Abstract
Purpose.
This study examined the association between peripapillary choroidal thickness (PCT) with age and race in a group of African descent (AD) and European descent (ED) subjects with normal eyes.
Methods.
Optic nerve head images from enhanced depth imaging spectral-domain optical coherence tomography of 166 normal eyes from 84 subjects of AD and ED were manually delineated to identify the principal surfaces of Bruch's membrane (BM), Bruch's membrane opening (BMO), and anterior sclera (AS). Peripapillary choroidal thickness was measured between BM and AS at increasing distance away from BMO. The mean PCT was compared between AD and ED subjects and generalized estimating equation (GEE) regression analysis was used to examine the association between race and PCT overall, in each quadrant, and by distance from BMO. Models were adjusted for age, BMO area, and axial length in the regression analysis.
Results.
Overall, the mean PCT increased from 63.9 μm ± 18.1 at 0 to 250 μm to 170.3 μm ± 56.7 at 1500 to 2000 μm from BMO. Individuals of AD had a greater mean PCT than those of ED at all distances from BMO (P < 0.05 at each distance) and in each quadrant (P < 0.05 in each quadrant). Results from multivariate regression indicate that ED subjects had significantly lower PCT compared to AD overall and in all quadrants and distances from BMO. Increasing age was also significantly associated with a lower PCT in both ED and AD participants.
Conclusions.
Peripapillary choroidal thickness varies with race and age, as individuals of AD have a thicker peripapillary choroid than those of ED. (ClinicalTrials.gov number, NCT00221923.)
Keywords: enhanced depth imaging SDOCT, peripapillary choroid, race, age
Peripapillary choroidal thickness, measured using enhanced depth imaging techniques with spectral-domain optical coherence tomography, in normal eyes is greater in individuals of African descent than those of European descent.
Introduction
The choroid is a multifunctional vascular tissue of the eye whose primary functions include supplying oxygen to the outer retina and optic nerve head (ONH), thermoregulation, growth factor secretion, and regulation of retinal position into a plane of focus by transient fluctuations in choroidal thickness.1 Current knowledge of the peripapillary choroid is limited by technical difficulties in visualizing the structure in vivo. Recent advances in imaging techniques such as the enhanced depth imaging (EDI) modality of spectral-domain optical coherence tomography (SDOCT) have provided the opportunity to better visualize and quantify the morphology of the choroid.2,3
Much attention has recently been focused on determining the role, whether primary or secondary, of the choroid in the pathogenesis of glaucoma. Intrascleral branches of the short posterior ciliary arteries (SPCA) perfuse both the lamina of the ONH and the peripapillary choroid, which in turn supplies the prelaminar region of the ONH. Speculation exists that changes in the choroidal vasculature, such as thinning from a loss of vascular tissue or damage to the SPCA, may be associated with glaucomatous optic neuropathy through an ischemic mechanism.4 However, there is inconsistent evidence regarding the association of choroidal thinning with the development of glaucoma. Histology has shown a thinned choroid in glaucomatous eyes,4 yet in vivo studies show conflicting associations with glaucoma, ranging from no association5–11 to an association with certain subtypes of glaucoma, such as normal tension glaucoma.12–15
It is well documented that with increased age, the peripapillary choroid decreases in thickness.2,5–14,16–18 While the association with age has been well described, prior literature has not evaluated differences of choroidal thickness across racial groups, and in particular in racial groups that are at especially high risk of developing glaucoma, such as seen in persons of African descent (AD).
In addition to the variation in peripapillary choroidal thickness (PCT) with age, PCT has been associated with the anterior to posterior position of Bruch's membrane opening (BMO).16 Thus, the deeper optic cup seen in individuals of AD compared to European descent (ED)19 may be due to differences in PCT across these racial groups. However, little is known about the variation in PCT with both age and race in normal eyes. The purpose of this study is to examine the association between PCT with age and race in a well-described group of AD and ED subjects without ocular disease.
Methods
Two National Institute of Health–funded cohort studies conducted at the University of Alabama at Birmingham (UAB), the African Descent and Glaucoma Evaluation Study (ADAGES),20 and the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR),21 provided the SDOCT data for this study. Only subjects without ocular diseases that would affect the optic nerve, retina, or choroid were included. The imaging methodologies followed in ADAGES and ALSTAR were identical. All participants from each study gave written informed consent. The UAB institutional review board approved the study methods and these methods adhered to the tenets of the Declaration of Helsinki.
