Abstract
Interleukin-18 (IL-18) is increased along with IL-1β by activation of the inflammasome and has been implicated in inflammatory and autoimmune diseases, but its role in the eye is uncertain. In patients with macular edema due to retinal vein occlusion, intraocular IL-18 levels increased significantly (p<0.001) after treatment with ranibizumab particularly in patients with high baseline IL-18 which correlated with good visual outcome (p<0.05). In mice with ischemic retinopathy, suppression of VEGF caused an increase in IL18 mRNA due to an increase in IL-18-positive myeloid cells. VEGF significantly and specifically inhibited IL-18 production by myeloid cells stimulated with lipopolysaccharide (p<0.001). Intraocular injection of IL-18 reduced VEGF-induced leakage and neovascularization, and reversed VEGF-induced suppression of Claudin5 expression and Claudin 5 labeling of vascular tight junctions. Injection of IL-18 also increased expression of Thrombospondin 1 and reduced ischemia-induced retinal neovascularization relevant to diabetic retinopathy and subretinal neovascularization relevant to neovascular age-related macular degeneration. Thus, VEGF and IL-18 suppress each other's production and effects on the vasculature suggesting that IL-18 may provide benefit in multiple retinal/choroidal vascular diseases.
Keywords: Vascular leakage, macular edema, ocular neovascularization, claudin 5
Introduction
Central and branch retinal vein occlusion are prevalent causes of visual impairment, second only to diabetic retinopathy among retinal vascular disorders (Rehak and Rehak, 2008). In patients with retinal vein occlusion there is reduced retinal perfusion, retinal ischemia, and excessive leakage from retinal vessels leading to macular edema, the predominant cause of reduced vision. Increased production of vascular endothelial growth factor-A (VEGF) is the major cause of macular edema in patients with retinal vein occlusion and intraocular injections of ranibizumab (Lucentis®; Genentech, Inc., South San Francisco, CA), a Fab that specifically binds all isoforms of VEGF (Ferrara et al., 2006), reduces edema and substantially improves vision in most patients (Brown et al., 2011; Brown et al., 2010; Campochiaro et al., 2011; Campochiaro et al., 2010a; Campochiaro et al., 2008; Campochiaro et al., 2010b). However, some patients have persistent macular edema and reduced vision despite aggressive treatment with ranibizumab suggesting that other factors may also contribute to leakage.
Bone marrow-derived cells and in particular activated myeloid cells have been implicated in the pathogenesis of ischemic retinopathies (Lima e Silva et al., 2007; Shen et al., 2007) and are a likely source of additional factors other than VEGF that contribute to vascular leakage and ocular neovascularization. Among the cytokines produced by activated myeloid cells are IL-1β, IL-12, TNF-α, and IL-18 (Boraschi and Dinarello, 2006; Wang et al., 2007). To determine whether any of these cytokines might contribute to residual macular edema after neutralization of VEGF in patients with retinal vein occlusion, we measured their levels in the aqueous of patients with retinal vein occlusion before and after multiple injections of ranibizumab. Unexpectedly, we observed that neutralization of VEGF was associated with a significant increase in IL-18 levels. The increase was greatest in eyes with relatively high IL-18 at baseline. There was a substantial improvement in mean best-corrected visual acuity in the entire ranibizumab-treated population, but visual outcome was significantly better in patients with relatively high intraocular levels of IL-18 at baseline compared to those with low IL-18. This led us to investigate the effects of IL-18 in several animal models of retinal vascular disease. Our results suggest a reciprocal relationship between VEGF and IL-18 in the eye with regard to levels and activities that could have therapeutic implications.
Materials and Methods
Subjects
The subjects for this study consisted of patients with macular edema due to retinal vein occlusion that were randomized to receive monthly injections of 0.3 or 0.5mg of ranibizumab at baseline, month 1, and month 2, with the primary endpoint at month 3. Patients were treated in compliance with the Declaration of Helsinki, US Code 21 of Federal Regulations, and the Harmonized Tripartite Guidelines for Good Clinical Practice (1996) and the protocol for the study was approved by the internal review board of the Johns Hopkins Medical Institutions. Thirty-nine of 40 patients had collection of aqueous samples at baseline, month 1, and month 2. The concentrations of IL-18, IL-1β, IL-6 and TNF-α, IL-6 were measured in aqueous samples or cell supernatants with ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Also after informed consent, 7 patients with macular pucker or macular hole who were undergoing vitreous surgery had an aqueous tap at the beginning of the procedure to serve as controls.
Cytokine production by lipopolysaccharide (LPS)-stimulated differentiated dendritic cell cultures
As previously described (Mimura et al., 2007; Takahashi et al., 2004) column sorted monocytes from freshly isolated peripheral blood monocytic cells from 4 donors were differentiated in culture with 5 ng/mL interleukin-4 (R&D Systems, Minneapolis, MN) and 20 ng/mL GM-CSF (R&D Systems, Minneapolis, MN). After 4 days, supernatants were isolated from some of the immature dendritic cell cultures and the remaining cultures were differentiated by addition of 100 ng/mL of LPS (Invivogen, San Diego, CA) alone (controls) or in combination with 50 ng/mL of recombinant human VEGF165 (R&D Systems, Minneapolis, MN). After 2 days of treatment (day 6), supernatants were collected and levels of IL-18, IL-12, and TNF-α were measured by ELISA. Cultured cells were analyzed by flow cytometry to determine the percentages of CD11c+ immature dendritic cells and CD80+ mature dendritic cells.
Cytokine measurements by ELISA
IL-1β, TNF-α, IL-6, and IL-18 were measured by ELISA per manufacturer protocol (R&D Systems, Minneapolis, MN). The assay manufacturer reports minimum detectable dose values of 1 pg/mL, 1.6 pg/mL, 0.70 pg/mL, 12.5 pg/mL for IL-1β, TNF-α, IL-6, and IL-18 respectively. Manufacturer's minimum detectable dose was determined by adding two standard deviations to the mean optical density value of standard replicates and calculating the corresponding concentration. These specifications were assessed in-house and minimum detectable dose for each assay was established using the same protocol as the manufacturer. Lower Limit of Quantification (LLQ) for each experiment is calculated as the minimum detectable dose x dilution factor of the sample. Aqeuous humor samples (100 μl) were diluted in kit assay buffer for ELISA measurements: 10X (IL-1β), 5X (TNF-α), 10X (IL-6), and 10X (IL-18) resulting in LLQ of 10 pg/mL, 8 pg/mL, 7 pg/mL, and 16 pg/mL respectively.
