Abstract
Background
The National Quality Forum has endorsed a 12 lymph node (LN) minimum as a surrogate measure of quality in colorectal cancer (CRC). The prognostic value of ultra-staging Hematoxylin and Eosin (H&E) negative LNs (N0) using pan-cytokeratin immunohistochemistry (pan-CK-IHC) is unknown.
Purpose
To assess the impact on survival of surgical quality and focused pathological analysis.
Patients and Methods
Between 2001 and 2007, 253 evaluable patients with resectable CRC were enrolled. Multiple sectioning and pan-CK-IHC was performed on N0 LNs (AJCC Stage II). Follow-up was performed at 6-month intervals with a 4-year disease free survival (DFS) primary end-point.
Results
There were 253 patients, 177 N0 and 76 N1/N2 patients, staged conventionally. Thirty-six (20%) N0 patients were upstaged using ultra-staging [N0→N0i+ (n=27) and N0→N1mi (n=9)]. At a mean follow up of 3.4±1.6 years, 38 (15%) have recurred. Only 3% (3/108) of patients with ≥ 12 LNs, negative by H&E and pan-CK-IHC (N0i-), compared to 18% (6/33) with <12 LNs/N0i- (6/33; p=0.0015) have recurred. Four-year DFS differed significantly according to surgical quality (<12 vs. ≥12 LNs) amongst Stage II patients only (DFS, <12 vs. ≥12 LNs: Stage I, 90.5% vs. 97.7%, p=0.22; Stage II, 67.5% vs. 94.7%, p=0.0036; Stage III, 61% vs. 61%, p=0.61).
Conclusion
This represents the first prospective report demonstrating that both surgical quality and nodal ultra-staging impacts survival in Stage II CRC. Patients with Stage II CRC having ≥12 LNs negative for micro-metastases (N0i-) are likely cured by surgery alone. Both surgical and pathological quality measures are imperative in early CRC in order to improve patient selection for adjuvant chemotherapy.
Keywords: colon cancer, staging, surgical quality, micrometastasis
Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed cancer and the second leading cause of cancer mortality in the United States 1. Within the AJCC cancer staging system regional nodal tumor dissemination in the absence of distant metastasis differentiates Stage III from Stage I/II disease 2. In fact, the staging of CRC as well as the decision to utilize adjuvant systemic therapy to a great extent relies on the tumor status of the regional lymph nodes (LNs) 3. In patients with non-metastatic disease, regional nodal dissemination is a principal determinant of cancer outcomes. Importantly, the number of mesenteric nodes removed surgically as well as the number of nodes assessed pathologically governs not only accuracy of tumor staging, but also overall survival 4-6.
The unacceptably high rate of disease recurrence in patients undergoing surgical resection of apparently LN-negative (N0) disease is attributable in part to incomplete resection of tumor-bearing regional LNs, possible stage migration (the Will Rogers Phenomenon) and under-treatment (failing to treat under-staged patients with systemic therapy) 2,5,7. There is wide variation in number of LNs retrieved surgically from patients with CRC. In a large population-based analysis in the United States we found that the median number of mesenteric LNs resected at time of colectomy in patients with colon cancer (CC) was nine and the mode, zero 4. Consensus-driven quality improvement initiatives in cancer care have emerged as a result of this and other studies pointing to unacceptably low nodal yield from surgical specimens. These multi-disciplinary, evidence-based practice management and quality improvement efforts involve the National Cancer Institute and Veteran Health Affairs (Cancer Care Outcomes Research and Surveillance, CanCORS), the American Society of Clinical Oncology (ASCO), RAND/Harvard and Commission on Cancer (CoC) of the American College of Surgeons (ACS), National Initiative on Cancer Care Quality (NICCQ), National Quality Forum (NQF), and, National Comprehensive Cancer Network (NCCN). The ACS CoC submitted consensus standards for the diagnosis and treatment of CRC to the NQF, which through collaboration with ASCO and NCCN developed two agreed upon national consensus quality standards for CRC: (1) ≥12 lymph nodes removed surgically and examined pathologically for resected colon cancer; and, (2) adjuvant systemic therapy considered or administered within 120 days of diagnosis for patients under age 80 with AJCC Stage III disease 8-11.
Under-staging may also reflect tumor biology as well as the inherent limitations of conventional pathological nodal assessment in detecting low volume disease: <1% of any given LN is assessed morphologically by standard hematoxylin-eosin (H&E) microscopy resulting in sampling error 4,14,15. These undetected micrometastases (MM) may account for the high (20-30%) rate of disease recurrence after surgical resection of Stage II colon cancer 12,13. The clinical relevance of MM detected by pancytokeratin immunohistochemistry (pan-CK-IHC) found to be negative by H&E staining in patients with colon cancer 16 have not however been uniformly accepted. Our group has demonstrated the utility of ultra-staging with nodal step sections and pan-CK-IHC for the detection of occult nodal disease, and has underscored the potential prognostic impact of MM in our ongoing efforts to not only standardize the surgical and pathological evaluation, but also improve patient selection for adjuvant therapy of Stage II colon cancer 12,13,17,18. The present analysis of two international prospective trials was undertaken to assess the impact of surgical quality and nodal ultra-staging on long-term disease-free survival (DFS) in early CRC.
Methods
Specific aims
We aimed to assess the impact on DFS of: (1) surgical quality (<12 versus 12 or more nodes resected surgically and assessed pathologically); and, (2) focused pathological assessment (step section and pan-CK-IHC detected presence or absence of MM disease amongst AJCC Stage II: N0i- versus N0i+) in enrolled study subjects with non-metastatic (AJCC Stage I-III) adenocarcinoma of the colon and rectum. A well characterized cohort with long-term follow up derived from two prospective observational trials of targeted nodal assessment in CRC was used for analysis. This study represents the work of a clinical research consortium including the United States Military Cancer Institute, Cancer Centers within the United States, Europe and Israel.
Primary endpoint
The primary outcome variable in this study was 4-year DFS. DFS was defined as time from study enrollment to the first documentation of disease recurrence or death.
