Highlights
-
•
Site-directed mutations of AUS1 around the CuA site were generated and verified.
-
•
All mutations led to loss of diphenolase activity with butein as substrate.
-
•
Exchange of histidines in the CuA resulted in enzymes containing only one Cu.
-
•
F273 mutation to alanine did not increase the monophenolase activity.
-
•
C97 mutation eliminated the diphenolase activity, but 2 Cu atoms were incorporated.
Abbreviations: AAS, atomic absorption spectroscopy; AmAS1, aureusidin synthase from Antirrhinum majus (protein); aoTYR, tyrosinase from Aspergillus oryzae (protein); bmTYR, tyrosinase from Bacillus megaterium (protein); cgAUS1, aurone synthase from Coreopsis grandiflora (protein); cgAUS1, aurone synthase from Coreopsis grandiflora (gene); F-AAS, flame atomic absorption spectroscopy; GF-AAS, graphite furnace atomic absorption spectroscopy; GST, glutathione S-transferase; ibCO, catechol oxidase from Ipomoea batatas (protein); IPTG, isopropyl-β-d-thiogalactopyranoside; LC, liquid chromatography; LC/MS, liquid chromatography / mass spectrometry; nanoUHPLC–ESI-MS/MS, ultra high performance liquid chromatography–electrospray tandem mass spectrometry; PES, poly(oxy-1,4-phenylsulfonyl-1,4-phenyl); PHC, 2′,3,4,4′,6′-pentahydroxychalcone; PMSF, phenylmethylsulfonylfluorid; PPO, polyphenol oxidase; PPO-6, dandelion PPO-6 from Taraxacum officinale (protein); TBC, 4-tert-butylcatechol; THC, 2′,4,4′,6′-tetrahydroxychalcone; vvCO, catechol oxidase from Vitis vinifera (protein)
Keywords: Type-3 copper protein, Polyphenol oxidase (PPO), Aurone synthase (AUS), Site-directed mutagenesis, 4-Deoxyaurone, Copper binding site
Abstract
Aurone synthase from Coreopsis grandiflora (cgAUS1), catalyzing conversion of butein to sulfuretin in a type-3 copper center, is a rare example of a polyphenol oxidase involved in anabolism. Site-directed mutagenesis around the CuA site of AUS1 was performed, and recombinant enzymes were analyzed by mass spectrometry. Replacement of the coordinating CuA histidines with alanine resulted in the presence of a single copper and loss of diphenolase activity. The thioether bridge-building cysteine and a phenylalanine over the CuA site, exchanged to alanine, have no influence on copper content but appear to play an important role in substrate binding.
1. Introduction
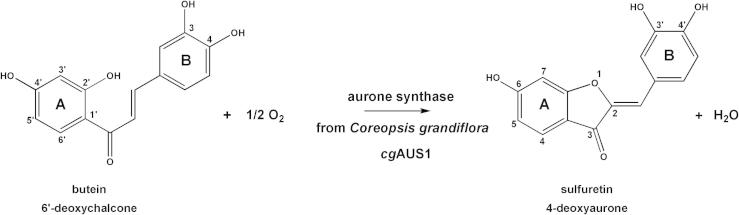
Type-3 copper enzymes contain two copper atoms (CuA and CuB) and bind dioxygen in a characteristic “side-on” bridging mode (μ-η2:η2) between both copper atoms [1]. Polyphenol oxidases (PPOs) are an important class of type-3 copper enzymes and are predominantly involved in pigment formation leading to browning of fruits and vegetables. They can also play a role during aurone formation or in the plant pigment biosynthesis [2–6]. The most prominent PPOs are classified by enzyme nomenclature into tyrosinases (monophenol, o-diphenol: oxygen oxidoreductase, EC 1.14.18.1), catechol oxidases (CO, o-diphenol:oxygen oxidoreductase, EC 1.10.3.1), aureusidin synthase (2′,4,4′,6′-tetrahydroxychalcone 4′-O-β-d-glucoside:oxygen oxidoreductase, EC 1.21.3.6) and laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) [2]. While tyrosinases catalyze both the hydroxylation of phenols to o-diphenols and the subsequent oxidation to their related, highly reactive o-quinones, catechol oxidases catalyze only the oxidation reaction of o-diphenols to o-quinones. Laccases are multicopper oxidase enzymes, which catalyze the oxidation of phenolic and non-phenolic compounds by withdrawing the electron from the substrate and converting them in free radicals, which then polymerize [7]. Aureusidin synthase (AmAS1) and the recently described aurone synthase are special PPOs as they are both involved in aurone formation [5,8]. Aureusidin synthase (Uniprot accession number Q9FRX6) was found in the 4-hydroxyaurone pathway of Antirrhinum majus (old Scrophulariaceae, now Plantaginaceae) and Helichrysum bracteatum (Asteraceae) and catalyzes two reactions, the hydroxylation and the oxidative cyclization of 2′,3,4,4′,6′-pentahydroxychalcone (PHC) and 2′,4,4′,6′-tetrahydroxychalcone (THC) into aureusidin and bracteatin, respectively [8–10]. The polyphenol oxidase homologue aurone synthase (Uniprot accession number A0A075DN54) in Coreopsis grandiflora (cgAUS1) is involved in the 4-deoxyaurone pathway and catalyzes only the conversion of chalcones to aurones, by oxidizing butein (6′-deoxychalcone) to sulfuretin (4-deoxyaurone) [5,11]. Thus, unlike aureusidin synthase, which accepts both monohydroxylated and dihydroxylated substrates, aurone synthase accepts only dihydroxylated substrates, with hydroxy groups in positions 3 and 4 of ring B (Fig. 1) [5,11].
Fig. 1.
Reaction catalyzed by cgAUS1 in the 4-deoxyaurone pathway. Butein (6′-deoxychalcone) is converted to sulfuretin (4-deoxyaurone). Note the differing atom numbering of the chalcone and the aurone.