Each participant in both ADAGES and ALSTAR underwent a complete ophthalmological exam, including medical history, Snellen best-corrected visual acuity, Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity, color vision, slit lamp biomicroscopy, applanation tonometry for IOP, central corneal thickness measurement, gonioscopy, axial length measurement, dilated funduscopy, stereoscopic ophthalmoscopy of the optic disc, and stereoscopic fundus photography. Standard Swedish Interactive Thresholding Algorithm (SITA) 24-2 perimetry was performed in both studies to define normality for inclusion (see below).
Inclusion/Exclusion Criteria
Participants were over 18 years of age for the ADAGES study and ≥60 years for the ALSTAR study. Eligible participants for both studies had open iridocorneal angles, a best-corrected acuity of 20/40 or better, and refractive error ≤ 50 diopters sphere and 3.0 diopters cylinder. Patients with diabetes and any retinal, corneal, or optic nerve disease were excluded. Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract surgery), elevated IOP (>22 mm Hg) at the time of the study, a history of elevated IOP, prior use of glaucoma medication, other intraocular eye disease, or other diseases affecting visual field. Further, patients were excluded if there was a diagnosis of any neurologic or psychiatric conditions that would prevent participation in a psychophysical test.
We required a reliable test on 24-2 standard automated perimetry (Carl Zeiss Meditec, Inc., Dublin, CA, USA) using the Swedish interactive threshold algorithm in both eyes at baseline. Reliability was defined as <33% false positives, false negatives, and fixation losses. A field was considered normal if the pattern standard deviation was not triggered at 5% or less, the Glaucoma Hemifield Test was within normal limits, and the field showed no sign of glaucomatous defect based on subjective evaluation from the enrolling clinician. A fellowship-trained glaucoma specialist assessed the stereoscopic photos of all optic nerves, and subjects with any structural finding suggestive of glaucoma (i.e., notching, rim thinning, disc hemorrhages, or retinal nerve fiber layer defects as judged) were excluded.
SDOCT Image Acquisition, Processing, and Quantification
All subjects underwent SDOCT imaging with OCT (Spectralis, Family Acquisition Module 5.4.8.0; Heidelberg Engineering, Heidelberg, Germany) with 48 radial volume scans (20 degree) centered on the ONH using the EDI mode.22 Scans were evaluated for imaging quality by the operator, and images were reacquired if there was improper B-scan positioning in the imaging frame, a quality score < 20, or poor centration of the ONH. A total of 168 radial volume scans from both eyes of 84 study subjects were included in the study. Scans were acquired using a signal averaging of 9 (n = 108; ADAGES) or 12 (n = 60; ALSTAR). All other characteristics of the imaging protocols were identical in each study.
In order to enhance the visibility of the deeper tissues, all B-scans from all SDOCT volumes were postprocessed using a customized adaptive compensation algorithm, with contrast and threshold exponents of 2.16,23,24 Two eyes were eliminated due to inadequate visualization of the anterior sclera (AS) following compensation. Adaptive compensation allowed an enhanced visualization and a more reliable delineation of characteristic structures such as Bruch's membrane (BM) and the AS.
Radial SDOCT scan volumes were loaded and aligned in custom software (based on the Visualization Toolkit [VTK]; Kitware, Inc., Clifton Park, NY) that was developed for three-dimensional delineation of histologic and OCT data as described in prior publications.25,26 Two trained observers masked to subject characteristics manually delineated 24 equally spaced radial sections of each eye. Reliability testing was performed by having each of the two observers delineate four different eyes (two AD eyes, two ED eyes) on three separate occasions. Intraclass correlations (ICC) were used to evaluate the within- and between-person variance of the manual delineation of scans by the two observers.27 The ICCs for mean PCT were very high overall (>0.75, excellent reliability), indicating that the measurements at a given location from BMO were very similar between observers. Additionally, ICCs were calculated based on race (AD eyes: 0.95, excellent reliability; ED eyes: 0.68, good reliability).