Study population
Two hundred fifty-three patients provided written informed consent and were enrolled over a 6-year study period in two prospective multi-center clinical trials of LN ultra-staging in CRC 12, 17. Eligibility criteria have been previously described 12, 17. In brief, study subjects were adults (18 years of age or older) with potentially curable primary non-metastatic colon (n=217) or rectal (n=36) carcinoma detected by endoscopy. Patients were considered non-eligible if they were found to have metastatic disease intra-operatively or failed to meet the major eligibility criteria. Patients with CRC were followed at six-month intervals for four years post-operatively. Colonoscopy was obtained 1 and 4 years after surgery and a chest radiograph and computed tomography scan (CT) of the abdomen and pelvis annually. An analysis of pooled data was then performed. Institutional Review Board approval for this study was provided by University of California Los Angeles, California (#09-09-088-01) and Hadassah Medical Organization, Jerusalem (#16-28-03-03).
Surgical technique and pathological nodal assessment
Surgeons enrolling patients in these trials were experienced surgical oncologists and colorectal surgeons that have demonstrated technical competence and perform at least twenty colorectal cancer operations a year. An oncologic resection was performed to include all regional mesenteric lymph nodes. Standard histopathological examination was performed on the resected colon and/or rectal specimen as well as the surrounding LNs. Ultra-staging of the H&E negative LNs by pan-CK-IHC was performed as previously described 12,17.
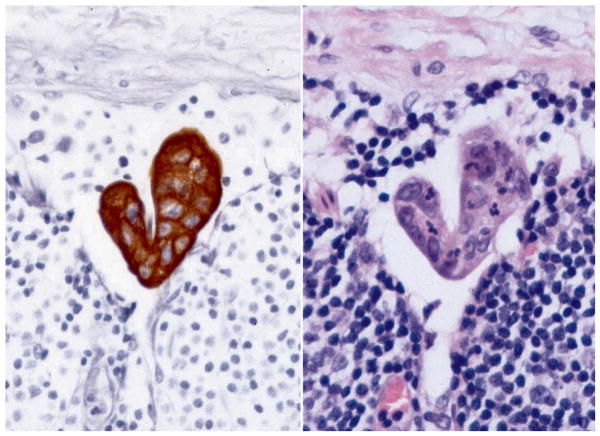
Serial step sectioning at 40-200 micrometer intervals was conducted on formalin-fixed-paraffin embedded LNs. Four 4-micrometer sections were stained with H&E (1st, 2nd, 3rd, and 4th sections) and two (2nd and 4th sections) were evaluated with pan-CK-IHC. The avidin-biotin-peroxidase complex method was used for the IHC according to a standardized protocol (Pan-keratin AE1/AE3, CAM 5.2, 35bH11; Ventana Medical Systems, Tucson, AZ) 12, 17, 19. In order for isolated tumor cells (ITCs) or cell clusters (CCs) within the LNs evaluated with pan-CK-IHC to be considered positive, two criteria had to be met: (1) cells had to stain strongly positive; and, (2) cells had to demonstrate anatomical and cytological features of carcinoma. A pan-CK-IHC-positive node was defined as a LN containing single cells or cell clusters (largest cluster ≤0.2mm) demonstrating morphological features consistent with CRC apparent on evaluation of H&E and/or pan-CK-IHC stained sections of the node. Tumor deposits within LNs were classified and staged according to the revised guidelines set by the American Joint Commission on Cancer (AJCC, 2002 6th Edition) and International Union Against Cancer (UICC) 20, 21. The largest LN cluster of cohesive aggregates of carcinoma cells was measured. Metastases less than 2 mm and greater than 0.2 mm (>0.2 to <2 mm) were considered MM (N1mi); isolated tumor cells or cell clusters up to 0.2 mm (≤ 0.2 mm) were usually detected by IHC (Figure 1) and classified (N0i+). Rare single cells staining positive with IHC that lacked cytological characteristics of malignancy were considered tumor-negative (N0i-).
Figure 1.
Ultra-staging of lymph nodes. Pan-CK-IHC analysis of H&E negative LN showing ITC's <0.2mm (N0i+).
Statistical Analysis
All data were reviewed and analyzed by the Department of Biostatistics at UCLA (DAE) and the Department of Clinical Investigation, Division of Biostatistics, Walter Reed Army Medical Center (RH). Summary statistics were obtained using established methods. The categorical variables were compared between groups using Fisher exact test or χ2 test as appropriate. Continuous data are presented as means and standard deviations (mean ± SD) and were compared using the two-sample t-test. If assumptions for normality were not satisfied (determined by the Shapiro-Wilk test) then data were summarized using the median and range and groups were compared using the Wilcoxon rank sum test. All tumor staging was conducted according to the American Joint Commission on Cancer (AJCC TNM) Staging (2002, 6th edition) criteria. The mean, median, and mode of the number of lymph nodes examined were determined. Based on the national guidelines, analysis was stratified by those with 12 or more, and those with fewer than 12 nodes resected surgically and examined pathologically, and in conventionally staged N0 (AJCC Stage I/II patients) stratified according to IHC results: N0(i-) [H&E(-)/pan-CK-IHC (-)] versus N0(i+) [H&E(-)/pan-CK-IHC (+)].
Demographic and clinical factors associated with surgical resection of 12 or more nodes were examined in a multivariate model using logistic regression. Variables which had a p value ≤0.20 in the univariate analysis were entered into the model. The final model included those variables which were significant at the p<0.05 level and are presented with odds ratios together with 95% confidence intervals.
The primary outcome variable in this study was DFS, which was defined as time from study enrollment to the first documentation of disease recurrence or death as a result of any cause, whichever came first. DFS analysis was undertaken using the Kaplan-Meier method. Survival differences were analyzed utilizing the Log-rank test. A Cox proportional hazards model was used for multivariate analysis. Factors potentially significant (p<0.05) on univariate analysis were entered into the multivariate model. The models were obtained by starting with all significant factors (univariate p<0.05) in the model and removing, stepwise, factors that were not significant in the multivariate analysis. Hence, a hierarchical forward stepwise methodology was used to screen clinical/pathological variables for model inclusion. Separate models were created for those with ≥12, and those with <12 LNs. We included variables at a significance level of p<0.05. Statistical analysis was performed using SPSS v17.0 (SPSS, Inc, Chicago IL). Significance levels were set at p<0.05. All tests were two sided.
