Abstract
Although Mycobacterium tuberculosis is the causative agent of pulmonary tuberculosis (PTB), environmental factors may influence disease progression. Ecologic studies conducted in countries outside the USA with high levels of air pollution and PTB have suggested a link between active disease and ambient air pollution. The present investigation is the first to examine the ambient air pollution/PTB association in a country, where air pollution levels are comparatively lower. We used Poisson regression models to examine the association of outdoor air pollutants, PM10 and PM2.5 with rates of PTB in North Carolina residents during 1993–2007. Results suggest a potential association between long-term exposure to particulate matter (PM) and PTB disease. In view of the high levels of air pollution and high rates of PTB worldwide, a potential association between ambient air pollution and tuberculosis warrants further study.
Keywords: pulmonary tuberculosis, air pollution, epidemiology, particulate matter
Introduction
It is estimated that one-third of the world’s population are infected with Mycobacterium tuberculosis and approximately 5–10% of those infected with tuberculosis during their lifetime (WHO 2012). In past years, air pollution has been identified as a possible risk factor for active tuberculosis (TB) disease. A cross-sectional study using the vital statistics in Japan found a correlation between total suspended particles in the air and TB disease (Iwai et al., 2005). Similarly, in an ecological analysis in China in 1992, the authors reported a significant association between daily mortality and daily ambient levels of total suspended particulates (TSP) and SO2 (Xu et al., 1995). Although these studies support the hypothesis that air pollution at the higher concentrations found in several Asian countries result in increased risk for TB, it is unknown whether this association is present in the U.S., where both the air pollution concentrations and the incidence of TB disease are lower.
More recently, Tremblay et al. (2007) compared statistics of TB disease and energy production, dating back to the 1940s, to assess the impact of environmental factors on tuberculosis. This study found that historical data on coal consumption and tuberculosis disease are correlated in the United States, Canada, and China. The authors suggest that pollution caused by the combustion of coal during industrialization has provoked previous epidemics in the West and may be the basis for the present TB epidemic in the developing world (Tremblay 2007).
Studies conducted in experimental models support the biologic plausibility of an association between ambient air pollution and TB (Saito et al. 2002a, 2002b). In a 2005 paper, Hiramatsu et al. examined effects of diesel exhaust inhalation on murine mycobacterium infection and found that after 6 months of exposure, the expression levels of interleukin (IL) -1β, IL-12, interferon (IFN) -γ, and iNOS mRNAs decreased considerably and the infection in the mice increased approximately four-fold (Hiramatsu et al. 2005). In a laboratory experiment, Saito, Azuma et al. (2002a) examined expression levels of several cytokines in murine cells to explore the effects of diesel exhaust particles on mycobacterium infection. After one month of diesel exhaust exposure there was a decrease in the levels of tumor necrosis factor (TNF)-α, IL-1β, and IL-12 mRNAs compared with those of cells treated with BCG alone and not exposed to diesel exhaust. These animal studies are noteworthy because IFN -γ and TNF-α are central to the defense against M. tuberculosis. IFN -γ induces macrophage activation to attack and kill the bacterium, while TNF-α is critical in granuloma formation to contain the infection. Moreover, the inhibition of TNF-α by drugs has been linked to reactivation of tuberculosis in epidemiological studies and in clinical trial (Maini et al. 1999; Flynn and Chan 2001; Mohan et al. 2004).
Given the high rates of TB infection in many countries and elevated levels of ambient air pollution in these same areas, the exploration of this potential link deserves further investigation. Additionally, laboratory experiments show an association between air pollution and TB is biologically plausible. However, studies in the U.S. addressing this hypothesis have not been previously reported. Effective control of TB requires a clear understanding of complex factors that influence disease in the community. Recent studies have focused on the influence of HIV, alcohol, and other behavioral risk factors, but have not assessed the impact of exposure to ambient air pollution. We conducted an ecologic investigation to explore our novel hypothesis -- namely the potential association between concentrations of ambient air pollutants and the rate of pulmonary tuberculosis (PTB) in North Carolina.
Materials and Methods
This study was approved by the institutional review board at the University of North Carolina at Chapel Hill.
