Abstract
Sympathetic Activation and Atrial Fibrillation.
Background
Chronic left ventricular myocardial infarction (LVMI) promotes atrial and pulmonary veins (PV) sympathetic nerve sprouting.
Objectives
To test the hypothesis that sympathetic stimulation with tyramine initiates atrial fibrillation (AF) by early afterdepolarization (EAD)-mediated triggered activity at the left atrial PV (LAPV) junction.
Methods
LVMI was created in 6 dogs and 6 dogs served as controls. Six to 8 weeks later the activation pattern of the isolated LAPV was optically mapped using dual voltage and intracellular Ca+2 (Cai2+)-sensitive epifluorescent dyes before and after tyramine (5 μM) perfusion.
Results
Tyramine initiated spontaneous AF in 5 of 6 atria but none in the control group (P < 0.01). The AF was initiated by late phase 3 EAD-mediated triggered activity that arose from the LAPV junction causing functional conduction block in LA, reentry, and AF. The AF was subsequently maintained by mixed reentrant and focal mechanisms. The EADs arose during the late phase 3, when the Cai2+ level was 64 ± 12% of the peak systolic Cai2+ transient amplitude, a property caused by tyramine's simultaneous shortening of the action potential duration and lengthening of the Cai2+ transient duration in the LVMI group but not in the control. Tyrosine hydroxylase and growth associated protein 43 positive nerve sprouts were significantly increased in the sinus node, LAA, and the LSPV in the LVMI group compared to control (P < 0.01).
Conclusions
Increased atrial sympathetic nerve sprouts after LVMI makes the LAPV junction susceptible to late phase 3 EAD-mediated triggered and AF during sympathetic stimulation with tyramine.
Keywords: atrial fibrillation, calcium transients, early afterdepolarization, myocardial infarction, optical mapping, pulmonary veins, sympathetic nerve sprouting, triggered activity, tyramine
Introduction
We have previously shown that chronic canine left ventricular myocardial infarction (LVMI) limited exclusively to the left ventricle causes an intense increase in atrial tyro-sine hydroxylase (TH) positive nerve endings1 a marker of sympathetic nerve sprouting.2,3 The potential role of these atrial sympathetic nerve sprouts in initiating spontaneous atrial fibrillation (AF; “adrenergic AF”), however, remains undefined. Adrenergic AF may be initiated by a focal firing from the left atrial pulmonary vein (LAPV) junction4,5 when the terminal repolarization phase of the action potential (AP) coincides in time with elevated cytosolic intracellular Ca+2 (Cai2+) levels.6 Such a condition is achieved when simultaneous AP duration (APD) shortening and Cai2+ transient duration prolongation develops promoting early after-depolarization (EAD)-mediated triggered activity and AF.6,7 Failed cardiac myocytes manifest slowed sarcoplasmic Cai2+ uptake rates causing Cai2+ transient duration prolongation,8 which when associated with an increase in sympathetic nerve activity shortens the APD,9,10 a functional substrate for triggering AF emerges by the EAD-mediated triggered mechanism. Low concentration of tyramine is known to activate the sympathetic neurons by rapidly releasing norepinephrine from their nerve endings.11 This indirectly acting sympathomimetic agent is found to be highly effective in assessing the functional integrity of the sympathetic nerve terminals in normal12 and in remodeled hearts caused by myocardial ischemia.13 Consequently, the first aim of this study was to determine if tyramine triggers AF in atria with intense sympathetic nerve sprouts. Determination of the mechanism and the site of the AF trigger, hypothesized to be caused by EAD-mediated triggered activity at the LAPV junction,7, 14-16 constitutes the second aim of this study.
Methods and Materials
Surgical Preparation
This study protocol was approved by the Institutional Animal Care and Use Committee and followed the guidelines of the American Heart Association. Twelve mongrel dogs of either sex, weighing 20–25 kg were studied (USDA-registered class A dealer). In 6 dogs, myocardial infarction was created by occluding the first diagonal branch as described before (LVMI group).1 Six dogs with thoracotomy but no left anterior descending coronary artery (LAD) occlusion (sham-operated) served as controls (Control group). Six to 8 weeks after the surgery, the hearts in both groups were isolated and perfused with Tyrode's solution for in vitro studies.
Isolated Atrial Tissue Preparations
The model is a modified version of our previously described isolated-perfused right and left atrial tissues.17 After infusion of 5,000 IU heparin, the whole heart and lungs were isolated and immediately immersed in the cold oxygenated Tyrode's solution (in mmol/L: 125.0 NaCl, 4.5 KCl, 0.5 MgCl2, 1.3 CaCl2, 1.8 NaH2PO4, 24.0 NaHCO3, and 5.5 glucose with 50 mg/L albumin; pH 7.35). The orifices of right coronary artery and left circumflex artery were cannulated with 6 Fr catheters and continuously perfused with warm (37 °C) oxygenated Tyrode's solution. The ventricles were cut and removed and all the cut arteries were occluded and cauterized. Part of the proximal lungs near the LAPV junction was preserved so to protect the integrity of this presumed arrhythmogenic site.6,15,18,19 Two bipolar electrodes (interelectrode distance 0.5 mm) were placed one on the LAPV junction and the second on the left atrial appendage (LAA) 1.5 cm away from the junction. Two widely spaced electrodes were positioned on the left and right atrium to record global atrial activity (pseudo-ECG). Finally, 2 coil electrodes 4 cm long were placed in the tissue bath 0.5 cm away from the left and right atria to deliver electrical shock to cardiovert sustained (>3 minutes) AF.
Simultaneous Dual Voltage-Cai2+ Optical Mapping
The isolated atrial tissues were simultaneously stained with voltage- (RH-237) and calcium-sensitive (rhod 2 AM) dyes (V-Cai2+) delivered by arterial perfusion.20,21 The double-stained atria were excited with a solid-state laser (532 nm, Verdi, Cohereht, Santa Clara, CA, USA), and V and Cai2+ fluorescence were recorded optically by a dual charge-coupled camera (CCD) cameras (CA-D1–0128T, Dalsa, Ontario, Canada) using a 690-nm long-pass filter for RH-237 and a 585 ± 20 nm filter for rhod 2. The 2 CCD cameras were carefully aligned to image the same region according to a reference grid placed in the optical field. Data were acquired at an acquisition rate of 250 frames/s. Spatial resolution was 128 × 128 pixels over a 35 × 35 mm2 area covering the LAPV junction (Fig. S1). Cytochalasin-D (10 μmol/L) was used to prevent motion artifact.20 Both snap shots of V-Cai2+ activation patterns and phase maps were used to identify focal firing and phase singularity during reentrant excitation.19,20 The influence of tyramine (5 μM),11,12,22 on the initiation of spontaneous AF was assessed for up to 40 minutes post-tyramine perfusion period.
