Abstract
Objectives
Patient adherence and persistence is important to improve outcomes in chronic conditions, including inflammatory and immunologic (I&I) diseases. Patient programs that aim at improving medication adherence or persistence play an essential role in optimizing care. This meta-analysis assessed the effectiveness of patient programs in the therapeutic area of I&I diseases.
Methods
A global systematic literature review was conducted with inclusion criteria of: patient programs in I&I diseases; published in English language between January 2008 and September 2013; and reporting measures of adherence or persistence, including medication possession ratio >80% and persistence rate. A meta-analysis was performed using a random effects model. Subgroup analyses based on the type of program was performed whenever feasible.
Results
Of 67 studies reviewed for eligibility, a total of 17 studies qualified for inclusion in the meta-analysis. Overall, patient programs increased adherence (odds ratio [OR]=2.48, 95% confidence interval [CI]=1.68–3.64, P<0.00001) as compared with standard of care. Combination patient programs that used both informational and behavioral strategies were superior in improving adherence (OR=3.68, 95% CI=2.20–6.16, P<0.00001) compared with programs that used only informational (OR=2.16, 95% CI=1.36–3.44, P=0.001) or only behavioral approaches (OR=1.85, 95% CI=1.00–3.45, P=0.05). Additionally, patients were more likely to be persistent (OR=2.26, 95% CI=1.16–4.39, P=0.02) in the intervention group as compared with the control group. Persistence (in days) was significantly (P=0.007) longer, by 42 additional days, in the intervention group than in the control group.
Conclusions
Patient programs can significantly improve adherence as well as persistence in the therapeutic area of I&I diseases. Programs employing a multimodal approach are more effective in improving adherence than programs with informational or behavioral strategies alone. This in turn may improve patient outcomes.
Keywords: systematic literature review, informational, behavioral, patient interventions
Introduction
Patient adherence and persistence to treatment are important for effective disease management, especially in chronic diseases that may become more severe over time, such as autoimmune and inflammatory conditions. Adherence refers to the act of conforming to recommendations made by the provider with respect to timing, dose, and frequency of administration.1 Persistence is defined as the duration of time from initiation to discontinuation of therapy.1 Significant evidence suggests that nonadherence is highly prevalent in medical care2 and is a rising concern to health care providers and payers because it increases the cost of care and results in poor patient outcomes.3 Patients are nonadherent to treatment due to various self-identified reasons, including fear of side effects; poor memory; inability to pay for medications; concerns about medications, due to little or no education regarding the disease or regimen; and lack of perceived need.4
Inflammatory and immunologic (I&I) diseases share common characteristics in that these disorders are caused by an immune system attack on the body’s own tissues, leading to increased inflammation. Prevalence rates of autoimmune diseases range from five to 500 per 100,000.5 Inflammatory diseases are a significant clinical burden due to the high prevalence and incidence rates and the chronic nature of these conditions. In the USA, over seven million individuals suffer from inflammatory rheumatic diseases, which are the most severe among the inflammatory diseases.6
Adherence and persistence to long-term treatment are commonly required for optimal disease management. However, patient adherence has been shown to be suboptimal in I&I diseases, such as psoriatic and rheumatic diseases, multiple sclerosis (MS), osteoporosis, and inflammatory bowel disease.7–10 Only about 50% of patients adhere to prescribed medications, while 30% of patients with I&I chronic diseases miss at least one dose intentionally or unintentionally.11 Nonadherence rates range from 43% to 72% in inflammatory bowel disease,12 from 14% to 67% in psoriasis,13 and from 30% to 80% in rheumatoid arthritis (RA).14,15 These rates highlight the need to improve adherence in patients who require long-term treatment.
A number of simple and complex programs have been developed to improve medication adherence and persistence, focusing on informational, behavioral, and combined strategies.16 Informational programs focus on increasing patient knowledge of their disease, treatments, and management tools, through educational brochures, group-based discussion sessions,17 and web-based presentations.9,18 Behavioral programs involve individually tailored adherence-focused sessions and nurse-assisted patient support programs.10,19
The effectiveness of these programs in improving adherence and persistence has not been previously assessed using a systematic approach. Therefore, a meta-analysis was undertaken to quantify and compare the impact of informational, behavioral, and combined patient programs on adherence and persistence.
Methods
Search strategy
Studies published between January 2008 and September 2013 were identified using the PubMed database. Key search terms and Medical Subject Headings (MeSH) terms (Table S1) for I&I (eg, “ankylosing spondylitis”, “psoriasis”, “psoriatic arthritis”, and “rheumatoid arthritis”) and type of adherence and persistence program interventions (eg, “compliance”, “medication adherence”, “behavioral intervention”, and “persistence”) were used to identify relevant studies. Additionally, an internet search targeting adherence and persistence programs was conducted, and conference proceedings in the I&I therapeutic area were searched.
Inclusion criteria included: (1) studies focused on diseases in the I&I therapeutic area; (2) studies reporting adherence or persistence outcomes; (3) patient programs or interventions comparing exposure with a control group; and (4) studies published in the English language. Studies were not limited to any age range. Two researchers screened the titles and abstracts to determine eligibility for full text review; any disagreements were resolved by consensus with a third researcher. All studies that met our inclusion criteria were reviewed via full-text screening.
Data abstraction
Data from selected studies were abstracted, and information was collected on the country of investigation, disease, study design, sample size in the intervention and control arms, program strategy in brief, study follow-up duration, adherence and persistence definitions, and adherence and persistence results.
