Abstract
Contact-dependent growth inhibition (CDI) is a mode of inter-bacterial competition mediated by the CdiB/CdiA family of two-partner secretion systems. CdiA binds to receptors on susceptible target bacteria, then delivers a toxin domain derived from its C-terminus. Studies with Escherichia coli suggest the existence of multiple CDI growth-inhibition pathways, whereby different systems exploit distinct target-cell proteins to deliver and activate toxins. Here, we explore the CDI pathway in Burkholderia using the CDIII Bp1026b system encoded on chromosome II of Burkholderia pseudomallei 1026b as a model. We took a genetic approach and selected Burkholderia thailandensis E264 mutants that are resistant to growth inhibition by CDIII Bp1026b. We identified mutations in three genes, BTH_I0359, BTH_II0599, and BTH_I0986, each of which confers resistance to CDIII Bp1026b. BTH_I0359 encodes a small peptide of unknown function, whereas BTH_II0599 encodes a predicted inner membrane transport protein of the major facilitator superfamily. The inner membrane localization of BTH_II0599 suggests that it may facilitate translocation of CdiA-CTII Bp1026b toxin from the periplasm into the cytoplasm of target cells. BTH_I0986 encodes a putative transglycosylase involved in lipopolysaccharide (LPS) synthesis. ∆BTH_I0986 mutants have altered LPS structure and do not interact with CDI+ inhibitor cells to the same extent as BTH_I0986+ cells, suggesting that LPS could function as a receptor for CdiAII Bp1026b. Although ∆BTH_I0359, ∆BTH_II0599, and ∆BTH_I0986 mutations confer resistance to CDIII Bp1026b, they provide no protection against the CDIE264 system deployed by B. thailandensis E264. Together, these findings demonstrate that CDI growth-inhibition pathways are distinct and can differ significantly even between closely related species.
Introduction
Contact-dependent growth inhibition (CDI) is a mechanism of inter-cellular competition used by some Gram-negative species to inhibit the growth of neighboring bacteria [1–3]. CDI is mediated by the CdiB/CdiA family of two-partner secretion proteins, which are distributed through α-, β- and γ-proteobacteria [4]. CdiB is an outer-membrane β-barrel protein that exports the CdiA toxic effector. CdiA proteins are very large (180–650 kDa depending on the species) and are predicted to form long β-helical filaments that extend from the surface of inhibitor cells [2,5]. During CDI, CdiA binds to specific receptors on susceptible bacteria and delivers a toxin domain derived from its C-terminal region (CdiA-CT). CdiA-CT sequences are highly variable between bacterial species and strains, but the N-terminal boundary of this region is typically delineated by a highly conserved VENN peptide motif [1,6]. CdiA-CT sequence diversity suggests a variety of toxin activities, and indeed most characterized CDI toxins are nucleases with different cleavage specificities for DNA, tRNA or rRNA [1,7–9]. Additionally, CdiA-CTEC93 from Escherichia coli EC93 appears to form pores in target-cell membranes [10], and sequence analysis suggests that other CDI toxins may have RNA deaminase and protease/peptidase activities [11]. CDI+ bacteria protect themselves from auto-inhibition by producing CdiI immunity proteins, which bind to CdiA-CT toxins and neutralize their activities.
CDI has been characterized most extensively in γ-proteobacteria, with E. coli EC93 and uropathogenic E. coli 536 (UPEC 536) serving as model systems. Studies with those systems have revealed that CDI exploits specific target-cell proteins to deliver growth inhibitory toxins [12,13]. Selections for mutants that are resistant to the E. coli EC93 system (CDIEC93) identified bamA and acrB mutations that protect target cells from growth inhibition [12]. BamA is an essential outer-membrane protein required for the assembly of all β-barrel proteins [14–17], and is specifically recognized as a target-cell receptor by CdiAEC93 [12,18]. AcrB is a trimeric integral membrane protein that functions together with AcrA and TolC as a multi-drug efflux pump [19]. However, the efflux function of AcrB is not required for CDIEC93 because ΔacrA and ΔtolC mutants are both fully sensitive to CDIEC93 [12]. Though the role of AcrB during CDIEC93 is not known, its localization suggests that it could facilitate assembly of the CdiA-CTEC93 pore-forming toxin into the target-cell inner membrane. Biochemical studies on CdiA-CT536 from UPEC 536 have shown that this toxin is a latent tRNase that only exhibits nuclease activity when bound to the cysteine synthase, CysK [13]. In accord with in vitro studies, E. coli ΔcysK mutants are completely resistant to inhibition by CDIUPEC536. Collectively, these findings indicate that CDI pathways can encompass at least three distinct steps: i) receptor-binding to identify target bacteria, ii) translocation of CdiA-CT toxin across the target-cell envelope, and iii) activation of the toxin in the target-cell cytoplasm. Notably, the protective effects of cysK and acrB mutations are specific to the CDIUPEC536 and CDIEC93 pathways, respectively [13]. These findings raise the possibility that each CDI system/toxin exploits a unique set of proteins to inhibit target-cell growth.
CdiB and CdiA share significant homology across the proteobacteria, but the CDI systems of Burkholderiales exhibit a number of differences compared to other bacteria. Firstly, the variable toxin region in Burkholderia CdiA is typically demarcated by the (E/Q)LYN peptide motif rather than the VENN sequence found in most other bacteria [9,20]. Burkholderia toxins are modular and can be exchanged readily between Burkholderia CdiA proteins [9], but chimeric E. coli CdiA proteins carrying Burkholderia CdiA-CTs fused at the VENN sequence are not functional in CDI [1]. Secondly, CDI genes are arranged as cdiAIB clusters in Burkholderia, Variovorax and Cupriavidus species rather than the cdiBAI order found in other bacteria. This alternative gene arrangement is also correlated with a lack of "orphan" cdiA-CT/cdiI gene pairs. Orphan modules resemble the displaced 3´-fragments of full-length cdiA genes together with their cognate cdiI immunity genes [3,21]. Tandem arrays of orphan cdiA-CT/cdiI gene pairs are commonly found downstream of cdiBAI loci in γ-proteobacteria, and all strains of Neisseria meningitidis also carry well-defined orphan toxin/immunity clusters [21,22]. Finally, many Burkholderia CDI systems encode a small predicted lipoprotein, BcpO, between the cdiI and cdiB genes [20]. The function of BcpO is not understood completely, but it is required for CdiA secretion in Burkholderia thailandensis E264 [20]. Collectively, these observations suggest that the mechanisms of CDI in Burkholderia species are fundamentally distinct from other bacteria.
Here, we begin exploring Burkholderia CDI pathways using the CDIII Bp1026b system encoded on chromosome II of Burkholderia pseudomallei 1026b as a model. We took a genetic approach and isolated transposon mutants of B. thailandensis E264 that are resistant to inhibition by the CDIII Bp1026b system. Independent selections identified multiple transposon insertions in three genes—BTH_I0359, BTH_II0599, and BTH_I0986, each of which confers resistance to CDIII Bp1026b. BTH_I0359 encodes a small cytosolic protein of unknown function, BTH_II0599 encodes an integral membrane protein from the major facilitator superfamily (MFS), and BTH_I0986 encodes a predicted lipopolysaccharide (LPS) transglycosylase. We find that LPS structure is altered in BTH_I0986 mutants, suggesting that LPS may function as a receptor or co-receptor for CdiAII Bp1026b. These results demonstrate that the CDIII Bp1026b is distinct from previously described E. coli pathways, suggesting that multiple pathways exist to translocate CDI toxins into target bacteria.