Study Population
The study population includes all North Carolina (NC) residents living in counties with available ambient particulate matter (PM) monitoring data for one or more years between 1993 and 2007 (see Exposure Assessment, below, for our rational for focusing on particulate matter as a proxy for ambient air pollution concentrations). Information on PTB for this analysis comes from the Tuberculosis Information Management System (TIMS) database and was provided by the NC Department of Health and Human Services. PTB cases in the TIMS database meet the clinical case definition or are laboratory confirmed. The clinical case definition of tuberculosis is a patient with a positive tuberculin skin test, other signs and symptoms compatible with tuberculosis disease (e.g., an abnormal, unstable chest radiograph, or clinical evidence of current disease), treatment with two or more anti-tuberculosis medications, and completed diagnostic evaluation. Laboratory criteria for diagnosis are isolation of M. tuberculosis from a clinical specimen, demonstration of M. tuberculosis from a clinical specimen by nucleic acid amplification test, or demonstration of acid-fast bacilli in a clinical specimen when a culture has not been or cannot be obtained (CDC 1997).
Exposure Assessment
For this ecologic analysis, we focused on PM air pollution. The extent of measured air pollutant data in NC makes PM10 and PM2.5 the best candidates for exploring the association with PTB. Of all ambient pollutants measured during the study period, PM was monitored most often. Moreover, PM is a constituent of total suspended particulates and diesel exhaust, and a bi-product of coal combustion, all pollutants for which previous epidemiologic studies conducted in Asia have suggested a possible relationship with TB.
Twenty-four hour averages of PM10 and PM2.5 were obtained from the U.S. EPA’s Aerometric Information Retrieval System (AIRS) from 1993–2007, for all monitoring sites in NC. PM2.5 is typically measured daily, while PM10 is typically measured every sixth day, though some sites have daily measures. Concentrations are rounded to the nearest 0.1 µg and annual values were computed from 24-hour values. Because monitor locations can change from year to year, this analysis used only data from counties with monitoring data available. These counties accounted for the majority of tuberculosis PTB cases and exhibited a wide range of air pollution levels. Air pollution exposure for the present study was defined as the yearly average of 24-hour PM10 and PM2.5 measurements averaged over all monitors within the county. PM10 measurements were available for the entire study period, 1993–2007; however, PM2.5 monitoring did not begin until 1999 so measurements were available only for 1999–2007.
Additional Data Required for the Statistical Analysis
Covariates that were considered as potential confounders in our analyses were obtained from the TIMS database. Individual-level covariates obtained from TIMS included age, gender, race, ethnicity, citizenship, previous TB diagnosis, occupation, homelessness, HIV status, or injection drug use. Using a directed acrylic graph (DAG) approach (Rothman 1998), and the known literature on the epidemiology of TB and air pollution concentrations in the U.S., we a priori selected as potential confounders, age, gender, race and ethnicity.
We used data on state and county population sizes obtained from the NC State Data Center to calculate state and county-specific PTB rates (see below). The NC State Data Center uses federal, state, and local government data sources along with mathematical computer models to create annual population estimates and population size projections for all NC counties by year. These annual estimates of NC county populations by age, race, and gender served as the denominator for calculating TB disease rates in this analysis.
Statistical Analysis
Poisson regression models were used to evaluate relationships between air pollution and PTB disease.
Non-linearity of the relation between model covariates and log rates was addressed using indicator variables and a scaled deviance parameter specification was used to account for overdispersion. Autocorrelation in model residuals was examined using the Durbin-Watson test and was determined not to be a concern within this data.
Covariates considered for inclusion in the models for the association between air pollution and PTB in this study included age, gender, race, and year of diagnosis (as discussed above). Each potential covariate was first tested for effect measure modification, however, but statistical interaction was not evident based on an a priori cut-off of p<0.15 for the likelihood ratio test. Covariates were then entered into the models as potential confounders screened with backwards elimination and a 10% change-in-estimate criterion. All were included in the analysis.
For the period when data for both PM10 and PM2.5 were available (1999–2007), single pollutant models were used. County population data were grouped by age (6 categories from 0 to 65+ years), gender, race (white vs. non-white), and year of diagnosis. PM was categorized into quintiles of exposure based on the distribution of county-years of air pollution levels, comparing the rates of PTB in counties with higher quintiles of pollution to those with the lowest quintile, while adjusting for confounders.