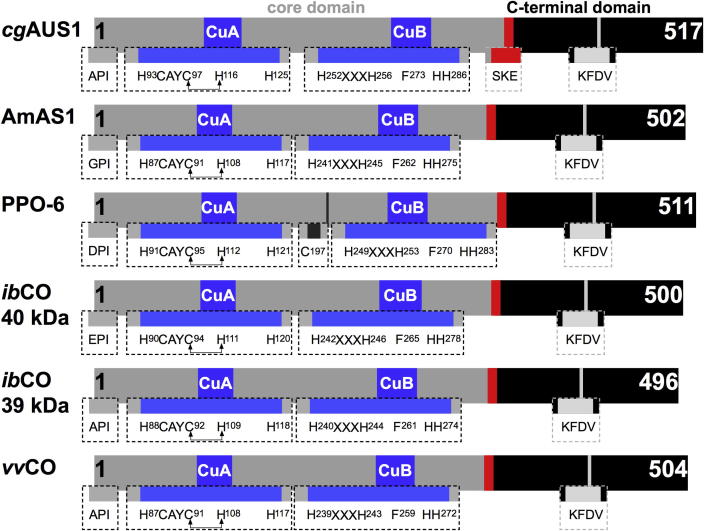
The total sequence (including the transit peptide) of aurone synthase (cgAUS1) contains 602 amino acids, corresponding to a mass of 68 kDa [5]. The pro-enzyme (also called latent enzyme, which contains no transit peptide) starts with the amino acids APITAPDI and consists of 517 amino acids, leading to a mass of 59 kDa (see Fig. 2, cgAUS1). Typically the transit peptide of plant PPOs is cleaved in vivo after transport to the target location (in case of AUS1 as in most plant PPOs the lumen of the thylakoid membranes in chloroplast), thus the enzyme is expressed in the latent form [5,12–14]. Proteolytic cleavage of the C-terminal domain results in an active enzyme (core domain), with a mass of 40 kDa [5]. In cgAUS1 the putative cleavage region is near the SKE motif, marked in red in Fig. 2. Typical highly conserved motifs in PPOs are the HCAYC motif around CuA, the HxxxH motif around CuB, as well as the seventh, conserved, non-copper coordinating histidine (H285 in the pro-enzyme of cgAUS1, see Fig. 2) around CuB and the KFDV motif in the C-terminal region [5]. The coordinating histidines around CuA in cgAUS1, shown in Fig. 2, are H93, H116 and H125. The second histidine (H116) builds a thioether bond with the highly conserved cysteine (C97) of the H93CAYC97 motif. This thioether-bridge was also found in both catechol oxidases of Ipomoea batatas (ibCO) (see Fig. 2) [15]. Gerdemann et al. [15] proposed a possible involvement of this bridge in the catalytic pathway, but a structural function was also under discussion [15]. In ibCO a phenylalanine residue (F265 or F261, see Fig. 2) is in the primary sequence region of CuB, however the crystal structure shows that the bulky side chain of this residue is positioned atop the CuA site [16]. This residue, which is also present in the amino acid sequence of cgAUS1 (F273, in the pro-enzyme, Fig. 2) [5], was described as being responsible for preventing substrate binding and therefore hindering the monophenolase activity [16–19]. A comparable thioether-bridge building cysteine (C97 in cgAUS1) is present in all plant PPOs (catechol oxidases and tyrosinase). The bulky, monophenolase-blocking phenylalanine (F273 in cgAUS1) is present in most plant PPOs, but in some species leucine is at this position instead of phenylalanine, such as pineapple PPO from Ananas comosus and bread wheat PPO from Triticum aestivum [20]. However, these structurally relevant residues are not found in human and fungal tyrosinases as in Agaricus bisporus or Neurospora crassa and the fungal catechol oxidase from Aspergillus oryzae, but a thioether-forming cysteine is found in fungal tyrosinases at a different sequence location than in plant PPOs (as CXH in abPPO4 and abPPO3; and C(X)16-19H in plant PPOs, see Fig. 2) [8,20–30].
Fig. 2.
Schematic alignment of plant PPO pro-enzymes (without transit peptide). cgAUS1: aurone synthase from Coreopsis grandiflora (Uniprot accession number A0A075DN54), AmAS1: aureusidin synthase from Antirrhinum majus (Uniprot accession number Q9FRX6), PPO-6: dandelion PPO from Taraxacum officinale (Uniprot accession number I7HUF2), ibCO 40 kDa: 40 kDa isoform of Ipomoea batatas (Uniprot accession number Q9MB14), ibCO 39 kDa is the 39 kDa isoform of Ipomoea batatas (Uniprot accession number Q9ZP19), vvCO: catechol oxidase from Vitis vinifera (Uniprot accession number P43311). The grey part is the core domain, the black part the C-terminal domain, the red part is the putative cleavage region.
Recently, aurone synthase from C. grandiflora (cgAUS1) was successfully heterologously expressed in Escherichia coli, but recombinant aureusidin synthase (AmAS1) has not been described so far [5,8]. Further, plant catechol oxidases as PPO-6 from Taraxacum officinale (see Fig. 2) [27], Malus pumila [31] and Physcomitrella patens [32] have been successfully expressed in E. coli. PPOs from Solanum melongena [33] and Camellia sinensis [34] were expressed in inclusion bodies and refolded, but not further purified. Active tyrosinase of Trifolium pratense was expressed in E. coli as well as in transgenic Medicago sativa [35]. A few fungal PPOs have been cloned and heterologously expressed in E. coli including tyrosinases from Verrucomicrobium spinosum [36], A. oryzae [37] and two tyrosinases from A. bisporus [23]. Recently the first fungal, extracellular catechol oxidase from A. oryzae was functional expressed and purified [38].
Mutagenesis studies of recombinant PPOs are even more rare. In plant PPOs, the first site-directed mutagenesis study was performed of dandelion PPO-6 from T. officinale [27]. In this study Dirks-Hofmeister et al. [27] analyzed a tetrameric PPO isoenzyme (PPO-6) from dandelion (T. officinale) heterologously expressed in E. coli and identified, through molecular modeling, a surface-exposed cysteine (C197 in the pro-enzyme see Fig. 2, which is not existing in cgAUS1). Site-directed mutagenesis of this cystein to a serine proved this amino acid residue to stabilize this tetramer via a disulfide linkage [27]. The serine mutant still formed a tetrameric structure but showed impaired enzymatic efficiency and cooperativity and a reduction in stability [27]. Site-directed mutagenesis on all copper coordinating histidines, the seventh, conserved non-coordinating histidine in CuB and the thioether-bridge building cysteine was performed on fungal tyrosinase from (aoTYR) A. oryzae [39]. Replacements of these amino acids with asparagine resulted in mutated enzymes exhibiting no activity and containing only one copper ion per molecule tyrosinase, indicating that these residues are essential for copper incorporation and activity. Investigations on the ratio of monophenolase/diphenolase activity of tyrosinase (bmTYR) of Bacillus megaterium were performed by Goldfeder et al. [19]. They suggested that the less bulky valine (V218), which does not occur in catechol oxidases, aureusidin synthase or aurone synthase [19], allows the hydroxylation of monophenols at CuA. Therefore, Goldfeder et al. [19] exchanged V218 with a phenylalanine (V218F, which corresponds to F273 in cgAUS1), expecting to have a decreased monophenolase activity. However, the monophenolase activity increased surprisingly [19].
The full-length sequence of cgAUS1 was previously described and the pro-enzyme was cloned in E. coli, thereby producing large amounts of pure, recombinant cgAUS1 [5]. cgAUS1 was isolated and purified from natural source as well and characterized and compared with the recombinant enzyme [40]. In order to investigate the structure and function deciding amino acids around CuA, this work reports on the first site-directed mutagenesis of cgAUS1 [5]. To demonstrate if all the three copper-coordinating histidines (H93, H116, H125 marked in dark red, red and orange, respectively in Fig. 3) are necessary for copper incorporation and folding, we mutated each histidine to alanine, as alanine is a non-polar amino acid, with a neutral, small side chain. Mutation of the phenylalanine (F273, marked in blue in Fig. 3) to alanine should clarify if the side chain of phenylalanine functions as a blocker residue in cgAUS1 and therefore influences the monophenolase/diphenolase ratio [18,19,41]. It has been suggested that the cysteine residue (C97, marked in violet in Fig. 3) forms a thioether bond with H116 [5,16]. Crystal structure investigations of ibCO revealed that the formed covalent bond increases structural restraints on the ligand sphere of the CuA center, therefore this cysteine (C97) was mutated to alanine in cgAUS1 [16]. However, Gerdemann et al. [15] supposed that this thioether-bridge does not seem to be essential, as it is absent from arthropodan HC crystal structures. Thus, a comprehensive investigation around the CuA site of aurone synthase from C. grandiflora (cgAUS1) is presented in this work. In order to investigate the activity of all coordinating histidines, the three histidines of CuB were mutated to alanine.