Results
Clinical and Pathological Characteristics
Of the 253 evaluable patients, 135 were female (53%) and 118 male (47%), with a median age of 71 years. Baseline demographic and clinical characteristics of the entire study population are shown in Table 1. Mean body mass index for the study population was 25.7 ± 4.4 kg/m2 and over half of the patients were either overweight (38%; BMI ≥ 25.0-29.9 kg/m2) or obese (15%; BMI ≥ 30 kg/m2). Most tumors were of colonic origin. Primary tumors were located in the colon in 217 (86%) patients, in the rectum in 36 (14%) patients.
Table 1. Characteristics of the study population (n=253).
| Surgical Quality Indicator | Total Patients | ||||||
|---|---|---|---|---|---|---|---|
| Number of Lymph Nodes | |||||||
| <12 (n=48) | ≥12 (n=205) | ||||||
| Characteristic | n= | % | n= | % | p= | n= | % |
| Gender | 0.99 | ||||||
| Female | 26 | 19.3 | 109 | 80.7 | 135 | 53.4 | |
| Male | 22 | 18.6 | 96 | 81.4 | 118 | 46.6 | |
| Mean age (years) ± Std Dev | 71.4 ± 11.7 | 67.9 ± 13.0 | 0.096 | 68.6 ± 12.8 | |||
| Body Mass Index (kg/m2) | 25.6 ± 3.9 | 25.8 ± 4.5 | 0.79 | 25.7 ± 4.4 | |||
| Primary Tumor Location | <0.001 | ||||||
| Colon | 33 | 15.2 | 184 | 84.8 | 217 | 85.8 | |
| Rectum | 15 | 41.7 | 21 | 58.3 | 36 | 14.2 | |
| Extent of resection | 0.036 | ||||||
| Segment | 47 | 20.7 | 180 | 79.3 | 227 | 89.7 | |
| > Segment | 1 | 3.8 | 25 | 96.2 | 26 | 10.3 | |
| Operation Category | 0.76 | ||||||
| Open | 44 | 18.7 | 191 | 81.3 | 235 | 92.9 | |
| Laparoscopic | 4 | 22.2 | 14 | 77.8 | 18 | 7.1 | |
| Mean tumor size (cm) ± Std Dev | 3.0 ± 1.9 | 3.9 ± 1.8 | 0.009 | 3.8 ± 1.9 | |||
| Tumor Stage Category | 0.023 | ||||||
| T1/T2 | 18 | 26.1 | 51 | 73.9 | 69 | 28.7 | |
| T3/T4 | 23 | 13.5 | 148 | 86.5 | 171 | 71.3 | |
| Lymph-vascular Invasion | 0.58 | ||||||
| Absent | 38 | 17.6 | 178 | 82.4 | 216 | 89.3 | |
| Present | 3 | 11.5 | 23 | 88.5 | 26 | 10.7 | |
| Mean number of nodes ± Std Dev | 8.0 ± 2.3 | 22.4 ± 11.1 | <0.001 | 19.6 ± 11.5 | |||
| Median positive nodes (range) | 0 (0-5) | 0 (0-40) | 0.016 | 0 (0-40) | |||
| Nodal Stage Category | 0.016 | ||||||
| N0 | 38 | 22.6 | 130 | 77.4 | 168 | 66.4 | |
| N1 | 9 | 15.3 | 50 | 84.7 | 59 | 23.3 | |
| N2 | 1 | 3.8 | 25 | 96.2 | 26 | 10.3 | |
| AJCC Stage | <0.001 | ||||||
| I | 24 | 35.3 | 44 | 64.7 | 68 | 26.9 | |
| II | 14 | 14.0 | 86 | 86.0 | 100 | 39.5 | |
| III (ultrastaged N0 to N1mi, n=9) | 10 | 11.8 | 75 | 88.2 | 85 | 33.6 | |
| Nodal Disease Volume | 0.019 | ||||||
| N0(i-) | 33 | 23.4 | 108 | 76.6 | 141 | 55.7 | |
| N0(i+); ≤ 0.2 mm | 5 | 18.5 | 22 | 81.5 | 27 | 10.7 | |
| N1mi; >0.2 to <2 mm | 3 | 33.3 | 6 | 66.7 | 9 | 3.6 | |
| N1/2; ≥ 2 mm | 7 | 9.2 | 69 | 90.8 | 76 | 30.0 | |
The majority of patients (93%) underwent open segmental resection of the colon. Types of resection performed included: right colectomy (n=114; 45%), sigmoid colectomy (n=50; 20%), low anterior resection (n=37; 14%), total colectomy (n=25; 10%), left colectomy (n=22; 9%), transverse colectomy (n=3; 1%), total proctocolectomy (n=1; <1%), and APR (1; < 1%).
Over 70% of tumors invaded through the muscularis propria and into the subserosa, peri-colonic tissues or adjacent organs (AJCC T3 or T4). Median primary tumor size was 3.6 cm and over 80% of tumors were of intermediate (67%) or high (16%) histological grade. Microscopically apparent lymphovascular invasion was identified in 11% of primary tumors.
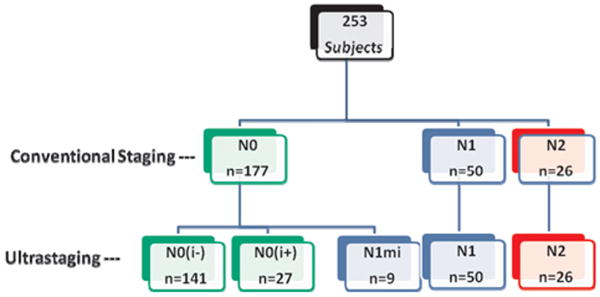
The mean number of LNs staged was 20 ± 12. Over 80% (205/253) of patients had 12 or more LNs resected surgically and evaluated pathologically, and a third (76/253; 30%) were node positive [AJCC N1 (n=50) or N2 (n=26); Figure 2] by conventional histopathology (H&E). Mean number of resected and mean number of positive nodes identified according to surgical quality indicator, < 12 versus ≥12 nodes were as follows: resected, 8.0 (< 12 nodes) versus 22.0 (≥12 nodes); and, mean # positive nodes, 0.6 (< 12 nodes) versus 2.0 (≥12 nodes). By multivariate logistic regression analysis (Table 2) only primary tumor size (p=0.01) and tumor location (colon vs. rectum) (p=0.013) were associated with increased LN retrieval (≥12 LNs).
Figure 2.