Results from the analyses are reported as incidence rate ratios with 95% confidence intervals. All data were analyzed using the SAS statistical package (version 9.2; SAS Institute Inc., Cary, NC, USA).
Results
Descriptive Characteristics
During 1993–2007 there were approximately 5319 cases of PTB disease in all 100 NC counties, including 4882 from counties with air pollution monitoring. After excluding two cases with data missing on age gender, race, or ethnicity, the total study population included 3028 PTB cases with exposure measurements for PM10 and 1853 cases with PM2.5. Of these cases, 68% were men, 11–12% were HIV positive, 10% were homeless, 34–38% were white, and 2–3% were injection drug users.
As shown in Table 1, the age, gender, and race distributions of the PTB cases in the counties and years with available PM10 and PM2.5 monitors are quite comparable to the PTB cases for NC as a whole. Cases of PTB with PM10 and PM2.5 monitoring data also did not differ significantly by citizenship, previous TB diagnosis, occupation, homelessness, HIV status, or by injection drug use from all cases of PTB in NC during the study period (data not shown).
Table 1.
Distribution of demographic characteristics among: (A) all cases of pulmonary tuberculosis in NC, (B) cases in counties with PM10 monitoring data available, and (C) cases with PM2.5 monitoring data available, 1993–2007.
| Frequency (%) |
|||
|---|---|---|---|
| Demographic Characteristics |
All NC cases During 1993–2007 |
(B)Cases in Counties with PM10 monitoring |
(C) Cases in Counties with PM2.5 monitoring |
| Gender | |||
| Male | 3649 (68.6) | 2067 (68.2) | 1253 (67.6) |
| Female | 1670 (31.4) | 963 (31.8) | 600 (32.4) |
| Age Group | |||
| 0–24 | 624 (11.7) | 391 (13.0) | 322 (17.5) |
| 25–44 | 1625 (30.6) | 1055 (34.8) | 678 (36.6) |
| 45–64 | 1558 (29.3) | 887 (29.3) | 508 (27.4) |