Mouse models
All studies involving mice were approved by the Animal Care and Use Committee of Johns Hopkins University and conformed to the recommendations of the Association for Research in Vision and Ophthalmology and the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Ischemic retinopathy was produced in neonatal C57BL/6 mice as previously described (Lima e Silva et al., 2007; Xie et al., 2008). At P15, retinas were stained with rabbit anti-mouse IL-18 antibody (Cat. 5180-R100, BioVision, Inc., Mountain View, CA) and Cy3-labeled goat anti-rabbit IgG. In some experiments retinas were double-labeled for IL-18 and FITC-conjugated Griffonia Simplicifolia lectin (GSA) to visualize retinal vessels, PE-conjugated anti-mouse F4/80 (1:200, eBioscience, Inc. San Diego, CA, USA) to visualize macrophages, or rat-anti-mouse CD45 antibody. Secondary antibodies for the latter 2 primary antibodies were Alexa 595 conjugated goat anti-rabbit antibody and Alexa 488 conjugated goat anti-rat antibody (1:500, Invitrogen, Carlsbad, CA). At P12, mice with ischemic retinopathy were given an intraocular injection of 1μg of anti-murine VEGF (Yu et al., 2008) some mice were euthanized at P15 and IL18 mRNA was measured by real time RT-PCR (see below). At P17, the remainder of the mice had NV stained by in vivo immunostaining for PECAM1 (Shen et al., 2007) and the area of NV was measured by image analysis (Image-Pro Plus; Media Cybernetics, Silver Spring, MD) by a masked observer (Shen et al., 2007; Xie et al., 2008). At P12, mice with ischemic retinopathy were given an intraocular injection of recombinant mouse IL-18 (MBL, International Co, Woburn, MA) in one eye and bovine serum albumin in the other eye. Injections were done with a Harvard pump and micropippettes as previously described (Mori et al., 2001b); the pressure for injection was set to give a 1 μl injection into air, but since injection into the closed eye compartment provides considerable back-pressure, a smaller but reproducible volume was injected into the eye. If the total volume could have been injected, the dose would have been 200 ng of IL-18, but our estimate of actual dose is in the low ng range. This dose was selected because dose-response experiments showed it to be the highest dose that caused no obvious inflammatory response or toxicity. At P15, 10 μm frozen ocular sections stained for Claudin 5 (Alexa 488 conjugated, Invitrogen), Occludin (Alexa 594 conjugated, Invitrogen), or ZO-1 (1:200, Invitrogen and donkey anti-rabbit polyclonal IgG antibody conjugated to Cy3, 1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA). Slides or retinas were washed and incubated in FITC-labeled GSA.
To assess the effect of IL-18 on VEGF-induced leakage, C57BL/6 mice were given an intraocular injection of VEGF, a mixture of VEGF and IL-18, or vehicle and after 6 hours they were perfused with PBS to wash out all intravascular albumin. If a full μl could have been injected, the dose would have been 200 ng of VEGF and IL-18, but we estimate actual doses were in the low ng range. Eyes were fixed in 10% formalin and retinas were immunostained with rabbit anti-rat albumin antibody that cross-reacts with mouse albumin (RARa/Alb, 1:200, Nordic Immunology Lab, Tilburg, Netherland) and Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA). The total area of albumin staining per retina was measured by image analysis by a masked investigator.
Transgenic mice in which the rhodopsin promoter drives expression of VEGF in photoreceptors (rho/VEGF mice) have the onset of VEGF expression at P7 and starting at P10 develop sprouts of NV from the deep capillary bed of the retina that grow through the photoreceptor layer and form an extensive network of new vessels in the subretinal space (Okamoto et al., 2006; Tobe et al., 1998a). Rho/VEGF mice were given an intraocular injection of IL-18 at P14 and were euthanized at different time points for tight-junction protein or albumin immunostaining, and for the measurement of neovascularization. At P20, rho/VEGF mice were given an intravitreous injection of VEGF, equivalent doses of VEGF plus IL-18, or PBS (see above for dose selection). After 24 or 48 hours immunostaining for albumin was done as described above. In some experiments immunoblotting for albumin was done on retinal homogenates.
Choroidal neovascularization was induced by laser photocoagulation-induced rupture of Bruch's membrane as previously described (Tobe et al., 1998b). After rupture of Bruch's membrane, mice were given an intraocular injection of IL-18 or vehicle and the area of choroidal NV was measured after 2 weeks as previously described (Mori et al., 2001a).
Quantitative real-time RT-PCR
Total retinal RNA was isolated using an RNeasy kit (QIAGEN, Valencia, CA), quantified with Gene SpecIII (Hitachi, Japan), 1 μg was treated with DNase I (Ambion, Austin, TX) to remove any contaminating genomic DNA, and cDNA was synthesized with reverse transcriptase (SuperScript III; Life Technologies, Gaithersburg, MD) and 5 μmol/L random hexamer. Real time quantitative PCR was performed and analyzed using the SYBR Green I format on the LightCycler (Roche, Indianapolis, IN, USA). Reactions were performed in a 20 μl volume using the SYBR Green master mixture with 0.5 mmol/L primers. Cyclophilin A was used as a standard for normalization. The sequences of the PCR primer pairs are shown in supplemental Table 1. Murine cDNA standards were synthesized by RT-PCR from mouse retinal total RNA using Pfu Taq polymerase (Stratagene, La Jolla, CA). The PCR products were purified with a gel extraction kit (QIAGEN) and used to generate standard curves for each gene for each real-time PCR reaction. Standard curves were used to calculate mRNA copy numbers for each retinal RNA sample and target gene mRNA copy numbers were normalized to 106 copies of cyclophilin A.
Transient transfections and reporter assays
Promoter fragments spanning −500/+144, −1000/+144 and −2000/+144 relative to the transcription start site of mouse Cldn5 gene cloned upstream of the luciferase reporter in pGL3 basic (Promega) (Aslam et al., 2011) were generously provided by Dr. Bernhard Hemmer (Munich, Germany). RPE19 cells (Dunn et al., 1996) were grown to confluence in 6-well plates, culture medium was replaced with 1.5 ml serum free medium, and then transient transfection of promoter constructs was performed with lipofectamine 2000 (Invitrogen). Cells were incubated for 24 hours in transfection medium containing following reagents: 5μg of promoter constructs (or empty pGL3 basic), 500 ng of renilla luciferase reporter plasmid pRL-SV40 (as normalization control), and 50 ng of venus-GFP expressing plasmid pCS2-venus (to monitor the transfection efficiency). Transfected cells were treated with IL-18 or IL-18 plus VEGF in serum free medium for 24h. Cells were then rinsed twice with PBS and lysed using passive lysis buffer (Promega). Renilla and firefly luciferase activity was measured in cell lysates using dual luciferase assay system (Promega, Madison, WI). Each experiment was done three times.
Statistics
Statistical analyses were performed using R version 2.15.1 (www.r-project.org). To compare month 2 (M2) with baseline levels (BL), values below the LLQ have been imputed at the respective assay's LLQ. Hypothesis testing was performed using the Wilcoxon signed-rank test utilizing the R package “coin” (Hothorn et al., 2008) to accommodate the presence of ties and matched samples. Spearman's rank sum correlation analysis was used to test for correlation. Wilcoxon/Kruskal-Wallis (Rank Sums) or the Peto & Peto modification of the Gehan-Wilcoxon test for left censored data was used to compare independent samples. Paired t-test of log transformed values was used to compare dependent samples dendritic cell experiments and average fold changes are reported as geometric mean.