Materials and Methods
Bacterial strains and growth conditions
Bacterial strains were derived from Burkholderia thailandensis E264 and are listed in Table 1. Bacteria were routinely cultured in LB media supplemented with the following antibiotics where appropriate: kanamycin (Kan) 500 μg/mL; tetracycline (Tet) 25 μg/mL; trimethoprim (Tp) 100 μg/mL; chloramphenicol (Cam) 34 μg/mL; and polymyxin B (PB) 100 μg/mL. CDIII Bp1026b competitions used Bt81 inhibitors, which are B. thailandensis E264 cells that express cdiAIB II Bp1026b from plasmid pJSW1–6 (Table 2) [9]. Bt81 inhibitors and target cells were grown individually for at least 48 h (to OD600 > 0.6) in M9-minimal media supplemented with 0.2% L-arabinose. Approximately 109 colony-forming units (cfu) of Bt81 inhibitors and 108 cfu of target cells were mixed in 150 μL of M9-minimal medium supplemented with 0.2% arabinose, 1 μg/mL thiamine and 0.3 μg/mL ferric citrate, and aliquots plated onto LB agar supplemented with Tet or Kan to enumerate viable inhibitors and targets (respectively) at time 0 h. The remaining cell mixture (100 μL) was spread onto M9-minimal medium agar supplemented with 0.2% L-arabinose, 1 μg/mL thiamine and 0.3 μg/mL ferric citrate and incubated for 24 h at 30°C. Cells were then harvested from the agar surface, and viable inhibitor and target cell counts were determined as total cfu on Tet and Kan (respectively) supplemented LB agar. The competitive index (C.I.) was calculated as the ratio of target cells to inhibitor cells at 24 h divided by the target to inhibitor ratio at time 0 h. CDIE264 competitions were conducted in a similar manner, except inhibitor and target cells were co-cultured on tryptone broth agar. For these latter competitions, the target cells were derived from strain Bt36, which carries a deletion of the entire cdiAIB E264 gene cluster [9]. CdiA-CTII Bp1026b toxicity was tested by expressing the toxin domain inside B. thailandensis cells. Plasmid pSCBAD-CTII1026b was introduced into E. coli DH5α and the resulting strain used in a four-parent mating with SM10λpir/pTNS3 [23,24], HB101 (pRK2013) [25] and B. thailandensis E264. Conjugation mixtures were split into two equal portions and plated on LB agar supplemented with PB, Tp and 0.2% D-glucose and LB agar supplemented with PB, Tp and 0.2% L-arabinose. The presence of exconjugants on plates supplemented with D-glucose and the simultaneous absence of exconjugant colonies after incubation at 37°C for 48 h on the L-arabinose containing plates was indicative of toxicity.
Table 1. Bacterial strains used in this study.
| Strains | Description | Source or Reference |
|---|---|---|
| B. thailandensis E264 | wild-type isolate | [54] |
| Bt5 | B. thailandensis E264 (pJSW2) | This study |
| Bt6 | glmS1::Tn7-Kan, KanR | [9] |
| Bt7 | glmS1::Tn7-cdiI 1026b-Kan, KanR | [9] |
| Bt28 | BTH_II0599::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 557 of coding sequence, TpR | This study |
| Bt29 | BTH_II0599::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 611 of coding sequence, TpR | This study |
| Bt30 | BTH_II0599::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 720 of coding sequence, TpR | This study |
| Bt32 | BTH_I0359::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 226 of coding sequence, TpR | This study |
| Bt33 | BTH_I0986::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 514 of coding sequence, TpR | This study |
| Bt35 | BTH_II0599::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 524 of coding sequence, TpR | This study |
| Bt36 | ΔcdiAIB glmS1::Tn7-kan, KanR | [9] |
| Bt45 | BTH_II0599::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 664 of coding sequence, TpR | This study |
| Bt47 | BTH_I0359::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 49 of coding sequence, TpR | This study |
| Bt49 | BTH_I0986::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 207 of coding sequence, TpR | This study |
| Bt50 | BTH_II0599::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 371 of coding sequence, TpR | This study |
| Bt51 | BTH_II0599::T23(ISlacZ-PrhaBo-FRT-Tp); T23 transposon inserted after nucleotide 521 of coding sequence, TpR | This study |
| Bt56 | ΔcdiAIB glmS1::Tn7-PrpsL-cdiI E264-kan, KanR | [9] |
| Bt79 | glmS1::Tn7-PrpsL-gfp-kan, KanR | T. Hoang |
| Bt81 | pJSW1–6, TetR | [9] |
| Bt83 | ΔcdiAIB glmS1::Tn7-kan ΔBTH_I0986, KanR | This study |
| Bt87 | glmS1::Tn7-kan ΔBTH_II0599, KanR | This study |
| Bt101 | glmS1::Tn7-kan pSCBAD::DsRed, KanR TpR | This study |
| Bt103 | glmS1::Tn7-kan ΔBTH_I0986, KanR | This study |
| Bt104 | ΔcdiAIB glmS1::Tn7-kan ΔBTH_II0599, KanR | This study |
| Bt111 | glmS1::Tn7-kan ΔBTH_I0986 pSCBAD::I0986, KanR TpR | This study |
| Bt121 | glmS1::Tn7-PrpsL-gfp-kan pJSW1–6, KanR TetR | This study |
| Bt123 | glmS1::Tn7-kan ΔBTH_I0986 pSCBAD::DsRed, KanR TpR | This study |
| Bt124 | glmS1::Tn7-kan ΔBTH_II0599 pSCBAD::DsRed, KanR TpR | This study |
| Bt132 | glmS1::Tn7-kan ΔBTH_I0359, KanR | This study |
| Bt134 | ΔcdiAIB glmS1::Tn7-kan ΔBTH_I0359, KanR | This study |
| Bt137 | glmS1::Tn7-kan ΔBTH_II0599 pSCBAD::II0599, KanR TpR | This study |
| Bt138 | glmS1::Tn7-kan ΔBTH_I0359 pSCBAD::I0359, KanR TpR | This study |
| Bt143 | glmS1::Tn7-kan ΔBTH_I0359 pSCBAD::DsRed, KanR TpR | This study |
Abbreviations: KanR, kanamycin-resistant; TetR, tetracycline-resistant; TpR, trimethoprim-resistant
Table 2. Plasmids used in this study.
| Plasmid | Description | Source or Reference |
|---|---|---|
| pEX18-Tp | Suicide vector containing pheS* gene for o-chlorophenylalanine counter-selection, TpR | [55] |
| pSCRhaB2 | Rhamnose-inducible promoter, TpR | [27] |
| pSCBAD | Derivative of pSCRhaB2 with E. coli araC and PBAD promoter, TpR | This study |
| pSCBAD-KX | Derivative of pSCRhaB2 with E. coli araC and PBAD promoter, TpR | This study |
| pJSW2 | Shuttle vector carrying oriVpVS1 oriVp15A oriT araC-PBAD,TetR | [9] |
| pJSW1–6 | pJSW2-cdiAIB II 1026b, expresses the Bp 1026b CDIII system under control of the arabinose-inducible PBAD promoter, TetR | [9] |
| pEX18-Tp:: ΔBTH_I0359 | BTH_I0359 deletion construct, TpR | This study |
| pEX18-Tp:: ΔBTH_II0599 | BTH_I0599 deletion construct, TpR | This study |
| pEX18-Tp:: ΔBTH_I0986 | BTH_I0986 deletion construct, TpR | This study |
| pSCBAD-KX::0359 | Arabinose-inducible expression of BTH_I0359, TpR | This study |
| pSCBAD::0599 | Arabinose-inducible expression of BTH_I0599, TpR | This study |
| pSCBAD::0986 | Arabinose-inducible expression of BTH_I0986, TpR | This study |
| pCH450-CTII 1026b | Arabinose-inducible expression of residues Met2821—Asn3122 of CdiAII Bp1026b, TetR | [9] |
| pSCBAD- CTII 1026b | Arabinose-inducible expression of residues Met2821—Asn3122 of CdiAII Bp1026b, TpR | This study |
| pTrc-DsRed | IPTG-inducible expression of DsRed, AmpR | [8] |
| pSCBAD::DsRed | Arabinose-inducible expression of DsRed, TpR | This study |
Abbreviations: AmpR, ampicillin-resistant; TetR, tetracycline-resistant; TpR, trimethoprim-resistant.