| 65+ | 1503 (28.3) | 695 (23.0) | 345 (18.6) |
| Race | |||
| Asian | 305 (5.7) | 247 (8.2) | 186 (10.0) |
| Black | 2912 (54.8) | 1735 (57.3) | 922 (49.8) |
| Haw./PI/Am. Ind/Alask. | 70 (1.3) | 21 (0.7) | 34 (1.8) |
| Multiracial | 14 (0.3) | 9 (0.3) | 9 (0.5) |
| White | 2018 (37.9) | 1018 (33.6) | 702 (37.9) |
| Ethnicity | |||
| Hispanic | 698 (13.1) | 372 (12.3) | 360 (19.4) |
| Non-Hispanic | 4621 (86.9) | 2658 (87.7) | 1493 (80.6) |
Air Pollution
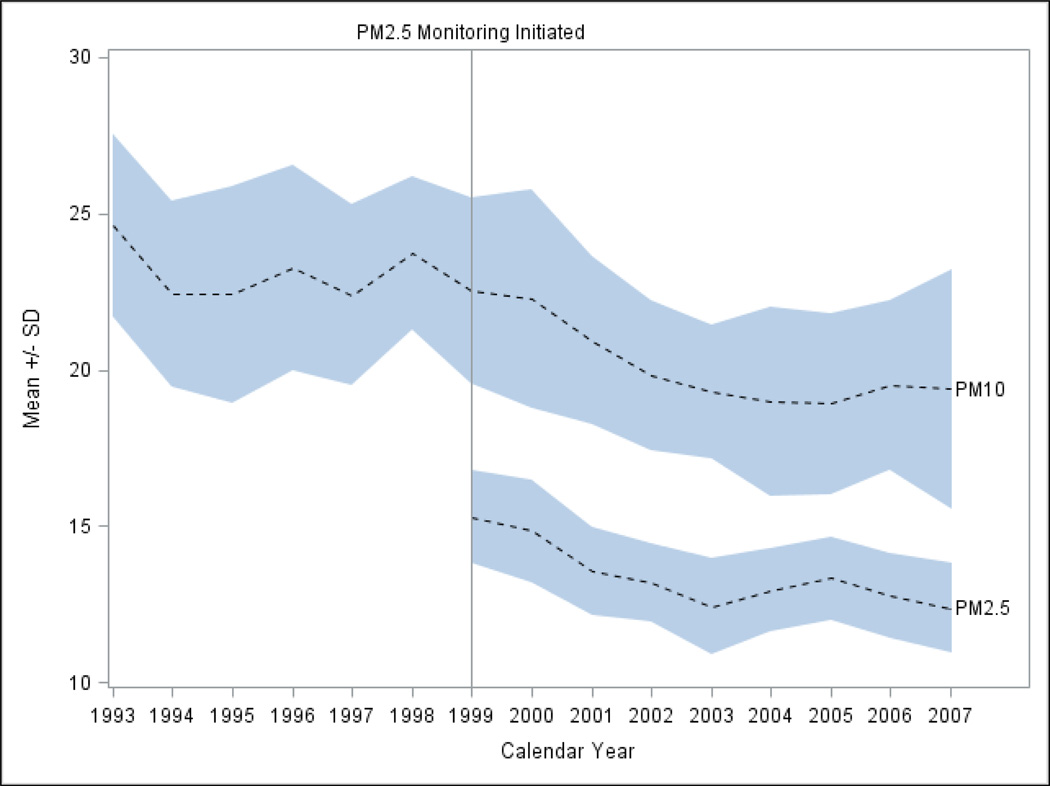
The number of counties with PM10 monitors varied during the period, with a maximum of 34 during 1995–1997 to 23 in 1998 and then 11–12 after 2002. The number of counties with PM2.5 monitoring varied less (29–31 except for 33 in 2003). As shown in Figure 1, the available data show a relatively steady decline of particulate air pollutants during the study period, from 24.63 in 1993 to 19.39 µg/m3 in 2007 for mean PM10, and from 15.30 in 1999 to 12.38 µg/m3 in 2007 for mean PM2.5. Throughout the study period annual PM10 and PM2.5 were both measured at levels well below current U.S. National Ambient Air Quality Standards (U.S. EPA).
Figure 1.
Mean ± standard deviation of particulate matter air pollution concentrations in North Carolina, 1993–2007.
Tuberculosis
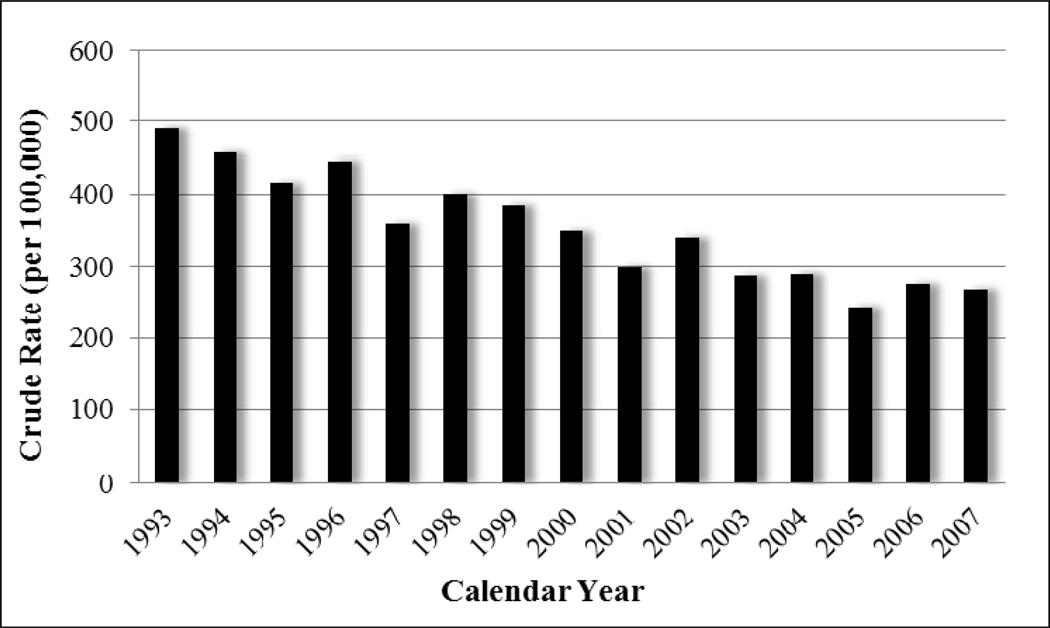
The overall rate of PTB disease in NC was 4.41 per 100,000 person-years from 1993 to 2007. Figure 2 illustrates how the rates and frequency of diagnosed PTB disease cases in NC have declined nearly monotonically since 1993. Crude PTB incidence rates were calculated by age, gender, race, year of diagnosis, and particulate air pollution level (divided at quintiles of the distribution of county-years). Rates were lower for whites and for women. With the exception of the 5–14 year age group, rates of reported disease increased with age.