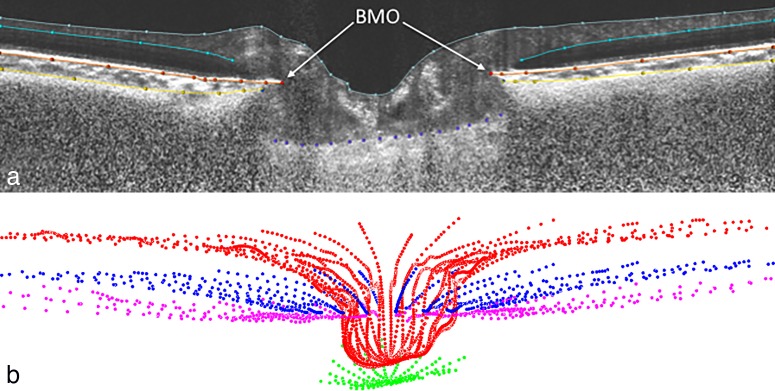
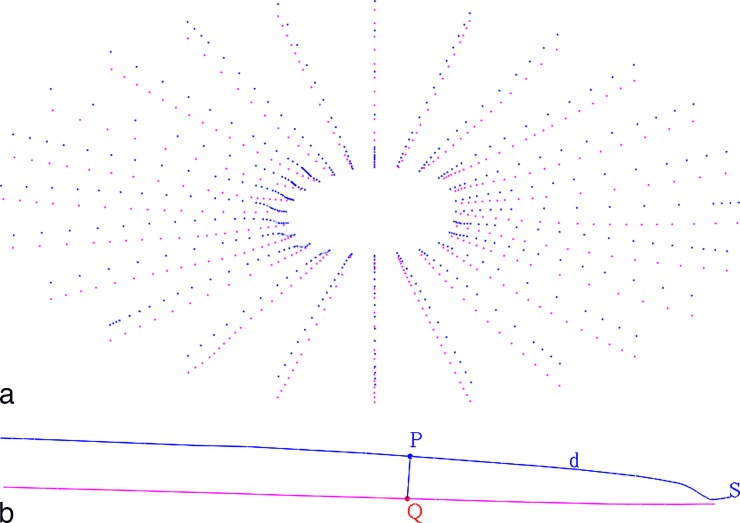
Principal surfaces delineated included BM, BMO, and AS (Fig. 1a). A mean of 490 points (range, 303–954) for BM and 505 points (range, 457–540) for AS were sampled (Fig. 1b). The methods for defining BMO have been published elsewhere.16 Peripapillary choroidal thickness is a measurement of the distance between BM and AS. Indeed, this measurement can be made at various offsets from BMO (Fig. 2a). Suppose that we want to compute PCT at an offset of d μm from BMO. On each BM half-section S, we walk d μm along the section away from the BMO to a point P of the BM, cast a ray along P's normal toward the sclera to a point Q of the AS, and compute the sectional PCT of S at d μm to be the distance between P and Q (Fig. 2b). Under these assumptions, PCT at d μm is the mean of all sectional PCTs at d μm. (Since the BM can exhibit local irregularities, yet is almost linear, we considered two alternatives for P's normal: the true normal of the BM section at P and the normal to a linear approximation of S. We found that these two choices yielded consistent results for thickness.)
Figure 1.
(a) Principal ONH surfaces delineated: internal limiting membrane (gray), BM (orange), AS (yellow), and anterior surface of the lamina cribrosa (purple) within a sample SDOCT radial section. Only BM surface and anterior scleral surface were used for this study. (b) Visualization of complete delineated surfaces: internal limiting membrane (red), BM (blue), AS (pink), and anterior surface of the lamina cribrosa (green).
Figure 2.
Measurement of PCT. (a) Point cloud of PCT measurements between BM (blue) and AS (pink), based on the original manual delineation. In sampling PCT over a range, a uniform sampling of the choroid from a reconstruction of this delineation is used. (b) Algorithm used to compute PCT: BM (blue) half-section S; point P is d μm away from BMO; a ray along P's normal toward the AS (pink) intersects at point Q; the PCT of S at d μm is the distance between P and Q.
Statistical Analysis
The mean PCT was calculated at five different distances away from BMO (0–250 μm, 250–500 μm, 500–1000 μm, 1000–1500 μm, and 1500–2000 μm) by uniformly sampling a reconstruction of the choroid sections over each range and was compared between AD and ED subjects. Distances were also grouped as a proximal distance of 0 to 1000 μm and a distal distance of 1000 to 2000 μm. The mean PCT was also compared between race (AD and ED) in four quadrants of the ONH (inferior, nasal, superior, and temporal). Generalized estimating equations (GEE) were used to evaluate the association between race with PCT in each quadrant and at different distances away from BMO. Generalized estimating equations allow the model to account for the within-subject correlation among fellow eyes from the same individual. An exchangeable working correlation matrix was specified because it minimized the difference in variance estimates between the empirical and model-based standard errors. Models were adjusted for the potentially confounding effects of age, axial length, and BMO area (a surrogate for optic disc size). An interaction term was added to the models to evaluate whether the effect of age on PCT varied by race. Age, axial length, and BMO area were continuous variables in the adjusted model; the ED group was the reference group. P values < 0.05 were considered statistically significant.