Selection of CDIR mutants
A library of random T23-TpR transposon-insertion mutants (>38,000 unique insertions) [26] was co-cultured with B. thailandensis Bt81 inhibitor cells [9]. Inhibitors and mutant target cells were mixed at a 10:1 ratio and plated onto M9 minimal agar medium supplemented with 0.2% L-arabinose 1 μg/mL thiamine and 0.3 μg/mL ferric citrate. After 24 h co-culture at 37°C, cells were harvested from the agar surface and surviving target cells isolated on Tp-supplemented LB agar. The target cells were pooled and subjected to two additional rounds of selection against CDIII Bp1026b expressing inhibitor cells. After enrichment, individual clones were selected and tested for CDI-resistance in competition co-cultures with Bt81 inhibitor cells. Transposon-insertion junctions were amplified by arbitrary PCR using primers LacZ-124L2, LacZ-148 and CEKG 2E/K/L (Table 3). The resulting products were sequenced with primers LacZ211 and CEKG4 to identify insertion sites (Table 3).
Table 3. Oligonucleotides used in this study.
| Oligonucleotide | Sequence a | Reference |
|---|---|---|
| 2725 | 5´—ATA Tcc cgg gTC ATC GAT CGG AGG TGT TCG | This study |
| 2729 | 5´—ATA Tcc cgg gTC ATC GCC CTC CGT TAC G | This study |
| 3103 | 5´—CAA CAA aag ctt CAT CGA CAC GCT CGT GGG AGA | This study |
| 3104 | 5´—GAT CGT ACT GGA TCG CTGC ACG CCA AAA ACC AAC GGC CGG ACC C | This study |
| 3105 | 5´—GCG TGC AGC GAT CCA GTA CGA TC | This study |
| 3106 | 5´—CAA CAA ggt acc CGT GTC GCC GAG CAA CAG ATG A | This study |
| 3182 | 5´—CAA CAA aag ctt CAT CAG CCG AAC CTG CGC AGC | This study |
| 3183 | 5´—GAT CGG AGG TGT TCG GCA GCT TCG CGG AAC CAC ACG TAG CCG G | This study |
| 3184 | 5´—GAA GCT GCC GAA CAC CTC CGA TC | This study |
| 3185 | 5´—CAA CAA ggt acc GAG CAG CGG CTT GTA CGC CTT | This study |
| 3258 | 5´—GCG Cga att cCG AGA CCC ACG CAT GCA AC | This study |
| 3259 | 5´—GCG Cga att cCA GGG CGC CAT TCG ATG AC | This study |
| 3296 | 5´—ATA Taa gct tCT GCG TGA TCG ACA AGA GC | This study |
| 3297 | 5´—CCG CCA TGC AAA TGA TCT ACA ACC CGT CGT TCT CCA CTG | This study |
| 3298 | 5´—CAG TGG AGA ACG ACG GGT TGT AGA TCA TTT GCA TGG CGG | This study |
| 3299 | 5´—GCG Ctc tag aGA TCG GCG ACG AAA CGA TCT | This study |
| CH1730 | 5´—GTA cca tgg TAC CTT CCT CCT GCT AGC | This study |
| CH2059 | 5´—AGT ggt acc ATG CAA ATG ATC TAC AAC AGC | This study |
| CH2799 | 5´—GAT atg cat AAT GTG CCT GTC AAA TGG | This study |
| CH2800 | 5´—TAC TGC AGC CCT CGA GTC AGT GGA GAA CGA CG | This study |
| LacZ-124L2 | 5´—CAG TCA CGA CGT TGT AAA ACG ACG | This study |
| LacZ-148 | 5´—GGG TAA CGC CAG GTT TTT CC | This study |
| LacZ-211 | 5´—TGC GGG CCT CTT CGC TAT TA | This study |
| CEKG 2E | 5´—GGC CAC GCT CGA CTA GTA CNN NNN NNN NNA TGT A | This study |
| CEKG 2K | 5´—GGC CAC GCG TCG ACT AGT ACN NNN NNN NNN AGT GC | This study |
| CEKG 2L | 5´—GGC CAC GCG TCG ACT ACN NNN NNN NNN CTG AG | This study |
| CEKG 4 | 5´—GGC CAC GCG TCG ACT AGT AC | This study |
aRestriction endonuclease sites are in lowercase; N indicates equal mixture of all four deoxyribonucleotides.
Construction of Plasmids and Chromosomal Deletions
Plasmid pSCBAD is a derivative of pSCRhaB2 [27]. The araC gene and araBAD promoter were excised from plasmid pCH450 [28] by NsiI/NcoI digestion and ligated to plasmid pSCRhaB2. This sub-cloning step replaces the original rhamnose-inducible promoter with an arabinose-inducible promoter. Plasmid pCH450 was amplified with primers CH1730/CH2799 and the resulting product cloned into pSCRhaB2 using NsiI/NcoI restriction sites to generate plasmid pSCBAD-KX. The BTH_II0599 and BTH_I0986 genes were amplified from chromosomal DNA using primers 3258/2725 and 3259/2729 (respectively), and the resulting products ligated to plasmid pSCBAD using EcoRI and XmaI restriction sites. BTH_I0359 was amplified using primers CH2059/CH2800 and ligated into pSCBAD-KX using KpnI and PstI restriction sites. The region encoding CdiA-CTII Bp1026b (residues Met2821—Asn3122 of full-length CdiAII Bp1026b) was subcloned from plasmid pCH450-CTII 1026b [9] into pSCBAD using NcoI and PstI restriction sites. The DsRed coding sequence was subcloned from plasmid pTrc-DsRed [8] into pSCBAD using NcoI and PstI restriction sites.
Gene deletions were constructed by allelic exchange as described previously [29]. DNA sequences upstream and downstream of the target gene were amplified and the two PCR products combined into one fragment using overlapping end PCR (OE-PCR) [30]. The OE-PCR products were ligated to plasmid pEX18-Tp (Table 2) using HindIII and KpnI/XbaI restriction sites. The BTH_I0359 deletion construct was generated using primer pairs 3296/3297 and 3298/3299; the BTH_II0599 deletion construct was generated using primer pairs 3182/3183 and 3184/3185; and the BTH_I0986 deletion construct generated using primer pairs 3103/3104 and 3105/3106 (Table 3).
Cell-cell adhesion
GFP-labeled B. thailandensis E264 [24] carrying plasmid pJSW1–6 (Bt121) or pJSW2 (Bt5) were grown overnight in tryptone broth, then diluted 1:50 in fresh tryptone broth and grown to OD600 ~0.5. DsRed-labeled target strains (Bt101, Bt123, Bt124 and Bt143) were grown in minimal M9-media supplemented with 0.2% L-arabinose for at least 48 h to OD600 ~ 0.5. Inhibitor and target cells were mixed at a 1:1 ratio and incubated for 30 min at room temperature to allow cell-cell binding. Cell suspensions were then diluted 1:50 into sterile filtered 1X phosphate buffered saline and analyzed by flow cytometry. Samples were run on an Accuri C6 flow cytometer using FL1 (533/30nm, GFP) and FL2 (585/40nm, DsRed) fluorophore filters. Cell-cell binding was measured as the percent of target cells in aggregates with inhibitor cells divided by the total number of target cells. Binding data were normalized to the level of cell-cell binding between wild-type target cells (Bt101) and CDIII Bp1026b expressing inhibitors (Bt121).