Fig. 3.
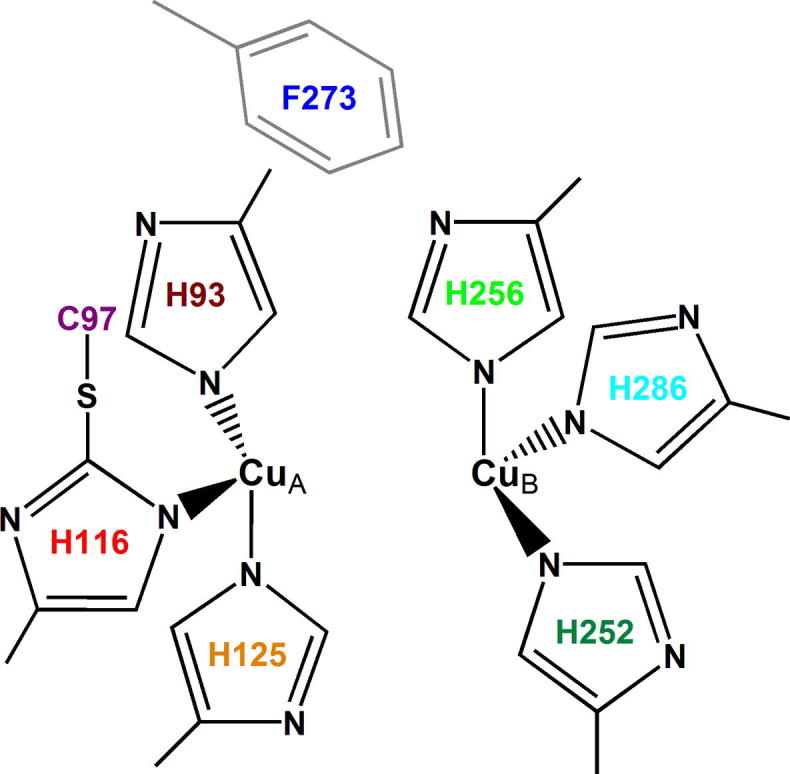
Schematic illustration of the type-3 copper center of cgAUS1.
2. Experimental procedure
2.1. Cloning and site-directed mutagenesis of cgAUS1
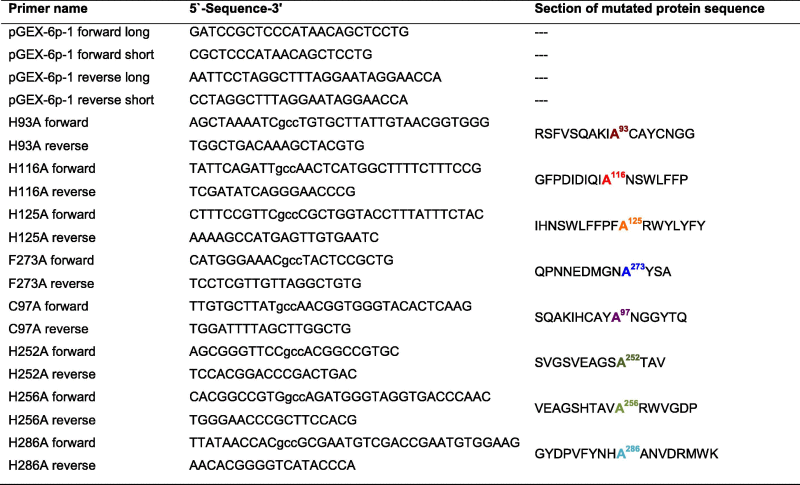
Isolation and cloning of aurone synthase (cgAUS1) gene from C. grandiflora into pTrcHis2 vector was previously described [5]. In order to simplify protein purification the gene was, in this work, recloned into pGEX-6P-1 (GE Healthcare, Munich, Germany), which contains a GST-tag, using BamHI and EcoRI as restriction sites and including the stop codon using sticky end PCR according to Walker et al. [42]. Primers used are listed in Table 1. Site-directed mutagenesis was performed using the Q5® Site-Directed Mutagenesis Kit according to the manufacturer manual. Plasmids were transformed into NEB 5-alpha competent cells and were sequenced by a commercial supplier.
Table 1.
Primers used for recloning into pGEX-6P-1 and site-directed mutagenesis.
 |
2.2. Heterologous expression of recombinant cgAUS1 in E. coli
Production of wild type and mutated cgAUS1 enzymes was performed by inoculating with overnight culture, growing at 37 °C in SB culture medium (3.2% tryptone, 2% yeast extract, 0.5% NaCl, supplemented with 100 μg/ml ampicillin), inducing with isopropyl-β-d-thiogalactopyranosid (IPTG) at an OD600 of 0.6 and harvesting the cells at an OD600 of ∼1.5, as described previously, [5]. The harvested cells were washed three times in 30 mM Tris–HCl, pH 8.5 by centrifugation at 10 000×g for 10 min and resuspended again in 10 ml 30 mM Tris–HCl, pH 8.5. The cells were lysed by three freeze–thaw cycles and by adding 0.3 mg/ml lysozyme (L6876, Sigma-Aldrich) and 1 mM phenylmethanesulfonylfluoride (PMSF). After adding 0.05 mg/ml DNaseI (SigmaAldrich) and 10 mM MgCl2 the lysate was centrifuged (25 000×g, 30 min, 4 °C) and directly loaded onto GSTrap FF.
2.3. Purification of recombinant cgAUS1 in E. coli
The GST-tagged cgAUS1 pro-enzymes (wild type and mutants) were purified by applying the lysate to 5 ml GSTrap FF column (GE Healthcare, Munich, Germany). All proteins were loaded onto the column using 30 mM Tris, 150 mM NaCl at pH 8.0 as binding buffer. cgAUS1 was eluted from the column using the same buffer supplemented with 10 mM reduced glutathione. The eluted peak was collected and buffer exchange to the binding buffer, to remove the glutathione, was performed with Vivaspin 20 30.000 MWCO PES (poly(oxy-1,4-phenylsulfonyl-1,4-phenyl)) (Sartorius Stedim Biotech GmbH, Göttingen, Germany). The GST-tag was cleaved by PreScission Protease (GE Healthcare, Munich, Germany) at 4 °C over night. The protein solution containing the GST-tag and the untagged, pure protein was loaded again onto the GSTrap FF column. GST-tag was bound onto the column and the untagged protein was eluted. The pure, untagged protein was concentrated with Vivaspin 20 30.000 MWCO PES and stored at 4 °C in 10 mM sodium acetate (pH 5.0) for further use.