Statistical analysis
Statistical analysis was performed using Review Manager 5.2. Cochran Q χ2 and I2 statistics were used to assess the heterogeneity among studies. Since included studies varied in the diseases studied, interventions utilized, study population, and other observable and unobservable factors, a random effects model was employed to allow study outcomes to vary assuming a normal distribution among study populations. If adherence or persistence was reported as a binary measure, then the effect of the intervention was measured as an odds ratio (OR); if it was reported as a continuous measure, then the effect was measured as the mean difference between the intervention and the control group. The pooled effect for each grouping of trials was derived from the OR for each separate trial, weighted by the inverse of the variance (1/standard error of the mean [SE]2), and 95% confidence intervals (CIs) were calculated. In studies reporting discontinuation rates, a persistence rate was calculated as 1 – discontinuation rate.20
Subgroup analyses were performed based on the type of strategy implemented in the patient programs (informational, behavioral, or combined). Informational interventions comprised educational materials administered via various means and formats: oral and telephone communication, written materials, audiovisual presentations, and mailed or emailed materials. Behavioral interventions comprised dosing change, dosing recommendations, and treatment reminders given by telephone or email.21,22
Publication bias was evaluated using funnel plots. The overall risk of bias for an individual study was categorized as low, unclear, or high, as per the Cochrane “Risk of bias” assessment tool in Review Manager.23
Results
Study selection
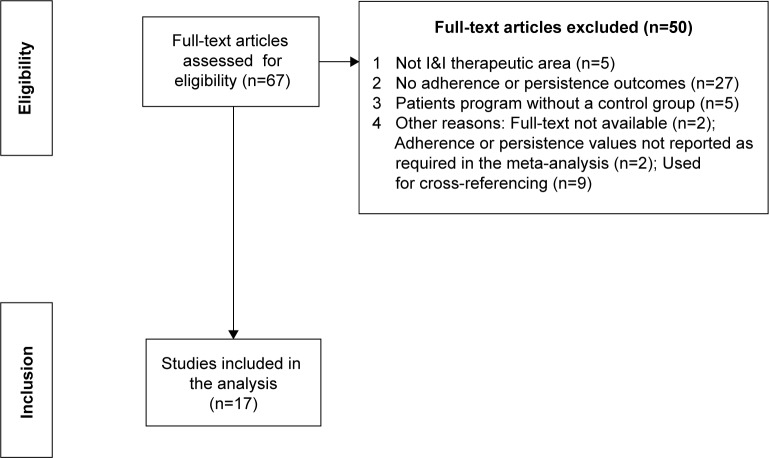
Of the 67 studies that qualified for full-text screening, 17 were included in the meta-analysis.7–9,17–19,24–34 A flow diagram summarizing the study selection and inclusion is reported in Figure 1.
Figure 1.
Flow diagram of systematic literature search.
Abbreviation: I&I, inflammatory and immunologic.
Study characteristics
Table 1 lists the characteristics of the 17 studies selected. Among these studies, eight were conducted in an osteoporosis patient population,9,17,24–26,31,33 four in ulcerative colitis (UC),18,19,29,30 two in MS,8,34 and one each in RA,32 childhood-onset systemic lupus erythematosus,28 and RA/psoriasis.7 Of these 17 studies, eight used informational interventions,7,9,17–19,24–26 two used behavioral strategies,27,28 and seven used combined strategies.8,29–34 Nine studies measured only adherence,7,17–19,25,26,28–30 while four studies measured only persistence,8,32–34 and four studies measured both adherence and persistence.9,24,27,31
Table 1.
Characteristics of included studies
| Study (country) | Study design | Disease area | Intervention | N | Comparison | N |
|---|---|---|---|---|---|---|
| Informational patient programs | ||||||
| Elkjaer et al (Denmark and Ireland)18 | RCT | Ulcerative colitis | Web-based patient education | 89 | Conventional treatment and follow up in the IBD out-patient clinic | 97 |
| Homer et al (UK)7 | RCT | Rheumatoid arthritis and psoriasis | Group counseling | 30 | Individual counseling | 32 |
| Lai et al (Malaysia)24 | RCT | Osteoporosis | Pharmaceutical care and counseling | 100 | No counseling | 98 |
| Montori et al (USA)9 | RCT | Osteoporosis | Decision aid | 52 | No decision aid | 48 |
| Moss et al (USA)19 | RCT | Ulcerative colitis | Nurse delivered patient support program | 15 | Standard medication refill and follow up | 36 |
| Nielsen et al (Denmark)17 | RCT | Osteoporosis | Group-based educational program | 136 | Standard prescribed therapy with visits at the general practitioner or clinic | 130 |
| Shu et al (USA)25 | RCT | Osteoporosis | Educational intervention | 593 | No intervention | 564 |
| Solomon et al (USA)26 | RCT | Osteoporosis | Telephone-based counseling | 1,046 | No counseling | 1,041 |
| Behavioral patient programs | ||||||
| Heilmann et al (USA)27 | Retrospective study | Osteoporosis | Pharmacy based management service with follow up | 291 | Nurse based service without follow up | 71 |
| Ting et al (USA)28 | RCT | Childhood-onset systemic lupus erythematosus | Cellular text messaging reminders | 19 | No reminders | 22 |
| Combined patient programs | ||||||
| Cook et al (USA)29 | RCT | Ulcerative colitis | Telephone nurse counseling | 278 | No counseling | 246 |
| Moshkovska et al (UK)30 | RCT | Ulcerative colitis | Tailored patient preference intervention | 37 | Standard care | 34 |
| Sewerynek et al (Poland)31 | Prospective study | Osteoporosis | Patient counseling, biochemical information, and nurse assistance | 29, 31, and 31 | No counseling, biochemical information, or assistance | 32 |
| Stockl et al (USA)32 | Retrospective study | Rheumatoid arthritis | DTM program | 244 | No DTM | 244 |
| Stockl et al (USA)8 | Retrospective study | Multiple sclerosis | DTM program | 156 | No DTM | 156 |
| Tamone et al (Italy)33 | RCT | Osteoporosis | Self-injection training and educational telephone follow-up program | 382 | No training, no follow up | 398 |
| Tan et al (USA)34 | Retrospective study | Multiple sclerosis | Specialty care management program | 3,125 | No care management | 868 |
Abbreviations: DTM, disease therapy management; IBD, Inflammatory bowel disease; RCT, randomized controlled trial.
Table 2 reports the adherence and persistence data, including their definitions, in the studies. The follow-up period in these studies ranged from 3 to 24 months. The majority of studies used a consistent approach to measure adherence or persistence within the study, except for two studies. Among these two studies, one study by Homer et al7 measured adherence in several ways: patients were asked to record how they took their medications in a dairy; the diaries were checked against pill counts at scheduled monthly follow-up appointments, and pills were counted by investigators. Patients who altered or stopped their medication as a result of contacting the Help line or of medical advice from their general practitioner were considered to be adherent. Another study by Lai et al24 measured adherence by three methods: direct reporting (asking participants how many doses they had missed); pill counts; and self-recording (participants recorded the date on which they took their dose). From both of these studies, only the adherence measures related to pill counts performed by the investigator was used in the meta-analysis.
Table 2.