Lipopolysaccharide (LPS) analysis
Bacteria were grown to OD600 > 0.6 in M9-minimal medium supplemented with 0.2% L-arabinose and LPS was harvested from an equivalent of 10 mL of OD600 = 1 culture using the LPS Extraction Kit (Boca Scientific, USA). Purified LPS was resolved on a 4–20% polyacrylamide Tris-glycine SDS gel (Thermo Scientific) and visualized using ProQ 300 Emerald LPS stain (Molecular Probes, USA).
Results
Isolation of CDIR mutants
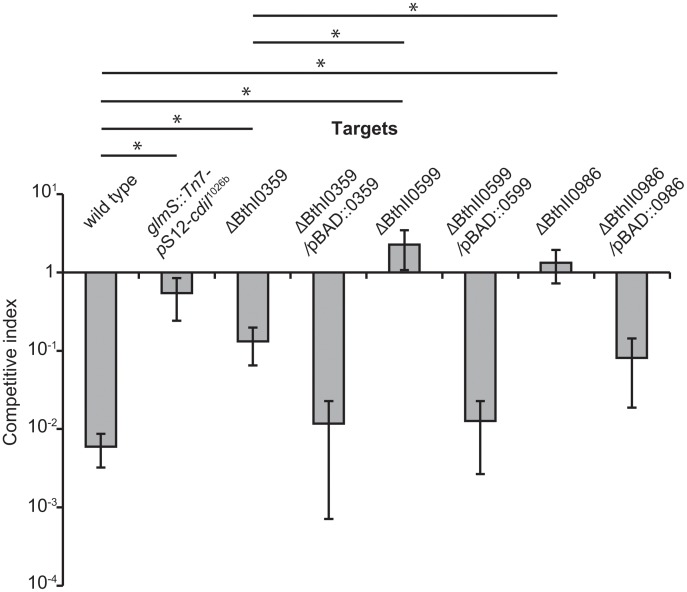
To gain insight into the CDI pathways in Burkholderia species, we used a genetic approach to identify target-cell genes that are required for growth inhibition. We reasoned that mutants with disruptions in the genes encoding the CDI receptor, toxin translocators and toxin activators would be CDI-resistant (CDIR). B. thailandensis E264 cells were subjected to random mutagenesis using a Tn5-based T23 transposon. Two independent T23 mutant pools were then co-cultured on solid media with B. thailandensis inhibitor cells that express the B. pseudomallei CDIII Bp1026 system from a plasmid vector (Bt81, Table 1). CDIR mutants were enriched through three cycles of co-culture with inhibitor bacteria, and 20 clones were selected for the identification of transposon insertion sites. Each mutant contained a T23 insertion within BTH_I0359, BTH_II0599 or BTH_I0986; corresponding to eleven unique insertion sites (Fig. 1A and Table 1). BTH_I0359 is located upstream of the genes for methionine biosynthesis and encodes a hypothetical protein of 85 amino acid residues (Fig. 1A). BTH_II0599 encodes a predicted major facilitator superfamily (MFS) protein and is likely to be an inner-membrane localized transporter. BTH_I0986 is annotated as lipooligosaccharide (LOS) glycosyl-transferase G and is located within an LPS biosynthesis operon on chromosome I (Fig. 1A). We picked two mutants for each disrupted gene and confirmed that each was 10- to 100-fold more resistant to the CDIII 1026b system than wild-type B. thailandensis (Fig. 1B).
Fig 1. Selection of CDIR mutants of B. thailandensis E264.
A) T23 transposon insertion sites were identified by semi-arbitrary PCR as described in Methods. Orange arrows indicate T23 insertions in the same transcriptional orientation of the disrupted gene and blue arrows indicate insertions in the opposite orientation. The corresponding CDIR mutant strain number is given above each arrow. Automated gene annotations are given below each ordered locus designation. GT-1, GT-2 and GT-9 indicate predicted glycosyltransferase families and DUF designations indicate domains of unknown function. B) The indicated B. thailandensis strains were co-cultured with Bt81 inhibitors (Table 1) that express the CDIII Bp1026b system for 24 h on solid medium, and the competitive index was calculated as described in Materials and Methods. The strain labeled cdiI 1026b expresses the cognate CdiIII Bp1026b immunity protein. Data represent the mean ± SEM for three independent experiments. Analysis of the data using Student’s t-test is shown at the top, with bars between samples that were statistically significant (* = p< 0.05).
Because multiple independent insertions were identified for each gene, it is likely that these mutations are directly responsible for the CDIR phenotype. However, it is possible that the mutant strains carry additional unidentified mutations that contribute to resistance. To ascertain the roles of BTH_I0359, BTH_I0986 and BTH_II0599 in CDI, we constructed in-frame deletions of each gene and tested the resulting mutants for CDIR. As expected, the deletion mutants each had CDIR phenotypes that were very similar to the originally isolated transposon-insertion mutants (Figs. 1B and 2). ΔBTH_I0986 and ΔBTH_II0599 mutants were fully resistant to CDIII Bp1026b, whereas the ΔBTH_I0359 mutant was only partially protected from inhibition (Fig. 2). These results strongly suggest that each gene is required for the CDIII Bp1026b inhibition pathway. We also showed that each deletion mutant was rendered sensitive to CDIII Bp1026b when complemented with a plasmid-borne copy of the appropriate gene (Fig. 2). These latter data exclude effects from transcriptional polarity and indicate that BTH_I0359, BTH_II0599 and BTH_I0986 are required for full sensitivity to the CDIII Bp1026b system.
Fig 2. Complementation of CDIR mutations.
The indicated B. thailandensis strains were co-cultured with Bt81 inhibitors (Table 1) that express the CDIII Bp1026b system for 24 h on solid medium, and the competitive index was calculated as described in Materials and Methods. The strain labeled cdiI 1026b expresses the cognate CdiIII Bp1026b immunity protein. Plasmid-borne copies of BTH_I0359, BTH_I0986 and BTH_II0599 genes were expressed from an L-arabinose inducible promoter. Data represent the mean ± SEM for three independent experiments. Sample values that were statistically different from one another (p < 0.05) are shown by bars with an asterisk (see Fig. 1).
Resistance mutations are specific for the CDIII Bp1026b system
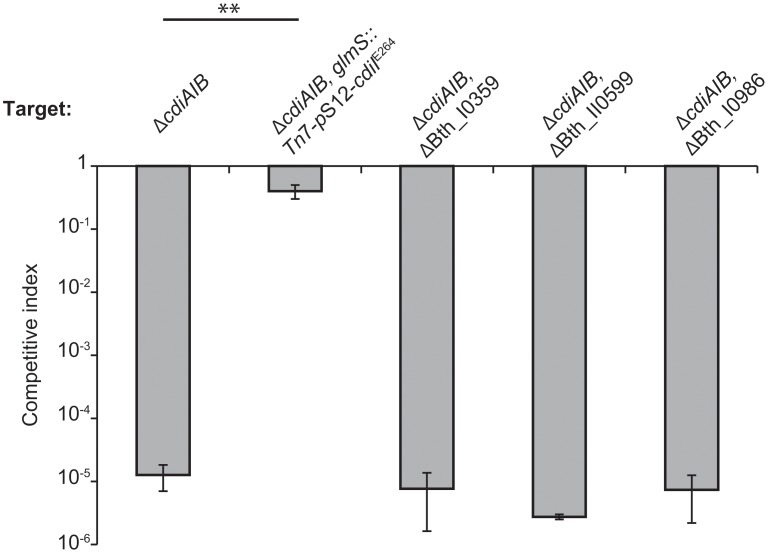
B. thailandensis E264 carries its own CDI system (CDIE264) and the CdiAE264 protein shares approximately 53% sequence identity with CdiAII Bp1026b. However, the CdiA-CTE264 and CdiA-CTII Bp1026b toxins are not homologous and have different nuclease activities [9], suggesting that the two toxin-delivery pathways could be distinct. Therefore, we asked whether mutations in BTH_I0359, BTH_II0599 and BTH_I0986 also provide resistance to CDIE264. We first confirmed that ΔcdiAIB E264 mutants, which lack immunity to CDIE264, are inhibited by wild-type CDI+ B. thailandensis cells as reported previously [9,20]. B. thailandensis ΔcdiAIB E264 targets were inhibited approximately 105-fold during co-culture with CDI+ cells (Fig. 3). This growth inhibition is attributable to CDIE264, because the target cells were fully protected when complemented with the cdiI E264 gene on a Tn7-based vector (Fig. 3). We then introduced ΔBTH_I0359, ΔBTH_II0599 and ΔBTH_I0986 mutations into the ΔcdiAIB E264 background and found that each of the resulting strains was still sensitive to CDIE264 (Fig. 3). These results demonstrate mutations in BTH_I0359, BTH_II0599 and BTH_I0986 specifically confer resistance to the CDIII Bp1026b system.