Study population distribution of conventional staging and ultra-staging
Table 2. Multivariate logistic regression analysis of factors associated with surgical quality (Odds of 12+ versus <12LNs).
| Odds Ratio (95% CI) | p= | |
|---|---|---|
| Tumor Location (Colon vs. Rectum) | 3.03 (1.27, 7.21) | 0.013 |
| Tumor Size (cm) | 1.32 (1.07, 1.64) | 0.01 |
Of the 177 H&E node negative (AJCC N0 by conventional staging) patients, 27 (15%) were found to have IHC-positive ITCs in ultra-staged nodes (N0→N0i+) Figure 2. Most (21/27; 78%) of these patients had T3 tumors. A majority of these upstaged patients did not have lymphovascular invasion (23/27; 85%) identified in the primary tumor.
Of the 177 H&E node negative (AJCC N0 by conventional staging) patients, 9 (5%) were found to have micrometastases (MM) by ultrastaging (N0→N1mi). Hence, nine (11%) of the overall 85 node positive patients (AJCC N1 or N2) were upstaged by nodal step sectioning and pan-CK-IHC from H&E N0 to nodal MM (N0→N1mi) (Figure 2). Most (7/9; 78%) of these patients had T3 or T4 tumors and none had lymphovascular invasion apparent on microscopic assessment of the primary tumor. The distribution of nodal disease volume is shown in Table 1, where ITCs or MM were identified in 14% (36/253) of patients with nodal ultra-staging. Thus, 20% (36/177) overall upstaging [N0→Ni+ (n=27) and N0→N1mi (n=9); Figure 2] was conferred by detailed nodal assessment (step section → H&E and pan-CK-IHC).
Follow up and Disease Free Survival
At a mean follow-up of 38.4 months (median 38 months), 38 of the 253 (15%) evaluable patients have experienced disease recurrence (8 local, 30 distant). Disease recurrence according to AJCC Stage is shown in Table 3 along with stage-specific adjuvant systemic therapy indicated. Four-year stage-specific DFS according to number of nodes resected surgically and examined pathologically is shown in Table 4. A statistically significant difference in 4-year DFS was found in all AJCC Stage II patients in relation to nodal yield (< 12 vs. ≥ 12 nodes: 68% vs. 95% DFS; p=0.0036). Only 3% (3/108) of N0 patients with ≥ 12 LNs, negative by both H&E and pan-CK-IHC (N0i-), compared to 18% (6/33) with <12 LNs, negative by both H&E and pan-CK-IHC (N0i-), have developed disease recurrence (p=0.0015) (Table 5).
Table 3. AJCC Stage-specific adjuvant treatment and recurrence data.
| AJCC Stage | Total Patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I (n=68) | II (n=100) | III (n=85)* | |||||||
| Characteristic | n= | % | n= | % | n= | % | p= | n= | % |
| Recurrence | <0.001 | ||||||||
| No | 65 | 30.2 | 92 | 42.8 | 58 | 27.0 | 215 | 85.0 | |
| Yes | 3 | 7.9 | 8 | 21.1 | 27 | 71.0 | 38 | 15.0 | |
| Type of Recurrence | 0.15 | ||||||||
| Local | 2 | 25.0 | 1 | 12.5 | 5 | 62.5 | 8 | 21.1 | |
| Distant | 1 | 3.3 | 7 | 23.3 | 22 | 73.3 | 30 | 78.9 | |
| Adjuvant systemic therapy | <0.001 | ||||||||
| No adjuvant systemic therapy | 64 | 43.2 | 78 | 52.7 | 6 | 4.1 | 148 | 60.9 | |
| Adjuvant systemic therapy | 4 | 4.2 | 22 | 23.2 | 69 | 72.6 | 95 | 39.1 | |
N1mi (n=9); N1(n=50); N2 (n=26); Systemic therapy information not available for 10 Stage III patients
Table 4. Four-year disease free survival AJCC Stage and surgical quality indicator (number of lymph nodes).
| Surgical Quality Indicator | ||||
|---|---|---|---|---|
| Number of Lymph Nodes | ||||
| <12 | ≥12 | |||
| AJCC Stage (n=253) | n= | 4 year DFS | 4 year DFS | p= |
| Stage I | 68 | 90.5% | 97.7% | 0.22 |
| Stage II | 100 | 67.5% | 94.7% | 0.0036 |
| Stage III | 85 | 61.0% | 61.0% | 0.61 |
Table 5. Disease recurrence according to anatomic site, nodal disease burden and surgical quality indicator amongst patients with AJCC N0 (n=168).
| N0, <12 LNs | N0, ≥ 12 LNs | |||||
|---|---|---|---|---|---|---|
| Study population (n=168) | n= | % Recurred | 4 yr DFS | % Recurred | 4 yr DFS | p= |
| N0i-: H&E (-) / IHC(-) | 141 | 6/33 (18.2%) | 78.4% | 3/108 (2.8%) | 97.1% | 0.0015 |
| N0i+: H&E (-) / IHC(+) | 27 | 0/5 (0%) | 100% | 2/22 (9.1%) | 89.5% | 0.46 |
| Colon Only (n=145) | ||||||
| N0i-: H&E (-) / IHC(-) | 119 | 4/22 (18.2%) | 76.9% | 3/97 (3.1%) | 96.7% | 0.0062 |
| N0i+: H&E (-) / IHC(+) | 26 | 0/5 (0%) | 100% | 2/21 (9.5%) | 88.9% | 0.45 |
| Rectum Only (n=23) | ||||||
| N0i-: H&E (-) / IHC(-) | 22 | 2/11 (18.2%) | 80.8% | 0/11 (0%) | 100% | 0.15 |
| N0i+: H&E (-) / IHC(+) | 1 | - | - | 0/1 (0%) | 100% | - |
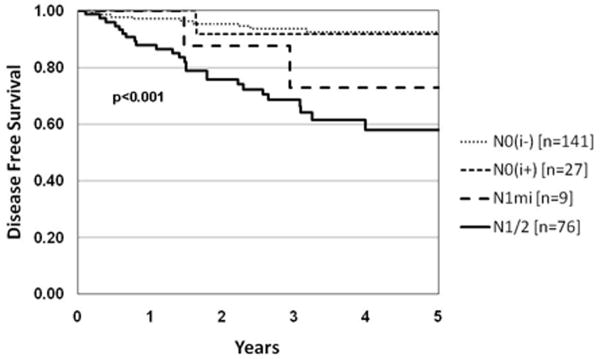
Disease recurrence varied significantly according to nodal disease volume: N0i-: 6%(9/141); N0i+: 7%(2/27); N1mi: 22%(2/9); N1/2: 33%(25/76); p<0.001 (Table 6; Figure 3). Hence, the finding of ITCs and cell clusters (≤0.2 mm) in LNs by nodal ultra-staging with pan-CK-IHC in otherwise conventionally staged N0 patients did not have an impact on DFS: disease recurrence, N0i- vs. N0i+: 9/141 (6%) vs. 2/27 (7%); p=0.92. However, 26% of patients with N0i+ received adjuvant chemotherapy vs. 11% of N0i- who did not (p = 0.054). In the N1/N2 group (Stage III), most (92%) patients were treated with adjuvant chemotherapy (Table 3). In the rectal cancer group 17/36 (47%) were treated with neoadjuvant radiation therapy.