Adherence and persistence outcomes
| Study (country) | Follow-up period (months) | Adherence | Persistence | Adherence outcomes (I vs C) | Persistence outcomes (I vs C) |
|---|---|---|---|---|---|
| Informational patient programs | |||||
| Elkjaer et al (Denmark and Ireland)18 | 12 | The compliance questionnaire included five questions with dichotomized answers: easy access to prescription, ability of relapse recognition, following the medical doctor’s advice, ability to self-initiate acute treatment, and adherence to 5-ASA treatment. In Denmark, patients’ answers regarding 5-ASA refill were compared with results from the e-prescription pharmacy database | Denmark: 73% vs 42% (P=0.005) Ireland: 73% vs 29% (P=0.03) |
NR | |
| Homer et al (UK)7 | 4 | Adherence was defined as: “the extent to which the patient’s behavior matches agreed recommendation from the prescriber” | – | 90% vs 69% (P=0.06) | NR |
| Lai et al (Malaysia)24 | 12 | Adherence was defined as the average percentage of participants who were both persistent (continued bisphosphonate therapy) and compliant (took medication in the correct manner on the scheduled day). Pill count (by counting the number of tablets left at each visit) was used to measure adherence | Persistence (defined as the time in days from the date of the first dose of bisphosphonate until discontinuation of treatment) was obtained from supply records, using the pharmacy information system | 97.70% vs 96.46% (P=0.322) | 87.0% vs 89.8% (P=0.481) |
| Montori et al (USA)9 | 12 | To assess medication adherence at 6 months, patients were telephoned and asked Haynes’ single-item adherence question (“Have you missed any of your pills in the last week?”). Pharmacy records were obtained to assess adherence and persistence | Persistence was estimated using proportion of patients who had ≥80% adherence | 100% vs 98.20% (P=0.09) | 170 (range: 30–180) days vs 180 (range: 28–180) days (P=0.38) |
| Moss et al (USA)19 | 6 | Adherence was calculated based on refill data from pharmacies according to Steiner’s formula. Only patients with adherence >80% were considered to be adherent | – | 67% vs 50% (P=0.3) | NR |
| Nielsen et al (Denmark)17 | 24 | Adherence was defined as patients taking their medicine correctly at the appropriate time; patients who changed to another osteoporosis drug were considered to be adherent. Data on adherence were obtained via self-completed questionnaires | – | 92% vs 80% (P=0.006) | NR |
| Shu et al (USA)25 | 3 | Adherence was measured as MPRb. A patient who consistently filled prescriptions and had medication available for each day was 100% adherent | – | 87.60% vs 88.80% (P=0.60) | Not used as it was not possible to determine the effect size, using the data given |
| Solomon et al (USA)26 | 12 | Adherence was measured as MPRb. Pharmacy claims data from the collaborating state run pharmacy benefits program was used to calculate the MPR | – | 49% vs 41% (P=0.07) | NR |
| Behavioral patient programs | |||||
| Heilmann et al (USA)27 | 6 | Adherence was defined as a MPRb of at least 80% | Medication persistence was defined based on the last medication purchase prior to the 365-day cutoff date. If the days’ supply, multiplied by 1.2 was equal to or greater than the number of days between the final prescription purchase and the cutoff date, then medication use was considered persistent. A factor of 1.2 was used to allow for an adherence rate of less than 100% but at least 80% | 46% vs 28% (P=0.007) | 54% vs 45% (P=0.19) |
| Ting et al (USA)28 | 12 | Pharmacy refill adherence, defined as the percentage of the number of doses dispensed divided by the number of doses prescribed for the period of time between study visits and pharmacy refill dates, with pharmacy refill information serving as primary measure of medication adherence. Patients with adherence >80% were considered adherent | – | 37% vs 27% | NR |
| Combined patient programs | |||||
| Cook et al (USA)29 | 6 | Adherence was defined as months of treatment completed. When participants reported adherence, RNs assessed what percent of the past month’s doses were taken as prescribed; Patients with adherence ≥80% were considered adherent | – | 88% vs 57% (P<0.001) | NR |
| Moshkovska et al (UK)30 | 12 | Nonadherence, based on analysis of urine samples, was defined as: • Complete nonadherence: undetectable (0 lg/mL) levels of 5-ASA or N-acetyl-5-ASA. • Partial nonadherence: 5-ASA <30 lg/mL and N-acetyl-5-ASA <90 lg/mL • Adherence: 5-ASA ≥30 lg/mL and N-acetyl-5-ASA ≥90 lg/mL In order to focus on identifying factors determining full adherence, partial and complete nonadherence were subsequently combined to form a single category for comparison with complete adherence |
– | 76% vs 32% (P=0.001) | NR |
| Sewerynek et al (Poland)31 | 12 | The refill adherence was measured as MPRb. Patients with adherence >80% were considered adherent | Persistence was defined as the time in days from the date of prescription to the “run out” date in the treatment period. Patients were defined as “persistent” until a gap of >90 days was reached between the end of one prescribed drug series and the date of subsequent prescription; or until the patient switched to another bisphosphonate; or had a refill gap >30 days between the end of one prescription series and the beginning of the subsequent one | Educational group: 75.71% vs 54.03% Biochemical information group: 68.29% vs 54.03% Nurse assistance group: 71.18% vs 54.03% |
Educational group: 269.72 (SEM 26.95) days vs 197.00 (SEM 26.91) days Biochemical information group: 249.19 (SEM 29.04) days vs 197.00 (SEM 26.91) days Nurse assistance group: 259.71 (SEM 25.10) days vs 197.00 (SEM 26.91) days |
| Stockl et al (USA)32 | 8 | – | Discontinuation was defined as a gap of >30 days between the depletion date (fill date + number of days’ supply) for the last filled prescription and the end of the postidentification period | NR | 89.30%a vs 59.40%a (P<0.001) |
| Stockl et al (USA)8 | 8 | – | Medication persistence was defined as the number of days on therapy until a gap of >30 days Medication discontinuation defined as the gap of >30 days past the end of supply date for the last filled prescription and the end of the “Post” period |
NR | 219.80 (SD 80.30) days vs 176.50 (SD 92.00) days (P<0.001) 92.30%a vs 71.80%a (P<0.001) |
| Tamone et al (Italy)33 | 18 | – | Persistence was defined as the number of patients continuing treatment until the end of the 18-month course | NR | 85.60% vs 77.40% (P=0.006) |
| Tan et al (USA)34 | 12 | – | Medication persistence was referred to the duration of time from initiation to discontinuation of therapy, while discontinuation was defined as failing to obtain any MS medication within 60 days after the depletion of the previous supply | NR | 306.10 (SD 84.10) days vs 246.90 (SD 129.60) days (P<0.001) |
Notes:
Persistence rate was calculated from the discontinuation rate using the formula: persistence rate =100 - discontinuation rate.