Fig 3. The CDIR phenotype is specific for CDIIIBp1026b.
The indicated B. thailandensis strains were co-cultured with wild-type (cdiAIB +) B. thailandensis E264 cells for 24 h on solid medium, and the competitive index was calculated as described in Materials and Methods The strain labeled cdiI E264 expresses the cognate CdiIE264 immunity protein. Data represent the mean ± SEM for three independent experiments. Sample values that were statistically different from one another (p < 0.01) are shown by a bar with a double asterisk (see Fig. 1).
CDIR genes are not required to activate the CdiA-CTII Bp1026b toxin
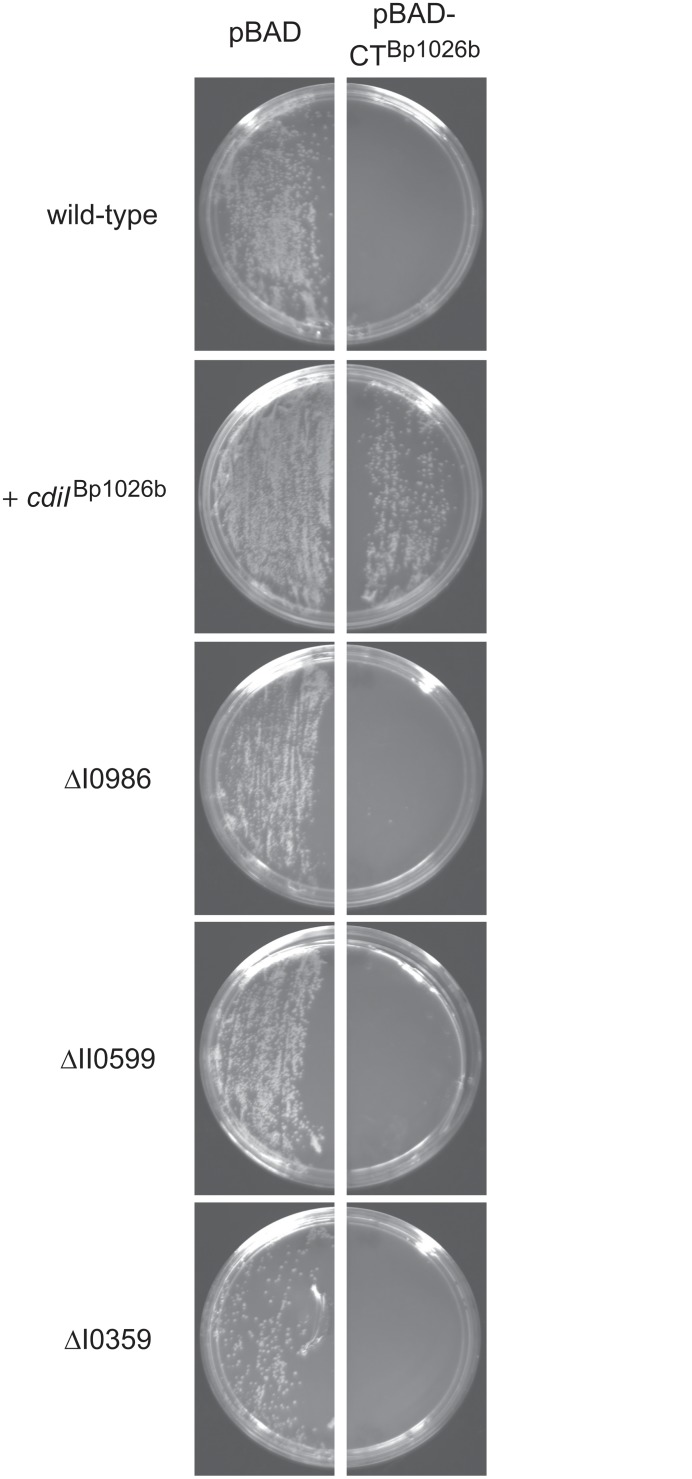
Work with the CDI536 system from UPEC 536 has shown that some CDI toxins must be activated by so-called "permissive" factors. CdiA-CT536 only has tRNase activity when bound to CysK, and therefore E. coli ΔcysK mutants are completely resistant to the toxin, even when produced at high levels inside the cell [13,31]. Based on the CDI536 paradigm, we asked whether any of the Burkholderia CDIR genes encode proteins with permissive factor function. We placed the cdiA-CT II Bp1026b coding sequence under control of an arabinose-inducible PBAD promoter and moved the construct onto a mobilizable plasmid. This plasmid can be stably maintained in E. coli cells under conditions that repress transcription from PBAD [9]. We then tested whether the cdiA-CT II Bp1026b plasmid could be introduced into B. thailandensis cells through tri-parental mating. No exconjugants were produced from matings to introduce the toxin plasmid into wild-type cells, but dozens of exconjugants were obtained when recipient cells expressed the cognate cdiI II Bp1026b immunity gene (Fig. 4). These results indicate that CdiA-CTII Bp1026b is toxic when expressed inside B. thailandensis cells and that CdiIII Bp1026b neutralizes the toxin to allow cell growth. We next performed matings with ΔBTH_I0359, ΔBTH_II0599 and ΔBTH_I0986 recipient strains, each of which produced no exconjugants (Fig. 4). Together, these results show that none of the CDIR mutations protect the cell from intracellular CdiA-CTII Bp1026b, indicating that the corresponding gene products do not function as CDI permissive factors.
Fig 4. Toxicity of CdiA-CTIIBp1026b expressed inside B. thailandensis cells.
Plasmids pSCBAD and pSCBAD::cdiA-CT II Bp1026b were introduced into the indicated B. thailandensis strains by conjugation as described in Materials and Methods. The mating mixtures were split into equal portions and plated onto LB agar with Polymyxin B and Trimethoprim supplemented with either D-glucose (left panels) or L-arabinose (right panels). See Materials and Methods.