Immunocytochemical Staining
Hearts were removed and fixed in 4% formalin for 1 hour and then stored in 70% alcohol. Five-micrometer-thick atrial tissue samples were taken from the sinus nodal, left superior pulmonary vein (LSPV), and the LAA. The samples were immunostained for TH (marker of sympathetic nerve) and for growth associated protein 43 (GAP43; marker of active nerve sprouting), and the density of nerves positive to TH and GAP43 were determined as we previously described.1,23 The entire sinus node (SN) region, the LSPV, and the LAAs were analyzed using 8–10 fields at ×20. Nerve density is expressed as the nerve area divided by the total area examined (μm2/mm2).1,23
Statistical Analysis
Paired 2-tailed Student's t-tests were used when comparing the effects of tyramine of V-Cai2+ parameters in each dog and ANOVA test was used for multiple comparisons. Significance of difference in AF incidence was determined using exact Fisher test. All results are presented as mean ± SD. P < 0.05 was considered significant.
Results
Effects of Sympathetic Nerve Activation by Tyramine on Atrial Rhythms
Isolated atria from sham-operated and LVMI groups were all in sinus rhythm at the onset of the perfusion with normal Tyrode's solution. The sinus rate, however, was significantly faster in the atria isolated from dogs with chronic LVMI compared to normal dogs both before and after tyra-mine (5 μM) perfusion (Table 1). Tyramine promoted sustained AF in 5 of 6 dogs in the LVMI group 16 ± 6 minutes after the onset of its perfusion. The mean cycle length (CL) of the AF was 108 ± 18 milliseconds (Fig. 1). In 3 of these 5 hearts, AF was cardioverted by electrical shocks after 4–5 minutes of AF onset, respectively. In the remaining 2 dogs, the AF terminated spontaneously after 2 minutes and 90 seconds of AF onset. AF reoccurred in all 5 dogs (2–3 episodes in each isolated atria) for a total of 13 AF episodes during the entire 40 minutes posttyramine study period. Figure 1 shows atrial electrograms 2 seconds after the onset of AF. The electrogram at the LAPV junction had a mean CL of activation 92 ± 14 milliseconds, significantly (P < 0.05) shorter than the mean CL of the AF. The bipolar electrogram at the LAPV junction often showed double potentials with intermittent conduction block to the adjoining LAA, a characteristic feature that results from the faster activation rates in the pulmonary veins (PV) and the complex anatomy at the LAPV junction.24 In none of the 6 sham-operated atria (Control) did tyramine (5 μM) perfusion initiate spontaneous AF for up to 1 hour of observation. To refute the possibility that tyramine-induced faster intrinsic sinus rates in the atria isolated from the LVMI group compared to sham-operated atria (Table 1) may be the cause of AF in the LVMI atria, we paced the sham-operated atria at rates similar (150 beats/min) or even higher (200 beats/min) than the intrinsic rates of LVMI atria after tyramine perfusion. No AF could be initiated with tyramine in any of the sham-operated atria during these relatively faster paced heart rates.
TABLE 1.
Effects of Tyramine on Heart Rate, AP and Cai2+ Duration
| Hear Rate | APD90 | CaD90 | |
|---|---|---|---|
| ControlBaseline | 90 ± 10 | 118 ± 16 | 110 ± 12 |
| ControlTyramine | 94 ± 12 | 116 ± 18 | 112 ± 16 |
| LVMlBaseline | 102 ± 4 | 122 ± 22 | 130 ± 15** |
| LVMlTyramine | 140 ± 3* | 90 ± 14* | 176 ± 18* |
P < 0.01 compared to baseline LVMI, ControlBaseline and ControlTyramine.
P < 0.05 compared to ControlBaseline and ControlTyramine.
Heart rate in beats/min; APD90 and CaD90 are action potential; Cai2+ transient durations to 90% relaxation respectively during pacing at a CL of 200 milliseconds.
All values are means ± SD.
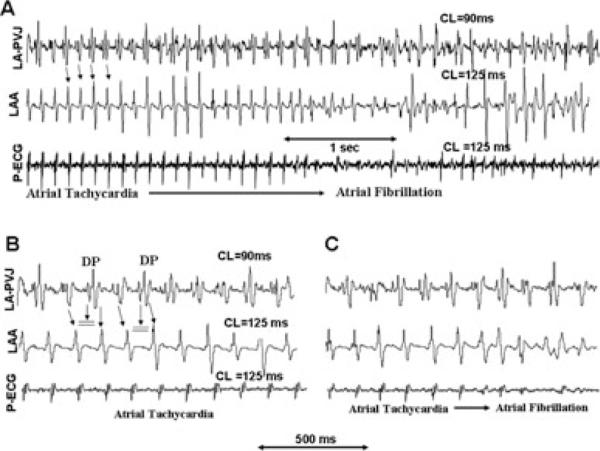
Figure 1.
Electrocardiographic recordings during tyramine-induced atrial tachycardia (AT) that degenerates to AF in a dog with chronic LVMI. Panel A shows that during the AF firing at the left atrial appendage (LAA)-LSPV junction with an average cycle length (CL) of 90 milliseconds is faster than the adjoining LAA. The rapid firing from the junction propagates (downward pointing arrows) to the LAA activating the LAA at a CL of 125 milliseconds with similar CL of global activation as recorded by the pseudo-ECG. Panel B is a faster sweep recording at the onset of the rapid pulmonary vein firing shown in panel A. Notice the presence of double potentials in LAA-LSPV bipolar electrogram separated by an interval of about 30 milliseconds with alternate failure to propagate to LA (double horizontal lines). Panel C is faster sweep recordings showing the last 4 beats of the AT that degenerates to AF.
