 99: 323–332. [DOI] [PubMed] [Google Scholar]
- 7. Vaudry H, Do Rego JC, Le Mevel JC, Chatenet D, Tostivint H, Fournier A, et al. Urotensin II, from fish to human. Ann N Y Acad Sci. 2010; 1200: 53–66. 10.1111/j.1749-6632.2010.05514.x [DOI] [PubMed] [Google Scholar]
- 8. Lihrmann I, Tostivint H, Bern HA, Vaudry H. Urotensin II and urotensin II-related peptides In: Kastin AJ, editor. Handbook of Biologically Active Peptides, 2nd edition Waltham: Elsevier Inc; 2013. pp. 957–965. [Google Scholar]
- 9. Sugo T, Murakami Y, Shimomura Y, Harada M, Abe M, Ishibashi Y, et al. Identification of urotensin II-related peptide as the urotensin II-immunoreactive molecule in the rat brain. Biochem Biophys Res Commun. 2003; 310: 860–868. [DOI] [PubMed] [Google Scholar]
- 10. Tostivint H, Lihrmann I, Joly L, Parmentier C, Lebon A, Morisson M, et al. Comparative genomics provides evidence for close evolutionary relationships between the urotensin II and somatostatin gene families. Proc Natl Acad Sci USA. 2006; 103: 2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konno N, Fujii Y, Imae H, Kaiya H, Mukuda T, Miyazato M, et al. Urotensin II receptor (UTR) exists in hyaline chondrocytes: a study of peripheral distribution of UTR in the African clawed frog, Xenopus laevis . Gen Comp Endocrinol. 2013; 185: 44–56. 10.1016/j.ygcen.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 12. Quan FB, Bougerol M, Rigour F, Kenigfest NB, Tostivint H. Characterization of the true ortholog of the urotensin II-related peptide (URP) in teleosts. Gen Comp Endocrinol. 2012; 177: 205–212. 10.1016/j.ygcen.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 13. Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999; 401: 282–286. [DOI] [PubMed] [Google Scholar]
- 14. Nothacker HP, Wang Z, McNeill AM, Saito Y, Merten S, O'Dowd B, et al. Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nat Cell Biol. 1999; 1: 383–385. [DOI] [PubMed] [Google Scholar]
- 15. Liu Q, Pong SS, Zeng Z, Zhang Q, Howard AD, Williams DL Jr, et al. Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem Biophys Res Commun. 1999; 266: 174–178. [DOI] [PubMed] [Google Scholar]
- 16. Mori M, Sugo T, Abe M, Shimomura Y, Kurihara M, Kitada C, et al. Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor, SENR (GPR14). Biochem Biophys Res Commun. 1999; 265: 123–129. [DOI] [PubMed] [Google Scholar]
- 17. Ohsako S, Ishida I, Ichikawa T, Deguchi T. Cloning and sequence analysis of cDNAs encoding precursors of urotensin II-alpha and-gamma. J Neurosci. 1986; 6: 2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ichikawa T, Ishida I, Ohsako S, Degushi T. In situ hybridization demonstrating coexpression of urotensins I, II-α and II-β in the caudal neurosecretory neurons of the carp, Cyprinus carpio . Gen Comp Endocrinol. 1988; 71: 493–501 [DOI] [PubMed] [Google Scholar]
- 19. Lu W, Dow L, Gumusgoz S, Brierley MJ, Warne JM, McCrohan CR, et al. Molecular characterisation and expression of urotensin II in the flounder (Platichthys flesus): a hormone system supporting body fluid homeostasis in euryhaline fish. Endocrinology. 2006; 147: 3692–370. [DOI] [PubMed] [Google Scholar]
- 20. Parmentier C, Hameury E, Lihrmann I, Taxi J, Hardin-Pouzet H, Vaudry H, et al. Comparative distribution of the mRNAs encoding urotensin I and urotensin II in zebrafish. Peptides. 2008; 29: 820–829. 10.1016/j.peptides.2008.01.023 [DOI] [PubMed] [Google Scholar]
- 21. Coulouarn Y, Lihrmann I, Jégou S, Anouar Y, Tostivint H, Beauvillain JC, et al. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc Natl Acad Sci USA. 1998; 95: 15803–15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coulouarn Y, Jegou S, Tostivint H, Vaudry H, Lihrmann I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett. 1999; 457: 28–32. [DOI] [PubMed] [Google Scholar]