MPR was defined as the number of days for which medication was available divided by number of days in the follow-up period.
Abbreviations: ASA, aminosalicylic acid; C, control group; I, intervention group; MPR, medication possession ratio; MS, multiple sclerosis; NR, not reported; RN, registered nurse; SD, standard deviation; SEM, standard error of mean.
Effect of patient programs on adherence measure
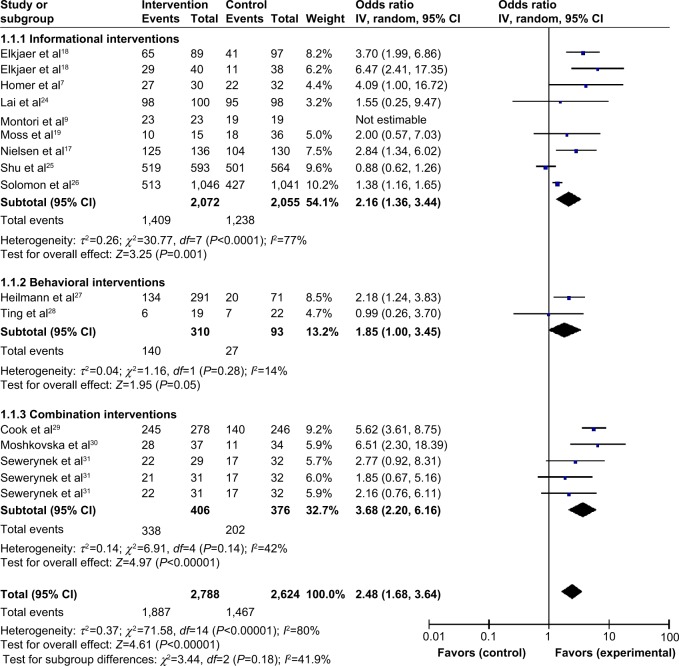
Adherence data (% adherent) were obtained from 13 studies, which were grouped by type of program: informational (n=8); behavioral (n=2); and combined (n=3). Forest plots (Figure 2) were developed in order to compare the ORs from each study. Overall, patient programs increased adherence (OR=2.48, 95% CI=1.68–3.64, P<0.00001) when compared with the control group (standard prescribed care or no intervention). Combination patient programs that used both informational and behavioral strategies were superior in improving adherence (OR=3.68, 95% CI=2.20–6.16, P<0.00001) compared with programs using only informational approaches (OR=2.16, 95% CI=1.36–3.44, P=0.001) or only behavioral approaches (OR=1.85, 95% CI=1.00–3.45, P=0.05). A random effects model was used due to the high level of heterogeneity in the overall analysis (I2=80%).
Figure 2.
Program effectiveness on adherence, by type of patient program.
Notes: The study by Sewerynek et al32 is one study with three intervention groups: (1) the patient counseling group; (2) the biochemical information groups; and (3) the nurse-assistance group. The squares in the lines represent the effect estimate, the lines represent the length of the confidence interval, the diamonds represents the overall result of the meta-analysis.
Abbreviations: CI, confidence interval; IV, inverse variance.
Informational patient programs
A total of seven out of eight studies reported higher adherence in the intervention group compared with the control group (Figure 2). Elkjaer et al18 employed a web-based patient education program to educate UC patients about their disease. Training was provided, and patients could ask their web doctor questions via email or text. During the training, investigators aimed to ensure that each patient understood the web-based training and education, could recognize a relapse, and was able to start the program-recommended treatment. Homer et al7 used group counseling to improve adherence in patients with RA or psoriasis. Patients were allowed to bring a relative, caregiver, or friend to these sessions. They were shown a presentation of “frequently asked questions”, and individualized advice was provided to each. Lai et al24 distributed a “counseling package” to the participants, which consisted of an explanation of osteoporosis, risk factors, lifestyle modifications, goals of osteoporosis therapy, side effects, and the importance of medication adherence. Verbal counseling was reinforced with an osteoporosis booklet, and pharmacists followed up with participants. Montori et al9 used a decision aid, which was a tailored pictographic that illustrated their 10-year fracture risk estimate, absolute risk reduction with bisphosphonates, side effects, and out-of-pocket costs. The decision aid also showed the absolute risk reduction in fracture risk with alendronate, assuming a treatment-related reduction in overall osteoporotic fracture risk of 40%. Moss et al19 assessed patients enrolled in the Script Assist program (an independent treatment adherence program that provides disease-specific information and promotion of medication adherence to patients). Patients received phone calls at 24 hours, 3 weeks, 7 weeks, 15 weeks, and 23 weeks after enrollment, from nurses who were trained to assess patient risk for noncompliance and to intervene with psychological techniques that could improve medication persistence. Nielsen et al17 conducted a group-based educational program in classes of eight to 12 patients each, lasting 3 to 4 hours a week over 4 weeks, conducted by a multidisciplinary team of physicians, dieticians, physiotherapists, and nurses, which were adjusted according to individual patient backgrounds and needs in order to strengthen competence and empowerment. Additionally, patients were invited to participate in a computerized support program, where patients were contacted once a month for 4 months and asked about pain, quality of life, and physical activity. Solomon et al26 sent out seven informational mailings regarding topics such as exercise, fall prevention, and recommended calcium intake to all the study patients. Additionally, the intervention group received ten motivational interview counseling sessions via telephone with a health educator, where each session had a specific educational topic (discussing medications with physician, calcium and vitamin D supplementation, fall prevention, managing adverse effects of medication, etc) and included a series of open-ended questions to elicit subjects’ attitudes toward medication adherence and to determine barriers to long-term osteoporosis medication use.
Shu et al25 reported lower adherence in the intervention group compared with the control group. In this study, randomly selected primary care physicians and their patients received education about osteoporosis diagnosis and treatment. The primary care physicians also received face-to-face education by trained pharmacists, while patients received letters outlining the importance of osteoporosis, its diagnosis, and appropriate treatment, and automated calls inviting them to undergo bone mineral density testing. The pharmacists had participated in a 1-day training session and several follow-up teleconferences about osteoporosis and the principles of one-to-one physician education. The control group received no education.