Results
Increased aqueous IL-18 from suppression of VEGF
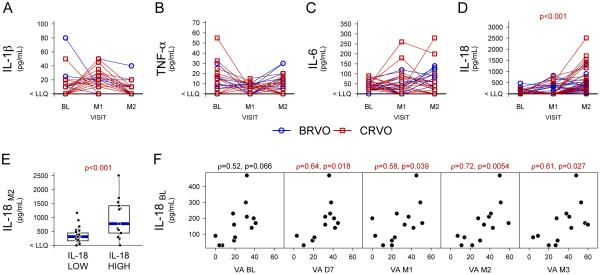
Twenty patients with macular edema due to central retinal vein occlusion and 20 patients with macular edema due to branch retinal vein occlusion were randomized to receive three monthly injections of 0.3 or 0.5 mg ranibizumab. Aqueous taps were done for all 20 of the central retinal vein occlusion patients and 19 of the branch retinal vein occlusion patients at baseline just prior to the first injection, at month 1 prior to the second injection, and at month 2 prior to the third injection. Aqueous humor levels of IL-1β, TNF-α, IL-6 and IL-18 were measured (Figures 1A-D), however many observations were below the LLQ (see Methods). Among the cytokines measured, we detected a significant increase in levels of only IL-18 (Figure 1D, p<0.001, Wilcoxon signed rank test) at month 2 as compared to baseline. Median levels of IL-18 at baseline and month 2 were below the LLQ (<16pg/mL) and 392.5 pg/mL (IQR 433.75), respectively. The increase occurred primarily in patients who had detectable IL-18 at baseline. While many patients with undetectable IL-18 at baseline who were categorized as “IL-18 low” had measurable levels at month 2, the levels in the IL-18 low group (median = 310 pg/mL, IQR = 282.5 pg/mL) was markedly less than that in patients who had detectable IL-18 at baseline who were categorized as “IL-18 high” (median = 980 pg/mL, IQR = 775 pg/mL) (Figure 1E, p<0.001, Peto & Peto modification of the Gehan-Wilcoxon test). Seven patients who underwent vitreous surgery for macular pucker or macular hole had aqueous taps just prior to surgery to measure IL-18 levels in patients without retinal vascular disease and levels in all 7 were < LLQ.
Figure 1. Aqueous levels of inflammatory cytokines in eyes of patients with retinal vein occlusion treated with ranibizumab.
Aqueous samples were obtained in 39 patients with macular edema due to central or branch retinal vein occlusion (CRVO or BRVO) at baseline (BL), 1 month after the first injection of ranibizumab (M1), and 1 month after the second injection (M2). Levels of interleukin (IL)-1β (A), tumor necrosis factor-α (TNF-α, B), IL-6 (C), and IL-18 (D) were measured by ELISA. Values below the lower limit of quantification (LLQ) are plotted at the y-axis lower limit (indicated as < LLQ). IL-18 levels are higher at M2 as compared to BL (p<0.001, Wilcoxon signed-rank test): BL and M2 median levels are < LLQ and 392.5 pg/mL respectively. Patients were categorized as “IL-18 high” (baseline level above the lower limit of quantification LLQ) or “IL-18 low” (below the LLQ) according to their IL-18 level at baseline and M2 IL-18 levels (IL-18M2) are plotted along the y-axis (E). IL-18 “high” patients had IL-18 levels that were significantly higher (p<0.001) than IL-18 “low” patients after 2 months of ranibizumab treatment. Baseline aqueous levels of IL-18 (IL-18BL) that were above the lower limit of quantification were plotted versus best-corrected visual acuity (VA; letters read at 4 meters on a standardized visual acuity chart from the Early Treatment Diabetic Retinopathy Study) and there was no correlation (F). However, IL-18BL levels correlated (Spearman's Rank Order p<0.05) with visual acuity at each of the time points after initiation of ranibizumab treatment, day 7 (D7), month 1 (M1), month 2 (M2), and month 3 (M3).
Positive correlation between baseline IL-18 and visual outcome
There was no correlation between visual acuity and IL-18 levels at baseline, but there was a significant positive correlation (p<0.05) between improvement in visual acuity at all subsequent time points, day 7 and months 1, 2, and 3, and IL-18 levels at baseline (Figure 1F). These data suggest that the IL-18 level at baseline may be a predictive marker for visual outcome in patients with macular edema due to retinal vein occlusion who are treated with ranibizumab.
Increase in IL-18-positive bone marrow-derived cells in mice with ischemic retinopathy after injection of anti-VEGF antibody
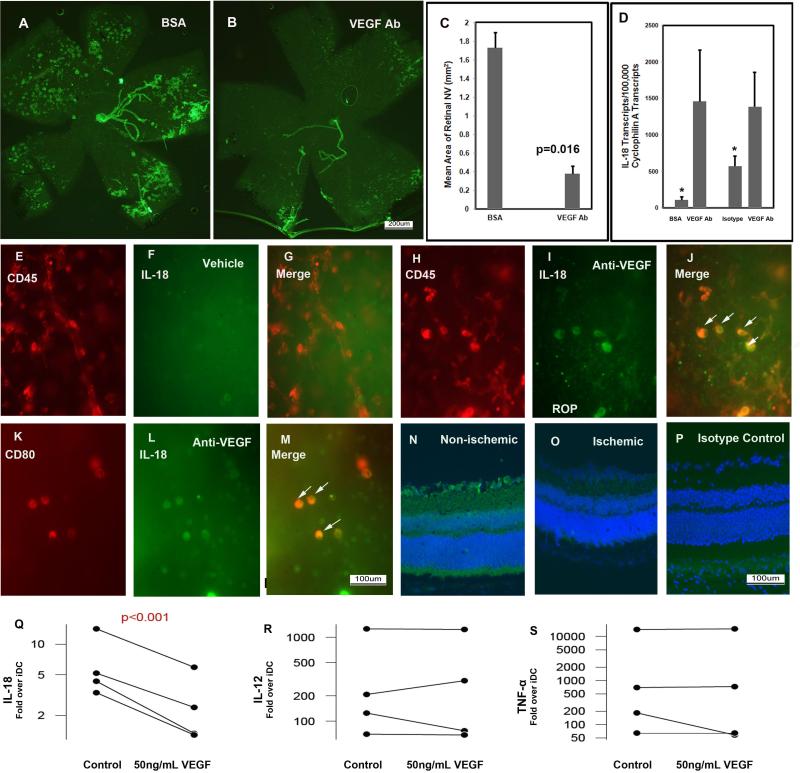
Like patients with retinal vein occlusion, mice with oxygen-induced ischemic retinopathy have areas of ischemic retina that release excessive amounts of VEGF resulting in leakage and retinal neovascularization (Pierce et al., 1995). Intraocular injection of 1 μg of an antibody directed against murine VEGF at postnatal day (P) 12, the beginning of the ischemic period, resulted in strong suppression of retinal neovascularization (Figure 2A-C) and also led to a significant increase in the mRNA for IL-18 (Figure 2D). In P17 mice with ischemic retinopathy that received an intraocular injection of PBS at P12, CD45+ bone marrow-derived cells, did not stain for IL-18 (Figure 2E-G). However, P17 mice with ischemic retinopathy that received an intraocular injection of 1 μg of anti-VEGF antibody at P12, showed staining for IL-18 in CD45+ cells (Figure 2H-J, arrows) and in a subset of CD45+ cells, CD80+ dendritic cells (Figure 2K-M, arrows). In the normal retina of P17 mice raised in room air, there was staining for IL-18 in ganglion cells (Figure 2N), but there was no detectable staining for IL-18 in ganglion cells within the ischemic retina of P17 mice with ischemic retinopathy (Figure 2O). In P17 mice raised in room air, there was no staining when control IgG was substituted for anti-IL-18 antibody (Figure 2P). Thus, in mice with ischemic retinas, high levels of VEGF reduce IL-18 and specific blockade of VEGF increases in IL-18, suggesting that VEGF suppresses production of IL-18.