2.4. Mono- and diphenolase activity of cgAUS1
The activity of the latent cgAUS1 enzymes was measured after cell lysis and monitored by spectrophotometric measurements (SHIMADZU UV-1800). Monophenolase activity was determined by the use of 1 ml of 10 mM sodium phosphate buffer, pH 6.5, 2.5 mM SDS as an activating agent, 33 μM l-tyrosine as substrate and up to 5 μL of enzyme solution (protein concentrations of the enzyme solutions are shown in Table 2). The increase of the absorbance at 305 nm was measured in a 1 cm quartz cuvette [43,44]. Diphenolase activity was determined with 150 μM butein, fisetin and TBC as substrate in a 125 mM sodium citrate buffer, pH 5.5, containing 2.5 mM SDS and 1 μg of enzyme solution (concentrations of cgAUS1 were determined by integrating the area of the collected peak after affinity chromatography, Fig. S1). Measurement was performed at 415 nm, 280 nm and 400 nm for butein, fisetin and TBC (4-tert-butylcatechol), respectively, in a 1 cm quartz cuvette [5,11].
Table 2.
Copper content of cgAUS1 wild type and mutants determined by atomic absorption spectroscopy (AAS). Protein concentration in mg/ml of cgAUS1 was determined after purification by UV at 280 nm; molecular weight (MW) and extinction coefficient for the protein concentration in μM was calculated by ProtParam [50]; copper concentration was determined by AAS; Copper/cgAUS1 is the determined ratio of copper per molecule AUS.
| Sample | Protein [mg/ml] | MW [g/mole] | Protein [μM] | Copper [μM] | Copper/cgAUS1 |
|---|---|---|---|---|---|
| AUS1 w/t | 0.63 | 85 761 | 7.39 | 15.42 | 2.1 |
| AUS1 H93A | 0.37 | 85 694 | 4.36 | 4.56 | 1.0 |
| AUS1 H116A | 1.09 | 85 694 | 12.67 | 14.16 | 1.1 |
| AUS1 H125A | 0.74 | 85 694 | 8.65 | 8.97 | 1.0 |
| AUS1 F273A | 0.27 | 85 684 | 3.17 | 7.40 | 2.3 |
| AUS1 C97A | 0.06 | 85 728 | 0.67 | 1.38 | 2.0 |
2.5. Denaturing and partially denaturing SDS–PAGE analysis of cgAUS1
Electrophoresis was performed by the method of Laemmli [45]. The 8% polyacrylamide gels were run in a Mini-PROTEAN Tetra Cell System (BioRad, Vienna, Austria) at a constant current of 120 mV. 2 μg of purified cgAUS1 (including the GST-tag) were mixed with reduced loading buffer and heated for 10 min before loading to the gel.
In-gel AUS activity was determined by applying partially 8% SDS–PAGE according to Cabanes et al. [46]. The samples were mixed with loading buffer containing no β-mercaptoethanol and loaded to the gel without heating. The gel was soaked in 125 mM sodium citrate pH 5.4 containing 41 μg/ml butein. The formation of sulfuretin, as shown in Fig. 1, was monitored by a Typhoon 8600 (GE Healthcare, Munich, Germany) in the fluorescence mode using green laser (532 nm) for excitation and 555BP20 as emission filter.
2.6. Chymotryptic digestion and peptide mass fingerprint of cgAUS1 mutants
2 μg of purified protein were applied to SDS–PAGE and visualized by coomassie staining followed by excision of gel bands corresponding to the protein. After band excision destaining, washing, reduction and carbamidomethylation of cysteines was performed. In-gel digestion was achieved using chymotrypsin at 37 °C over night. Peptides were recovered from the gel using ultrasonication and dried completely by vacuum centrifugation. The samples were stored at −20 °C prior to LC–MS/MS analysis. Dried peptide samples were dissolved in 5 μL 30% formic acid (Fluka) and diluted with 40 μL eluent A (97.9% H2O, 2% acetonitrile, 0.1% formic acid).
Analysis of the peptide samples was carried out on a nanoUHPLC–ESI-MS/MS system using a high resolution orbitrap mass spectrometer (Dionex Ultimate 3000 RSLCnano, LTQ Velos orbitrap, Thermo Scientific). Data analysis was performed via Proteome Discoverer 1.4 by searching against the corresponding mutant sequences using Sequest as search engine. Peptide mass tolerance was 5 ppm and the fragment mass tolerance 0.5 Da. Modifications applied for each search were carbamidomethylation as fixed modification for cysteines and methionine oxidation as variable modification. For high confidence of the MS data, the false discovery rate (FDR) of the peptide spectrum matches (PSM) was set to <0.01 (Proteome Discoverer). Details about applied parameters can be found in Tables S1 and S2.
2.7. Quantification of copper content
Pure cgAUS1 wild type and mutants (Table 2) were used to determine the copper content by atom absorption spectroscopy (AAS). 3 ml of the aqueous protein solutions (concentrations are listed in Table 2) were acidified with 1 ml of 69% HNO3 (TraceSELECT®, Fluka, Sigma–Aldrich, Vienna, Austria) and digested for one hour at 100 °C in the oven. After cooling to room temperature the loss of liquid was filled up with deionized water. Samples were then measured directly with F-AAS (AAnalyst 300 by Perkin-Elmer, Brunn am Gebirge, Austria). Samples containing less than 0.2 mg/L Cu were measured by means of GF-AAS (PinAAcle 900Z by Perkin-Elmer, Brunn am Gebirge, Austria) using a Pd-Mg modifier. For both methods, element concentrations of each sample were calculated from the corresponding regression lines (correlation factor > 0.9995) using five different dilutions of a standard solution (Fluka, Sigma–Aldrich, Vienna, Austria). The range of calibration was 0.2–5.0 mg/L for F-AAS and 1.00–50.0 μg/L for GF-AAS, respectively.
3. Results and discussion
3.1. Heterologous expression and yield of cgAUS1 mutants and wild type
As a result of recloning cgAUS1 proenzyme into pGEX-6P-1 the recently developed method could be advanced to successfully produce large amounts of pure, active and soluble cgAUS1 wild type (58 kDa + 26 kDa GST tag) in E. coli and simplify the purification process by a reduction of the four ion exchange purification steps to only one affinity chromatography step [5]. Purification was performed under non-denaturating conditions using GSTrap FF affinity chromatography. An exemplary chromatogram of cgAUS1 wild type is shown in Fig. S1, where cgAUS1 was eluted by the use of reduced glutathione. All cgAUS1 mutants (H93A, H116A, H125A, H252A, H256A, H286A C97A and F273A) were expressed and purified under identical conditions, using wild type cgAUS1 as control sample. The amount of soluble, expressed and purified enzyme, shown in Table 2, revealed that all mutants, except the C97A mutant, showed acceptable concentrations in comparison with the wild type cgAUS1. The C97A mutant showed almost a less than one-tenth ratio (0.06 mg/ml) followed by F273A (0.27 mg/ml), H286A (0.33 mg/ml), H93A (0.37 mg/ml), H125A (0.74 mg/ml), H116A (1.09 mg/ml), H256A (1.20 mg/ml) and H252A (1.24 mg/ml) compared to cgAUS1 wild type (0.63 mg/ml). The significantly lower concentration of C97A may be due to the missing thioether bond, between C97 and H116. In contrast, Gerdemann et al. [15] proposed that this cysteine–histidine thioether bond seems not to be essential, as it is absent in arthropodan HC crystal structures. However, we suppose that this bond seems to be structurally important during expression and accurate folding. Furthermore, a thioether bond is present in all so far determined crystal structures of the type 3 copper proteins catechol oxidase, tyrosinase and molluscan hemocyanin and all of these proteins have in common two major domains (a N-terminal core domain containing the copper center and a C-terminal domain which shields the active site) [47]. In all of these structures the core domain showed a similar fold. Arthropod hemocyanin consists of three different domains (a N-terminal domain shielding the entrance of the active site, a central domain containing the active site and a immunoglobulin-like C-terminal domain). This significant structural difference might be a reason for the absence of the thioether bond in arthropod hemocyanins [48].