Activation Maps at the Onset of Spontaneous AF
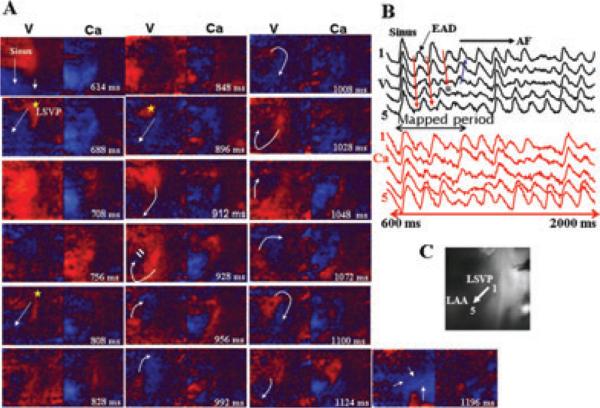
We captured by our optical mapping system the onset of 3 episodes of spontaneous AF that arose suddenly during the sinus rhythm allowing us to analyze in detail the wavefront dynamics associated with the transition of sinus rhythm to AF. Figure 2 illustrates a spontaneous episode of AF initiated by an EAD-mediated triggered focal activity that arises from the LSPV 74 milliseconds after the last sinus beat and propagates to the LAA appendage (Fig. 2A). After 124 milliseconds another focal activity arises from the same site, which is followed after 88 milliseconds by a third focal beat (Fig. 2A). The third focal beat undergoes conduction block as it propagates to the recovering LAA (Fig. 2A, frame 928 milliseconds). The block leads to the formation of 2 consecutive clockwise reentrant wavefronts over the LAA (CLs of 100 milliseconds), which then becomes irregular signaling the onset of the AF (snap shot 1,196 milliseconds in Fig. 2A). The onset of the EAD at the site of its origination near the LSVP (Fig. 2B, site 1) had an underlying Cai2+ that was 65% of the peak systolic Cai2+ transient amplitude. However, as the triggered EADs propagated toward the LAA (i.e., from site 1 to site 5, Fig. 2C), the underlying Cai2+ were progressively decreased to <10% of the peak systolic Cai2+ transient amplitude (site #5, Fig. 2B,C).
Figure 2.
Dual V-Cai2+ optical snap shots at the onset of spontaneous initiation of AF in an atrium isolated from a dog with chronic LVMI and perfused with tyramine (5 μM). Panel A shows snap shots of the last sinus beat followed by 3 focal activations (yellow asterisk) originating from the LSPV. The third activation undergoes wavebreak (frame 928 milliseconds) leading to 2 full clockwise reentrant activation wavefronts with subsequent emergence of irregular activation wavefronts (snap shot 1,196 milliseconds) indicative of AF. (The time during the first sinus beat is arbitrarily chosen as 600 milliseconds). White arrows in panel A indicate the direction of propagation. Panel B shows 5 consecutive simultaneous optical V-Cai2+ recordings from sites numbered 1-5 and shown on the atrial silhouette in panel C. The black double-headed arrow in panel B shows the mapped period, in panel A and the 3 downward pointing red arrows indicate the direction of the propagating EAD-mediated triggered beats. The double horizontal blue lines indicate the site of the block (also shown in Fig. 2A, frame 928 milliseconds), which is then followed by propagation in a direction opposite to the direction of the previous two triggered beats (upward pointing blue arrow in Fig. 2B). Notice the shorter optical APD of the last sinus beat (panel B, V, sites 1-5) relative to its underlying Cai2+ transient duration (panel B, Ca, site 1-5).
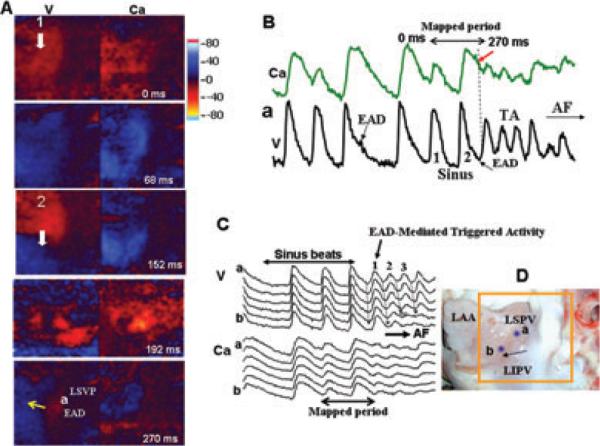
Figure 3A illustrates another spontaneous episode of AF initiated by an early afterdepolarization (EAD)-mediated triggered activity arising from the LSVP-LAA junction (Fig. 3B). The first 2 triggered beats propagate uninterrupted toward the LAA (Fig. 3C); however, the third beat undergoes conduction block over the LAA signaling the onset of AF (Fig. 3C). Figure 3D shows the location of the optical signals shown in Figure 3B,C. The onset of the EAD at the site of its origination (site a close to LSVP, Fig. 3D) had an underlying Cai2+ level that was 62% of the peak systolic Cai2+ transient amplitude (Fig. 3B,C). As the distance from the LSVP increased (sites away from site a toward site b, Fig. 3C,D), the underlying Cai2+ level in the LAA of the triggered beats progressively decreased reaching to 10–25% of the peak systolic Cai2+ transient amplitude. The third spontaneous tyramine-induced AF episode that we captured and analyzed had comparable wavefront dynamics as the AF episode shown in Figure 2.
Figure 3.
Dual V-Cai2+ optical snap shots at the onset of spontaneous AF initiated with tyramine (5 μM) in an atrium isolated from a dog with chronic LVMI. Panel A shows dual V-Cai2+ snap shots during the last 2 sinus beats (beats #1 and 2) that propagate over the left atrial appendage (LAA). A focal activity originates 78 milliseconds after the second sinus beat from the LSPV (a in panels A and D) triggering the onset of AF shown in panel B and C. The time during the first sinus beat is arbitrarily chosen as 0 milliseconds and the white arrows indicate the direction of propagation. Panel B shows simultaneous dual V-Cai2+ signals at pixel labeled a located at the LSVP (shown in panel C). Notice that initially the site a manifests a small (subthreshold) EAD that fails to trigger (downward pointing black arrow, in panel B); however, 3 beats later the late EAD (upward pointing black arrow, sinus beat #2 in panel B) succeeds in causing triggered activity that degenerates to AF. Notice the longer duration of the Ca transient signal compared to the optical action potentials both in panels B and C and that the origination of the EAD (upward pointing black arrow in B) occurs when the underlying Cai2+ level is 75% of the peak systolic calcium transient amplitude (dashed vertical line and downward pointing red arrow in panel B). Panel C shows 5 consecutive simultaneous V-Cai2+ recordings (labeled a to b) located at sites shown in panel D. Notice that V-Cai2+ activation during the sinus rhythm is almost simultaneous (double headed arrow top of panel C) which then becomes sequential from a to b (downward pointing black arrows in panel C and labeled 1 to 3) as the focal activity originating from the LSPV and propagates toward the LAA. The 2 double-headed black at the bottom of C indicate the mapped period shown in panel A.