Table 6. Disease recurrence according to anatomic site and nodal disease volume.
| Nodal Disease Volume | ||||||
|---|---|---|---|---|---|---|
| N0i- | N0i+ | N1mi | N1/2 | |||
| n= | % Recurred 4 year DFS | % Recurred 4 year DFS | % Recurred 4 year DFS | % Recurred 4 year DFS | p= | |
| Study population | 253 | 9/141 (6.4%) 92.4% | 2/27 (7.4%) 91.7% | 2/9 (22.2%) 72.9% | 25/76 (32.9%) 57.9% | <0.001 |
| Colon only | 217 | 7/119 (5.9%) 92.7% | 2/26 (7.7%) 91.3% | 2/9 (22.2%) 72.9% | 22/63 (34.9%) 55.5% | <0.001 |
| Rectal only | 36 | 2/22 (9.1%) 89.4% | 0/1 (0%) 100% | – | 3/13 (23.1%) 67.5% | 0.39 |
Figure 3.
Kaplan Meier DFS demonstrating difference between LN macrometastases (macro) N1/2, micrometastases (micro) N1mi, isolated tumor cells (ITCs) N0i+ and N0i- (H&E -/IHC-) p<0.001.
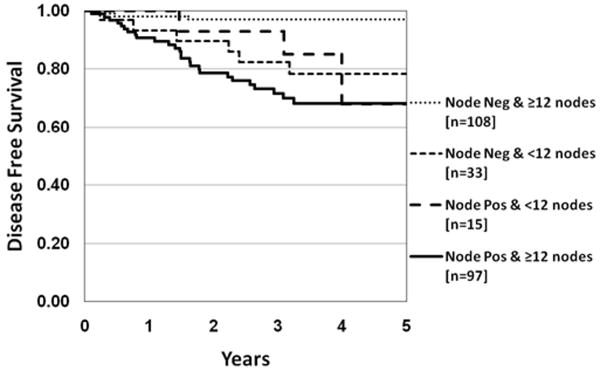
When recurrence according to nodal disease volume was assessed on a site-specific (colon versus rectum) basis, the same finding of disease recurrence varying significantly with nodal disease volume was demonstrated in the colon (p<0.001), but not the rectal cancer population, although the latter subset was decidedly small (Table 6). Disease recurrence between patients found to be node negative by step section and pan-CK IHC (N0i-) was 6% (9/141) compared to 11% for upstaged N0 patients (N0→N0i+ and N0→N1mi; 4/36). Hence, there was no significant difference between patients upstaged by nodal ultra-staging, N0i+ and N1mi, and those that remained node negative (N0i-) following detailed pathological assessment (p=0.41). Disease recurrence according to anatomic site (colon and rectum, colon only, rectum only), nodal disease burden [N0i-: H&E(-)/pan-CK-IHC(-) vs. N0i+: H&E(-)/pan-CK-IHC (+)] and surgical quality indicator (< 12 vs. ≥ 12 nodes) amongst patients with AJCC N0 stage disease is shown in Table 5. A significant difference in rate of disease recurrence was identified amongst patients with negative nodal ultra-staging [AJCC N0i-: H&E (-) / pan-CK-IHC (-)] according to surgical quality or nodal yield for the entire group (Figure 4) as well as the colon-only study population. By multivariate analysis LN number (≥ 12/<12) and LN status interaction effect (N0i-: H&E(-) / pan-CK-IHC(-) vs. other groups) were independent significant factors (p=0.01), Table 7.
Figure 4.
Kaplan Meier DFS of LN number and tumor volume. A large DFS difference is demonstrated in patients with ≥12 LN's negative for metastases (H&E -/IHC -; N0i-) vs. ≥ 12 LNs with metastases or <12LNs with or without metastases p<0.001.
Table 7. Multivariate analysis for DFS according to LN number and LN status interaction effect (N0i-: H&E(-) / pan-CK-IHC(-) vs. other groups).
| HR DFS (95% CI) | p= | |
|---|---|---|
| Total # Nodes (12+/<12) | 1.44 (0.91, 2.27) | 0.12 |
| Any node status (N0i- vs. else) | 1.81 (1.14, 2.86) | 0.01 |
| #Nodes by Node Status interaction effect | 0.55 (0.35, 0.86) | 0.01 |
Discussion
This study addresses an imperative in colon cancer - that of improving the accuracy of nodal pathological assessment, recognizing that this assessment is critical to disease staging and treatment planning. The present analysis of two international prospective trials was undertaken to assess the impact of surgical quality (< 12 vs. ≥ 12 nodes) and nodal ultra-staging (step section → H&E and pan-CK-IHC) on DFS in early CRC. DFS differed significantly according to surgical quality in this study amongst AJCC Stage II patients, emphasizing that both surgical and pathological quality measures are critical in early CRC in order to facilitate judicious adjuvant treatment decisions.