Figure 2. Blockade of VEGF in ischemic retina causes elevation of IL-18 in myeloid cells.
Mice with oxygen-induced ischemic retinopathy (n= 5) were given an intraocular injection of 1μg of an antibody directed against murine VEGF in one eye and bovine serum albumin (BSA) in the fellow eye or control IgG at postnatal day 12, the onset of the ischemic period. At P15, some of the mice were used to isolate total retinal RNA and real-time RT-PCR was used to quantify mRNA for IL-18 and Cyclophilin-A. At P17, in vivo immunostaining for PECAM1 showed extensive NV on the surface of retina (the large vessels are hyaloid vessels and everything else is NV) in BSA-injected eyes (A) and little NV on the surface of retina in eyes injected with the anti-VEGF antibody (B); image analysis confirmed a significant reduction in area of NV (C, n=5 for each group, p=0.016). There was a significant increase in mRNA for IL-18 in retinas from eyes injected with anti-VEGF antibody compared to those injected with BSA or isotype control IgG (D; n=5 for each, *p<0.049 by unpaired t-test). Flat mounted ischemic retinas double-labeled for IL-18 (green) and CD45 (red) showed that in vehicle-treated eyes, CD45-positive cells did not stain for IL-18 (E-G)), while many CD45-positive cells stained for IL-18 in eyes treated with anti-VEGF antibody (H-J, arrows). CD80-positive cells also stained for IL-18 in ischemic retina of eyes treated with an anti-VEGF antibody (K-M, arrows) In P17 mice raised in room air, there was staining for IL-18 in ganglion cells (N), but in P17 mice with ischemic retinopathy, no staining for IL-18 could be detected in ganglion cells (O). In P17 mice raised in room air, there was no staining when control IgG was substituted for anti-IL-18 antibody (P). Blood was obtained from 4 human donors and immature dendritic cells (iDC) were prepared as described in Methods. Supernatants were collected from some of the iDC cultures and the remainder of the cultures was activated by incubation with lipopolysaccharide (LPS) alone or in combination with 50ng/mL of VEGF. The levels of IL-18, IL-12, and TNF-α were measured in the supernatants by ELISA and the levels obtained in activated cultures were divided by the levels obtained from corresponding iDC cultures. The increase in cytokine (fold increase above level in corresponding iDC) induced by LPS alone (controls) compared to LPS plus VEGF is plotted for cultures from each of the 4 donors and the mean is represented by the green line. VEGF significantly reduced IL-18 levels (Q; p=0.03), but not IL-12 (R) or TNF-α (S).
IL-18 production by activated myeloid cells suppressed by VEGF
To directly test the effect of VEGF on IL-18 production, we utilized an in vitro cell culture model of LPS-activated dendritic cells differentiated from freshly isolated human monocytes (Mimura et al., 2007; Takahashi et al., 2004). Based on FACS analysis, in vitro culture of freshly isolated peripheral blood monocytes treated with IL-4 and GM-CSF typically yielded greater that 85% CD11c+ immature dendritic cells (data not shown). Subsequent activation by the addition of 100 ng/mL LPS for 3 additional days typically yielded greater than 85% CD80+ mature dendritic cells. In 4 independent experiments utilizing monocytes isolated from 4 separate donor peripheral blood monocyte preparations, addition of 50 ng/mL VEGF165 during maturation had no significant effect on the frequency of CD80+ mature dendritic cells. In this study, cytokine levels were normalized for each donor by the respective concentration produced by immature dendritic cells prior to maturation. IL-1β levels were not significantly induced by LPS treatment nor were they affected by the addition of VEGF165 (data not shown). In contrast, treatment with LPS caused average (geometric mean) increases of 5.7-fold, 219-fold, and 584-fold in IL-18, IL12, and TNF-α respectively. VEGF165 significantly attenuated the LPS-stimulated increase in IL-18 by 60% (geometric mean, p<0.001, paired t-test on log transformed data, Figure 2Q), but had no detectable effect on the LPS-induced increases in IL-12 or TNF-α (Figure 2R,S).