Activation Maps During AF
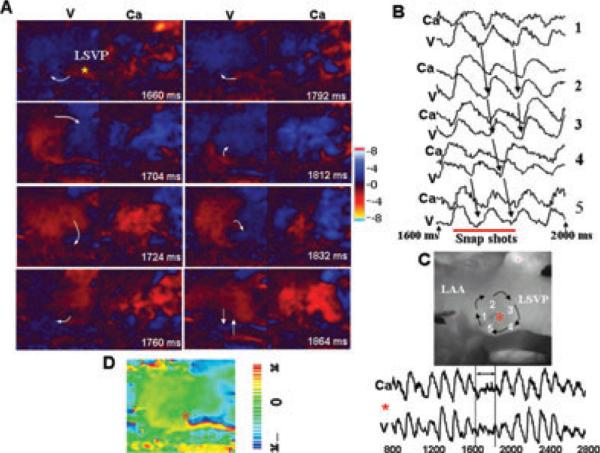
We analyzed 8 episodes of AF 10 seconds after its onset by optical mapping of activation pattern over the LAPV junction. The onset of spontaneous AF was characterized by an initial focal activity arising from the LSVP followed by reentry over the LAPV junction and the LAA. During the AF we found that both reentrant and focal activity arising from the LSPV and the left inferior pulmonary vein (LIPV) remain active contributing to the maintenance of AF. Figure 4A illustrates an example of an ongoing AF episode with a focal activity arising from the LSPV, which leads to a complete clockwise reentrant excitation over the LAA at a CL of 104 milliseconds. However, upon the second rotation the reentrant wavefront encounters another wavefront that enters the mapping field from the bottom causing collision and coalescence of the 2 wavefronts (Fig. 4A frame 1,864). Notice that the APD was shorter than the underlying Cai2+ transient duration as shown in Figure 4B (sites 1–5, locations shown in Fig. 4C). A plot of the voltage phase map during the first reentrant excitation shows a singularity point (core of the reentry) (Fig. 4D) that was located at the LSVP-LAA junction also shown in Figure 4C. During the AF, focal activity also arose from the LIPV as shown in Figure 4E. The focal activity originating from the LIPV collided and coalesced with another wavefront entering the mapped region from the opposite direction (Fig. 4E, frame 32 milliseconds). During the recovery and 104 milliseconds after the collision-coalescence another focal activity suddenly arises from the same site in the LIPV, which undergoes conduction block at the junction of the LIPV and LAA (Fig. 4, frame 136 milliseconds) causing a transient period of quiescence (Fig. 4E, frame 172). The mapped field was then invaded 16 milliseconds later by 2 wavefronts entering the mapped field from the top and the bottom (Fig. 4E, frame 188 milliseconds). Mixed focal and reentrant activation wavefronts were seen during the maintenance of AF in all other 8 episodes analyzed.
Figure 4.
Activation pattern during the maintenance of tyramine-induced AF. Panel A shows 2 consecutive clockwise rotating reentrant wavefront around the LSVP (onset time arbitrarily chosen as 1,660 milliseconds to reflects data acquired during the 1,600 milliseconds to 2,000 milliseconds shown in panel B). During the third rotation a wavefront enters from the bottom of the mapped field (frame 1,864 milliseconds) disrupting the free rotation of the reentrant wavefront (the white arrows indicate the direction of propagation). Panel B shows 5 simultaneous V-Cai2+ optical recordings from sites 1 to 5 indicated on the atrial silhouette shown in panel C. The double-headed red arrow indicates the mapped period shown in panel A. The red line in panel B shows the mapped period shown in panel A and the downward black arrows show the sequence of activation during the reentry. The simultaneous dual V-Cai2+ beneath the panel C shows that when the core of the reentry visits the pixel identified with a red asterisk, a sudden low level voltage and Cai2+ oscillations emerge (i.e., the core of the functional reentry). Panel D is an actual voltage phase map of the reentry indicating that the red asterisk showing in panel C correspond to the point of phase singularity (red asterisk in panel D). Panel E shows snap shots of simultaneous V-Cai2+ activation dynamics 10 seconds after the onset of tyramine-mediated AF. Two consecutive focal activations arise from the left inferior pulmonary vein (LIPV, frames 20 milliseconds and 136 milliseconds marked with an asterisk) and propagate in the direction of recovering tissue toward the LAA. The front of the first focal activation suddenly encounters an incoming front from the opposite direction (frame 32 milliseconds) resulting in collision and coalescence (frame 44 milliseconds) resulting in the depolarization of virtually the entire mapped surface (frame 60 milliseconds). The second focal activity arises from the same site (LIPV, frame 136 milliseconds) and propagates toward the recovering LAA. The front, however, undergoes conduction block (frame 136 milliseconds, double vertical line) resulting in a transient period of quiescence (frame 172). Within 16 milliseconds however, 2 wavefronts enter the mapped region one from the top and one from the bottom (frame 188 milliseconds). The presence of firing foci from (LSPV and LIPV) and reentry indicate that both reentrant and focal mechanisms contribute to the maintenance of AF. The bottom tracing is a bipolar electrogram from the LAA and the double-headed red arrow indicates the mapped period.
Effects of Tyramine on Cai2+ and AP
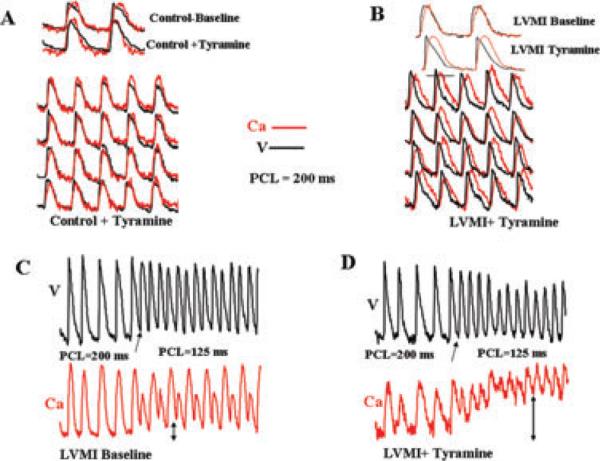
There were no significant differences between the durations of the atrial APs and the Cai2+ transient in the sham-operated group either at baseline or after tyramine (Fig. 5 and Table 1). However, in the LVMI group the baseline atrial Cai2+ transient duration was significantly longer than the sham-operated Control group, however the APD remained unchanged (Table 1). Tyramine perfusion further prolonged the Cai2+ transient duration but at the same time causing a significant shortening of the APD (Fig. 5B and Table 1). The disparate effects of tyramine on the Cai2+ transient duration and the APD caused the Cai2+ transient duration to significantly (P < 0.01) outlast the APD, i.e., 62 ± 14 versus 8 ± 4 milliseconds (Figs. 2–5 and Table 1).