- 23. Pelletier G, Lihrmann I, Dubessy C, Luu-The V, Vaudry H, Labrie F. Androgenic down-regulation of urotensin II precursor, urotensin II-related peptide precursor and androgen receptor mRNA in the mouse spinal cord. Neuroscience. 2005; 132: 689–696. [DOI] [PubMed] [Google Scholar]
- 24. Dubessy C, Cartier D, Lectez B, Bucharles C, Chartrel N, Montero-Hadjadje M, et al. Characterization of urotensin II, distribution of urotensin II, urotensin II-related peptide and UT receptor mRNAs in mouse: evidence of urotensin II at the neuromuscular junction. J Neurochem. 2008; 107: 361–374. 10.1111/j.1471-4159.2008.05624.x [DOI] [PubMed] [Google Scholar]
- 25. Nobata S, Donald JA, Balment RJ, Takei Y. Potent cardiovascular effects of homologous urotensin II (UII)-related peptide and UII in unanaesthetized eels after peripheral and central injections. Am J Physiol Regul Integr Comp Physiol. 2011; 300: R437–446 10.1152/ajpregu.00629.2010 [DOI] [PubMed] [Google Scholar]
- 26. Parmentier C, Hameury E, Dubessy C, Quan FB, Habert D, Calas A, et al. Occurrence of two distinct urotensin II-related peptides in zebrafish provides new insight into the evolutionary history of the urotensin II gene family. Endocrinology. 2011; 152: 2330–2341. 10.1210/en.2010-1500 [DOI] [PubMed] [Google Scholar]
- 27. Tostivint H, Quan FB, Bougerol M, Kenigfest N.B, Lihrmann I. Impact of gene/genome duplications on the evolution of the urotensin II and somatostatin families. Gen Comp Endocrinol. 2013; 188: 110–117. 10.1016/j.ygcen.2012.12.015 [DOI] [PubMed] [Google Scholar]
- 28. Tostivint H, Ocampo Daza D, Bergqvist CA, Quan FB, Bougerol M, Lihrmann I, et al. Molecular evolution of somatostatin and urotensin II systems. J Mol Endocrinol. 2014; 52: T61–86. 10.1530/JME-13-0274 [DOI] [PubMed] [Google Scholar]
- 29. Leprince J, Oulyadi H, Vaudry D, Masmoudi O, Gandolfo P, Patte C, et al. Synthesis, conformational analysis and biological activity of cyclic analogs of the octadecaneuropeptide ODN. Design of a potent endozepine antagonist. Eur J Biochem. 2001; 268: 6045–6057 [DOI] [PubMed] [Google Scholar]
- 30. Chatenet D, Dubessy C, Boularan C, Scalbert E, Pfeiffer B, Renard P, et al. Structure-activity relationships of a novel series of urotensin II analogs: identification of a urotensin II antagonist. J Med Chem. 2006; 49: 7234–7238. [DOI] [PubMed] [Google Scholar]
- 31. Chatenet D, Dubessy C, Leprince J, Boularan C, Carlier L, Ségalas-Milazzo I, et al. Structure-activity relationships and structural conformation of a novel urotensin II-related peptide. Peptides. 2004; 25: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 32. Labarrère P, Chatenet D, Leprince J, Marionneau C, Loirand G, Tonon MC, et al. Structure-activity relationships of human urotensin II on rat aortic ring contraction. J Enz Inhib Med Chem. 2003; 18: 77–88. [DOI] [PubMed] [Google Scholar]
- 33. Devos N, Deflorian G, Biemar F, Bortolussi M, Martial JA, Peers B, et al. Differential expression of two somatostatin genes during zebrafish embryonic development. Mech Dev. 2002; 115: 133–117. [DOI] [PubMed] [Google Scholar]
- 34. Yang L, Rastegar S, Strähle U. Regulatory interactions specifying Kolmer-Agduhr interneurons. Development. 2010; 137: 2713–2722. 10.1242/dev.048470 [DOI] [PubMed] [Google Scholar]