Behavioral patient programs
Heilmann et al27 used a pharmacy-based management service, where a clinical pharmacist developed a therapeutic plan for treatment recommendations (bone marrow density screening, initiation of osteoporosis therapy, and calcium and vitamin D supplementation) after reviewing the medical history of patients. This plan was then approved by the primary care provider before implementation with patients. Ting et al28 sent daily text messages to patients three times a week prior to each scheduled follow-up clinic appointment. These texts were individualized for each patient by including the scheduled time of the upcoming clinic appointment. Messages were also sent in cases when patients failed to schedule a follow-up visit. A standardized daily reminder was sent to patients based on the prescription (eg once or twice per day), and also received printed information about the benefits and the side effects of the medication.
Combined patient programs
Studies conducted by Sewerynek et al,31 Cook et al,29 and Moshkovska et al30 evaluated the impact of combined strategies on patient adherence. In the study conducted by Cook et al29 telephone follow-up calls were made to UC patients. Within a day of referral, patients received a call from a trained registered nurse who provided an introduction to the program, a preliminary assessment, and offer of a patient program, based on their concerns and readiness for change, using cognitive-behavioral and motivational interviewing counseling techniques. All patients were given a toll-free number for questions, and the referring health care practitioner received a progress note after each call, with notes on the participant’s adherence level and concerns. Moshkovska et al30 employed a tailored patient preference program in which UC patients were given one-on-one education and motivational sessions to deliver individualized support, motivation, and education. At week 4, a brief follow-up telephone call was made to the patient, and at week 24, a 10-minute reinforcement session was held to stress the importance of adherence to medication, to reassess beliefs regarding medicine-taking, and to discuss practical problems. At the end of the session, patients were offered an educational leaflet and a choice of three practical adherence-enhancing patient programs that included medication reminder charts, visual medication reminders for refrigerators and bedside cabinets, daily or weekly electronic pill box organizers with alarms, and a mobile telephone alarm setup.
Sewerynek et al31 randomized patients into four groups, receiving: patient counseling; biochemical information; nurse assistance; or no intervention (control). In the nurse assisted group, a follow-up phone contact was made after 3 and 9 months of treatment, to improve monitoring. In the counseled group, patients were educated and interviewed for 30 minutes about osteoporosis, diagnostic methods, treatment, and preventative behavior. In the biochemical group, patients were educated about serum levels of calcium, phosphorus, alkaline phosphatase, and of urinary calcium and phosphorus concentration levels and diurnal excretion rates.
Effect of patient programs on persistence
Persistence in studies was measured as either percent of patients who were persistent or number of days persistent; the former was a dichotomous variable, while the latter was a continuous variable. As shown in Figure 3, patients were more likely to be persistent (OR=2.26, 95% CI=1.16–4.39, P=0.02) in the intervention group compared with the control group. A subgroup analysis was not feasible due to the small number of studies reporting persistence. A random effects model was used to adjust for the presence of heterogeneity (I2=86%).
Figure 3.
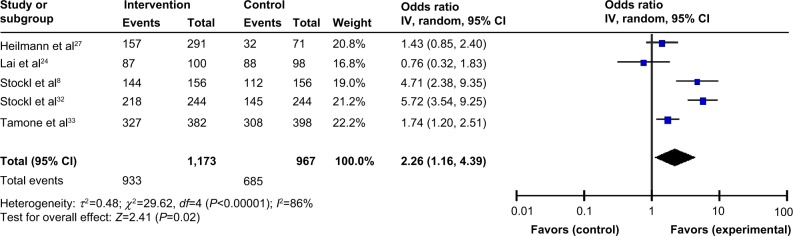
Program effectiveness measured as % persistence.
Notes: The squares in the lines represent the effect estimate, the lines represent the length of the confidence interval, the diamonds represent the overall result of the meta-analysis.
Abbreviations: CI, confidence interval; IV, inverse variance.
Five studies measured persistence in terms of percent of patients persistent over follow up. Stockl et al8 studied MS patients in a disease therapy management (DTM) program, who received telephone consultations, mailed care plans, and educational materials, based on a predefined schedule for level of intensity of the program (regular-intensity versus high-intensity). In another study by Stockl et al patients with RA were enrolled in a DTM program in which they were given a brochure detailing medication ordering and storage, monitoring, proper disposal of ancillary supplies, mail service medication delivery, refill reminders by patient care coordinators, and access to a pharmacist 24 hours a day, 7 days a week.32 The program used a patient-centric approach providing education and support, to assist patients in developing self-management skills for symptom and treatment management. Each patient was assigned a clinician for the entirety of the program and received telephone consultations (licensed pharmacist or registered nurse) providing education on the medical condition and treatment options, and promoting medication adherence. Tamone et al33 implemented an educational telephone program. At the beginning of treatment, nurses trained patients on self-injection, then, every 2 months, nurses gave new drug pens to the patients; this guaranteed the surveillance of compliance. Nurses called patients to help resolve any issues, schedule the next visit, and, if applicable, collect adverse events information, dates, and reasons for treatment discontinuation.
In a small number of studies, persistence was also reported as number of days persistent (Figure 4). When measured as persistent days, persistence was significantly longer, by 41.96 additional days (P=0.007), in the intervention group than in the control group.
Figure 4.
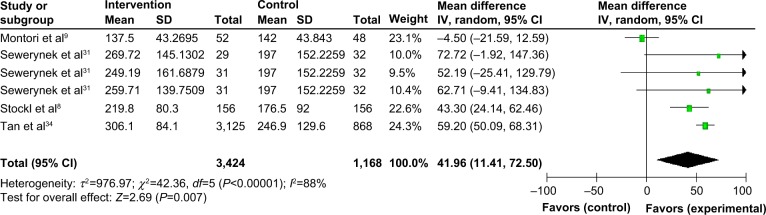
Effect of intervention vs control on persistence (in days) in I&I.
Notes: The squares in the lines represent the effect estimate, the lines represent the length of the confidence interval, the diamonds represent the overall result of the meta-analysis.
Abbreviations: CI, confidence interval; I&I, inflammatory and immunologic; SD, standard deviation; IV, inverse variance.
As described above, Montori et al9 employed a pictographic decision aid; Sewerynek et al31 employed three intervention groups in which patients received either nurse assistance, information about their biochemical charts, or counseling; and the study by Stockl et al8 of (MS), utilized a DTM program. Tan et al34 provided a specialty care management program service, including mail order medications, disease-specific patient education materials, refill reminder calls, and assessment calls by nurses at the beginning of the program and at months 3, 6, and 12, and every 12 months thereafter.