Figure 5.
Dual optical and Cai2+ transient recordings during pacing in Control (A) and LVMI (B) atria at baseline and after tyramine perfusion. Notice the differential effect of tyramine on the APD (shortening) and Cai2+ transient duration (lengthening) by tyramine in the LVMI but not in Control (sham-operated) atria. Panel C shows that further acceleration of the pacing rate from 200 to 125 milliseconds promotes considerable elevation of the diastolic Cai2+ levels after tyramine in the atria of LVMI dog compared to baseline, 90% versus 10% at baseline (compare double-headed vertical arrows in panels C and D).
Atrial nerve sprouting
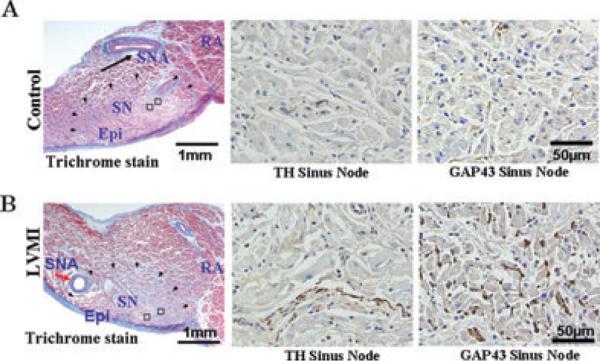
The density of TH- and GAP43-positive nerve were significantly increased in the SN, the LSPV, and the LAA in the atria isolated from LVMI dogs (Table 2 and Fig. 6), consistent with our previous report.1 As in our earlier findings, the density of the GAP43 was significantly (P < 0.01) higher than the density of TH-positive nerves (Table 2 and Fig. 6).
TABLE 2.
TH and GAP43 Positive Staining in Sinus Node and LSVP
| Control | LVMI | |
|---|---|---|
| Sinus node (TH) | 1,100 ± 250 | 5,200 ± 2,500* |
| Sinus node (GAP43) | 5,100 ± 2,200 | 17,500 ± 4,000* |
| LSVP (TH) | 210 ± 20 | 1,300 ± 120* |
| LSVP (GAP43) | 850 ± 200 | 5,500 ± 500* |
| LAA (TH) | 600 ± 100 | 4,300 ± 1,200* |
| LAA (GAP43) | 1,800 ± 300 | 7,500 ± 2,000* |
P < 0.01. All values are in μ2/mm2;
TH = tyrosine hydroxylase; GAP43 = growth associated protein43;
LSVP = left superior pulmonary vein; LAA = left atrial appendage.
Figure 6.
Tyrosine hydroxylase (TH) and growth associated protein43 (GAP43)-positive staining nerves. Panels A and B are in the sinus node region and panels C and D are in the LSPV and the LAA. The open squares indicate the regions of immuno-stained histological sections shown to the right of each panel. Notice the considerable increase in both TH and GAP43 positive nerves in atria isolated from dogs with chronic LVMI compared to atria isolated from sham-operated dogs at all 3 sites, sinus node (panel A and B), the LSVP and the LAA (panels C and D). Epi epicardium; RA right atrium; SN = sinus node; SNA = sinus node artery.
Discussion
Major Findings
To our knowledge this is the first demonstration of spontaneous AF initiation by a focal firing from the LSPV and LAPV junction in response to sympathetic activation with tyramine in atria with increased sympathetic nerve sprouting caused by chronic LVMI. Tyramine-mediated AF via release of norepinephrine from the sympathetic nerve endings13,22 indicates that the TH-positive nerve endings in neurally remodeled atria by chronic LVMI respond to sympathetic stimulation as do mature functional sympathetic nerve endings.13,22 Importantly, upon activation of these nerve endings EAD-mediated triggered and AF emerges perhaps mimicking “adrenergic AF.”4,5,15, 25-28 The functional integrity of the sympathetic nerve sprouts is further emphasized by the significantly greater acceleration of the sinus nodal rate in response to tyramine in the atria with sympathetic nerve sprouts compared to normally innervated (Control) atria (Table 1).
We do not know the mechanism(s) by which the nerves in the atria sprout in response to an evolving isolated LVMI with no atrial infarction. It is likely that the LVMI-induced increase in the level of circulating nerve growth factor29,30 perhaps combined with the LVMI-mediated atrial stretch31 contribute to the increased atrial nerve sprouting.