3.2. Copper incorporation
The purified cgAUS1 samples were used to determine the incorporated copper content by AAS (Table 2). The copper content of the wild type cgAUS1 was determined as being approx. 2 Cu per molecule cgAUS1, as expected. All three histidine mutants of CuA (H93A, H116A, H125A) contained only 1 Cu per molecule enzyme. For the F273A mutant, 2 Cu per molecule cgAUS1 could be determined, as it is the case in the wild type. The C97A mutant contained as well two Cu per molecule cgAUS1.
In aoTYR the substitution of cysteine (C82) with alanine as well as substitution of the three copper coordinating histidines (H63, H84, H93) in the CuA site decreased copper binding to 50%, indicating that these mutants contain only 1 Cu per molecule [39]. This is in contrast to cgAUS1, where mutation of the cysteine residue did not decrease the copper content, indicating that the thioether bond seems to be structurally important for expression and folding, as well as for enzymatic catalysis (Fig. 4 and Table 3), but not essential for copper incorporation. The three copper coordinating histidines in aoTYR were confirmed as essential residues for copper binding and catalysis, due to the decrease of the copper content to one copper atom and the loss of mono- and diphenolase activity with l-tyrosine and l-dopa, respectively [39]. In fact, the decrease of copper content in the histidine mutants (H93A, H116A, H125A) confirms that these residues are essential for copper binding in cgAUS1.
Fig. 4.
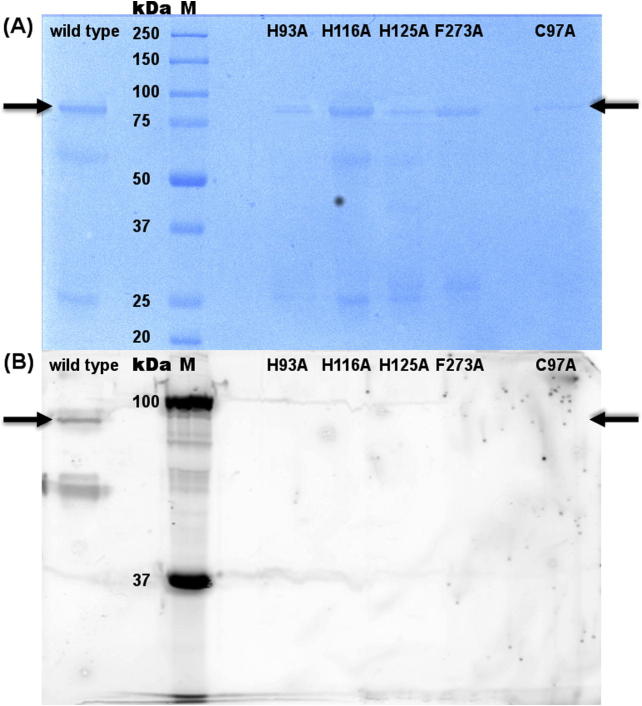
SDS–PAGE of cgAUS1 wild type and mutants, after purification through GSTrap FF. (A) Gel was stained with Coomassie. (B) Gel was soaked with butein. The arrows indicate the calculated size of 84 kDa, corresponding to cgAUS1 pro-enzyme (58 kDa) including the GST-tag (26 kDa).
Table 3.
Diphenolase activity of cgAUS1 wild type and mutants determined by spectrophotometric measurements [40]. Values are calculated based on three independent measurements.
| Sample | Enzymatic activity [μmol/(l∗min)] |
||
|---|---|---|---|
| Butein | Fisetin | TBC | |
| w/t | 11055 | 22137 | 125533 |
| H93A | 62 | 92 | 218 |
| H116A | 0 | 0 | 0 |
| H125A | 0 | 0 | 0 |
| F273A | 4 | 0 | 0 |
| C97A | 0 | 0 | 16 |
| H252A | 0 | 0 | 0 |
| H256A | 27 | 0 | 0 |
| H286A | 0 | 0 | 0 |
3.3. Verification of cgAUS1 wild type and mutants
The mass of the purified cgAUS1 wild type and mutants were determined by SDS–PAGE. Fig. 4(A) shows samples under reduced conditions, stained with Coomassie. All samples showed a band at 84 kDa, corresponding to the calculated size of cgAUS1 pro-enzyme including a 26 kDa GST-tag. The smaller bands with sizes of 58 kDa and 26 kDa correlate to the cgAUS1 pro-enzyme without the GST-tag and the GST-tag itself, due to digestion during the cell lysis, in spite of the use of the protease inhibitor PMSF. These 84 kDa bands were excised and sequenced by nanoUHPLC–ESI-MS/MS and the identified peptides were summarized in Fig. 5 (lists of all peptides for each enzyme are shown in Tables S3–S8). Mass spectrometry sequence analysis demonstrated that all mutated cgAUS1 enzymes could be established. The sequence coverage was 51%, 53%, 53%, 51%, 59% and 57% for cgAUS1 wild type, H93A, H116A, H125A, F273A and C97A, respectively. For the mutants H93A (bold and dark red in Fig. 5(B)), H116A (bold and red in Fig. 5(C)) and F273A (bold and blue in Fig. 5(E)) peptides in the region of the mutated amino acids could be identified, verifying that the expressed protein contains the desired mutation. In contrast, the two H125A and the C97A mutants (in Fig. 5(D) and (F), respectively) showed an identical peptide pattern as cgAUS1 enzyme. A peptide containing the mutation site could not be determined, neither in the wild type enzyme nor in any other mutated enzyme. To further proof, signals on the MS level matching the m/z values of mutation-specific peptides were identified. Mutant C97A was identified, by a peak at 19.97 min with m/z 819.8839, matching the peptide VSQAKIHCAYA97NGGY (m/z 819.8883 at charge + 2, marked in yellow in Fig. 5(F)) and H125A showed a peak at 42.16 min with m/z 542.2969 matching the peptide LFFPFA116RW (m/z 542.2923 at charge + 2, marked in yellow in Fig. 5(D)). The absence of a peptide containing the third histidine of CuA is probably due to a low grade of ionization, as described previously [5,29]. All mutations were, however, verified by DNA sequencing, just as reported for tyrosinase mutants from A. oryzae, tyrosinase from Bacillus megaterium or PPO-6 from T. officinale [19,27,39].