Risk of bias in the reviewed studies
The common types of biases evaluated in this analysis were selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases inherent in interventional study designs. Selection bias was determined if patients were not assigned to an intervention or control group using random sequence generation and if the allocation of participants were not concealed. Performance bias referred to the lack of blinding of participants and personnel – blinding ensures that the control group receives similar attention, treatment, and diagnostic investigations as the intervention group. Detection bias referred to blinding of investigators, which reduces confounding related to the knowledge of intervention assignment. Reporting bias is often related to selective reporting of study measures (publications more often report statistically significant differences than nonsignificant differences, leading to reporting bias), while attrition bias is due to incomplete outcomes data, due to omission of some participants from the reports of analyses. Other biases included bias due to study designs, and inclusion and exclusion of patients.
As depicted in Figure 5, six8,27,29,32–34 out of the 17 studies did not perform random sequence generation, and eleven8,17,19,24–28,32–34 did not conceal the allocation of participants, resulting in selection bias. Although blinding of participants and personnel, as well as that of outcomes assessed in the study, is not usually possible in patient programs, it was addressed by two studies.9,18 There was no attrition or reporting bias in the selected studies. However, other types of biases related to study designs were observed in three studies.
Figure 5.
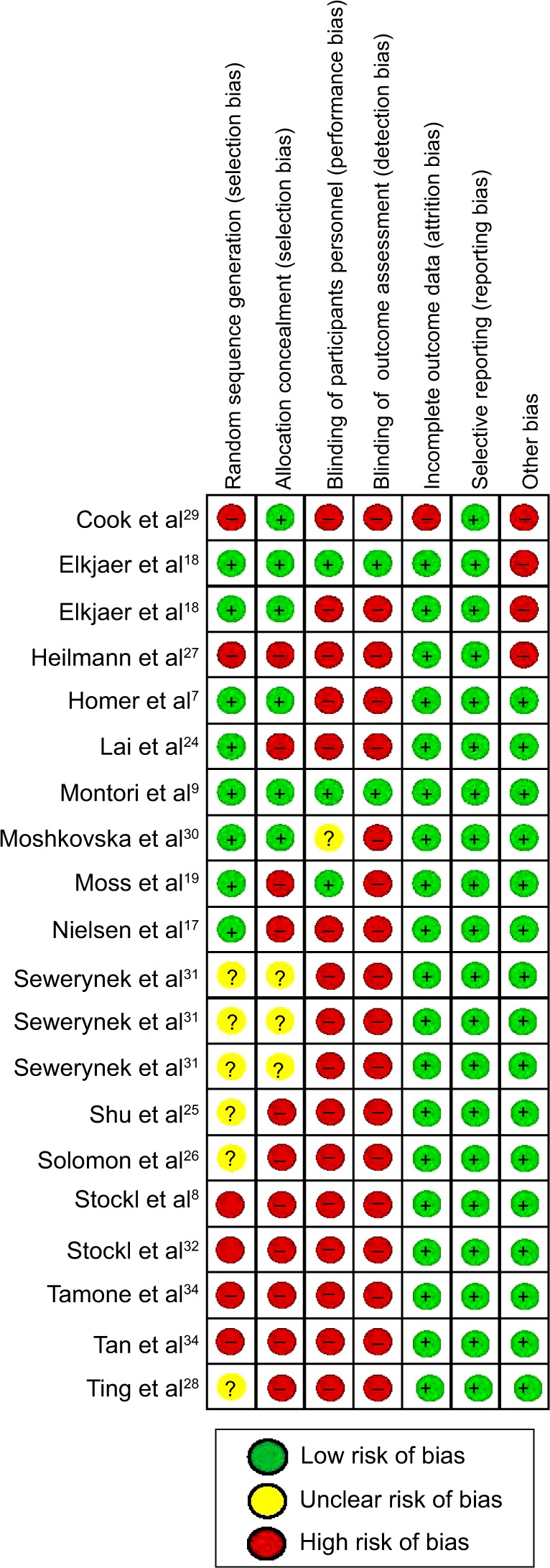
Risk of bias summary.
Among these studies, Cook et al29 divided the intervention population into two groups: with high risk and low risk. However, the results were aggregated for the two groups and were not presented separately for the high- and low-risk patients. The study by Elkjaer et al18 only selected patients who suffered from mild to moderate UC; those with severe UC were not included, and yet, they are an important target population for adherence. Participants in the study conducted by Heilmann et al27 required continuous enrollment in their health plan (no membership gaps greater than 45 days), therefore, members who did not have continuous membership were not included in the study.
Discussion
Key findings
Though various patient programs have been developed with the goal of improving adherence and persistence, little has been published on the overall effectiveness of these programs in the therapeutic area of I&I diseases. The results of this meta-analysis show that patient programs incorporating combined strategies (informational and behavioral techniques together) were more likely to improve adherence compared with informational or behavioral strategies alone.
Among the combination patient programs, two out of three studies significantly increased adherence, using cognitive-behavioral and motivational interview counseling over the telephone and tailoring the program as per patient preference to include individualized education, support sessions, and problem solving.29,30 Patient counseling and motivational sessions formed a major part of all combination patient programs, suggesting that a greater degree of communication may have existed between patients and health care practitioners in these instances. Combined strategies also focused on providing education about the disease and treatment. Therefore, behavior modification as well disease knowledge was targeted to improve adherence.
Among the eight informational patient programs, three significantly increased adherence in the intervention group as compared with the control group. These programs included strategies for web-based patient education, group counseling, and group-based education.7,17,18 We found that patient education and counseling, more so in a group-based setting, can be an effective strategy to enhance adherence. One study reported lower adherence in the intervention group as compared with the control group.25 This intervention focused on physicians more than patients and suggested that adherence programs might be more effective when they are patient-centric. In the study, researchers suspected that their inability to observe a difference in adherence was due to a high rate of baseline adherence in the control group, and the 10-month follow-up period. In the study by Homer et al7 reports by the participants – the use of diary and self-reports of pill count – were used, which may lead to biases. Hence, the pill count data recorded by the health care professional, considered to be unbiased, was used for the meta-analysis.