Results
The final study sample consisted of 166 eyes from 84 subjects. Sixty percent of those in the sample were ED, and the remaining were AD. Subjects ranged in age from 29 to 92 years, with a mean age of 58 years; however, those in the AD group were significantly younger (mean 50.4 years, SD 11.3) than those in the ED group (mean 63.4, SD 15.6; P < 0.0001) (Table 1). The average axial length was 23.7 mm (SD 1.0), and BMO area was 1.9 μm2 × 106 (SD 0.4).
Table 1.
Demographic and Ocular Characteristics of the Study Sample by Race (N = 166 Eyes)
|
Variable |
Overall |
AD, N = 64 |
ED, N = 102 |
PValue |
| Age, y | 58.4 (15.5) | 50.4 (11.3) | 63.4 (15.6) | <0.0001 |
| Axial length, mm* | 23.7 (1.0) | 23.6 (0.7) | 23.8 (1.1) | 0.54 |
| BMO area, μm2 × 106 | 1.9 (0.4) | 2.1 (0.5) | 1.7 (0.3) | <0.0001 |
All values are mean (SD).
12 missing data.
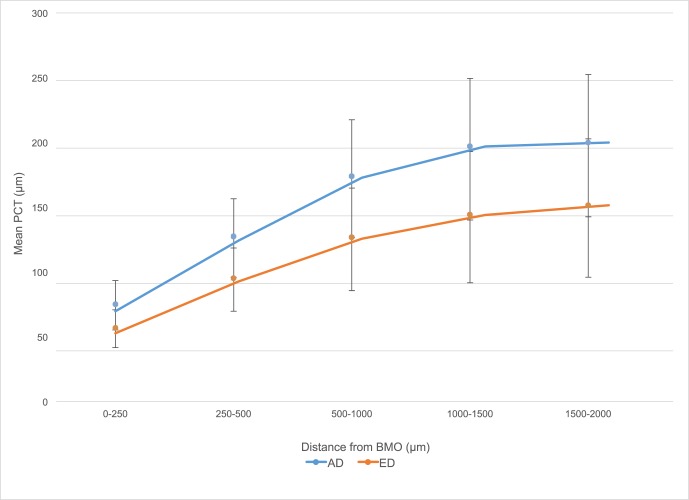
Overall, the choroid was thinnest at 0 to 250 μm from the BMO (63.9 μm ± 18.1) and thickest at a distance of 1500 to 2000 μm from the BMO (170.3 μm ± 56.7; Fig. 3). Based on visual inspection, AD and ED participants had a similar pattern of increase from 0 to 2000 μm (Fig. 4). Table 2 compares PCT in the four peripapillary quadrants as well as by distance from BMO between AD and ED subjects. The mean PCT was relatively similar within the superior, nasal, and temporal quadrants and was slightly lower in the inferior quadrant. The choroid was significantly thicker among AD than ED participants in each quadrant and at all distances from BMO (P < 0.05 at each distance; Fig. 5).
Figure 3.
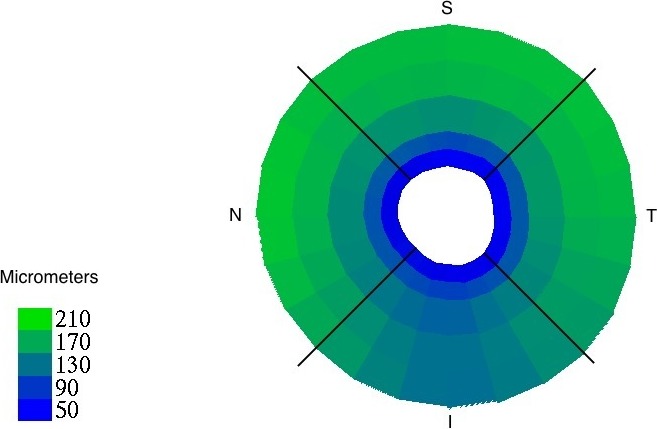
Overall mean PCT, shown by distance from BMO (concentric bands at 0–250 μm, 250–500 μm, 500–1000 μm, 1000–1500 μm, and 1500–2000 μm) and optic nerve quadrant (I, N, S, T), with thickness in micrometers denoted by color (lower left key).
Figure 4.
Mean (SD) PCT and distance from BMO by race. For all points, P < 0.05.
Table 2.