- 35. Alunni A, Krecsmarik M, Bosco A, Galant S, Pan L, Moens CB, et al. Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development. 2013; 140: 3335–3347. 10.1242/dev.095018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012; 9:676–82. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wulliman MF, Rupp B, Reichert H. Neuroanatomy of the zebrafish brain: a topological atlas. 1st edition Birkhäuser: Basel; 1996. [Google Scholar]
- 38. Mueller T, Vernier P, Wullimann MF. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio . Brain Res. 2004; 1011: 156–169. [DOI] [PubMed] [Google Scholar]
- 39. Bernhardt RR, Patel CK, Wilson SW, Kuwada JY. Axonal trajectories and distribution of GABAergic spinal neurons in wildtype and mutant zebrafish lacking floor plate cells. J Comp Neurol. 1992; 326: 263–272. [DOI] [PubMed] [Google Scholar]
- 40. Martin SC, Heinrich G, Sandell JH. Sequence and expression of glutamic acid decarboxylase isoforms in the developing zebrafish. J Comp Neurol. 1998; 396: 253–266. [PubMed] [Google Scholar]
- 41. Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankne D, et al. The cells and logic for mammalian sour taste detection. Nature. 2006; 442: 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Djenoune L, Khabou H, Joubert F, Quan FB, Nunes Figueiredo S, Similowski T, et al. Investigation of spinal cerebrospinal fluid-contacting neurons expressing PKD2L1: evidence for a conserved system from fish to primates. Front Neuroanat. 2014; 8: 26 10.3389/fnana.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Orts-Del’Immagine A, Kastner A, Tillement V, Tardivel C, Trouslard J, Wanaverbecq N. Morphology, distribution and phenotype of polycystin kidney disease 2-like 1-positive cerebrospinal fluid contacting neurons in the brainstem of adult mice. PLoS one. 2014; 9: e87748 10.1371/journal.pone.0087748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafishexpressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000; 20: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohata S, Kinoshita S, Aoki R, Tanaka H, Wada H, Tsuruoka-Kinoshita S, et al. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development. 2009; 136: 1653–1663. 10.1242/dev.033290 [DOI] [PubMed] [Google Scholar]
- 46. Clemente D, Porteros A, Weruaga E, Alonso JR, Arenzana FJ, Aijón J, et al. Cholinergic elements in the zebrafish central nervous system: Histochemical and immunohistochemical analysis. J Comp Neurol. 2004; 474: 75–107. [DOI] [PubMed] [Google Scholar]
- 47. Yulis CR, Lederis KL. Extraurophyseal distribution of urotensin II immunoreactive neuronal perikarya and their processes. Proc Natl Acad Sci USA. 1986; 83: 7079–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kolmer W. Das “Sagittalorgan” der Wirbeltiere. Z. Anat Entwicklungs. 1921; 60: 652–717. [Google Scholar]
- 49. Agduhr E. Über ein Zentrales Sinnesorgan (?) bei den Vertebraten. Z Anat Entwicklungs. 1922; 66: 223–360. [Google Scholar]
- 50. Vigh B, Vigh-Teichmann I. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc Res Tech. 1998; 41: 57–83. [DOI] [PubMed] [Google Scholar]
- 51. Dale N, Roberts A, Ottersen OP, Storm-Mathisen J. The development of a population of spinal cord neurons and their axonal projections revealed by GABA immunocytochemistry in frog embryos. Proc R Soc Lond B Biol Sci. 1987; 232: 205–215. [DOI] [PubMed] [Google Scholar]
- 52. Dale N, Roberts A, Ottersen OP, Storm-Mathisen J. The morphology and distribution of “Kolmer-Agduhr cells”, a class of cerebrospinalfluid-contacting neurons revealed in the frog embryo spinal cord by GABA immunocytochemistry. Proc R Soc Lond B Biol Sci. 1987; 232: 193–203. [DOI] [PubMed] [Google Scholar]