Figure 2.
Overall annual rate of pulmonary TB in NC, 1993–2007.
Table 2 shows rate ratios for PTB in relation to PM10 and PM2.5 before and after adjustment for age, gender, race, and year of diagnosis. Most of the ratios are modestly elevated, with relatively narrow confidence intervals. Elevated PM2.5 tended to have larger incidence rate ratios than PM10, although there was inconsistency across the highest PM categories. Prior to adjustment for confounding variables, all quintiles of particulate air pollution above the lowest had elevated incidence rate ratios.
Table 2.
Crude and adjusted* incidence rate ratios of pulmonary TB by ambient PM exposure concentrations in North Carolina.
| Crude IRR (95%CI) |
Adjusted* IRR (95%CI) |
||||
|---|---|---|---|---|---|
| PM10† | <19.05 | Ref | Ref | ||
| (µg/m3) | 19.06–21.08 | 1.20 | (1.07, 1.34) | 1.21 | (0.97, 1.51) |
| 21.09–22.78 | 1.09 | (0.97, 1.23) | 0.96 | (0.76, 1.20) | |
| 22.79–24.92 | 1.17 | (1.05, 1.31) | 1.00 | (0.79, 1.26) | |
| ≥24.93 | 1.44 | (1.30, 1.60) | 1.22 | (0.98, 1.52) | |
| PM2.5‡ | <11.94 | Ref | Ref | ||
| (µg/m3) | 11.95–12.91 | 1.38 | (1.15, 1.66) | 1.29 | (0.97, 1.71) |
| 12.92–13.75 | 1.28 | (1.09, 1.50) | 1.27 | (1.00, 1.62) | |
| 13.76–14.68 | 1.13 | (0.96, 1.32) | 1.09 | (0.86, 1.39) | |
| ≥14.68 | 1.34 | (1.14, 1.56) | 1.06 | (0.80, 1.40) | |
Analysis includes years 1993 – 2007;
Analysis includes years 1999 – 2007;
Adjusted for age, gender, race, and year of diagnosis
In the crude models, the highest quintiles of exposure yielded incidence rate ratios of 1.44 (95%CI: 1.30, 1.60) and 1.34 (95%CI: 1.14, 1.56), for PM10 and PM2.5 respectively. Adjustment for potential confounders for the most part resulted in weaker rate ratios. The only statistically significant association seen in the adjusted models, 1.27 (95%CI: 1.00, 1.62), occurred in the third quintile of PM2.5 exposure. There is no indication of a clear dose-response relation for either the crude or adjusted estimates, making causal interpretation more uncertain.
Discussion
This is the first U.S.-based study to report to present ecologic data suggestive of an association between the geographic distribution of ambient air pollution levels and the distribution of active PTB cases. Our findings support results of other epidemiologic studies previously conducted in Asia and elsewhere (Iwai et al., 2005; Xu et al., 1995), where both the disease rates and the exposure levels are much higher. Thus, we are the first to report that an air pollution-TB link in an area with lower air pollution concentrations and lower PTB disease rates.
We utilized an ecologic study design to address our study hypothesis. This approach is commonly used to generate hypotheses of possible determinants of disease and, thus, was chosen appropriately here as the first tool with which to explore this biologically plausible hypothesis in a U.S. population. Our descriptive study design is also an efficient and economical method, which makes use of existing data. For example, to assess exposure we utilized EPA surveillance data, and to identify our study population we drew upon the resources of the NC TIMS database. This disease registry is a result of mandatory reporting of all tuberculosis disease to the NC state health department by all physicians, and is the most complete and comprehensive database on cases of TB disease in NC. Thus, the use of the ecological study design offered an efficient strategy to investigate a possible relationship between air pollution and tuberculosis in a cost-effective manner.