BTH_I0986 influences the binding of inhibitor and target cells
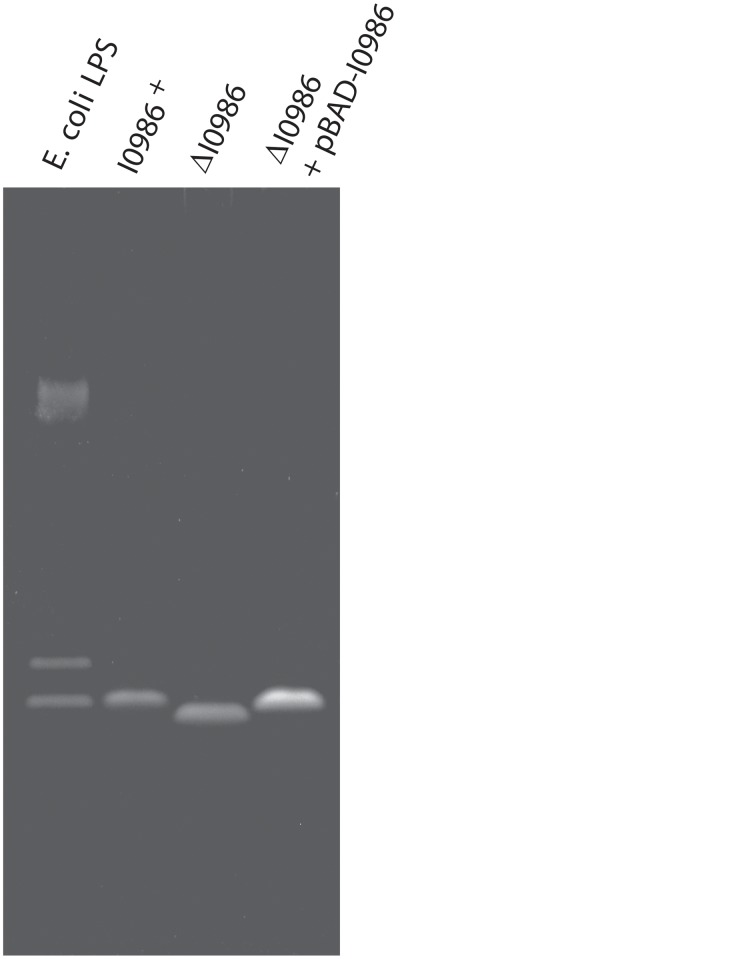
We next considered the possibility that the CDIR genes may influence the recognition of target cells. The BTH_I0986 mutation is of particular interest because this gene belongs to the GT1 family of glycosyltransferases and is predicted to function in lipopolysaccharide (LPS) biosynthesis. Thus, the BTH_I0986 mutation could alter LPS structure, thereby preventing CDIII Bp1026b inhibitor cells from recognizing and/or binding to target bacteria. To determine whether BTH_I0986 influences LPS structure, we used SDS-PAGE to analyze LPS isolated from wild-type and ΔBTH_I0986 cells. Surprisingly, we found that LPS isolated from wild-type B. thailandensis E264 cells lacked polymeric O antigen (Fig. 5), in contrast to previous reports [32,33]. The LPS from ΔBTH_I0986 mutants also lacked an O-antigen ladder, but migrated more rapidly during electrophoresis than LPS from BTH_I0986+ cells (Fig. 5). Complementation with plasmid-borne BTH_I0968 restored mutant LPS to the wild-type mobility (Fig. 5). Therefore, disruption of BTH_I0986 alters the target-cell surface by changing LPS structure.
Fig 5. Lipopolysaccharide (LPS) analysis.
LPS was isolated from the indicated B. thailandensis strains and analyzed by SDS-PAGE using fluorescent detection. The LPS standard is from Escherichia coli serotype 055:B5.
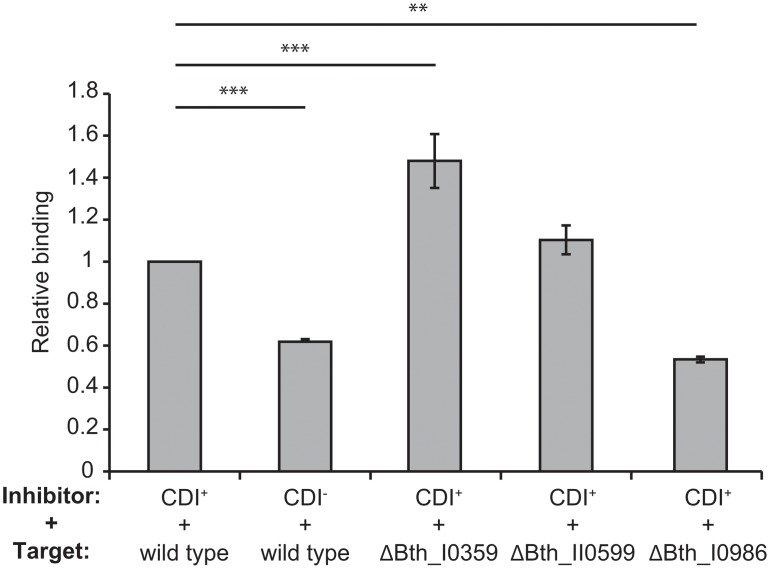
In the E. coli EC93 system, inhibitor cells bind stably to target bacteria and the resulting cell aggregates can be detected and quantified using flow cytometry [12,18]. Therefore, we used the same approach to examine the binding of CDIII Bp1026b inhibitors to different target cell strains. We mixed GFP-labeled CDIII Bp1026b inhibitors at a 1:1 ratio with DsRed-labeled target cells and analyzed the suspensions by flow cytometry to detect events with both green and red fluorescence, which correspond to aggregates containing both inhibitor and target cells. This analysis showed that approximately 40% more target cells bind to CDIII Bp1026b inhibitors compared to CDI– mock inhibitors (Fig. 6).
Fig 6. Cell-cell binding.
CDI+ (Bt81) and CDI– (wild-type B. thailandensis) cells were labeled with GFP and mixed with the indicated DsRed-labeled target cells, then analyzed by flow cytometry to detect and quantify cell-cell aggregates. Binding was normalized to 1.0 for the interaction between Bt81 and wild-type B. thailandensis cells. Sample values that were statistically different from one another are shown by bars; ** = p < 0.01, and *** = p < 0.001 (see Fig. 1). We then tested the three CDIR target strains and found that ΔBTH_I0986 targets interacted poorly with inhibitor cells, similar to the level observed with CDI– mock inhibitors (Fig. 6). In contrast, the ΔBTH_II0599 mutant showed wild-type binding levels, and ΔBTH_I0359 targets showed increased binding to inhibitor cells (Fig. 6). Together, these results suggest that mutations in BTH_I0986 confer CDIR by altering the cell surface to prevent stable associations with CDIII Bp1026b inhibitor cells.
Discussion
The results presented here show that at least three genes, BTH_I0359, BTH_I0986 and BTH_II0599, are required for B. thailandensis cells to be fully inhibited by the CDIII Bp1026b system. We identified each gene in two independent selection experiments, suggesting that they represent the major non-essential genes required for the CDIII Bp1026b pathway. Indeed, BTH_II0599 and BTH_I0986 are particularly critical because deletion of either gene provides full resistance to target bacteria. Notably, the three B. thailandensis genes identified here are distinct from those previously identified in E. coli within the CDIEC93 growth inhibition pathway [12]. These results suggest that the CDIII Bp1026b and CDIEC93 systems deliver toxins through different pathways. CDI is initiated through direct binding interactions between CdiA and receptors on the surface of target bacteria. CdiAEC93 uses the E. coli BamA protein as a receptor and appears to bind specific epitopes within extracellular loops eL6 and eL7 [12,18]. Our results here suggest that B. pseudomallei CdiAII Bp1026b may exploit LPS as a target-cell receptor. BTH_I0986 is a predicted transglycosylase and mutants lacking this enzyme have altered LPS structure (Fig. 5). Moreover, the ΔBTH_I0986 mutant shows defects in binding to CDIII Bp1026b inhibitor cells (Fig. 6), consistent with a role in receptor function. Surprisingly, we also found that our B. thailandensis E264 isolate lacks a detectable O-antigen ladder. This could account for the fact that we did not identify any additional LPS biosynthesis genes in independent selections. It is unclear whether the rough LPS phenotype reflects phase variation [34–36], or whether a rough-strain mutant was selected through laboratory passage. In either event, it will be important to determine how O-antigen influences CDI susceptibility in Burkholderia species. Although our results do not support a role for BamA in Burkholderia CDI, we acknowledge that CDIR alleles of the essential bamA gene would be difficult to isolate using a transposon mutagenesis approach. If Burkholderia BamA does function as a CDI receptor, then the interactions must be distinct from the CDIEC93 system, because BamA loops eL6 and eL7 loops differ significantly between E. coli and Burkholderia species (Fig. 7) [37].