Fig. 5.
Identified peptide regions/peptides (in green) of cgAUS1 wild type and mutants determined by nanoUHPLC–ESI-MS/MS, including the mutated amino acids framed and colored. (A) cgAUS1 wild type (B) cgAUS1 H93A mutant (C) cgAUS1 H116A mutant (D) cgAUS1 H125A mutant, identified peak matching the peptide which contains the mutation is marked in yellow (E) cgAUS1 F273A mutant (F) cgAUS1 C97A, identified peak matching the peptide which contains the mutation is marked in yellow.
3.4. Activity of cgAUS1 wild type and mutants
Fig. 4(B) shows SDS–PAGE of non-reduced samples, soaked with butein, to detect the formation of sulfuretin, see Fig. 1. Table 3 shows AUS1 activity of purified cgAUS1 (see also Fig. S2). The wild type cgAUS1 was expressed as soluble and functional enzyme, showing diphenolase activity, due to the presence of SDS as activating agent for latent enzymes in the gel [46]. All mutants of cgAUS1 showed no diphenolase activity with any dihydroxylated substrates such as butein, fisetin or TBC [5]. Monophenolase activity could not be observed neither for the wild type, which was expected, nor for any mutant.
The bulky CuA-site blocking phenylalanine residue (F273) was assumed to hinder monophenolated substrates in catechol oxidases [16–18]. Mauracher et al. [18] compared the crystal structure of ibCO with the crystal structure of tyrosinase (abPPO4) from A. bisporus [49]. A superimposition of the active sites showed that, an alanine residue is located in abPPO4 at the position of the phenylalanine in ibCO, which endorsed the assumption of a blocker residue [18]. In contrast, the lack of monophenolase and diphenolase activity of the F273A mutant in cgAUS1 disproves this role for the phenylalanine, as blocking residue for monophenolated substrates. This indicates, that phenylalanine 273 plays a role in substrate binding. Investigation on a less bulky valine, of bmTYR, at the same position of the phenylalanine showed an increase of the monophenolase activity after mutation of this valine to a phenylalanine [19]. Thus, the analysis of this bmTYR mutant does as well contradict a previously suggested proposal of phenylalanine as a general blocking residue in PPOs, which would eliminate the ability of the PPO to perform hydroxylation of monophenols [19,41].
In aoTYR site-directed mutagenesis of all copper coordinating histidines to asparagine, a single-essential cysteine residue to alanine (equivalent to C97 in cgAUS1) and the non-coordinating histidine in CuB as well to asparagine showed no detectable activity after mutation [39]. Nakamura et al. [39] indicated that these residues (H63, H84, H93, H290, H294, H332, H333, C82) are essential for activity, which corresponds to the results obtained with cgAUS1. Furthermore, both single mutations (the C97A and the H116A) demonstrated in cgAUS1 that this thioether bond is essential for the diphenolase activity [47,48].
4. Conclusion
A summary of the presented results is shown in Table 4. All selected amino acids (H93, H116, H125, F273, C97, H252, H256, H286) are essential for the full function of the enzyme and therefore essential for substrate binding. Besides the crucial presence of the histidines for Cu coordination, the F273 and C97 are presumably essential either for the substrate to bind at all, or to bind in the proper orientation for the reaction. Moreover the amino acid C97, forming a thioether bond with H116, seems to be necessary for expression as soluble enzyme in E. coli. We propose in vivo a twofold importance for C97: for proper folding of the protein structure and for diphenolase activity. The decrease of the copper content in the histidine mutants (H93A, H116A, H125) was expected, but surprisingly the loss of one copper coordinating histidine had no influence on the expression as soluble protein. However, we demonstrate that ligation of the copper at CuA site is only mediated by the three histidine residues (H93A, H116A, H125A), but is not influenced by the thioether bond. The assumption that the F273 residue acts as a blocker residue for substrates or hinders the monophenolase activity could be disproved for cgAUS1, as it still contains two copper atoms per molecule but lost its total activity [18,41]. We propose for cgAUS1 that the amino acid F273 is essential for substrate binding, but is not able to change the reaction mechanism to a tyrosinase-like enzyme.
Table 4.
Summary of the results obtained with various mutants in comparison to the wild type enzyme. Yield is the protein content after expression and purification; activity was determined by observing the formation of sulfuretin from butein by spectrophotometric measurements; copper incorporation was determined by AAS.
| Feature | Yield [%] | Diphenolase activity [%] | Copper incorporation [%] | |
|---|---|---|---|---|
| w/t | – | 100 | 100.0 | 100 |
| H93A | Copper coordinating | 59 | 0.6 | 50 |
| H116A | Copper coordinating | 171 | 0.0 | 54 |
| H125A | Copper coordinating | 117 | 0.0 | 50 |
| F273A | Blocker residue atop the CuA site | 43 | 0.0 | 112 |
| C97A | Thioether bridge with H116 | 9 | 0.0 | 98 |
Conflict of interest
The authors declare no competing financial interest.
Acknowledgements
The research was funded by the Austrian Science Fund (FWF): P25217-N28 and P24331-B16. The authors are grateful to Univ.-Prof. Dr. Christopher Gerner for access to nanoUHPLC-ESI-MS/MS measurements at the Mass Spectrometry Centre, University of Vienna. Thanks to Dipl.−Chem. Christian Molitor and Dipl.−Ing. Matthias Pretzler for valuable discussions regarding this work and Amir Blazevic, MSc. for proofreading this manuscript.
Contributor Information
Cornelia Kaintz, Email: cornelia.kaintz@univie.ac.at.
Rupert L. Mayer, Email: rupert.mayer@univie.ac.at.
Franz Jirsa, Email: franz.jirsa@univie.ac.at.
Heidi Halbwirth, Email: hhalb@mail.zserv.tuwien.ac.at.
Annette Rompel, Email: annette.rompel@univie.ac.at, http://www.bpc.univie.ac.at.