Despite the biologic plausibility of a possible air pollution-PTB association, our results must be interpreted with caution given the inherent limitations associated with the use of the ecological study design, which reflects group-level data. An issue commonly seen in ecologic studies is the inability to control for some individual-level confounding factors. This problem arose in our investigation as we obtained data on the study population from the TIMS database, which does not include individual-level information on active or passive smoking. Exposure to cigarette smoke is potentially a key confounder on the relationship between air pollution and tuberculosis (Bates et al., 2007) and should be accounted for in future investigations. Another concern with utilizing an ecologic study design is the ecological fallacy. The fallacy of this study type assumes that each individual within a group possesses the average characteristics of the entire group, thus residing in a specific county assumes that all residents within that county are exposed to the same levels of air pollution. While it is likely that individuals travel throughout the county and are exposed to similar levels of air pollution, the association detected at the group-level does not necessarily apply for an individual within the group. Further, because we relied on group-level data, commonly used biostatistical approaches for analyzing longitudinal data could not be used in our study analysis; therefore additional epidemiologic studies, that employ a more sophisticated approach, are needed to clarify the association between air pollution and tuberculosis.
It should also be noted that the true effects of air pollution on PTB may be other than estimated here, for numerous reasons. Importantly, individuals are typically exposed to a mixture of air pollutants, whereas we analyzed only PM10 and PM2.5. To the extent that these two pollutants are imprecise indicators of relevant pathogenic constituents of air pollution, the observed association would be attenuated. Furthermore, although an ecological study was the next logical step for investigating this association, county level measures, adapted from local monitors, are an imprecise measure of PM10 and PM2.5. Information is also lacking in this study concerning the length of residence in a particular county. Available data could not account for individuals migrating in and out of a county and varying air pollutant exposures as a result. This history is important, because the effects of air pollution on the risk of PTB disease could be cumulative over time. Temporal ambiguity complicated by an unknown latency period should be noted, however, the trends for PM and reported PTB disease during the period both declined similarly throughout the period. These exposure measures can potentially mask spatial heterogeneity in exposure to air pollution and population mobility. The lack of variability in air pollution concentrations from 1993–2007 could result in exposure misclassification. However, this misclassification would likely be non-differential and would therefore bias results towards the null.
Our analysis was also hampered by the lack of PM monitors within NC. Official monitoring of PM2.5 by the U.S. EPA in the state did not occur routinely until 1999, and as a result, no data for this pollutant could be analyzed before that year. The number of PM10 monitors in NC also declined by one-third in 1998 and then by half in 2003. Considering the lack of available air pollutant data for PM2.5 during previous study years, building a reliable model to account for missing pollutant data during the study period was not feasible.
Consistent with the intriguing observations in past air pollutant studies conducted in countries with high levels of air pollution and numbers of active PTB cases, the present results, from an American-based study conducted in an area with comparatively lower air pollution and disease levels, provide limited evidence for long-term exposure to PM and increased susceptibility to the development of PTB disease. Although, we have not observed a monotonic, statistically significant dose-response association between long-term exposure to air pollution and PTB disease, there does appear to be some association. All of the estimates for PM2.5 and most of those for PM10 showed higher PTB rates for all air pollution levels above the lowest quintile, and no rate ratios were meaningfully below 1.0.
Tuberculosis persists as a major cause of morbidity and mortality throughout the world (Schachter 2004; Hopewell and Pai 2005; WHO 2012). Since air pollution is known to have adverse health effects in humans (Dockery et al. 1993; Wei et al. 2001; Pope et al. 2002; Chen et al. 2004; Downs et al. 2007), and both laboratory experiments and epidemiological studies have raised the possibility that air pollution may increase risk of tuberculosis disease (Mishra et al. 1999; Saito et al. 2002a, 2002b; Hiramatsu et al. 2005; Iwai et al. 2005; Fullerton et al. 2008), it is critical to examine the link between PTB and ambient air pollution in analytical epidemiologic studies that take into account all major potential confounders and use study designs more suited to testing hypotheses.
Conclusions
In this first U.S.-based ecologic analysis of air pollution and PTB, we found suggestive evidence for an association between PTB in NC and two indices of air pollution, PM10 and PM2.5. Although the causative agent of PTB has long been known, the factors that influence susceptibility to tuberculosis disease are complex and not entirely understood. Our findings of a potential air pollution-PTB link are consistent with experimental data in animal models and with ecologic analyses conducted in areas with substantially higher PTB and air pollution levels. This intriguing hypothesis deserves to be investigated with an analytic epidemiologic approach.