Fig 7. Alignment of BamA proteins.
The β-barrel portion of BamA proteins from E. coli K-12 (Uniprot: P0A940), Enterobacter cloacae ATCC 13047 (D5CHY0), Vibrio cholerae ATCC 39315 (Q9KPW0), B. thailandensis E264 (Q2SWZ0) and B. pseudomallei 1026b (I1WHZ2). Sequences that correspond to extracellular loops (eL) are indicated above the alignment and are based on the crystal structures of BamA from Neisseria gonorrhoeae and Haemophilus ducreyi [37]. The alignment was rendered using Jalview 2.8 [53] at 30% sequence identity.
Because CdiA-CTII Bp1026b is a tRNase, this toxin must be transported into the target-cell cytoplasm to reach its substrate. CDI toxin translocation is poorly understood, but our recent work with E. coli indicates that transport across the target-cell outer membrane is energy-independent, whereas translocation into the cytoplasm requires the proton-motive force [38]. These findings raise the possibility that BTH_II0599, a predicted MFS transporter, is co-opted to translocate the tRNase domain across the target-cell inner membrane. In this model, periplasmic toxin would bind to BTH_II0599 and be driven into the cytoplasm by either the chemical or electrical potential of the pmf. These interactions are specific because the ΔBTH_II0599 mutation provides no protection against the B. thailandensis CDIE264 system, suggesting that the CdiA-CTE264 toxin must exploit another entry pathway. Although MFS proteins harness chemiosmotic gradients to transport a variety of metabolites [39,40], it seems unlikely that the transporter could translocate a folded nuclease domain in the same manner as small solutes. One possibility is that CdiA-CTII Bp1026 has an autonomous membrane translocation activity, but requires BTH_II0599 as a receptor to facilitate insertion into the inner membrane. This model is similar to that proposed by Kleanthous and colleagues for the translocation of colicin nuclease domains, some of which interact with phospholipids and form pores in membranes [41–43].
The role of BTH_I0359 in the CDIII Bp1026b pathway remains enigmatic, in part because the function of this gene is unknown. BTH_I0359 encodes a DUF3567 family member (PF12091, http://pfam.xfam.org/family/PF12091), which is only found within the order Burkholderiales. The gene neighborhood of BTH_I0359 includes the downstream metH a and metH b (which encode a split methionine synthase) and an upstream DUF3108 family member. DUF3567 and DUF3108 genes are linked throughout all the Burkholderiales, whereas linkage to metH is limited to Burkholderia, Ralstonia and Cupriavidus species. DUF3108 genes encode outer-membrane β-barrel proteins with a characteristic YmcC fold (PDB: 3FZX). Although strong genetic linkage is often indicative of a functional relationship, we did not isolate BTH_I0360 mutations in our CDIR selections, even though this gene is not essential for B. thailandensis growth [26]. We have also excluded a "permissive" factor function for BTH_I0359. Permissive factors are target-cell proteins that are required to activate CdiA-CT toxins in the target-cell cytoplasm [13]. This conclusion is also supported by previous studies showing that purified CdiA-CTII Bp1026b has tRNase activity in vitro, and therefore does not require an additional factor for activation [8].
All B. pseudomallei strains contain at least one CDI system, and some isolates carry up to three loci [9]. Each system can be placed into one of 10 different toxin/immunity groups [9,20], suggesting that CDI mediates competition between different B. pseudomallei strains. Using B. thailandensis as a model, Cotter and colleagues have recently demonstrated that such competition does in fact occur in mixed-strain biofilms, and that CDI influences the composition of these communities [20,44]. Additionally, there are indications that B. pseudomallei and B. thailandensis do not co-inhabit the same environmental niches [45], again suggesting that anti-bacterial competition systems shape their environmental distributions. If Burkholderia species do in fact directly antagonize one another in the environment, then type VI secretion systems (T6SS) are more likely to effect this competition. B. thailandensis and B. pseudomallei strains all carry multiple T6SS, which have been shown to deploy toxins against both bacteria and eukaryotic targets [46–49]. Moreover, a given T6SS is capable of killing many different species of Gram-negative bacteria [50–52]. In contrast, CDI is a receptor-mediated process, and therefore variations in the cell-surface receptor epitopes restrict inhibition activity to a subset of bacteria [18]. In accord with this general model, data presented here show that CDIE264 is significantly more effective against B. thailandensis targets than CDIII Bp1026b. Together, these observations indicate that CDI is used primarily to differentiate sibling cells from other closely related bacteria.
Data Availability
All relevant data are within the paper.
Funding Statement
This work received support from the following sources: National Institutes of Health U54 AI065359 (DAL); National Institutes of Health UO1 GM102318 (CSH); Santa Barbara Cottage Hospital (SK), http://cottagehealthsystem.org/tabid/1026/Default.aspx; Carl Tryggers Stiftelse (SK); Wenner-Gren Foundation (SK), http://www.swgc.org/welcome.aspx. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aoki SK, Diner EJ, de Roodenbeke CT, Burgess BR, Poole SJ, Braaten BA, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 2010; 468: 439–442. 10.1038/nature09490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. Contact-dependent inhibition of growth in Escherichia coli . Science 2005; 309: 1245–1248. [DOI] [PubMed] [Google Scholar]
- 3. Ruhe ZC, Low DA, Hayes CS. Bacterial contact-dependent growth inhibition. Trends Microbiol 2013; 21: 230–237. 10.1016/j.tim.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayes CS, Koskiniemi S, Ruhe ZC, Poole SJ, Low DA. Mechanisms and biological roles of contact-dependent growth inhibition systems. Cold Spring Harb Perspect Med 2014; 4 pii: a010025 10.1101/cshperspect.a010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kajava AV, Cheng N, Cleaver R, Kessel M, Simon MN, Willery E. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol Microbiol 2001; 42: 279–292. [DOI] [PubMed] [Google Scholar]
- 6. Zhang D, Iyer LM, Aravind L. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res 2011; 39: 4532–4552. 10.1093/nar/gkr036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck CM, Morse RP, Cunningham DA, Iniguez A, Low DA, Goulding CW. CdiA from Enterobacter cloacae delivers a toxic ribosomal RNase into target bacteria. Structure 2014; 22: 707–718. 10.1016/j.str.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morse RP, Nikolakakis KC, Willett JL, Gerrick E, Low DA, Hayes CS, et al. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc Natl Acad Sci U S A 2012; 109: 21480–21485. 10.1073/pnas.1216238110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikolakakis K, Amber S, Wilbur JS, Diner EJ, Aoki SK, Poole SJ, et al. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol Microbiol 2012; 84: 516–529. 10.1111/j.1365-2958.2012.08039.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aoki SK, Webb JS, Braaten BA, Low DA. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli . J Bacteriol 2009; 191: 1777–1786. 10.1128/JB.01437-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct 2012; 7: 18 10.1186/1745-6150-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aoki SK, Malinverni JC, Jacoby K, Thomas B, Pamma R, Trinh BN, et al. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol 2008; 70: 323–340. 10.1111/j.1365-2958.2008.06404.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diner EJ, Beck CM, Webb JS, Low DA, Hayes CS. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI). Genes Dev 2012; 26: 515–525. 10.1101/gad.182345.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 2003; 299: 262–265. [DOI] [PubMed] [Google Scholar]
- 15. Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol 2004;164: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli . Cell 2005; 121: 235–245. [DOI] [PubMed] [Google Scholar]