Appendix A. Supplementary data
References
- 1.Kitajima N., Fujisawa K., Moro-oka Y., Toriumi K. μ-η2:η2-Peroxo binuclear copper complex, [Cu(HB(3,5-iPr2pz)3)]2(O2) J. Am. Chem. Soc. 1989;111:8975–8976. [Google Scholar]
- 2.Mayer A.M. Polyphenol oxidases in plants and fungi: going places? a review. Phytochemistry. 2006;67:2318–2331. doi: 10.1016/j.phytochem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Mayer A.M., Harel E. Polyphenol oxidases in plants. Phytochemistry. 1979;18:193–215. [Google Scholar]
- 4.Strack D., Schliemann W. Bifunctional polyphenol oxidases: novel functions in plant pigment biosynthesis. Angew. Chem. Int. Ed. Engl. 2001;40:3791–3794. doi: 10.1002/1521-3773(20011015)40:20<3791::aid-anie3791>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Kaintz C., Molitor C., Thill J., Kampatsikas I., Michael C., Halbwirth H., Rompel A. Cloning and functional expression in E. coli of a polyphenol oxidase transcript from Coreopsis grandiflora involved in aurone formation. FEBS Lett. 2014;588:3417–3426. doi: 10.1016/j.febslet.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho M., Moinuddin S., Helms G., Hishiyama S., Eichinger D., Davin L., Lewis N. (+)-Larreatricin hydroxylase, an enantio-specific polyphenol oxidase from the creosote bush (Larrea tridentata) Proc. Natl. Acad. Sci. U.S.A. 2003;100:10641–10646. doi: 10.1073/pnas.1934562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwivedi U.N., Singh P., Pandey V.P., Kumar A. Structure–function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011;68:117–128. [Google Scholar]
- 8.Nakayama T., Yonekura-Sakakibara K., Sato T., Kikuchi S., Fukui Y., Fukuchi-Mizutani M., Ueda T., Nakao M., Tanaka Y., Kusumi T., Nishino T. Aureusidin synthase: a polyphenol oxidase homolog responsible for flower coloration. Science. 2000;290:1163–1166. doi: 10.1126/science.290.5494.1163. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama T. Enzymology of aurone biosynthesis. J. Biosci. Bioeng. 2002;94:487–491. doi: 10.1016/s1389-1723(02)80184-0. [DOI] [PubMed] [Google Scholar]
- 10.Ono E., Fukuchi-Mizutani M., Nakamura N., Fukui Y., Yonekura-Sakakibara K., Yamaguchi M., Nakayama T., Tanaka T., Kusumi T., Tanaka Y. Yellow flowers generated by expression of the aurone biosynthetic pathway. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11075–11080. doi: 10.1073/pnas.0604246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miosic S., Knop K., Hölscher D., Greiner J., Gosch C., Thill J., Kai M., Shrestha B.K., Schneider B., Crecelius A.C., Schubert U.S., Svatoš A., Stich K., Halbwirth H. 4-Deoxyaurone formation in Bidens ferulifolia (Jacq.) DC. PLoS ONE. 2013;8:e61766. doi: 10.1371/journal.pone.0061766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaintz C., Mauracher S.G., Rompel A. Advances in Protein Chemistry and Structural Biology. Academic Press; 2014. Type-3 copper proteins: recent advances on polyphenol oxidases; pp. 1–35. [DOI] [PubMed] [Google Scholar]
- 13.Tran L.T., Taylor J.S., Constabel C.P. The polyphenol oxidase gene family in land plants: lineage-specific duplication and expansion. BMC Genomics. 2012;13:395–407. doi: 10.1186/1471-2164-13-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gelder C.W.G., Flurkey W.H., Wichers H.J. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry. 1997;45:1309–1323. doi: 10.1016/s0031-9422(97)00186-6. [DOI] [PubMed] [Google Scholar]
- 15.Gerdemann C., Eicken C., Krebs B. The crystal structure of catechol oxidase: new insight into the function of type-3 copper proteins. Acc. Chem. Res. 2002;35:183–191. doi: 10.1021/ar990019a. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde T., Eicken C., Sacchettini J.C., Krebs B. Crystal structure of a plant catechol oxidase containing a dicopper center. Nat. Struct. Biol. 1998;5:1084–1090. doi: 10.1038/4193. [DOI] [PubMed] [Google Scholar]
- 17.Matoba Y., Kumagai T., Yamamoto A., Yoshitsu H., Sugiyama M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006;281:8981–8990. doi: 10.1074/jbc.M509785200. [DOI] [PubMed] [Google Scholar]
- 18.Mauracher S.G., Molitor C., Al-Oweini R., Kortz U., Rompel A. Latent and active abPPO4 mushroom tyrosinase cocrystallized with hexatungstotellurate(VI) in a single crystal. Acta Crystallogr. 2014;D70:2301–2315. doi: 10.1107/S1399004714013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldfeder M., Kanteev M., Adir N., Fishman A. Influencing the monophenolase/diphenolase activity ratio in tyrosinase. Biochim. Biophys. Acta. 2013;1834:629–633. doi: 10.1016/j.bbapap.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Marusek C.M., Trobaugh N.M., Flurkey W.H., Inlow J.K. Comparative analysis of polyphenol oxidase from plant and fungal species. J. Inorg. Biochem. 2006;100:108–123. doi: 10.1016/j.jinorgbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Giebel L.B., Strunk K.M., Spritz R.A. Organization and nucleotide sequences of the human tyrosinase gene and a truncated tyrosinase-related segment. Genomics. 1991;9:435–445. doi: 10.1016/0888-7543(91)90409-8. [DOI] [PubMed] [Google Scholar]
- 22.Kupper U., Niedermann D.M., Travaglini G., Lerch K. Isolation and characterization of the tyrosinase gene from Neurospora crassa. J. Biol. Chem. 1989;264:17250–17258. [PubMed] [Google Scholar]
- 23.Wichers H.J., Recourt K., Hendriks M., Ebbelaar C.E.M., Biancone G., Hoeberichts F.A., Mooibroek H., Soler-Rivas C. Cloning, expression and characterisation of two tyrosinase cDNAs from Agaricus bisporus. Appl. Microbiol. Biotechnol. 2003;61:336–341. doi: 10.1007/s00253-002-1194-2. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Chen H., Gao J., Liu X., Cheng W., Ma X. Cloning, characterization and expression of two new polyphenol oxidase cDNAs from Agaricus bisporus. Biotechnol. Lett. 2010;32:1439–1447. doi: 10.1007/s10529-010-0329-2. [DOI] [PubMed] [Google Scholar]
- 25.Fujita Y., Uraga Y., Ichisima E. Molecular cloning and nucleotide sequence of the protyrosinase gene, melO, from Aspergillus oryzae and expression of the gene in yeast cells. Biochim. Biophys. Acta. 1995;1261:151–154. doi: 10.1016/0167-4781(95)00011-5. [DOI] [PubMed] [Google Scholar]
- 26.Machida M., Asai K., Sano M., Tanaka T., Kumagai T., Terai G., Kusumoto K.-I., Arima T., Akita O., Kashiwagi Y., Abe K., Gomi K., Horiuchi H., Kitamoto K., Kobayashi T., Takeuchi M., Denning D.W., Galagan J.E., Nierman W.C., Yu J., Archer D.B., Bennett J.W., Bhatnagar D., Cleveland T.E., Fedorova N.D., Gotoh O., Horikawa H., Hosoyama A., Ichinomiya M., Igarashi R., Iwashita K., Juvvadi P.R., Kato M., Kato Y., Kin T., Kokubun A., Maeda H., Maeyama N., Maruyama J.-I., Nagasaki H., Nakajima T., Oda K., Okada K., Paulsen I., Sakamoto K., Sawano T., Takahashi M., Takase K., Terabayashi Y., Wortman J.R., Yamada O., Yamagata Y., Anazawa H., Hata Y., Koide Y., Komori T., Koyama Y., Minetoki T., Suharnan S., Tanaka A., Isono K., Kuhara S., Ogasawara N., Kikuchi H. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 27.Dirks-Hofmeister M.E., Inlow J.K., Moerschbacher B.M. Site-directed mutagenesis of a tetrameric dandelion polyphenol oxidase (PPO-6) reveals the site of subunit interaction. Plant Mol. Biol. 2012;80:203–217. doi: 10.1007/s11103-012-9943-9. [DOI] [PubMed] [Google Scholar]