Acknowledgements
This research was supported in part by awards from the National Institute of Environmental Health Sciences (ES07018 and ES10126).
References
- Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of Tuberculosis From Exposure to Tobacco Smoke: A Systematic Review and Meta-analysis. Arch Intern Med. 2007 doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep. 1997;46:1–55. [PubMed] [Google Scholar]
- Chen B, Hong C, Kan H. Exposures and health outcomes from outdoor air pollutants in China. Toxicology. 2004;198:291–300. doi: 10.1016/j.tox.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, Gerbase MW, Keller R, Kunzli N, Leuenberger P, Probst-Hensch NM, Tschopp JM, Zellweger JP, Rochat T, Schwartz J, Ackermann-Liebrich U. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357:2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102:843–851. doi: 10.1016/j.trstmh.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Saito Y, Sakakibara K, Azuma A, Takizawa H, Sugawara I. The effects of inhalation of diesel exhaust on murine mycobacterial infection. Exp Lung Res. 2005;31:405–415. doi: 10.1080/01902140590918786. [DOI] [PubMed] [Google Scholar]
- Hopewell PC, Pai M. Tuberculosis, vulnerability, and access to quality care. JAMA. 2005;293:2790–2793. doi: 10.1001/jama.293.22.2790. [DOI] [PubMed] [Google Scholar]
- Iwai K, Mizuno S, Miyasaka Y, Mori T. Correlation between suspended particles in the environmental air and causes of disease among inhabitants: cross-sectional studies using the vital statistics and air pollution data in Japan. Environ Res. 2005;99:106–117. doi: 10.1016/j.envres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. The Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Mishra VK, Retherford RD, Smith KR. Biomass cooking fuels and prevalence of tuberculosis in India. Int J Infect Dis. 1999;3:119–129. doi: 10.1016/s1201-9712(99)90032-2. [DOI] [PubMed] [Google Scholar]
- Mohan AK, Timothy RC, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the Use of Etanercept, a Tumor Necrosis Factor Inhibitor. Clinical Infectious Diseases. 2004;39:295–299. doi: 10.1086/421494. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K, Greenland S. Modern Epidemiology. 2nd edition. Philadelphia, PA: Lippincott, Williams and Wilkins; 1998. [Google Scholar]
- Saito Y, Azuma A, Kudo S, Takizawa H, Sugawara I. Effects of diesel exhaust on murine alveolar macrophages and a macrophage cell line. Exp Lung Res. 2002a;28:201–217. doi: 10.1080/019021402753570509. [DOI] [PubMed] [Google Scholar]
- Saito Y, Azuma A, Kudo S, Takizawa H, Sugawara I. Long-term inhalation of diesel exhaust affects cytokine expression in murine lung tissues: comparison between low- and high-dose diesel exhaust exposure. Exp Lung Res. 2002b;28:493–506. doi: 10.1080/01902140290096764. [DOI] [PubMed] [Google Scholar]
- Schachter EN. Tuberculosis: a global problem at our doorstep. Chest. 2004;126:1724–1725. doi: 10.1378/chest.126.6.1724. [DOI] [PubMed] [Google Scholar]
- Tremblay GA. Historical statistics support a hypothesis linking tuberculosis and air pollution caused by coal [Review Article] The International Journal of Tuberculosis and Lung Disease. 2007;11:722–732. [PubMed] [Google Scholar]
- Wei FS, Hu W, Wu GP, Teng EJ, Zhang J, Chapman RS. Analysis of relation between air pollution and children's lung function indexes. China Environmental Science. 2001;21:385–389. [Google Scholar]
- World Health Organization (WHO) [Accessed 5 May 2012];Tuberculosis Fact Sheet no. 104. 2012 from the website: http://www.wpro.who.int/mediacentre/factsheets/20120306_tuberculosis/en/index.html.
- Xu Z, Chen B, Kjellstrom T, Xu X, Lin Y, Yu D. Study of severe air pollution and mortality in Shenyang, China, Air Pollution and its health effects in China. Geneva: World Health Organization; 1995. pp. 47–88. [Google Scholar]