IL-18 blocks VEGF-induced leakage
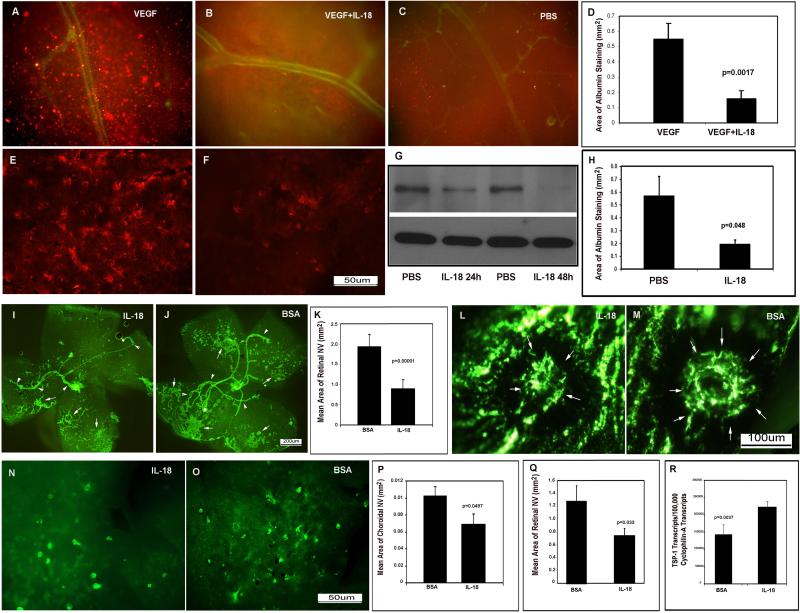
Injection of VEGF into the vitreous cavity of mice results in excessive vascular permeability that is quantifiable by visualization of the serum protein albumin (Saishin et al., 2003b). Mice were given an intraocular injection of VEGF with or without an equivalent dose of murine IL-18 and immunostaining for serum albumin showed numerous aggregates of albumin throughout retinas from eyes injected with VEGF alone (Figure 3A), that were reduced in retinas from eyes injected with both VEGF and IL-18 (Figure 3B) and similar to control eyes that were not injected with VEGF (Figure 3C). The total area of albumin staining per retina was significantly less in eyes injected with both VEGF and IL-18 compared to that in eyes injected with VEGF alone (Figure 3D). Thus, IL-18 substantially counteracts vascular leakage from retinal vessels induced by VEGF. To determine if IL-18 has any effect on leakage from neovascularization, we used transgenic mice with expression of VEGF in photoreceptors driven by the rhodopsin promoter (rho/VEGF mice), which develop numerous areas of subretinal NV by postnatal day (P) 21 (Okamoto et al., 1997; Tobe et al., 1998a). At P21, rho/VEGF mice had intraocular injection of IL-18 in one eye and vehicle in the fellow eye and 24 hours after injection, retinal flat mounts showed prominent albumin staining around tufts of neovascularization in vehicle-injected eyes (Figure 3E), but little albumin staining in IL-18-injected eyes (Figure 3F). Immunoblots showed significantly less albumin 24 or 48 hours after injection of IL-18 (Figure 3G) and the area of albumin staining was significantly less in IL-18 injected eyes compared to fellow eyes (Figure 3H). These data indicate that IL-18 reduces excessive leakage from new vessels as well as pre-existent retinal vessels.
Figure 3. IL-18 prevents VEGF-induced vascular leakage and inhibits retinal and choroidal neovascularization.
Adult C57BL/6 mice were given an intraocular injection of VEGF (A), VEGF + IL-18 (B), or vehicle (C). Equivalent doses of VEGF and IL-18 were given and after 6 hours, mice were perfused with PBS to remove all blood from the vasculature and retinas were stained for albumin (red) and PECAM-1 (green). Retinal flat mounts from eyes injected with VEGF alone (n=8) showed numerous clumps of albumin throughout the retina (A), while those from eyes injected with IL-18 + VEGF (n=7) showed relatively few clumps of albumin (B) and were similar in appearance to retinas from eyes injected with vehicle (C). The mean (±SEM) area of albumin staining per retina was significantly less in eyes injected with IL-18 +VEGF compared with eyes injected with VEGF alone (D). At P21, rho/VEGF mice had injection of IL-18 in one eye and vehicle in the fellow eye and after 24 hours, there was prominent immunoreactive albumin around tufts of NV in vehicle-injected eyes (E), but very little albumin in IL-18-injected eyes (F). Immunoblots for albumin (G, upper panel) showed that 24 or 48 hours after injection of IL-18 there was much less albumin in retinas from IL-18-injected eyes compared to vehicle-injected eye, and reprobing of blots for β-actin demonstrated equal loading (G, lower panel). The area of albumin staining per retina was also significantly reduced 48 hours after injection of IL-18 compared to vehicle-injected fellow eye (H, n=4). At P12, mice with ischemic retinopathy (n=6) were given an intraocular injection of IL-18 in one eye and bovine serum albumin (BSA) in the other eye. At P17, immunostaining for PECAM-1 showed that retinal flat mounts from eyes injected with IL-18 (I) had less NV than those from eyes injected with BSA (J,K). In mice with laser-induced rupture of Bruch's membrane (n=15), eyes injected with IL-18 had significantly less choroidal NV than that seen in eyes injected with BSA (L-M). At P21, eyes of rho/VEGF mice (n=5) that had injection of IL-18 at P14 had less subretinal NV than eyes injected with BSA (N-Q). Mice with ischemic retinopathy were given an intraocular injection of IL-18 at P12 and at P15 total real time RT-PCR showed a significant increase in mRNA for thrombospondin-1 in IL-18-injected eyes compared to BSA-injected eyes (R).
IL-18 suppresses retinal and subretinal neovascularization
High intraocular levels of VEGF contribute to retinal neovascularization as well as excessive vascular permeability in ischemic retinopathies such as diabetic retinopathy. In mice with ischemic retinopathy, injection of IL-18 caused a significant reduction in the amount of neovascularization (Figure 3I) compared to albumin-injected eyes (Figure 3J). Subretinal neovascularization occurs in patients with neovascular age-related macular degeneration and can originate from the choroid, in which case it is labeled choroidal neovascularization, or it can originate from the deep capillary bed of the retina which is labeled retinal angiomatous proliferation (Yannuzzi et al., 2001). Choroidal neovascularization is modeled by laser-induced rupture of Bruch's membrane in mice (Tobe et al., 1998b) and retinal angiomatous proliferation is modeled in rho/VEGF mice (Okamoto et al., 1997). VEGF is a critical stimulus for choroidal neovascularization and retinal angiomatous proliferation in these models (Kwak et al., 2000; Saishin et al., 2003a) and in patients with neovascular age-related macular degeneration (Brown et al., 2006; Rosenfeld et al., 2006). Intraocular injection of IL-18 caused a significant reduction in choroidal neovascularization (Figure 3L) compared to controls (Figure 3M, P) or subretinal neovascularization (Figure 3N) compared to controls (Figure 3O,Q). It has been previously demonstrated that IL-18 increases expression of the anti-angiogenic protein thrombospondin 1 in gastric cancer cells (Kim et al., 2006) and therefore we assessed the expression of thrombospondin 1 in the retina after intraocular injection of IL-18. Compared to mice with ischemic retinopathy given an intraocular injection of bovine serum albumin, those injected with IL-18 showed a significant increase in mRNA for thrombospondin 1 in the retina (Figure 3R).