Mechanisms of Tyramine-Mediated AF
Although sympathetic hyperinneravtion was diffusely present in the LSPV and the LAA, the focal EAD-mediated triggered activity preferentially arose from the LAPV junction (namely the LSPV and LIPV) and not the LAA. This suggests that in addition to the increased sympathetic nerve sprouts the local anatomical and electrophysiological features at the LAPV junction may play an important role in the genesis of EADs and EAD-mediated triggered activity. Our extensive previous quantitative histoanatomical analyses of the canine LAPV junction showed the presence of considerable muscle bundle narrowing as the PVs connect to the left atrial (LA)24 along with complex fiber orientation at the LAPV junction.24,32 These anatomical features alter PV-LA conduction pattern including conduction block with the characteristic emergence of double potentials on the bipolar electrograms at the LAPV junction as seen in this and our previous study.24 The influence of selective narrowing of the PV atrial muscle bundles at the LAPV junction down to ~250 μM,24 however, does not alter only the conduction between the PV and the LA, but also overcomes the robust protective effects of the source-to-sink mismatch typically seen in well-coupled tissues. Such an anatomical feature (narrowing of the muscle bundles in the PV) favors the emergence and the propagation of EADs as the sink (repolarizing) effect diminishes at this junction. Under well-coupled conditions as soon as the APs of affected cells generate EADs, electrotonic current flows into the EAD-generating myocytes through the gap junctions from neighboring cells normally repolarizing myocytes suppressing the EADs. If the majority of the neighboring cells are not predisposed to having an EAD on the same beat, the majority will prevail, forcing the EAD-susceptible myocyte to repolarize along with its unsusceptible neighbors preventing triggered activity.33 Our simulation studies showed that as the electrical sink imposed on the EAD generating cells decreases, the number of contiguous cells needed to generate a propagating triggered beats decreases.34 The narrowing of the muscle bundle at the PV-LA junction down to ~250 μM successfully provides this platform (“first hit”),34 which, when combined with the sympathetic nerve stimulation at this junction (“second hit”), promotes EADs and rapid triggered activity that propagates to the LAA causing AF. In addition to complex anatomical factors, the possible distinct electrophysiological feature of the PV cells may also contribute to the genesis of focal activity. For example, the presence of periodic acid-Schiff-positive cells in the PVs (“Purkinje-like” cells) with their inherent potential to act like nodal pacemaker cells may also promote focal discharge by the PV muscle cells.19
The Cellular Mechanisms of EAD Formation in the Remodeled Atria
Sympathetic stimulation is known to shorten the APD,9,10 increase Cai2+ transient amplitude, and also accelerate Cai2+ uptake by the sarcoplasmic reticulum. Although, in this study we have seen a considerable decrease of the APD in the remodeled atria upon sympathetic stimulation with tyramine, the Cai2+ transient duration, however, lengthened instead of shortening (Figs. 2–5 and Table 1). We do not know the mechanism of this “paradoxical” lengthening of the Cai2+ duration with tyramine-mediated sympathetic stimulation. The significantly longer Cai2+ transient duration in the chronic LVMI remodeled atria at baseline compared to sham-operated atria (Table 1) suggests deficient calcium uptake by the sarcoplasmic/endoplasmic reticulum Cai2+-ATPase2a (SERCA2a) pump.35 It is also possible that the apparent Cai2+ affinity of the Ca pump may be reduced, as in the failing hearts, because of an increased expression or reduced phosphorylation of phospholamban and its homo-logue sarcolipin, thus inhibiting the SERAC2a pump.36 Furthermore, increased sympathetic stimulation increases Cai2+ entry into the cell via the L-type Ca channels (ICa−L) causing greater Cai2+ entry with each beat eventually leading to Cai2+ accumulation and subsequent lengthening of the Cai2+ transient duration. More work is needed to clarify this point. Nevertheless, by whatever mechanism(s), the combined lengthening of the Cai2+ transient duration and shortening of the APD is known to favor EAD formation in the PVs. Patterson et al. have shown in canine PVs that increasing the duration of contractile force, a surrogate of Cai2+ transient duration, promotes an inward depolarizing current via the activation of the sodium-calcium exchanger current that in cells with shortened APD helps the reactivation of ICa−L causing EAD-mediated triggered activity.6,7,37. Similar cellular mechanism of EAD-mediated triggered activity has also been suggested to be operative in remodeled ventricles as well.38,39 Consistent with our present findings, the recent studies using direct nerve recordings from ambulatory dogs with remodeled atria showed simultaneous sympathovagal discharge just before the onset of AF.40 The combined sympathovagal activation may cause simultaneous shortening of the APD (vagal effect) and the lengthening of the Cai2+ transient duration (sympathetic activation of ICa-L). Indeed, stimulation of the isolated LAPV tissues with combined norepinephrine + acetyl choline challenge mimicking sympatho-vagal activation is shown to shorten the APD and lengthen the Cai2+ transient duration promoting EAD-mediated triggered activity and AF.6 The pioneering findings of simultaneous sympathovagal discharge by direct nerve recording in the canine model provided strong mechanistic evidence of previous clinical studies showing a primary increase in the adrenergic tone followed by an abrupt shift toward vagal predominance just before the onset of human AF.41
From EAD-Mediated Triggered Activity to Reentry and AF Maintenance
We previously have shown in this canine model that pacing the atria at CLs <120, i.e., at CLs similar to the intrinsic rates of EAD-mediated triggered activity at the LSPV and LIPV, leads to conduction block and reentry over the LAA leading to AF.1 The interaction between the focal firing and reentry seen at the onset of the AF was also present during the maintenance of AF. This interaction seems to provide a positive feedback mechanism that sustains the AF. The rapid reentrant atrial activations maintains the diastolic Cai2+ elevated favoring the genesis of EAD-mediated triggered activity that in turn leads to reentry (positive feedback). This positive feedback mechanism (triggered activity-reentry-triggered activity) seems to maintain the AF for long duration often requiring electrical shock for termination.
Clinical Impact
It has been shown that tyramine releases norepinephrine in human hearts42-45 and consistent with our findings case reports have shown that tyramine promotes AF in patients with isolated chronic LVMI.46,47 It is therefore tempting to suggest that the mechanistic dynamic scenario seen with tyramine-mediated initiation and maintenance of AF in our model may also be applicable in neurally remodeled human atria with a positive feedback mechanism between triggered activity and reentry. In fact, focal firing from LAPV junction with subsequent formation of reentry in the adjoining LA has been demonstrated in human patients with paroxysmal AF.7 Furthermore, the demonstration of increased atrial sympathetic nerve sprouts in human atria with a history of paroxysmal48 and persistent49 AF, and the high efficacy of LAPV junctional ablation in preventing the recurrences of paroxysmal AF in humans50,51 suggest a causal association between the sympathetic activation and the initiation of paroxysmal AF in man by an EAD-mediated triggered activity from LAPV junction.
Limitations
It may be argued that the electrical remodeling that develops in our model may lead to reentry that may act as a trigger for AF instead of EAD-mediated triggered activity. However, our previous studies have shown that wavebreak and reentry in this model develops only at CLs <120 milliseconds1 and the shortest intrinsic sinus CL in our isolated perfused atria was longer than 400 milliseconds making wavebreak and reentry unlikely trigger for AF. The unpredictable occur-rences of spontaneous AF prevented us to image the onset of every AF episode with our optical mapping system. It is possible that some of those missed AF episodes could have originated from sites other than the LAPV junction. However, the uniformly faster rates of activation seen on the bipolar electrograms recorded from the LAPV junction compared to the adjoining LAA indicate that the focal firing form LAPV junction acts as a trigger for AF. Finally, it may be argued that tyramine's lack of efficacy in control atria could result from tachyphylaxis (“acute tolerance”) because of tyramine-induced depletion of norepinephrine from the sympathetic nerve endings. However, the presence of positive response in the neurally remodeled atria within 20 minutes, a period long before tachyphylaxis develops52 makes this an unlikely event.