- 28.Gerdemann C., Eicken C., Magrini A., Meyer H.E., Rompel A., Spener F., Krebs B. Isozymes of Ipomoea batatas catechol oxidase differ in catalase-like activity. Biochim. Biophys. Acta. 2001;1548:94–105. doi: 10.1016/s0167-4838(01)00219-9. [DOI] [PubMed] [Google Scholar]
- 29.Zekiri F., Molitor C., Mauracher S.G., Michael C., Mayer R.L., Gerner C., Rompel A. Purification and characterization of tyrosinase from walnut leaves (Juglans regia) Phytochemistry. 2014;101:5–15. doi: 10.1016/j.phytochem.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virador V.M., Reyes Grajeda J.P., Blanco-Labra A., Mendiola-Olaya E., Smith G.M., Moreno A., Whitaker J.R. Cloning, sequencing, purification, and crystal structure of Grenache (Vitis vinifera) polyphenol oxidase. J. Agric. Food Chem. 2010;58:1189–1201. doi: 10.1021/jf902939q. [DOI] [PubMed] [Google Scholar]
- 31.Haruta M., Murata M., Hiraide A., Kadokura H., Yamasaki M., Sakuta M., Shimizu S., Homma S. Cloning genomic DNA encoding apple polyphenol oxidase and comparison of the gene product in Escherichia coli and in apple. Biosci. Biotechnol. Biochem. 1998;62:358–362. doi: 10.1271/bbb.62.358. [DOI] [PubMed] [Google Scholar]
- 32.Richter H., Lieberei R., Strnad M., Novak O., Gruz J., Rensing S.A., von Schwartzenberg K. Polyphenol oxidases in Physcomitrella: functional PPO1 knockout modulates cytokinin-dependent development in the moss Physcomitrella patens. J. Exp. Bot. 2012;63:5121–5135. doi: 10.1093/jxb/ers169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shetty S.M., Chandrashekar A., Venkatesh Y.P. Eggplant polyphenol oxidase multigene family: cloning, phylogeny, expression analyses and immunolocalization in response to wounding. Phytochemistry. 2011;72:2275–2287. doi: 10.1016/j.phytochem.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y.L., Pan L.P., Yu S.L., Li H.H. Cloning, microbial expression and structure–activity relationship of polyphenol oxidases from Camellia sinensis. J. Biotechnol. 2010;145:66–72. doi: 10.1016/j.jbiotec.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan M.L., Hatfield R.D., Thoma S.L., Samac D.A. Cloning and characterization of red clover polyphenol oxidase cDNAs and expression of active protein in Escherichia coli and transgenic alfalfa. Plant Physiol. 2004;136:3234–3244. doi: 10.1104/pp.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren Q., Henes B., Fairhead M., Thöny-Meyer L. High level production of tyrosinase in recombinant Escherichia coli. BMC Biotechnol. 2013;13:18. doi: 10.1186/1472-6750-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujieda N., Murata M., Yabuta S., Ikeda T., Shimokawa C., Nakamura Y., Hata Y., Itoh S. Multifunctions of MelB, a fungal tyrosinase from Aspergillus oryzae. ChemBioChem. 2012;13:193–201. doi: 10.1002/cbic.201100609. [DOI] [PubMed] [Google Scholar]
- 38.Gasparetti C., Faccio G., Arvas M., Buchert J., Saloheimo M., Kruus K. Discovery of a new tyrosinase-like enzyme family lacking a C-terminally processed domain: production and characterization of an Aspergillus oryzae catechol oxidase. Appl. Microbiol. Biotechnol. 2010;86:213–226. doi: 10.1007/s00253-009-2258-3. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M., Nakajima T., Ohba Y., Yamauchi S., Lee B.R., Ichishima E. Identification of copper ligands in Aspergillus oryzae tyrosinase by site-directed mutagenesis. Biochem. J. 2000;350:537–545. [PMC free article] [PubMed] [Google Scholar]
- 40.Molitor C., Mauracher S.G., Pargan S., Mayer R.L., Halbwirth H., Rompel A. Latent and active aurone synthase from petals of C. grandiflora: a PPO with unique characteristics. Planta. 2015;2:10–20. doi: 10.1007/s00425-015-2261-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujieda N., Yabuta S., Ikeda T., Oyama T., Muraki N., Kurisu G., Itoh S. Crystal Structures of copper-depleted and copper-bound fungal pro-tyrosinase: insights into endogenous cystein-dependent copper incorporation. J. Biol. Chem. 2013;288:22128–22140. doi: 10.1074/jbc.M113.477612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker A., Taylor J., Rowe D., Summers D. A method for generating sticky-end PCR products which facilitates unidirectional cloning and the one-step assembly of complex DNA constructs. Plasmid. 2008;59:155–162. doi: 10.1016/j.plasmid.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-López J., Bañón-Arnao M., Martinez-Ortiz F., Tudela J., Acosta M., Varón R., García-Cánovas F. Catalytic oxidation of 2,4,5-trihydroxyphenylalanine by tyrosinase: identification and evolution of intermediates. Biochim. Biophys. Acta. 1992;1160:221–228. doi: 10.1016/0167-4838(92)90011-2. [DOI] [PubMed] [Google Scholar]
- 44.Sato T., Nakayama T., Kikuchi S., Fukui Y., Yonekura-Sakakibara K., Ueda T., Nishino T., Tanaka Y., Kusumi T. Enzymatic formation of aurones in the extracts of yellow snapdragon flowers. Plant Sci. 2001;160:229–236. doi: 10.1016/s0168-9452(00)00385-x. [DOI] [PubMed] [Google Scholar]
- 45.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 46.Cabanes J., Escribano J., Gandia-Herrero F., Garcia-Carmona F., Jimenez-Atienzar M. Partial purification of latent polyphenol oxidase from peach (Prunus persica L. Cv. Catherina). Molecular properties and kinetic characterization of soluble and membrane-bound forms. J. Agric. Food Chem. 2007;55:10446–10451. doi: 10.1021/jf072165y. [DOI] [PubMed] [Google Scholar]
- 47.Decker H., Tuczek F. Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem. Sci. 2000;25:392–397. doi: 10.1016/s0968-0004(00)01602-9. [DOI] [PubMed] [Google Scholar]
- 48.Jaenicke E., Büchler K., Markl J., Decker H., Barends T.R.M. Cupredoxin-like domains in haemocyanins. Biochem. J. 2010;426:373–378. doi: 10.1042/BJ20091501. [DOI] [PubMed] [Google Scholar]
- 49.Mauracher S.G., Molitor C., Al-Oweini R., Kortz U., Rompel A. Crystallization and preliminary X-ray crystallographic analysis of latent isoform PPO4 mushroom (Agaricus bisporus) tyrosinase. Acta Crystallogr. F. 2014;70:263–266. doi: 10.1107/S2053230X14000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasteiger E., Hoogland C., Gattiker A., Duvaud S.e., Wilkins M.R., Appel R.D., Bairoch A. The Proteomics Protocols Handbook. Humana Press; 2005. Protein Identification and Analysis Tools on the ExPASy Server; pp. 571–607. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.