IL-I8 prevents VEGF-induced reduction of claudin 5 at vascular tight junctions in the retina
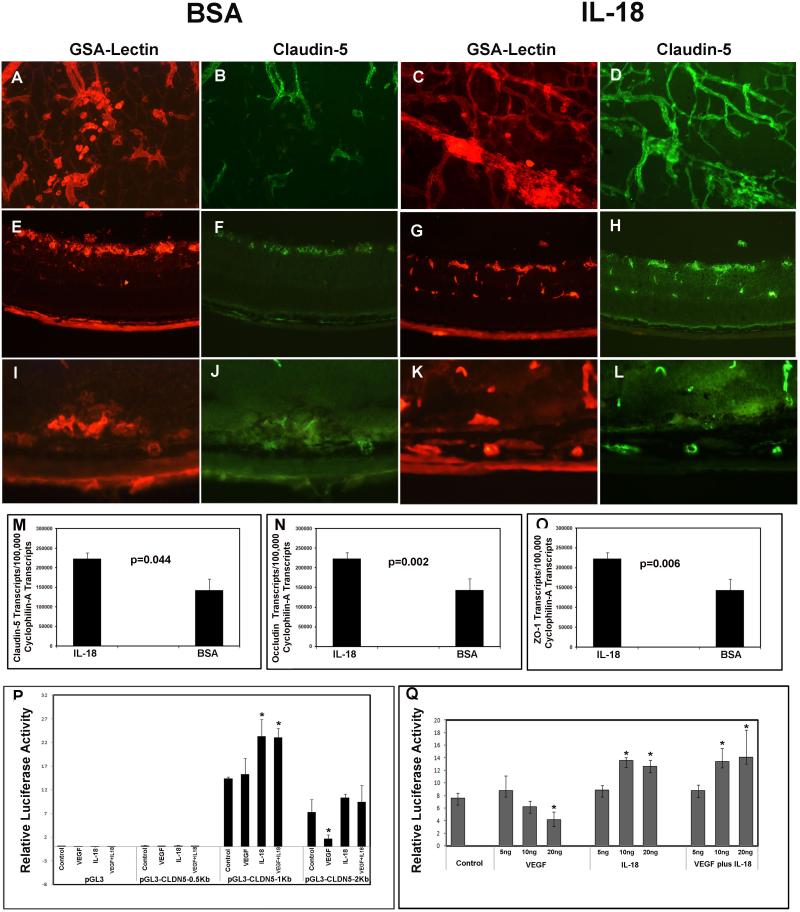
At P17 in mice with ischemic retinopathy, 2 days after intraocular injection of IL-18 in one eye and bovine serum albumin in the fellow eye, retinal flat mounts from albumin-injected eyes showed retinal neovascularization on the surface of the retina and underlying pre-existent vessels (Figure 4A). There was weak staining for claudin 5 in some pre-existent blood vessels and some tufts of neovascularization in albumin-injected eyes (Figure 4B). In contrast, there was strong staining for claudin 5 in neovascularization and pre-existent retinal vessels in IL-18-injected eyes (Figure 4C, D). In ocular sections, compared to albumin-injected eyes in which there was faint claudin 5 labeling of vascular structures at the surface of the retina (Figure 4E, F), there was strong claudin 5 labeling of all vascular structures in the retina of IL-18-injected eyes (Figure 4G, H). Retinas from control P21 rho/VEGF mice showed little claudin 5 staining of subretinal neovascularization (Figure 4I, J), while retinas from eyes injected with IL-18 at P15 showed light claudin 5 staining of subretinal neovascularization and strong staining of feeder vessels extending from the deep capillary bed to the subretinal space (Figure 4L). In mice with ischemic retinopathy, real time RT-PCR showed that compared to retinas from albumin-injected eyes, retinas from eyes injected with IL-18 contained significantly less mRNA for claudin 5 (Figure 4M), occludin (Figure 4N), and ZO-1 (Figure 4O).
Figure 4. IL-I8 prevents VEGF-induced reduction of claudin 5 at vascular tight junctions in the retina.
At P15, mice with ischemic retinopathy (n=5) were given an intraocular injection of IL-18 in one eye and BSA in the fellow eye. At P17 intact retinas or ocular frozen sections were double-stained with GSA lectin and anti-claudin 5. The GSA-stained vasculature in control retinas (A) showed faint staining for claudin 5 (B), whereas the vasculature in IL-18-injected eyes (C) showed much stronger staining for claudin 5 (D). Likewise, ocular sections from control retinas showed vasculature (E) that stained faintly for claudin 5 (F), while the vasculature IL-18-injected eyes (G) showed robust staining for claudin 5 (H). Retinas from control P21 rho/VEGF mice showed little claudin 5 staining of the vasculature (I, J), while retinas from eyes injected with IL-18 at P15 showed strong claudin 5 staining (K,L); arrowhead show claudin 5 in feeder vessels and arrows show claudin 5 in subretinal neovascularization. In mice with ischemic retinopathy (n=5), real time RT-PCR showed that compared to retinas from BSA-injected eyes, retinas from eyes injected with IL-18 contained significantly more mRNA for claudin 5 (M), occludin (N), and ZO-1 (O). Cultured retinal pigmented epithelial (RPE) cells were transfected with the luciferase (Luc) reporter plasmid, pGL4, or pGL4 containing 0.5kb, 1kb, or 2kb of DNA upstream of the transcription start site in the Claudin5 gene. In cells transfected with constructs containing 2kb, but not 0.5 or 1kb, of upstream DNA , the relative expression of firefly:renilla Luc was reduced by treatment with 20ng/ml of VEGF (P), while cells transfected with 2 or 1kb, but not 0.5kb, showed an increase in relative firefly:renilla Luc expression when treated with 20ng/ml of IL-18 (P). Cells transfected with constructs containing 2kb of upstream DNA showed a reduction in relative expression of firefly:renilla Luc when treated with 10 or 20ng of VEGF and an increase in relative expression when treated with 10 or 20ng of IL-18 alone or in the presence of 10 or 20ng of VEGF (Q).
To investigate the effect of VEGF and IL-18 on transcription of Claudin5, cultured retinal pigmented epithelial cells were transfected with the luciferase reporter plasmid, pGL4, or pGL4 containing 0.5kb, 1kb, or 2kb of DNA upstream of the transcription start site in the Claudin5 gene. In cells transfected with constructs containing 2kb, but not 0.5 or 1kb, of upstream DNA, the relative expression of firefly:renilla luciferase was reduced by treatment with 20 ng/ml of VEGF (Figure 4P), while cells transfected with 2 or 1kb, but not 0.5 kb, showed an increase in relative firefly:renilla luciferase expression when treated with 20 ng/ml of IL-18 (Figure 4P). Cells transfected with constructs containing 2kb of upstream DNA showed a reduction in relative expression of firefly:renilla luciferase when treated with 10 or 20 ng/ml of VEGF and an increase in relative expression when treated with 10 or 20 ng/ml of IL-18 alone or in the presence of 10 or 20 ng/ml of VEGF (Figure 4Q).