The incidence of node-negative (N0) CRC is increasing in the United States because of improved public awareness and greater access to screening colonoscopy, which enables earlier detection of disease. Despite this, up to one third of patients recur, possibly due to inadequate lymphadenectomy or inaccurate standard pathological staging techniques leading to overlooked nodal metastases. This has resulted in considerable debate about the utility of adjuvant chemotherapy in Stage II colon cancer. In a recent review of 37 randomized trials and 11 meta-analyses in over 20,000 patients 22 with AJCC Stage II (N0) colon cancer, there was a 5-10% improvement in DFS with adjuvant chemotherapy, but this did not translate into a statistically significant improvement in overall survival. Within these trials however, there were certain subsets of patients with survival similar to Stage III (node-positive, N1/N2) colon cancer. These largely represented patients with incomplete surgical resection and/or pathological assessment (< 12 nodes). This is further supported by pooled analyses of data from randomized trials 23, 24 which demonstrated a strong correlation between survival and number of LNs examined independent of other known prognostic factors. In a large Intergroup Trial analysis (INT 0089), an improvement in 5-year survival from 73% to 87% was reported in Stage II colon cancer when the number of LNs recovered increased from <10 to >20,6 a larger impact than any adjuvant treatment has attained to date.
Both the AJCC and the UICC recommend the examination of at least 12 LNs per specimen20,21 and this has been endorsed nationally as a benchmark for hospital-based performance25,26. The ACS CoC endorses the NQF consensus standard for CRC: surgical retrieval and pathological evaluation of ≥12 LNs. This does not however address inconsistencies in pathological evaluation, LN sampling errors in the face of small nodal disease deposits, and the significant limitations of conventional H&E to detect occult nodal metastases.
This analysis of two prospective trials was performed to evaluate whether surgical quality (≥12 LNs) and focused pathological analysis using multiple nodal sectioning and pan-CK-IHC improves the detection of occult metastases and impacts 4-year DFS. Overall, 36 of 177 patients (20%) were found to have nodal MM (N0→N1mi, n=9) and ITCs (N0→N0i+, n=27), which were not detected by conventional (H&E) staging methods (Figure 2). Nine of 85 (11%) Stage III patients were upstaged with nodal ultra-staging from N0 to N1mi, potentially impacting adjuvant therapy decisions. The 4-year DFS was significantly higher (95%) in Stage II patients with ≥12 nodes compared with <12 nodes (68%). Only 3% of N0 patients with ≥12 LNs, negative by both H&E and pan-CK-IHC have recurred compared to 18% with <12 LNs and N0i. Nodal yield did not impact survival in Stage III colon cancer in this study.
Colon cancer recurrence varied significantly according to nodal disease volume (Table 5, Figure 3), but the finding of ITCs and cell clusters (≤0.2 mm) alone detected by pan-CK-IHC (N0i+) did not impact recurrence; however, these patients were twice as likely to receive adjuvant systemic therapy compared to N0i- patients. However, when surgical quality (≥12 LNs) was combined with pathological ultra-staging [step section → H&E and pan-CK-IHC: H&E (-) / IHC(-): N0i-] there was a significant improvement in DFS amongst node negative (N0) patients (Table 6, Figure 4). These data suggest that patients who meet the 12-node minimum benchmark, and are negative by both H&E and pan-CK-IHC step section assessment are likely cured by surgery alone (Table 7), and will not benefit from adjuvant chemotherapy; however the impact of ultrastaging on Stage III colon cancer is less apparent. This raises several important questions. Does the improvement in survival reflect Stage migration (i.e. the “Will Rogers” phenomenon), the response to adjuvant systemic therapy in those patients that are upstaged with nodal ultra-staging, the resection of micro-metastases (suggesting micro-metastases have prognostic value), better surgery alone or a combination thereof?
Stage migration suggests that the higher the number of nodes examined the greater the chance of finding positive LNs, thereby improving the selection of patients for adjuvant systemic chemotherapy. In a series of 35,787 cases of Stage II colon cancer from the National Cancer Data Base (NCDB), the 5-year survival for Stage II patients was 64% if only one or two LNs were examined versus 86% if more than 25 LNs were examined27. The NCDB investigators concluded that at least 13 lymph nodes should be retrieved and declared negative for an accurate diagnosis of Stage II disease to be attained. In our review of the Surveillance, Epidemiology, and End Results (SEER) database in more than 82,896 patients treated between 1988-2000, the 5-year survival in Stage II colon cancer was 78% when ≥15 nodes were evaluated compared with 70% for 8-14 nodes and 66% for 1-7 nodes (p<0.001). For all colon cancer stages increased nodal sampling was associated with improved survival4. The resection of at least 15 lymph nodes was associated with significantly prolonged median overall survival by 11 months in patients with Stage I disease, by 54 months in Stage II, and by 21 months in Stage III disease. Interestingly, in our prospective trial LN number did not impact survival in Stage III colon cancer. This may reflect that the majority of patients received more effective oxaliplatin-based chemotherapy, which was not available during the data collection period of the SEER review, where adjuvant therapy was largely 5-Fluorouracil and Leucovorin, or it may reflect limited sample size on which the analysis was based.
The potential prognostic value of nodal MM detected by ultra-staging in N0 (by conventional H&E) colon cancer remains unresolved. In the current study ITCs detected by pan-CK-IHC failed to significantly impact 4-year DFS in patients with LNs otherwise staged negative by conventional pathological evaluation (H&E). This may be a consequence of modest sample size and associated lack of statistical power, or the influence of nodal MM alone (ITCs) on decisions regarding the administration of adjuvant chemotherapy. In this study, treating physicians were not blinded to the pathology reports and this likely reflects the number of N0 patients with ITCs (N0i+) who subsequently received adjuvant chemotherapy. Even though no clear benefit of chemotherapy has been demonstrated in this setting, this group may be among those who derive benefit from adjuvant therapy.
Alternatively, tumor cells detected by IHC may not be as prognostically relevant as those detected by molecular assays including quantitative reverse transcriptase-polymerase chain reaction assay (qRT-PCR). The prognostic value of micrometastatic lymphatic disease was evaluated in a meta-analysis of all N0 colon cancer between 1991 and 200228 that reported overall survival. All studies identified MM after subjecting N0 LNs (by H&E) to greater pathological scrutiny. Molecular techniques using qRT-PCR upstaged 37% of patients from N0 to N0mol+ and were associated with an absolute survival difference at 3 years of 19%. Overall survival at 3 years was 78% for patients with molecularly detected MM N0mol+ and 97% for patients without molecularly detected MM [N0mol-; p<0.001]. Histological techniques including serial sectioning with IHC staining upstaged 32% of pN0 patients (N0→N0i+). Although MM identified with IHC techniques appeared to adversely affect survival, the differences were not statistically significant, possibly due to variations in IHC techniques. These variations included differences in the nodal counts per specimen, nodal sections analyzed with IHC per specimen, volume of nodal analysis, the range of anti-cytokeratin antibodies used and the different definitions used to describe MM.
