Abstract
Introduction
Correct target positioning is crucial for accurate dose delivery in breast radiotherapy resulting in utilisation of daily imaging. However, the radiation dose from daily imaging is associated with increased probability of secondary induced cancer. The aim of this study was to quantify doses associated with three imaging modalities and investigate the correlation of dose and varying breast size in breast radiotherapy.
Methods
Planning computed tomography (CT) data sets of 30 breast cancer patients were utilised to simulate the dose received by various organs from a megavoltage computed tomography (MV-CT), megavoltage electronic portal image (MV-EPI) and megavoltage cone-beam computed tomography (MV-CBCT). The mean dose to organs adjacent to the target volume (contralateral breast, lungs, spinal cord and heart) were analysed. Pearson correlation analysis was performed to determine the relationship between imaging dose and primary breast volume and the lifetime attributable risk (LAR) of induced secondary cancer was calculated for the contralateral breast.
Results
The highest contralateral breast mean dose was from the MV-CBCT (1.79 Gy), followed by MV-EPI (0.22 Gy) and MV-CT (0.11 Gy). A similar trend was found for all organs at risk (OAR) analysed. The primary breast volume inversely correlated with the contralateral breast dose for all three imaging modalities. As the primary breast volume increases, the likelihood of a patient developing a radiation-induced secondary cancer to the contralateral breast decreases. MV-CBCT showed a stronger relationship between breast size and LAR of developing a radiation-induced contralateral breast cancer in comparison with the MV-CT and MV-EPI.
Conclusions
For breast patients, imaging dose to OAR depends on imaging modality and treated breast size. When considering the use of imaging during breast radiotherapy, the patient's breast size and contralateral breast dose should be taken into account.
Keywords: Breast radiotherapy, breast size, imaging, lifetime attributable risk, secondary cancer
Introduction
Volumetric anatomical images have become routine in image-guided radiotherapy (IGRT) for a number of tumour sites, as they provide three-dimensional soft tissue information. Daily volumetric imaging has been shown to be effective in reducing systematic and random uncertainties in patient positioning for various tumour sites1–3 and improved dose delivery.4,5 Volumetric imaging may offer additional information to improve the setup accuracy in breast radiotherapy;6–8 however, daily imaging with ionising radiation can increase the dose to organs at risk (OAR) significantly,9–11 potentially increasing the patients’ likelihood of developing a secondary cancer.12
Numerous studies have reported the dose to OAR from various image guidance procedures.9,10,13–16 A weak correlation between body mass index (BMI) and imaging dose was determined for megavoltage cone-beam CT (MV-CBCT) imaging of the pelvis, chest and intracranial region.15 However, the relationship, if any, between breast size and imaging dose has not been investigated.
During breast radiotherapy, two-dimensional orthogonal electronic portal imaging (EPI) has traditionally been used to verify patient setup.17 However, for complex breast radiotherapy techniques such as partial breast irradiation, EPI has been found to be inadequate.7,8 This is attributed to the EPI providing limited soft tissue information, requiring surrogate structures such as bony landmarks or implanted radio-opaque markers to acquire positional information of the target volume. Currently, three-dimensional computed tomography (CT) imaging within the treatment room is becoming more available with various vendors providing different options, including the MV-CBCT available on Siemens linear accelerators (Siemens Medical Solutions, Erlangen, Germany) and the helical megavoltage CT (MV-CT) available on Tomotherapy units (Accuray Inc., Sunnyvale, CA).
The dose and Biological Effects of Ionising Radiation (BEIR VII) lifetime attributable risk (LAR) for standard and complex breast radiotherapy treatments with kilovoltage cone-beam CT imaging was recently investigated.18 BEIR VII provides comprehensive risk estimates for cancer and other health effects from exposure to ionising radiation. In this study, the increased risk of developing secondary cancer in the contralateral breast due to MV imaging modalities, compared to that associated with treatment alone, is evaluated using the BEIR VII model.19 The aim of this study was to compare the dose received by OAR from the MV-CT, MV-EPI and MV-CBCT with consistent imaging parameters for each modality in patients with varying breast sizes.
Materials and Methods
Patient data sets
Thirty patient data sets were retrospectively evaluated for this study. These patients previously underwent breast radiotherapy between April 2010 and May 2011. Patients were treated in the supine position (14 left sided and 16 right sided) after breast conservation surgery. Patients were selected consecutively until 30 cases were accrued. Approval for the study was granted by South Western Sydney Human Research Ethics Committee in August 2011, and the study was conducted from September 2011 to February 2012.