Discussion
It is well-established that VEGF plays an important role in macular edema due to retinal vein occlusion and specific antagonism of VEGF by intraocular injections of ranibizumab provide benefit, (Brown et al., 2011; Brown et al., 2010; Campochiaro et al., 2011; Campochiaro et al., 2010a; Campochiaro et al., 2008; Campochiaro et al., 2010b) but some patients have an incomplete response suggesting that other mediators may also be involved. We explored the possible involvement of IL-1β, TNF-α, IL-6, and IL-18 and compared to eyes with resolved edema after 3 injections of ranibizumab, those with residual edema did not show higher levels of any of the cytokines (Campochiaro et al., 2009). Those data failed to support our hypothesis that one of these cytokines might contribute to residual edema, but there was a surprising and striking finding regarding IL-18. Aqueous levels of IL-18 were variable at baseline, but were significantly increased after injections of ranibizumab with the greatest increases in patients who had relatively high IL-18 at baseline. Furthermore, there was a positive correlation between baseline level of IL-18 and good visual outcome. Similar to the findings in patients with retinal ischemia from retinal vein occlusion, mice with ischemic retinopathy that received an intraocular injection of an anti-VEGF antibody, showed an increase in IL18 mRNA in the retina and staining for IL-18 in CD45+, CD-80+ bone marrow-derived cells, while there was no detectable staining for IL-18 in ischemic retina in the absence of anti-VEGF treatment In normal non-ischemic retina, there was staining for IL-18 in ganglion cells which was eliminated in the setting of ischemia and high levels of VEGF. Activated myeloid cells from human donors exhibited VEGF-induced suppression of IL-18. Thus, VEGF suppresses IL-18 production by activated myeloid cells and ganglion cells, and neutralization of VEGF releases the suppression causing increased levels of IL-18 in the eye. Our findings are consistent with the observation of a negative correlation between VEGF and IL-18 levels during retinal vascular development and in a model of ischemia-induced retinal neovascularization (Qiao et al., 2007; Qiao et al., 2004). The suppression is reciprocal, because in the cornea, increased expression of IL-18 resulted in reduced expression of VEGF (Kim et al., 2005) and incubation of cultured RPE cells with IL-18 causes reduced production of VEGF (Doyle et al., 2012).
In addition to opposing each other's production, IL-18 and VEGF oppose each other's effects on retinal vasculature. VEGF causes leakage from retinal vessels, retinal neovascularization, and subretinal neovascularization. In mice with ischemic retinopathy or rho/VEGF transgenic mice, there is VEGF-induced neovascularization and VEGF-induced leakage from neovascularization and pre-existent retinal vessels. Intraocular injection of IL-18 prevented neovascularization when given early in the process and when given for a brief interval late in the process, it acutely reduced vascular leakage. The reduced leakage is consistent with the observation that IL-18 has an anti-permeability effect in rat brain in which it reduces edema associated with status epilepticus (Jung et al., 2012). With regard to neovascularization, the effects of IL-18 appear to be context-dependent, because it inhibits neovascularization in some tissues (Cao et al., 1999; Coughlin et al., 1998; Kim et al., 2005), but is proangiogenic in joint synovium (Amin et al., 2007; Cho et al., 2006; Park et al., 2001). With regard to effects in the eye, mice deficient in IL-18 have abnormal development of the retinal vasculature characterized by areas of poor vascularization, increased vascular leakage, and ectopic vessels at postnatal day P14 with gradual remodeling and normalization of the vasculature by P84 (Qiao et al., 2004). Intraperitoneal injections of recombinant IL-18 failed to reduce ischemia-induced retinal neovascularization at P17 (Qiao et al., 2007), which differs from our results, possibly due to the different site of injection; the suppressive effects of IL-18 may require direct administration into the eye. Injection of an IL-18 binding protein at P17 in mice with ischemic retinopathy resulted in persistent areas of nonperfusion and neovascularization at P24 and it was concluded that endogenous IL-18 promotes regression of neovascularization (Qiao et al., 2007). Our findings are consistent with the recent demonstration that adult IL18−/− mice develop more choroidal neovascularization at Bruch's membrane rupture sites compared to wild type mice (Doyle et al., 2012).
Claudin 5 is a tight junction protein that plays a critical role in maintenance of the blood-brain barrier and blood-retinal barrier (Campbell et al., 2009; Nitta et al., 2003). Intracranial injection of VEGF causes blood-brain barrier breakdown and reduces claudin 5 staining in brain microvessels, and treatment of brain endothelial cell cultures with VEGF decreases expression of claudin 5 (Argaw et al., 2008). In mice with ischemic retinopathy or rho/VEGF transgenic mice, there was weak staining for claudin 5 in retinal vessels or neovascularization that was markedly increased by intraocular injection of IL-18. Claudin5 promoter-luciferase reporter assays showed IL-18-stimulation of expression with constructs containing 1kb or 2kb upstream fragments, while VEGF caused repression of expression of constructs containing 2kb but not 1kb upstream fragments suggesting different regulatory elements for the two effects. When cells transfected with a construct containing a 2kb fragment of the Claudin5 promoter were treated with both VEGF and IL-18, there was stimulation of expression similar to that seen with IL-18 alone. Incubation of gastric cancer cells with IL-18 stimulates expression of the antiangiogenic protein thrombospondin 1 (Kim et al., 2006). Intraocular injections of IL-18 increased expression of thrombospondin 1 in the retina which likely contributes to the antiangiogenic activity of IL-18, but there may also be other ways by which IL-18 suppresses neovascularization that are independent of thrombospondin 1.
Taken as a whole, the data from patients with retinal vein occlusion and mouse models suggest the following. Bone marrow-derived cells traffic through the retina under normal circumstances and if stimulated may produce angiogenic and pro-permeability factors. This can be countered by production of IL-18 by the bone marrow derived cells and by retinal ganglion cells, which suppresses leakage and neovascularization helping to maintain a quiescent state. However, when VEGF is increased by ischemia or other stimuli, IL-18 production by myeloid cells and retinal ganglion cells is suppressed and the reduction in IL-18 accentuates the pro-permeability and angiogenic effects of VEGF. Blockade of VEGF with ranibizumab allows levels of IL-18 to rise which helps to reverse the proangiogenic and propermeability effects of VEGF. Relatively high pre-treatment levels of IL-18 despite suppression by VEGF in patients with macular edema due to retinal vein occlusion predict robust elevation of IL-18 when VEGF is blocked and greater visual acuity after treatment with ranibizumab. These data suggest that IL-18 levels in aqueous may provide predictive value regarding the likely response to VEGF suppression and also suggest that administration or upregulation of IL-18 may provide a new therapeutic approach for macular edema and ocular neovascularization.
Supplementary Material
Acknowledgments
Funding: Contract grant sponsor: National Eye Institute Contract grant number: EY012609, Contract Grant Sponsor: Genentech, Investigator Initiated Study grant
Footnotes
Competing interests: None of the authors have financial interest related to IL-18. DFC and JRA are employees of Genentech.