Supplementary Material
Acknowledgments
Supported in part by the American Heart Association Western States Affiliate (0555057Y, Grant-in-Aid), NIH Grants P01 HL078931, R01 HL103662, the Laubisch, Kawata, Medtronic-Zipes Endowments and the Electrocardiographic Heart Beat Organization, Los Angeles, CA. Dr. Chen reports support in the form of research equipment from Medtronic, Cryocath, and St. Jude Medical. Other authors: No disclosures.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Miyauchi Y, Zhou S, Okuyama Y, Miyauchi M, Hayashi H, Hamabe A, Fishbein MC, Mandel WJ, Chen LS, Chen PS, Karagueuzian HS. Altered atrial electrical restitution and heterogeneous sympathetic hyper-innervation in hearts with chronic left ventricular myocardial infarction: Implications for atrial fibrillation. Circulation. 2003;108:360–366. doi: 10.1161/01.CIR.0000080327.32573.7C. [DOI] [PubMed] [Google Scholar]
- 2.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 3.Zhou S, Cao JM, Swissa M, Gonzalez-Gomez I, Chang CM, Chien K, Miyauchi Y, Fu KJ, Yi J, Asotra K, Karagueuzian HS, Fishbein MC, Chen PS, Chen LS. Low-affinity nerve growth factor receptor p75NTR immunoreactivity in the myocardium with sympathetic hyperinnervation. J Cardiovasc Electrophysiol. 2004;15:430–437. doi: 10.1046/j.1540-8167.2004.03517.x. [DOI] [PubMed] [Google Scholar]
- 4.Doshi RN, Wu TJ, Yashima M, Kim YH, Ong JJC, Cao JM, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen PS. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 5.Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: Implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 6.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: An arrhythmia trigger within pulmonary veins 1. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Patterson E, Jackman WM, Beckman KJ, Lazzara R, Lockwood D, Scherlag BJ, Wu R, Po S. Spontaneous pulmonary vein firing in man: Relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J Cardiovasc Electrophysiol. 2007;18:1067–1075. doi: 10.1111/j.1540-8167.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 8.Ng GA, Cobbe SM, Smith GL. Non-uniform prolongation of intracellular Ca2+ transients recorded from the epicardial surface of isolated hearts from rabbits with heart failure. Cardiovasc Res. 1998;37:489–502. doi: 10.1016/s0008-6363(97)00255-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: Role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 10.Ng GA, Mantravadi R, Walker WH, Ortin WG, Choi BR, de Groat W, Salama G. Sympathetic nerve stimulation produces spatial heterogeneities of action potential restitution. Heart Rhythm. 2009;6:696–706. doi: 10.1016/j.hrthm.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Burke MN, McGinn AL, Homans DC, Christensen BV, Kubo SH, Wilson RF. Evidence for functional sympathetic reinnervation of left ventricle and coronary arteries after orthotopic cardiac transplantation in humans. Circulation. 1995;91:72–78. doi: 10.1161/01.cir.91.1.72. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki T, Akiyama T, Kitagawa H, Takauchi Y, Kawada T, Sunagawa K. A new, concise dialysis approach to assessment of cardiac sympathetic nerve terminal abnormalities. Am J Physiol. 1997;272:H1182–H1187. doi: 10.1152/ajpheart.1997.272.3.H1182. [DOI] [PubMed] [Google Scholar]
- 13.Lameris TW, de Zeeuw S, Alberts G, Boomsma F, Duncker DJ, Verdouw PD, Veld AJ, van Den Meiracker AH. Time course and mechanism of myocardial catecholamine release during transient ischemia in vivo. Circulation. 2000;101:2645–2650. doi: 10.1161/01.cir.101.22.2645. [DOI] [PubMed] [Google Scholar]
- 14.Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P, Chang MS, Lin CI. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: Implication in initiation of atrial fibrillation. Circulation. 2001;104:2849–2854. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- 15.Miyauchi Y, Hayashi H, Miyauchi M, Okuyama Y, Mandel WJ, Chen PS, Karagueuzian HS. Heterogeneous pulmonary vein myocardial cell repolarization implications for reentry and triggered activity. Heart Rhythm. 2005;2:1339–1345. doi: 10.1016/j.hrthm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Nattel S. Basic electrophysiology of the pulmonary veins and their role in atrial fibrillation: Precipitators, perpetuators, and perplexers. J Cardiovasc Electrophysiol. 2003;14:1372–1375. doi: 10.1046/j.1540-8167.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawase A, Ikeda T, Nakazawa K, Ashihara T, Namba T, Kubota T, Sugi K, Hirai H. Widening of the excitable gap and enlargement of the core of reentry during atrial fibrillation with a pure sodium channel blocker in canine atria. Circulation. 2003;107:905–910. doi: 10.1161/01.cir.0000050148.72502.3a. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S, Chang CM, Wu TJ, Miyauchi Y, Okuyama Y, Hamabe A, Omichi C, Hayashi H, Brodsky LA, Mandel WJ, Ting CT, Fishbein MC, Karagueuzian HS, Chen PS. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2002;283:H1244–H1252. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]
- 19.Chou CC, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, Fishbein MC, Lin SF, Chen PS. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 20.Ono N, Hayashi H, Kawase A, Lin SF, Li H, Weiss JN, Chen PS, Karagueuzian H. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. Am J Physiol Heart Circ Physiol. 2007;292:639–648. doi: 10.1152/ajpheart.00445.2006. [DOI] [PubMed] [Google Scholar]
- 21.Omichi C, Lamp ST, Lin SF, Yang J, Baher A, Zhou S, Attin M, Lee MH, Karagueuzian HS, Kogan B, Qu Z, Garfinkel A, Chen PS, Weiss JN. Intracellular Ca dynamics in ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2004;286:H1836–H1844. doi: 10.1152/ajpheart.00123.2003. [DOI] [PubMed] [Google Scholar]
- 22.Lameris TW, van Den Meiracker AH, Boomsma F, Alberts G, de Zeeuw S, Duncker DJ, Verdouw PD, Veld AJ. Catecholamine handling in the porcine heart: A microdialysis approach. Am J Physiol. 1999;277:H1562–H1569. doi: 10.1152/ajpheart.1999.277.4.H1562. [DOI] [PubMed] [Google Scholar]