- 53. Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009; 461: 407–410. 10.1038/nature08323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yulis CR, Lederis KL. Occurrence of an anterior spinal, cerebrospinal fluid-contacting, urotensin II neuronal system in various fish species. Gen Comp Endocrinol. 1988; 70: 301–311. [DOI] [PubMed] [Google Scholar]
- 55. Buchanan JT, Brodin L, Hokfelt T, Van Dongen PA, Grillner S. Survey of neuropeptide-like immunoreactivity in the lamprey spinal cord. Brain Res.1987; 408: 299–302. [DOI] [PubMed] [Google Scholar]
- 56. LaMotte CC, Shapiro CM. Ultrastructural localization of substance P, met-enkephalin, and somatostatin immunoreactivity in lamina X of the primate spinal cord. J Comp Neurol. 1991; 306: 290–306. [DOI] [PubMed] [Google Scholar]
- 57. Jalalvand E, Robertson B, Wallén P, Hill RH, Grillner S. Laterally projecting cerebrospinal fluid-contacting cells in the lamprey spinal cord are of two distinct types. J Comp Neurol. 2014; 522: 1753–1768. 10.1002/cne.23542 [DOI] [PubMed] [Google Scholar]
- 58. Yulis CR, Lederis K. Relationship between urotensin II- and somatostatin-immunoreactive spinal cord neurons of Catostomus commersoni and Oncorhynchus kisutch (Teleostei). Cell Tissue Res. 1988b; 254: 539–542. [DOI] [PubMed] [Google Scholar]
- 59. Park HC, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. 2004; 131: 5959–5969. [DOI] [PubMed] [Google Scholar]
- 60. Pelletier G, Lihrmann I, Vaudry H. Role of androgens in the regulation of urotensin II precursor mRNA expression in the rat brainstem and spinal cord. Neuroscience. 2002; 115: 525–532. [DOI] [PubMed] [Google Scholar]
- 61. Lancien F, Leprince J, Mimassi N, Mabin D, Vaudry H, Le Mével JC. Central effects of native urotensin II on motor activity, ventilatory movements, and heart rate in the trout Oncorhynchus mykiss . Brain Res. 2004; 1023: 167–174. [DOI] [PubMed] [Google Scholar]
- 62. Le Mével JC, Olson KR, Conklin D, Waugh D, Smith DD, Vaudry H, et al. Cardiovascular actions of trout urotensin II in the conscious trout, Oncorhynchus mykiss . Am J Physiol. 1996; 271: R1335–R1343. [DOI] [PubMed] [Google Scholar]
- 63. Le Mével JC, Lancien F, Mimassi N, Leprince J, Conlon JM, Vaudry H. Central and peripheral cardiovascular, ventilatory, and motor effects of trout urotensin-II in the trout. Peptides. 2008; 29: 830–837. [DOI] [PubMed] [Google Scholar]
- 64. Le Mével JC, Lancien F, Mimassi N, Conlon JM. Brain neuropeptides in central ventilatory and cardiovascular regulation in trout. Front Endocrinol. 2012; 3: 124 10.3389/fendo.2012.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997; 377: 577–595. [DOI] [PubMed] [Google Scholar]
- 66. Kinney WA, Almond HR Jr, Qi J, Smith CE, Santulli RJ, de Garavilla L, et al. Structure-function analysis of urotensin II and its use in the construction of a ligand-receptor working model. Angew Chem Int Ed Engl. 2002; 41: 2940–2944. [DOI] [PubMed] [Google Scholar]
- 67. Lavecchia A, Cosconati S, Novellino E. Architecture of the human urotensin II receptor: comparison of the binding domains of peptide and non-peptide urotensin II agonists. J Med Chem. 2005; 48: 2480–2492. [DOI] [PubMed] [Google Scholar]
- 68. Boucard AA, Sauvé SS, Guillemette G, Escher E, Leduc R. Photolabelling the rat urotensin II/GPR14 receptor identifies a ligand-binding site in the fourth transmembrane domain. Biochem J. 2003; 370: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boivin S, Segalas-Milazzo I, Guilhaudis L, Oulyadi H, Fournier A, Davoust D. Solution structure of urotensin-II receptor extracellular loop III and characterization of its interaction with urotensin-II. Peptides. 2008; 29: 700–710. 10.1016/j.peptides.2008.02.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.