Behavioral techniques suggest a positive impact on adherence benefits when data were pooled, though our finding is based on only two studies. Of the two studies, Ting et al28 did not show a positive benefit; however, the study included a small sample size, of 19 patients in the intervention group and 22 patients in the control group. In contrast, Heilmann et al27 did show a significant improvement in adherence, and the study was weighted more heavily due to the much larger sample size (N=362). The strategy included pharmacy-based management services that provided recommendations on screening and medication. Also, Heilmann et al27 followed patients for 6 months, while Ting et al28 followed their patients for 12 months. The longer follow-up period may have influenced level of adherence. There was also a major difference in the patient population, with the study by Heilmann et al27 consisting of elderly and potentially more severe patients. Heilmann et al27 studied women patients suffering from osteoporosis, aged 67 years or older, while Ting et al28 included patients with childhood-onset systemic lupus erythematosus, aged between 13 and 25 years, with unlimited access to cellular text messaging.
Persistence could be enhanced significantly by DTM programs, self-injection training, disease and treatment education over the telephone, and specialty care management. DTM, as well as specialty care management programs, involve a combination of services, such as consultation, care plans, educational material mailings, reminders, mail-service medication delivery, access to the pharmacist, and thorough follow-up.8,32–34 Hence, they prove to be exhaustive programs that can improve persistence.
Overall, programs that empower patients, through counseling, education, reminders, and support, improve adherence and persistence. Additionally, active participation of pharmacists, registered nurses, and primary care physicians can further enhance adherence. Follow up is an important part of patient programs as it gives patients a chance to ask questions and resolve any issues. A systematic review conducted by Haynes et al21 confirms our findings and reported that patient programs that consisted of both informational and behavioral techniques, such as counseling, reminders, reinforcement, and individualized plans, commonly improved patient adherence. However, the review by Haynes et al21 only included randomized controlled trials and made no attempt to pool data to evaluate the effectiveness of these programs in a meta-analysis.
Strengths and limitations of the study
This meta-analysis adds to the current body of evidence by assessing the effectiveness of programs on adherence and persistence outcomes in diseases that require long-term management. One of the strengths of this study is the comprehensive, structured, and systematic approach undertaken to search the literature and conference proceedings to identify all studies that assessed adherence-focused patient programs. Furthermore, to our knowledge, this is the first meta-analysis in the therapeutic area of I&I diseases to study the effect of such programs, based on the type of the patient program used.
Although this review provides a comprehensive understanding of the effect of patient programs on adherence and persistence, some limitations should be considered when interpreting our findings. Although osteoporosis has not been categorized as an I&I disease, emerging molecular and clinical evidence highlights that inflammation exerts significant influence on bone turnover, which induces osteoporosis.35 Transplantation was not included in this analysis, due to the unique clinical and treatment characteristics in the transplant population, which may not be generalizable and comparable to populations with chronic I&I disease. A limitation inherent in meta-analyses is that we could not control for potential confounding variables such as age, socioeconomic level, education level, disease severity, and comorbidities. Also, studies that reported persistence as a medication possession ratio were not included in the meta-analysis. However, excluding these studies did not likely bias our finding for an overall benefit because these studies reported a significant increase in the medication possession ratio in the intervention group compared with the control group. Additionally, a number of studies focused on a specific population segment, for example, patients with internet access or unlimited access to cellular text messaging, and results may not be generalizable to the general population. Although these patient programs were associated with improvements in adherence and persistence, it is worth noting that they were conducted in controlled settings. The actual effect of such complex patient programs on patients may be different in real-world clinical practice.
Overall, we found that patient programs can significantly improve adherence as well as persistence, in I&I diseases. Programs employing a multimodal approach seem to be the most effective, given that they address multiple aspects of treatment management; however, informational or behavioral strategies alone also appear to be beneficial by themselves. Supporting and implementing similar patient programs may in turn improve patient outcomes in those with chronic I&I disease.
Supplementary material
Table S1.
Search terms
| Primary search terms | Secondary search terms |
|---|---|
| Allergies | Compliance |
| Ankylosing spondylitis | Medication adherence |
| Asthma | Adherence program |
| Behcet’s disease | Adherence intervention |
| Bursitis | Behavioral intervention |
| Celiac disease | Capacitance |
| Chronic pain | Compliance program |
| Crohn’s disease | Compliance intervention |
| Gout | Concordance |
| Idiopathic thrombocytopenic purpura | Medication possession ratio |
| Inflammatory bowel disease | Persistence |
| Multiple sclerosis | Persistence program |
| Osteoarthritis | Proportion of days covered |
| Osteoporosis | |
| Psoriasis | |
| Psoriatic arthritis | |
| Rheumatoid arthritis | |
| Sarcoidosis | |
| Scleroderma | |
| Sjögren’s syndrome | |
| Systemic lupus | |
| Systemic sclerosis | |
| Tendonitis | |
| Ulcerative colitis | |
| Vasculitis |
Footnotes
Disclosure
Market Access Solutions, LLC, a consulting company, received funding from Celgene Corporation to conduct the research, analysis, and develop the manuscript. Zeba M Khan and Satyin Kaura are employees of Celgene Corporation. Chakkarin Burudpakdee was formerly employed by Market Access Solutions, LLC, and is currently a Principal at IMS Health. Smeet Gala and Merena Nanavaty are employed at Market Access Solutions, LLC. The authors report no other conflicts of interest.