Mean PCT for Each Quadrant and Distance From BMO by Race
|
Overall |
AD |
ED |
PValue |
|
| Quadrant, μm | ||||
| Inferior | 114.8 (53.2) | 142.9 (56.1) | 97.1 (42.6) | <0.0001 |
| Nasal | 135.9 (62.8) | 158.6 (68.6) | 119.9 (58.8) | 0.0053 |
| Superior | 135.2 (65.1) | 155.0 (65.0) | 123.8 (58.3) | 0.050 |
| Temporal | 134.8 (65.85) | 161.7 (69.6) | 118.6 (56.0) | 0.0029 |
| Distance from BMO, μm | ||||
| 0–250 | 63.9 (18.1) | 75.1 (18.3) | 56.9 (14.0) | 0.0003 |
| 250–500 | 107.7 (30.1) | 127.5 (29.0) | 95.2 (23.4) | <0.0001 |
| 500–1000 | 145.1 (46.2) | 174.0 (43.5) | 126.9 (38.0) | 0.0006 |
| 1000–1500 | 164.8 (56.1) | 197.1 (52.4) | 144.4 (48.5) | 0.0087 |
| 1500–2000 | 170.3 (56.7) | 199.9 (52.7) | 151.6 (51.2) | 0.048 |
| Proximal | 105.6 (47.1) | 125.5 (51.5) | 93.0 (39.3) | <0.0001 |
| Distal | 167.5 (56.4) | 198.5 (52.3) | 148.0 (49.9) | 0.021 |
All values are mean micrometers (SD). Generalized estimating equation models were used to obtain P values comparing mean PCT between AD and ED. Models were adjusted for age, axial length, and BMO area. Proximal defined as 0 to 1000 μm from BMO. Distal defined as 1000 to 2000 μm from BMO.
Figure 5.
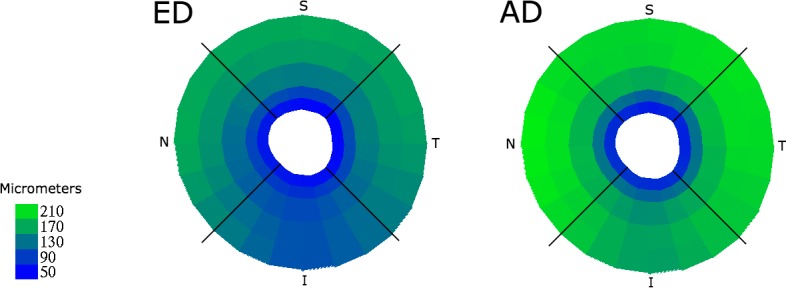
Mean PCT by race (ED, AD), shown by distance from BMO (concentric bands at 0–250 μm, 250–500 μm, 500–1000 μm, 1000–1500 μm, and 1500–2000 μm) and optic nerve quadrant (I, N, S, T), with thickness in micrometers denoted by color (lower left key).
Those of AD had a significantly thicker choroid compared to those of ED, even after adjusting for the potentially confounding effects of age, axial length, and BMO area (Table 3). The analysis was repeated excluding ED participants over age 78 so that the AD and ED groups had comparable age ranges; the pattern of results was unchanged and remained consistent with Table 3. In addition, increasing age was associated with a significant decrease in PCT in each quadrant as well as in the proximal and distal regions. Separate models were used to examine the association at the proximal and distal distances from BMO for each quadrant (Table 2). The associations between age and race with PCT were similar at the proximal location within each quadrant; however, race was not statistically significant at the distal location within the nasal and superior quadrants. An interaction term was added to each model to test if the association between age and PCT differed by race; however, the interaction term was not statistically significant in any of the models (Table 4).
Table 3.
Adjusted Association Between Race With Mean PCT by Proximal and Distal Location From BMO
|
Proximal |
Distal |
|||||
|
Coefficient |
SE |
PValue |
Coefficient |
SE |
PValue |
|
| Race, ED ref | 24.18 | 6.05 | <0.0001 | 26.29 | 11.4 | 0.021 |
| Age | −0.73 | 0.18 | <0.0001 | −1.89 | 0.36 | <0.0001 |
| Axial length | −1.04 | 1.86 | 0.58 | −4.28 | 0.80 | <0.0001 |
| BMO area | −3.02 | 5.57 | 0.59 | −6.83 | 7.39 | 0.36 |
Generalized estimating equation models were used to obtain parameter estimates and P values. The model included age and race and was further adjusted for axial length and BMO area. Proximal defined as 0 to 1000 μm from BMO. Distal defined as 1000 to 2000 μm from BMO. ED ref indicates the ED group was the reference group.
Table 4.