Changes in the AJCC 6th addition Cancer Staging Manual20 and the identification of MM in the sentinel node(s) from patients with melanoma and breast cancer have provided standardized terminology that has decreased technical variations in pathological assessment among subsequent studies. Although the sentinel node concept has been effectively applied to CRC17,19 it will largely remain investigational until the biological relevance of MM has been definitively established. Once this is established, targeted nodal assessment in CRC may ultimately prove to be a sensitive, expedient and cost-effective technique for the evaluation of nodal MM.
Primary tumor characteristics in addition to nodal evaluation are also an essential component in establishing a prognostic profile and predicting disease behavior. The presence of primary satellite tumor deposits has been associated with higher incidence of metastatic recurrence and therefore was recently incorporated into the revised AJCC 7th edition as N1c disease29. Intra-tumoral molecular profiling and specific gene signatures (18q loss of heterozygosity, DNA microsatellite instability, p27, KRAS mutation and thymidylate synthase) may prove to be associated with patient prognosis or response to therapy independent of nodal status30-32. Although these factors may become part of a more comprehensive staging system, lymph node evaluation continues to be the most important prognostic factor, and clinical decision determinant in colon cancer. For this reason it is imperative for the surgeon to apply oncological principles to optimize nodal yield and for the pathologist to utilize techniques to improve the identification of smaller lymph nodes and nodal tumor deposits - micrometastases.
Continued emphasis must be placed on standardizing the pathological and surgical evaluation of patients with N0 CRC. Whether examining a larger number of LNs with ultra-staging techniques minimizes the false-negative results associated with standard H&E assessment and improves staging and prognosis remains to be determined. This study represents the first prospective trial to confirm that the “12 lymph node benchmark” is an important prognostic factor in N0 colon cancer and that patients who meet this quality measure and have LNs negative for MM are likely cured by surgery alone. Our ongoing international prospective multicenter trial (2RO1CA090848) will establish a prognostic profile combining molecular signatures and nodal ultra-staging in Stage II colon cancer. Patients in this trial will not receive adjuvant chemotherapy which will allow us to further improve risk stratification and provide individualized clinical decision support.
Acknowledgments
Supported by grant 2RO1CA090848-05A2 from the National Cancer Institute and the Joyce E and Ben B Eisenberg Foundation, The Hearst Foundation, The Davidow Charitable Fund, The Rod Fasone Memorial Cancer Fund, Mrs. Ruth Weil, the Sequoia Foundation for achievement in the arts and education, Nancy and Bruce Newberg Charitable Fund and Mrs Marguerite Perkins Mautner
Abbreviations
- ACoS
American College of Surgeons
- AJCC
American Joint Commission on Cancer
- ASCO
American Society of Clinical Oncology
- CanCORS
Cancer Care Outcomes Research and Surveillance
- CC
cell cluster
- CoC
Committee on Cancer
- CRC
Colorectal cancer
- CK
Cytokeratin
- DFS
Disease free survival
- H&E
Hematoxylin & Eosin
- IHC
Immunohistochemistry
- ITC
isolated tumor cell
- LN
Lymph node
- MM
Micrometastasis
- N0
Node negative
- N1
Node positive
- NCCN
National Comprehensive Cancer Network
- NICCQ
National Initiative on Cancer Care Quality
- NQF
National Quality Forum
- Pan-CK-IHC
pan-cytokeratin immunohistochemistry
Footnotes
Presented at the American Surgical Association, April 8th 2010, Chicago
Author contributions:
Conception and design: Bilchik, Peoples, Stojadinovic
Acquisition of data: Bilchik, Shen, McCarter, Protic, Nissan
Analysis and interpretation of data: Bilchik, Wainberg, Protic, Howard, Elashoff, Tyler, Peoples, Stojadinovic
Drafting of manuscript: Bilchik, Howard, Stojadinovic
Critical revision: Bilchik, Nissan, Wainberg, Shen, McCarter, Protic, Tyler, Peoples, Stojadinovic
Statistical expertise: Howard, Elashoff
Supervision: Bilchik, Stojadinovic
Disclaimer: The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense or the United States Government.
Some of the contributing authors are military service members (or employees of the U.S. Government: RH, JT, GEP, AS), and this work was prepared as part of their official duties. Title 17 U.S.C. 105 provides the “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
We certify that all individuals who qualify as authors have been listed; each has participated in one or more of the following areas: conception and design of this work, the acquisition and/or analysis of data, the writing, and/or critical revision of the document, and supervision of this cooperative research effort. All contributing authors approve of the submission of this version of the manuscript and assert that the document represents valid work. If information derived from another source was used in this manuscript, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document. All contributing authors take public responsibility for this work.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Green FL, Compton CC, Fitz AG, Shah J, Winchester DP, editors. AJCC Cancer Staging Atlas. Lippincott Rave Publishers; PA, USA: 2006. IX.IBSN: 978-0-387-29014-0. [Google Scholar]
- 3.Cohen AM, Tremiterra S, Candela F, et al. Prognosis of node-positive colon cancer. Cancer. 1991;67:1859–1861. doi: 10.1002/1097-0142(19910401)67:7<1859::aid-cncr2820670707>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III colon cancer: A population-based study. Ann Surg. 2006 Oct;244(4):602–10. doi: 10.1097/01.sla.0000237655.11717.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prandi M, Lionetto R, Bini A, et al. Prognostic evaluation of Stage B colon cancer patients is improved by an adequate lymphadenectomy: Results of a secondary analysis of a large scale adjuvant trial. Ann Surg. 2002 Apr;235(4):458–63. doi: 10.1097/00000658-200204000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeVoyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer is associated with increasing number of lymph nodes analyzed: A secondary survey of Intergroup Trial INT-0089. J Clin Oncol. 2003;21:2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon: Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. NEJM. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 8.President's Cancer Panel Report: Cancer care issues in the United States: Quality of care, quality of life, National Cancer Program. National Cancer Institute; 1998. jan 1, 1997 – 31 dec. [Google Scholar]
- 9.Schneider EC, Epstein AM, Malin JL, et al. Developing a system to assess the quality of care: ASCOs National Initiative on Cancer Quality Care. J Clin Oncol. 2004 Aug 1;22(15):2985–91. doi: 10.1200/JCO.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, Landrum MB, Klabunde CN, Fletcher RH, Rogers SO, Doucette WR, Tisnado D, Clauser S, Kahn KL. Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity. J Clin Oncol. 2008 May 20;26(15):2532–7. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 11.National Quality Forum Endorsed Commission on Cancer Measures for Quality of Cancer Care for Breast and Colorectal Cancers. first posted April 12, 2007 World Wide Web based press release available at http://www.facs.org/cancer/qualitymeasures.html.