- 23.Chang CM, Wu TJ, Zhou SM, Doshi RN, Lee MH, Ohara T, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–25. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 24.Hamabe A, Okuyama Y, Miyauchi Y, Zhou S, Pak HN, Karagueuzian HS, Fishbein MC, Chen PS. Correlation between anatomy and electrical activation in canine pulmonary veins. Circulation. 2003;107:1550–1555. doi: 10.1161/01.cir.0000056765.97013.5e. [DOI] [PubMed] [Google Scholar]
- 25.Arora R, Verheule S, Scott L, Navarrete A, Katari V, Wilson E, Vaz D, Olgin JE. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation. 2003;107:1816–1821. doi: 10.1161/01.CIR.0000058461.86339.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayachandran JV, Sih HJ, Hanish S, Winkle W, Arnett CJ, Zipes DP, Hutchins GD, Olgin JE. Heterogeneous sympathetic innervation of the atria in atrial fibrillation–autonomic remodeling with rapid atrial rates. Pacing and Cardiac Electrophysiology. 1998;21:831–831. [Google Scholar]
- 27.Wu TJ, Ong JJC, Chang CM, Doshi RN, Yashima M, Huang HLA, Fishbein MC, Ting CT, Karagueuzian HS, Chen PS. Pulmonary veins and ligament of Marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation. 2001;103:1157–1163. doi: 10.1161/01.cir.103.8.1157. [DOI] [PubMed] [Google Scholar]
- 28.Chen PS, Wu TJ, Hwang C, Zhou S, Okuyama Y, Hamabe A, Miyauchi Y, Chang CM, Chen LS, Fishbein MC, Karagueuzian HS. Thoracic veins and the mechanisms of non-paroxysmal atrial fibrillation. Cardiovasc Res. 2002;54:295–301. doi: 10.1016/s0008-6363(01)00554-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fish-bein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 30.Govoni S, Pascale A, Amadio M, Calvillo L, D'Elia E, Cereda C, Fantucci P, Ceroni M, Vanoli E. NGF and heart: Is there a role in heart disease? Pharmacol Res. 2011;63:266–277. doi: 10.1016/j.phrs.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Rana OR, Schauerte P, Hommes D, Schwinger RH, Schroder JW, Hoffmann R, Saygili E. Mechanical stretch induces nerve sprouting in rat sympathetic neurocytes. Auton Neurosci. 2010;155:25–32. doi: 10.1016/j.autneu.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Hocini M, Ho SY, Kawara T, Linnenbank AC, Potse M, Shah D, Jais P, Janse MJ, Haissaguerre M, de Bakker JM. Electrical conduction in canine pulmonary veins: Electrophysiological and anatomic correlation. Circulation. 2002;105:2442–2448. doi: 10.1161/01.cir.0000016062.80020.11. [DOI] [PubMed] [Google Scholar]
- 33.Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early after-depolarizations and cardiac arrhythmias. Heart Rhythm. 2010;7:1891–1899. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: Requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99:1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lokuta AJ, Maertz NA, Meethal SV, Potter KT, Kamp TJ, Valdivia HH, Haworth RA. Increased nitration of sarcoplasmic reticulum Ca2+− ATPase in human heart failure. Circulation. 2005;111:988–995. doi: 10.1161/01.CIR.0000156461.81529.D7. [DOI] [PubMed] [Google Scholar]
- 36.Bhupathy P, Babu GJ, Periasamy M. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J Mol Cell Cardiol. 2007;42:903–911. doi: 10.1016/j.yjmcc.2007.03.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa M, Morita N, Tang L, Karagueuzian HS, Weiss JN, Lin SF, Chen PS. Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm. 2009;6:784–792. doi: 10.1016/j.hrthm.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita N, Lee JH, Bapat A, Fishbein MC, Mandel WJ, Chen PS, Weiss JN, Karagueuzian HS. Glycolytic inhibition causes spontaneous ventricular fibrillation in aged hearts. Am J Physiol Heart Circ Physiol. 2011;301:H180–H191. doi: 10.1152/ajpheart.00128.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 42.Gilliam LK, Palmer JP, Taborsky GJ., Jr Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J Clin Endocrinol Metab. 2007;92:4035–4038. doi: 10.1210/jc.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leis S, Drenkhahn S, Schick C, Arnolt C, Schmelz M, Birklein F, Bickel A. Catecholamine release in human skin–a microdialysis study. Exp Neurol. 2004;188:86–93. doi: 10.1016/j.expneurol.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Jacob G, Costa F, Vincent S, Robertson D, Biaggioni I. Neurovascular dissociation with paradoxical forearm vasodilation during systemic tyramine administration. Circulation. 2003;107:2475–2479. doi: 10.1161/01.CIR.0000065605.37863.C0. [DOI] [PubMed] [Google Scholar]
- 45.Forman MB, Robertson D, Goldberg M, Bostick D, Uderman H, Perry JM, Robertson RM. Effect of tyramine on myocardial catecholamine release in coronary heart disease. Am J Cardiol. 1984;53:476–480. doi: 10.1016/0002-9149(84)90015-8. [DOI] [PubMed] [Google Scholar]
- 46.Tiller JW, Dowling JT, Tung LH, Maquire KP, Rand MJ, Davies BM. Tyramine-induced cardiac arrhythmias. N Engl J Med. 1985;313:266–267. doi: 10.1056/nejm198507253130415. [DOI] [PubMed] [Google Scholar]
- 47.Jacob LH, Carron DB. Atrial fibrillation precipitated by tyramine containing foods. Br Heart J. 1987;57:205–206. doi: 10.1136/hrt.57.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen BL, Fishbein MC, Chen LS, Chen PS, Masroor S. Histopatho-logical substrate for chronic atrial fibrillation in humans. Heart Rhythm. 2009;6:454–460. doi: 10.1016/j.hrthm.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gould PA, Yii M, McLean C, Finch S, Marshall T, Lambert GW, Kaye DM. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:821–829. doi: 10.1111/j.1540-8159.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 50.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 51.Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 52.Axelrod J, Gordon E, Hertting G, Kopin IJ, Potter LT. On the mechanism of tachyphylaxis to tyramine in the isolated rat heart. Br J Pharmacol Chemother. 1962;19:56–63. doi: 10.1111/j.1476-5381.1962.tb01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.