Age and Race Effects on Mean PCT by Proximal and Distal Location From BMO
|
Proximal |
Distal |
|||||
|
Coefficient |
SE |
PValue |
Coefficient |
SE |
PValue |
|
| Race, ED ref | 41.54 | 20.55 | 0.043 | 63.50 | 43.87 | 0.15 |
| Age | −0.64 | 0.22 | 0.0031 | −1.69 | 0.41 | <0.0001 |
| Race * age | −0.33 | 0.37 | 0.37 | −0.70 | 0.81 | 0.38 |
| Axial length | −1.26 | 1.89 | 0.51 | −4.37 | 0.86 | <0.0001 |
| BMO area | −1.86 | 5.45 | 0.73 | −5.96 | 7.22 | 0.41 |
Generalized estimating equation models were used to obtain parameter estimates and P values. The model included age, race, and an interaction term between age and race (Race * age), and was further adjusted for axial length and BMO area. Proximal defined as 0 to 1000 μm from BMO. Distal defined as 1000 to 2000 μm from BMO.
Discussion
Determining the variation in PCT in normal eyes due to aging and race is important in order to clarify the relationship, whether primary or secondary, between changes in PCT and the development of glaucomatous optic neuropathy. The results of this study illustrate that PCT is lower proximal to BMO and progressively increases up to 1000 μm away from BMO, where it then levels off. Additionally, this study indicates that in all quadrants and distances from BMO, individuals of AD have a significantly greater PCT than do those of ED. When examined by quadrant adjacent to the ONH, PCT has a similar thickness in the superior, nasal, and temporal quadrants, but is lowest inferiorly. Our analysis also suggests that both AD and ED groups exhibit a decrease in PCT with aging and AD individuals maintain a greater PCT compared to ED individuals at all ages. The interaction between age and race would suggest that individuals of AD have a decrease in mean PCT with age similar to that of individuals of ED. However, given the low numbers of older AD individuals, it is certainly possible that some difference in the aging effects exist between racial groups at older ages. There are numerous recent papers addressing choroidal thickness, but to the best of our knowledge, this is the first study to examine PCT in normal eyes across both age and race. Further, the image quantification methods used in this study allow for detailed characterization of the entire peripapillary region up to 2000 μm from BMO, unlike prior studies that, in general, assessed only limited, specific measurement regions.
While it is well known that age and axial length are associated with choroidal thickness, there is limited data on the association with race, specifically the AD group at high risk for glaucoma. As PCT decreases with age, it may cause BMO position to migrate posteriorly, leading to a deeper optic cup depth, as seen in individuals of AD.19 Even with aging in normal eyes, the AD group maintains a greater PCT than the ED group, yet both groups exhibit similar regional variation in PCT thickness. Our results show that even in young, normal eyes, the inferior choroid is thinner than the other regions. In both normal and glaucomatous eyes, studies have shown regional variation in other ONH parameters with age: The inferotemporal neuroretinal rim area shows the greatest rate of thinning over time,28 and scleral strain on the ONH is highest in the inferotemporal region.29 These regional ONH parameters of neuroretinal rim thinning and high scleral strain could result in or be caused by a thinner choroid or variation in intrascleral vascular anatomy. Additionally, it has been noted that during embryonic development, the optic fissure closes in the inferotemporal region of the ONH, with defects in closure resulting in colobomas of the ONH in this region. Thus the inferior choroid may be thinner than the other regions of peripapillary choroid as a result of embryologic development processes.
Similar to the present study, the majority of previous studies investigating the PCT were conducted using the Heidelberg Spectralis SDOCT. As opposed to the present study that sampled nearly 500 data points ranging from 0 to 2000 μm from BMO to calculate mean PCT, many other studies used the Spectralis standard 12° circle scan (3.4-mm diameter) centered on the ONH to image the peripapillary choroid,9,13,14,17 thus obtaining a measurement of PCT at an isolated circumferential location of approximately 1000 μm from BMO, assuming the radius of the optic disc is 0.75 mm.17 Recent work by Lee and colleagues11 used a custom-designed swept-source OCT to measure PCT in 10 normal individuals at 250, 750, 1250, and 1750 μm from BMO and found a similar pattern of greater PCT in the nasal, superior, and temporal quadrants compared to inferiorly. Ho et al.3 used the Cirrus HD-OCT (Carl Zeiss Meditec, Inc.) to measure PCT in 36 normal individuals at 500-μm segments from 500 to 2000 μm from BMO along a single vertical and horizontal 6-mm raster scan through the ONH. Their results also showed an increase in PCT linearly away from BMO as well as the geographic difference of a thinner inferior quadrant, similar to the present work. Park and coworkers12 measured mean PCT of 48 Korean adults without glaucoma at 300-μm segments from BMO in 12 sectors around the ONH and also found the inferior region to be the lowest. Rossou et al.30 studied the PCT of 63 normal eyes, with an average age of 22 years old, using only the horizontal raster scan of the Cirrus HD-OCT in 500-μm segments from 0 to 1000 μm from the ONH in order to analyze the papillomacular region. None of the above studies analyzed PCT variance by race, and most of the studies did not identify race in the study demographics. Given that race was not identified in many of the previous studies, it is difficult to directly compare the absolute values of PCT measurements to the present study.