- 12.Bilchik AJ, Hoon DS, Saha S, Turner RR, Wiese D, DiNome M, Koyanagi K, McCarter M, Shen P, Iddings D, Chen SL, Gonzalez M, Elashoff D, Morton DL. Prognostic impact of micrometastases in colon cancer: interim results of a prospective multicenter trial. Ann Surg. 2007 Oct;246(4):568–75. doi: 10.1097/SLA.0b013e318155a9c7. Discussion 575-7. [DOI] [PubMed] [Google Scholar]
- 13.Koyanagi K, Bilchik AJ, Saha S, Turner RR, Wiese D, McCarter M, Shen P, Deacon L, Elashoff D, Hoon DS. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res. 2008 Nov 15;14(22):7391–6. doi: 10.1158/1078-0432.CCR-08-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera-Ornelas L, Justiniano J, Castillo N, Petrelli NJ, Stulc JP, Mittelman A. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987 Nov;122(11):1253–6. doi: 10.1001/archsurg.1987.01400230039006. [DOI] [PubMed] [Google Scholar]
- 15.Compton CC. Updated protocol for the examination of specimens from patients with carcinomas of the colon and rectum, excluding carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix: a basis for checklists. Cancer Committee. Arch Pathol Lab Med. 2000 Jul;124(7):1016–25. doi: 10.5858/2000-124-1016-UPFTEO. [DOI] [PubMed] [Google Scholar]
- 16.Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW., Jr Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer. 1994 Feb 1;73(3):563–9. doi: 10.1002/1097-0142(19940201)73:3<563::aid-cncr2820730311>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Stojadinovic A, Nissan A, Protic M, Adair CF, Prus D, Usaj S, Howard RS, Radovanovic D, Breberina M, Shriver CD, Grinbaum R, Nelson JM, Brown TA, Freund HR, Potter JF, Peretz T, Peoples GE. Prospective randomized study comparing sentinel lymph node evaluation with standard pathologic evaluation for the staging of colon carcinoma: results from the United States Military Cancer Institute Clinical Trials Group Study GI-01. Ann Surg. 2007 Jun;245(6):846–57. doi: 10.1097/01.sla.0000256390.13550.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nissan A, Protic M, Bilchik A, Eberhardt J, Peoples GE, Stojadinovic A. Predictive Model of Outcome of Targeted Nodal Assessment in Colorectal Cancer. Ann Surg. 2010 Feb;251(2):265–74. doi: 10.1097/SLA.0b013e3181bd5187. [DOI] [PubMed] [Google Scholar]
- 19.Bilchik AJ, DiNome M, Saha S, Turner RR, Wiese D, McCarter M, Hoon DS, Morton DL. Prospective multicenter trial of staging adequacy in colon cancer: preliminary results. Arch Surg. 2006 Jun;141(6):527–33. doi: 10.1001/archsurg.141.6.527. discussion 533-4. [DOI] [PubMed] [Google Scholar]
- 20.Green FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th. New York: Springer Verlag; 2002. [Google Scholar]
- 21.Sobin LH, Wittekind C, editors. International Union Against Cancer (UICC) TNM classification of malignant tumors. 6th. New York: Wiley; 2002. [Google Scholar]
- 22.Figuerdo A, Charette ML, Maroun J, et al. Adjuvant therapy for stage II colon cancer: A systematic review from the Cancer Care Ontario Program in Evidence-Based Care's Gastrointestinal Cancer Disease Site Group. J Clin Oncol. 2004;22(16):3395–407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 23.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller GD, Shepherd LE, Seitz JF, Francini G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 24.Gill S, Loprinzi C, Sragent DJ, Thome SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J, Labianca R, Chen W, Cha SS, Heldbrant MP, Golderg RM. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 25.Wong S, Ji H, Hollenbeck B, et al. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007 Nov 14;298(No 18):2149–2154. doi: 10.1001/jama.298.18.2149. [DOI] [PubMed] [Google Scholar]
- 26.Pinkowish MD. Lymph Node Evaluation as a Colon Cancer Quality Measure. CA Cancer J Clin. 2009;59:2–4. doi: 10.3322/caac.20012. [DOI] [PubMed] [Google Scholar]
- 27.Swanson RS, Compton CC, Stewart AK, Bland KI. The Prognosis of T3N0 Colon Cancer Is Dependent on the Number of Lymph Nodes Examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 28.Iddings D, Ahmad A, Elashoff D, Bilchik A. The prognostic effect of micrometastases in previously staged lymph node negative (NO) Colorectal Carcinoma: A Meta-analysis. Ann Surg Oncol. 2006;13:1386–1392. doi: 10.1245/s10434-006-9120-y. [DOI] [PubMed] [Google Scholar]
- 29.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th. New York: Springer Verlag; 2010. [Google Scholar]
- 30.Allegra CJ, Jessup JM, Somerfield MR. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations with metastatic colorectal carcinoma to predict response to anti epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–6. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 31.Goel A, Arnold CN, Niedzwiecki D, Chang DK, Ricciardiello L, Carethers JM, Dowell JM, Wasserman L, Compton C, Mayer RJ, Bertagnolli MM, Boland CR. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003;63:1608–14. [PubMed] [Google Scholar]
- 32.Sinicrope FA, Rego RL, Foster N, Sargent DJ, Windschitl HE, Burgart LJ, Witzig TE, et al. Microsatellite Instability Accounts for Tumor Site-Related Differences in Clinicopathologic Variables and Prognosis in Human Colon Cancers. Gastroenterol. 2006;101(12):2818–25. doi: 10.1111/j.1572-0241.2006.00845.x. [DOI] [PubMed] [Google Scholar]