References
- 1.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284–290. doi: 10.1007/s11606-010-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 4.McHorney CA, Spain CV. Frequency of and reasons for medication non-fulfillment and non-persistence among American adults with chronic disease in 2008. Health Expect. 2011;14(3):307–320. doi: 10.1111/j.1369-7625.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 6.Helmick CG, Felson DT, Lawrence RC, et al. National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 7.Homer D, Nightingale P, Jobanputra P. Providing patients with information about disease-modifying anti-rheumatic drugs: Individually or in groups? A pilot randomized controlled trial comparing adherence and satisfaction. Musculoskeletal Care. 2009;7(2):78–92. doi: 10.1002/msc.141. [DOI] [PubMed] [Google Scholar]
- 8.Stockl KM, Shin JS, Gong S, Harada AS, Solow BK, Lew HC. Improving patient self-management of multiple sclerosis through a disease therapy management program. Am J Manag Care. 2010;16(2):139–144. [PubMed] [Google Scholar]
- 9.Montori VM, Shah ND, Pencille LJ, et al. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am J Med. 2011;124(6):549–556. doi: 10.1016/j.amjmed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Hommel KA, Herzer M, Ingerski LM, Hente E, Denson LA. Individually tailored treatment of medication nonadherence. J Pediatr Gastroenterol Nutr. 2011;53(4):435–439. doi: 10.1097/MPG.0b013e3182203a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber N, Parsons J, Clifford S, Darracott R, Horne R. Patients’ problems with new medication for chronic conditions. Qual Saf Health Care. 2004;13(3):172–175. doi: 10.1136/qshc.2003.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawthorne AB, Rubin G, Ghosh S. Review article: medication non-adherence in ulcerative colitis – strategies to improve adherence with mesalazine and other maintenance therapies. Aliment Pharmacol Ther. 2008;27(12):1157–1166. doi: 10.1111/j.1365-2036.2008.03698.x. [DOI] [PubMed] [Google Scholar]
- 13.Thorneloe RJ, Bundy C, Griffiths CE, Ashcroft DM, Cordingley L. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol. 2013;168(1):20–31. doi: 10.1111/bjd.12039. [DOI] [PubMed] [Google Scholar]
- 14.van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351. doi: 10.1586/eci.12.23. [DOI] [PubMed] [Google Scholar]
- 15.Barton JL, Criswell LA, Kaiser R, Chen YH, Schillinger D. Systematic review and metaanalysis of patient self-report versus trained assessor joint counts in rheumatoid arthritis. J Rheumatol. 2009;36(12):2635–2641. doi: 10.3899/jrheum.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen D, Ryg J, Nielsen W, Knold B, Nissen N, Brixen K. Patient education in groups increases knowledge of osteoporosis and adherence to treatment: a two-year randomized controlled trial. Patient Educ Couns. 2010;81(2):155–160. doi: 10.1016/j.pec.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Elkjaer M, Shuhaibar M, Burisch J, et al. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59(12):1652–1661. doi: 10.1136/gut.2010.220160. [DOI] [PubMed] [Google Scholar]
- 19.Moss AC, Chaudhary N, Tukey M, et al. Impact of a patient-support program on mesalamine adherence in patients with ulcerative colitis – a prospective study. J Crohns Colitis. 2010;4(2):171–175. doi: 10.1016/j.crohns.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82(12):1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 21.Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet. 1996;348(9024):383–386. doi: 10.1016/s0140-6736(96)01073-2. [DOI] [PubMed] [Google Scholar]
- 22.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–665. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011. [Accessed December 24, 2014]. [updated March 2011]. Available from: www.cochrane-handbook.org. [Google Scholar]
- 24.Lai P, Chua SS, Chan SP. A systematic review of interventions by healthcare professionals on community-dwelling postmenopausal women with osteoporosis. Osteoporos Int. 2010;21(10):1637–1656. doi: 10.1007/s00198-010-1199-0. [DOI] [PubMed] [Google Scholar]
- 25.Shu AD, Stedman MR, Polinski JM, et al. Adherence to osteoporosis medications after patient and physician brief education: post hoc analysis of a randomized controlled trial. Am J Manag Care. 2009;15(7):417–424. [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–483. doi: 10.1001/archinternmed.2011.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heilmann RM, Friesleben CR, Billups SJ. Impact of a pharmacist-directed intervention in postmenopausal women after fracture. Am J Health Syst Pharm. 2012;69(6):504–509. doi: 10.2146/ajhp110309. [DOI] [PubMed] [Google Scholar]
- 28.Ting TV, Kudalkar D, Nelson S, et al. Usefulness of cellular text messaging for improving adherence among adolescents and young adults with systemic lupus erythematosus. J Rheumatol. 2012;39(1):174–179. doi: 10.3899/jrheum.110771. [DOI] [PubMed] [Google Scholar]
- 29.Cook PF, Emiliozzi S, El-Hajj D, McCabe MM. Telephone nurse counseling for medication adherence in ulcerative colitis: a preliminary study. Patient Educ Couns. 2010;81(2):182–186. doi: 10.1016/j.pec.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Moshkovska T, Stone MA, Smith RM, Bankart J, Baker R, Mayberry JF. Impact of a tailored patient preference intervention in adherence to 5-aminosalicylic acid medication in ulcerative colitis: results from an exploratory randomized controlled trial. Inflamm Bowel Dis. 2011;17(9):1874–1881. doi: 10.1002/ibd.21570. [DOI] [PubMed] [Google Scholar]
- 31.Sewerynek E, Horst-Sikorska H, Stępień-Kłos W, et al. The role of counselling and other factors in compliance of postmenopausal osteoporotic patients to alendronate 70 therapy. Arch Med Sci. 2013;9(2):288–296. doi: 10.5114/aoms.2013.34575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockl KM, Shin JS, Lew HC, et al. Outcomes of a rheumatoid arthritis disease therapy management program focusing on medication adherence. J Manag Care Pharm. 2010;16(8):593–604. doi: 10.18553/jmcp.2010.16.8.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamone C, Fonte G, Panico A, Molinatti PA, D’Amelio P, Isaia GC. Impact of a phone follow-up program on persistence with teriparatide or PTH(1–84) treatment. Calcif Tissue Int. 2012;90(4):272–278. doi: 10.1007/s00223-012-9574-9. [DOI] [PubMed] [Google Scholar]
- 34.Tan H, Yu J, Tabby D, Devries A, Singer J. Clinical and economic impact of a specialty care management program among patients with multiple sclerosis: a cohort study. Mult Scler. 2010;16(8):956–963. doi: 10.1177/1352458510373487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2(1):14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Search terms
| Primary search terms | Secondary search terms |
|---|---|
| Allergies | Compliance |
| Ankylosing spondylitis | Medication adherence |
| Asthma | Adherence program |
| Behcet’s disease | Adherence intervention |
| Bursitis | Behavioral intervention |
| Celiac disease | Capacitance |
| Chronic pain | Compliance program |
| Crohn’s disease | Compliance intervention |
| Gout | Concordance |
| Idiopathic thrombocytopenic purpura | Medication possession ratio |
| Inflammatory bowel disease | Persistence |
| Multiple sclerosis | Persistence program |
| Osteoarthritis | Proportion of days covered |
| Osteoporosis | |
| Psoriasis | |
| Psoriatic arthritis | |
| Rheumatoid arthritis | |
| Sarcoidosis | |
| Scleroderma | |
| Sjögren’s syndrome | |
| Systemic lupus | |
| Systemic sclerosis | |
| Tendonitis | |
| Ulcerative colitis | |
| Vasculitis |