There are several limitations of this study that are important to address. Even though the SDOCT data used in the study was obtained from two different trials, ADAGES and ALSTAR, both studies used the same imaging protocols, similar inclusion and exclusion criteria, and acquired subjects from the same source populations. The cross-sectional nature of the study prevents the longitudinal assessment of PCT change with age and race. Thus, additional evaluation of the study cohort over time is needed to confirm the aging effect that is strongly suggested by the significant associations seen in this and prior studies. While the sample size was somewhat small, particularly in the AD older age group, it was larger than the previous studies, and statistically significant associations were observed between age, race, and PCT. Given the lower number of older AD individuals, it is possible that there may be a significant interaction between age and race that was not detected by the study. Thus, while the study clearly demonstrates the global and regional differences in the choroid with age and across racial groups, it is not powered enough to determine if these aging effects are similar among older individuals. Due to the vascular nature of the choroid, other studies have noted variation in choroidal thickness with time of day and the cardiac cycle.5,31–34 The SDOCT images obtained in this study were taken at various times during the day, and elements of the patient's cardiac cycle (such as diastolic blood pressure and ocular perfusion pressure) were not collected. However, any effect from these two aspects would be random and would have biased the results away from significance. The retinal pigment epithelium may have created a shadow, preventing adequate visualization of the AS, particularly in individuals of AD, which may have impacted the measurement of PCT; yet, only two eyes had to be excluded from the analysis due to poor visualization of the AS. Lastly, the study included only healthy patients, and future research including patients with glaucoma is necessary to better understand the role of PCT in glaucoma.
The use of EDI with SDOCT has allowed greater visibility of the choroid than previously possible; however, our view of the peripapillary choroid closest to the ONH, namely the area from 0 to 250 μm from BMO, remains difficult to visualize consistently. It is possible that this area of peripapillary choroidal vasculature is the most crucial to the health of the ONH. Damage in this proximal region may either primarily lead to the susceptibility of the ONH to glaucomatous damage or may be secondarily affected by structural changes to the ONH. The difficulty in accurately visualizing and measuring this key proximal region of choroid could explain why previous studies have found such disparate results as to the association of choroidal thickness and glaucoma.
In summary, the present study suggests a consistent variation in PCT with race, an important demographic risk factor for glaucoma, as individuals of AD have a thicker choroid in all quadrants and at all distances from BMO than do those of ED. As future studies examine the relationship, be it primary or secondary, of PCT to the development and progression of glaucomatous optic neuropathy, inherent PCT variation between people of AD and ED will be important to consider.
Acknowledgments
The authors thank Claude Burgoyne, MD, and the Optic Nerve Head Research Laboratory of the Devers Eye Institute (Portland, OR, USA) for providing the Multiview delineation software used in this study.
Supported by grants from National Eye Institute (R01 EY018926, U10 EY14267, EY019869; Bethesda, MD, USA); National Institute on Aging (R01 AG04212; Bethesda, MD, USA); Eyesight Foundation of Alabama (Birmingham, AL, USA); Research to Prevent Blindness (New York, NY, USA); Alfreda J. Schueler Trust (Chicago, IL, USA); and a National University of Singapore Young Investigator Award (NUSYIA_FY13_P03; Singapore). The authors alone are responsible for the content and writing of the paper.