- 17. Werner J, Misra R. YaeT (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli . Mol Microbiol 2005; 57: 1450–1459. [DOI] [PubMed] [Google Scholar]
- 18. Ruhe ZC, Wallace AB, Low DA, Hayes CS. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. MBio 2013; 4: 00480–13 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli . Mol Microbiol 1995; 16: 45–55. [DOI] [PubMed] [Google Scholar]
- 20. Anderson MS, Garcia EC, Cotter PA. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent growth inhibition systems. PLOS Genet 2012; 8: e1002877 10.1371/journal.pgen.1002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poole SJ, Diner EJ, Aoki SK, Braaten BA, t'Kint de Roodenbeke C, Low DA, et al. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLOS Genet 2011; 7: e1002217 10.1371/journal.pgen.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arenas J, Schipper K, van Ulsen P, van der Ende A, Tommassen J. Domain exchange at the 3' end of the gene encoding the fratricide meningococcal two-partner secretion protein A. BMC Genomics 2013; 14: 622 10.1186/1471-2164-14-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR . J Bacteriol 1988; 170: 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi KH, Mima T, Casart Y, Rholl D, Kumar A, Beacham IR, et al. Genetic tools for select-agent-compliant manipulation of Burkholderia pseudomallei . Appl Environ Microbiol 2008. 74: 1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ditta G, Stanfield S, Corbin D, Helinski DR. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A 1980; 77: 7347–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gallagher LA, Ramage E, Patrapuvich R, Weiss E, Brittnacher M, Manoil C. Sequence-defined transposon mutant library of Burkholderia thailandensis . MBio 2013; 4: e00604–00613. 10.1128/mBio.00604-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cardona ST, Valvano MA. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia . Plasmid 2005; 54: 219–228. [DOI] [PubMed] [Google Scholar]
- 28. Hayes CS, Sauer RT. Cleavage of the A site mRNA codon during ribosome pausing provides a mechanism for translational quality control. Mol Cell 2003; 12: 903–911. [DOI] [PubMed] [Google Scholar]
- 29. Barrett AR, Kang Y, Inamasu KS, Son MS, Vukovich JM, Hoang TT, et al. Genetic tools for allelic replacement in Burkholderia species. Applied and Environmental Microbiology 2008; 74: 4498–4508. 10.1128/AEM.00531-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aiyar A, Xiang Y, Leis J. Site-directed mutagenesis using overlap extension PCR. Methods Mol Biol. 1996; 57: 177–191. [DOI] [PubMed] [Google Scholar]
- 31. Beck CM, Diner EJ, Kim JJ, Low DA, Hayes CS. The F pilus mediates a novel pathway of CDI toxin import. Mol Microbiol 2014; 93: 276–290. 10.1111/mmi.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stone JK, Mayo M, Grasso SA, Ginther JL, Warrington SD, Allender CJ, et al. Detection of Burkholderia pseudomallei O-antigen serotypes in near-neighbor species. BMC Microbiol 2012; 12: 250 10.1186/1471-2180-12-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tuanyok A, Stone JK, Mayo M, Kaestli M, Gruendike J, Georgia S, et al. The genetic and molecular basis of O-antigenic diversity in Burkholderia pseudomallei lipopolysaccharide. PLOS Negl Trop Dis 2012; 6: e1453 10.1371/journal.pntd.0001453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Broadbent SE, Davies MR, van der Woude MW. Phase variation controls expression of Salmonella lipopolysaccharide modification genes by a DNA methylation-dependent mechanism. Mol Microbiol 2010; 77: 337–353. 10.1111/j.1365-2958.2010.07203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cota I, Blanc-Potard AB, Casadesus J. STM2209-STM2208 (opvAB): a phase variation locus of Salmonella enterica involved in control of O-antigen chain length. PLOS One 2012; 7: e36863 10.1371/journal.pone.0036863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seed KD, Faruque SM, Mekalanos JJ, Calderwood SB, Qadri F, Camilli A. Phase variable O antigen biosynthetic genes control expression of the major protective antigen and bacteriophage receptor in Vibrio cholerae O1. PLOS Pathog 2012; 8: e1002917 10.1371/journal.ppat.1002917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noinaj N, Kuszak AJ, Gumbart JC, Lukacik P, Chang H, Easley NC, et al. Structural insight into the biogenesis of beta-barrel membrane proteins. Nature 2013; 501: 385–390. 10.1038/nature12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruhe ZC, Nguyen JY, Beck CM, Low DA, Hayes CS. The proton-motive force is required for translocation of CDI toxins across the inner membrane of target bacteria. Mol Microbiol 2012; 94: 466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Law CJ, Maloney PC, Wang DN. Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol 2008; 62: 289–305. 10.1146/annurev.micro.61.080706.093329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fluman N, Bibi E. Bacterial multidrug transport through the lens of the major facilitator superfamily. Biochim Biophys Acta 2009; 1794: 738–747. 10.1016/j.bbapap.2008.11.020 [DOI] [PubMed] [Google Scholar]
- 41. Mosbahi K, Lemaitre C, Keeble AH, Mobasheri H, Morel B, James R,et al. The cytotoxic domain of colicin E9 is a channel-forming endonuclease. Nat Struct Biol 2002; 9: 476–484. [DOI] [PubMed] [Google Scholar]
- 42. Mosbahi K, Walker D, James R, Moore GR, Kleanthous C. Global structural rearrangement of the cell penetrating ribonuclease colicin E3 on interaction with phospholipid membranes. Protein Sci 2006; 15: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mosbahi K, Walker D, Lea E, Moore GR, James R, Kleanthous C. Destabilization of the colicin E9 Endonuclease domain by interaction with negatively charged phospholipids: implications for colicin translocation into bacteria. J Biol Chem 2004; 279: 22145–22151. [DOI] [PubMed] [Google Scholar]
- 44. Anderson MS, Garcia EC, Cotter PA. Kind discrimination and competitive exclusion mediated by contact-dependent growth inhibition systems shape biofilm community structure. PLOS Pathog 2014; 10: e1004076 10.1371/journal.ppat.1004076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trakulsomboon S, Vuddhakul V, Tharavichitkul P, Na-Gnam N, Suputtamongkol Y, Thamlikitkul V. Epidemiology of arabinose assimilation in Burkholderia pseudomallei isolated from patients and soil in Thailand. Southeast Asian J Trop Med Public Health 1999; 30: 756–759. [PubMed] [Google Scholar]
- 46. Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLOS Pathog 2010; 6: e1001068 10.1371/journal.ppat.1001068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burtnick MN, Brett PJ, Harding SV, Ngugi SA, Ribot WJ, Chantratita N, et al. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun 2011; 79: 1512–1525. 10.1128/IAI.01218-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toesca IJ, French CT, Miller JF. The Type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species. Infect Immun 2014; 82: 1436–1444. 10.1128/IAI.01367-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwarz S, Singh P, Robertson JD, LeRoux M, Skerrett SJ, Goodlett DR, et al. VgrG-5 is a Burkholderia type VI secretion system-exported protein required for multinucleated giant cell formation and virulence. Infect Immun 2014; 82: 1445–1452. 10.1128/IAI.01368-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010; 7: 25–37. 10.1016/j.chom.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng J, Ho B, Mekalanos JJ. Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae . PLOS One 2011; 6: e23876 10.1371/journal.pone.0023876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A 2010; 107: 19520–19524. 10.1073/pnas.1012931107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009; 25: 1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brett PJ, DeShazer D, Woods DE. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol 1998; 48 Pt 1: 317–320. [DOI] [PubMed] [Google Scholar]
- 55. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 1998; 212: 77–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.