References
- Amin MA, Mansfield PJ, Pakozdi A, Campbell PL, Ahmed S, Martinez RJ, Koch AE. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthritis Rheum. 2007;56:1787–1797. doi: 10.1002/art.22705. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, Gareth JR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-branin barrier breakdown. Proc Natl Acad Sci USA. 2008;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Ahmad N, Srivastava R, Hemmer B. TNF-alpha induced NFkappaB signaling and p65 (RelA) overexpression repress Cldn5 promoter in mouse brain endothelial cells. Cytokine. 2011;57:269–275. doi: 10.1016/j.cyto.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Boraschi D, Dinarello CA. IL-18 in autoimmunity: a review. Eur Cytokine Netw. 2006;17:224–252. [PubMed] [Google Scholar]
- Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, Adamis AP, Rubio RG, Murahashi WY. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–1602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Brown DM, Campochiaro PA, Singh RP, Gray S, Rundle AC, Li Z, Rubio RG, Murahashi WY, Group CS. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion:6-month results of the phase III CRUISE study. Ophthalmology. 2010;117:1124–1133. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Anchor Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Campbell M, Nguyen AT, Kiang AS, Tam LC, Goobo OL, Kerskens C, Ni Dhubhghaill S, Humphries MM, Farrar GJ, Kenna PF, Humphries P. An experimental platform for sytemic drug delivery to the retina. Proc Natl Acad Sci USA. 2009;106:17817–17822. doi: 10.1073/pnas.0908561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA, Brown DM, Awh CC, Lee Y, Gray S, Saroj N, Murahashi WY, Rubio RG. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–2049. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Choy DF, Do DV, Hafiz G, Shah SM, Nguyen QD, Rubio R, Arron JR. Monitoring ocular drug therapy by analysis of aqueous samples. Ophthalmology. 2009;116:2158–2164. doi: 10.1016/j.ophtha.2009.04.038. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Hafiz G, Channa R, Shah SM, Nguyen QD, Ying H, Do DV, Zimmer-Galler I, Solomon SD, Sung JU, Syed B. Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusion: two-year outcomes. Ophthalmology. 2010a;117:2387–2394. doi: 10.1016/j.ophtha.2010.03.060. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV, Quinlan E, Zimmer-Galler I, Haller JA, Solomon S, Sung JU, Hadi Y, Janjua KA, Choy DF, Arron JR. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther. 2008;16:791–799. doi: 10.1038/mt.2008.10. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG, Group BS. Ranibizumab for macular edema following branch retinal vein occlusion: 6-month primary endpoint results of a phase III study. Ophthalmology. 2010b;117:1102–1112. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J. 1999;13:2196–2202. doi: 10.1096/fasebj.13.15.2195. [DOI] [PubMed] [Google Scholar]
- Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH, Park SH, Cho CS, Jue DM, Kim HY. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett. 2006;103:159–166. doi: 10.1016/j.imlet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SL, Campbell M, Ozaki E, Salomon RG, More A, Kenna PF, Farrar GJ, Klang AS, Humphries MM, Lavelle EC, O'Neill LA, Hollyfield JG, Humphries P. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:792–799. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. J Statistical Software. 2008;28:1–23. [Google Scholar]
- Jung HK, Ryu HJ, Kim M-J, Won IK, Choi HK, Choi H-C, Song H-K, Jo S-M, Kang T-C. Interleukin-18 attenuates disruption of the blood-brain barrier induced by status epilepticus within the rat piriform cortex in interferon-gamma independent pathway. Brain Res. 2012;1447:126–134. doi: 10.1016/j.brainres.2012.01.057. [DOI] [PubMed] [Google Scholar]
- Kim B, Lee S, Suvas S, Rouse BT. Application of plasmid DNA encoding IL-18 diminishes development of herpetic stromal keratitis by antiangiogenic effects. J Immunol. 2005;175:509–516. doi: 10.4049/jimmunol.175.1.509. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim C, Kim TS, Sa IB, Yang Y, Park H, Cho D. IL-18 enhances thrombospondin-1 production in human gastric cancer via JNK pathway. Biochem Biophys Res Comm. 2006;344:1284–1289. doi: 10.1016/j.bbrc.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara C, McLauer T, Aslam S, Gong YY, Xiao WH, Khu NH, Thut C, Campochiaro P. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Canc Immunol Immunother. 2007;56:761–770. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Ando A, Gehlbach P, Nesbitt D, Takahashi K, Goldsteen D, Penn M, Chen CT, Melia M, Phipps S, Moffat D, Brazzell K, Liau G, Dixon KH, Campochiaro PA. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol. 2001a;159(1):313–320. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001b;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5 deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A, Iwamoto Y, Maru Y. Oxidative stress-responsivetranscriptionfactor ATF3 potentially mediates diabetic angiopathy. Mol Cell Biol. 2006;26:108710–108797. doi: 10.1128/MCB.26.3.1087-1097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151(1):281–291. [PMC free article] [PubMed] [Google Scholar]
- Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Sonoda KH, Ikeda Y, Yoshimura T, Hijioka K, Jo YJ, Sassa Y, Tsutsumi-Miyahara C, Hata Y, Akira S, Ishibashi T. Interleuikin-18 regulates pathological intraocular neovascularization. J Leukoc Biol. 2007;81:1012–1021. doi: 10.1189/jlb.0506342. [DOI] [PubMed] [Google Scholar]
- Qiao H, Sonoda KH, Sassa Y, Hisatomi T, Yoshikawa H, Ikeda Y, Murata T, Akira S, Ishibashi T. Abnormal retinal vascular development in IL-18 knockout mice. Lab Invest. 2004;84:973–980. doi: 10.1038/labinvest.3700115. [DOI] [PubMed] [Google Scholar]
- Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33:111–131. doi: 10.1080/02713680701851902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Marina Study Group Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takahashi K, Lima Silva R, Hylton D, Rudge J, WS J, Campochiaro PA. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003a;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takahashi K, Melia M, Vinores SA, Campochiaro PA. Inhibition of protein kinase C decreases prostaglandin-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003b;195:210–219. doi: 10.1002/jcp.10238. [DOI] [PubMed] [Google Scholar]
- Shen J, Xie B, Dong A, Swaim M, Hackett SF, Campochiaro PA. In vivo immunostaining demonstrates macrophages associate with growing and regressing vessels. Invest Ophthalmol Vis Sci. 2007;48:4335–4341. doi: 10.1167/iovs.07-0113. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not proinflammatory cytokines. Canc Immunol Immunother. 2004;53:543–550. doi: 10.1007/s00262-003-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, Zack DJ, Campochiaro PA. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci. 1998a;39(1):180–188. [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998b;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang X, Hussain S, Zheng Y, Sanjabi S, Ouaaz F, Beg AA. Distinct roles of different NF-kappa B subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J Immunol. 2007;178:6777–6776. doi: 10.4049/jimmunol.178.11.6777. [DOI] [PubMed] [Google Scholar]
- Xie B, Shen J, Dong A, Swaim M, Hackett SF, Wyder L, Worpenberg S, Barbieri S, Campochiaro PA. An Adam15 amplification loop promotes vascular endothelial growth factor-induced ocular neovascularization. FASEB J. 2008;22:2775–2783. doi: 10.1096/fj.07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannuzzi LA, Negrao S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter JS, Freund KB, Sorenson J, Orlock D, Borodoker N. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–434. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, Fuh G, Lowman H, Ferrara N. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008;49:522–527. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.