Disclosure: L.A. Rhodes, None; C. Huisingh, None; J. Johnstone, None; M.A. Fazio, None; B. Smith, None; L. Wang, None; M. Clark, None; J.C. Downs, None; C. Owsley, None; M.J.A. Girard, None; J.M. Mari, None; C.A. Girkin, None
References
- 1. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147: 811–815. [DOI] [PubMed] [Google Scholar]
- 3. Ho J, Branchini L, Regatieri C, Krishnan C, Fujimoto JG, Duker JS. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011; 118: 2001–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin ZQ, Vaegan , Millar TJ, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997; 6: 23–32. [PubMed] [Google Scholar]
- 5. Maul EA, Friedman DS, Chang DS, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011; 118: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 3430–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suh W, Cho HK, Kee C. Evaluation of peripapillary choroidal thickness in unilateral normal-tension glaucoma. Jpn J Ophthalmol. 2014; 58: 62–67. [DOI] [PubMed] [Google Scholar]
- 8. Chung HS, Sung KR, Lee KS, Lee JR, Kim S. Relationship between the lamina cribrosa, outer retina, and choroidal thickness as assessed using spectral domain optical coherence tomography. Korean J Ophthalmol. 2014; 28: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Bian A, Zhou Q, Mao J. Peripapillary choroidal thickness in both eyes of glaucoma patients with unilateral visual field loss. Am J Ophthalmol. 2013; 156: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 10. Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011; 92: 189–194. [DOI] [PubMed] [Google Scholar]
- 11. Lee S, Han SX, Young M, Beg MF, Sarunic MV, Mackenzie PJ. Optic nerve head and peripapillary morphometrics in myopic glaucoma. Invest Ophthalmol Vis Sci. 2014; 55: 4378–4393. [DOI] [PubMed] [Google Scholar]
- 12. Park HY, Lee NY, Shin HY, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014; 23: 225–231. [DOI] [PubMed] [Google Scholar]
- 13. Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012; 12: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts KF, Artes PH, O'Leary N, et al. Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Arch Ophthalmol. 2012; 130: 980–986. [DOI] [PubMed] [Google Scholar]
- 15. Usui S, Ikuno Y, Miki A, Matsushita K, Yasuno Y, Nishida K. Evaluation of the choroidal thickness using high-penetration optical coherence tomography with long wavelength in highly myopic normal-tension glaucoma. Am J Ophthalmol. 2012; 153: 10–16. [DOI] [PubMed] [Google Scholar]
- 16. Johnstone J, Fazio M, Rojananuangnit K, et al. Variation of the axial location of Bruch's membrane opening with age, choroidal thickness, and race. Invest Ophthalmol Vis Sci. 2014; 55: 2004–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang W, Wang W, Zhou M, et al. Peripapillary choroidal thickness in healthy Chinese subjects. BMC Ophthalmol. 2013; 13: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang W, Wang W, Gao X, et al. Choroidal thickness in the subtypes of angle closure: an EDI-OCT study. Invest Ophthalmol Vis Sci. 2013; 54: 7849–7853. [DOI] [PubMed] [Google Scholar]
- 19. Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. 2010; 128: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009; 127: 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Owsley C, Huisingh C, Jackson GR, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014; 55: 4776–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee EJ, Kim TW, Weinreb RN, Park KH, Kim SH, Kim DM. Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2011; 152: 87–95 e81. [DOI] [PubMed] [Google Scholar]
- 23. Girard MJ, Strouthidis NG, Ethier CR, Mari JM. Shadow removal and contrast enhancement in optical coherence tomography images of the human optic nerve head. Invest Ophthalmol Vis Sci. 2011; 52: 7738–7748. [DOI] [PubMed] [Google Scholar]
- 24. Mari JM, Strouthidis NG, Park SC, Girard MJ. Enhancement of lamina cribrosa visibility in optical coherence tomography images using adaptive compensation. Invest Ophthalmol Vis Sci. 2013; 54: 2238–2247. [DOI] [PubMed] [Google Scholar]
- 25. Strouthidis NG, Yang H, Downs JC, Burgoyne CF. Comparison of clinical and three-dimensional histomorphometric optic disc margin anatomy. Invest Ophthalmol Vis Sci. 2009; 50: 2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Downs JC, Yang H, Girkin C, et al. Three-dimensional histomorphometry of the normal and early glaucomatous monkey optic nerve head: neural canal and subarachnoid space architecture. Invest Ophthalmol Vis Sci. 2007; 48: 3195–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fleiss JL. The Design and Analysis of Clinical Experiments. New York: John Wiley Sons; 1986. [Google Scholar]
- 28. See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009; 116: 840–847. [DOI] [PubMed] [Google Scholar]
- 29. Fazio MA, Grytz R, Morris JS, et al. Age-related changes in human peripapillary scleral strain. Biomech Model Mechanobiol. 2014; 13: 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossou E. Abegao Pinto L, Vandewalle E, Cassiman C, Willekens K, Stalmans I. Choroidal thickness of the papillomacular region in young healthy individuals. Ophthalmologica. 2014; 232: 97–101. [DOI] [PubMed] [Google Scholar]
- 31. Brown JS, Flitcroft DI, Ying GS, et al. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009; 50: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee SW, Yu SY, Seo KH, Kim ES, Kwak HW. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy Korean subjects. Retina. 2014; 34: 385–393. [DOI] [PubMed] [Google Scholar]
- 33. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 261–266. [DOI] [PubMed] [Google Scholar]
- 34. Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012; 53: 2300–2307. [DOI] [PubMed] [Google Scholar]