The treatment planning CT data sets were acquired on a Siemens Somatom Sensation 4 (Siemens Medical Solutions, Germany) with 0.25 cm slice thickness. Delineation of the breasts, heart, lungs and spinal cord was completed by a senior radiation therapist with Focal v4.40 (Elekta AB, Stockholm, Sweden) and checked by a radiation oncologist. All delineation was completed according to the predetermined breast and heart delineation protocols.20,21
Imaging dose
The XiO treatment planning system (Elekta AB, Stockholm, Sweden) was utilised to simulate and estimate the radiation dose from the orthogonal MV-EPI and MV-CBCT. A prototype Tomotherapy planning station (Accuray Inc., Sunnyvale, CA) was used to simulate and estimate the dose from a MV-CT using TomoDirect pattern.
The MV-CT technique used a 3.5 MV beam and a predefined scan selection of normal (pitch of 2). The scan range/length was adjusted according to breast size with 1 cm margin superiorly and inferiorly. For the orthogonal MV-EPI, an anterior–posterior (0°) and a lateral field (270° and 90° for right and left breast respectively) were created with a 6 MV beam, each with varying field sizes according to the patient's breast size, and a beam-on time of 2 and 3 monitor units (MU) respectively. The MV-EPI fields were delivered to the isocentre. The MV-CBCT was created with a 6 MV, 200° beam arc, with a standard field width of 27.4 cm and field length adjusted for individual patients according to their breast size with 1 cm margin. The rotational beam began at 270°, rotating 200° clockwise to finish at 110°, and was divided into 200 subfields at 1° intervals. The MV-CBCT was centred on the midline to avoid gantry–couch collisions and an 8 MU protocol was used as this is the minimum deliverable MU.
To assess the overall significance of daily imaging (25 scans) for each image modality, the OAR dose from the patient's original tangential wedged breast radiotherapy plan was calculated. For each patient, mean OAR dose was recorded for the three imaging modalities and the radiotherapy plan. Using SPSS software (IBM SPSS Statistics for Windows, Version 20.0.; IBM Corp., Armonk, NY), a Pearson correlation analysis was performed to determine the relationship between imaging dose, primary breast volume and BMI for all OAR.
Secondary cancer risk
The LAR of developing radiation-induced contralateral breast cancer was estimated using BEIR VII methodology19:
where M (D, e, a) is the excess absolute risk for breast cancer, and S(a)/S(e) is the probability of the patient surviving from their age at exposure to their attained age. The LAR model was chosen as it includes age at exposure, a latency period, attained age since exposure, gender and organ-specific parameters. The contralateral breast LAR was assessed for women exposed at the age of 50 years and for the following protocols: treatment plus daily MV-CT imaging (25 scans), treatment plus daily MV-EPI imaging and treatment plus daily MV-CBCT imaging.
Results
Imaging dose
The mean patient age was 57.8 years (standard deviation ± 8.8 years). Mean volume of the primary breast was 1006.5 cm3 (range, 215.3–2144.5 cm3). All patients received a treatment dose of 50 Gy in 25 fractions. The range of differences in breast volume is shown in Figure1. The mean OAR dose and range for daily imaging with the MV-CT, MV-EPI and MV-CBCT for the 30 patients are displayed in Table1. The mean cumulative dose to OAR from treatment with no imaging and treatment plus daily image verification for the three imaging modalities are displayed in Figure2 for the 30 patients. The highest cumulative dose was from the prescribed treatment plus daily MV-CBCT, followed by MV-EPI and MV-CT for all OAR. On average, a single MV-CBCT, MV-EPI and MV-CT scan contributed ∽10%, 3% and 1%, respectively, in proportion to the total treatment dose for the contralateral breast. A similar trend was found for contralateral lung, spinal cord and heart.
Figure 1.
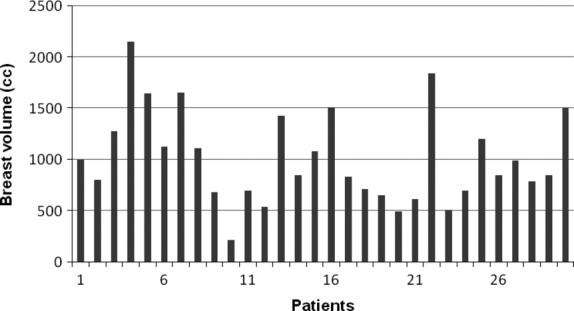
The range of breast volume for the 30 patients.
Table 1.
Mean organ at risk dose (Gy) and range for the 30 patients from daily imaging (25 scans) with the MV-CT, MV-EPI and MV-CBCT
| Mean dose in Gray (range) | |||
|---|---|---|---|
| Organ at risk | MV-CT | MV-EPI | MV-CBCT |
| Contralateral breast | 0.11 (0.08–0.13) | 0.22 (0.16–0.34) | 1.79 (1.53–2.13) |
| Ipsilateral lung | 0.08 (0.07–0.12) | 0.84 (0.64–1.53) | 1.49 (1.12–1.81) |
| Contralateral lung | 0.08 (0.07–0.12) | 0.33 (0.22–0.42) | 1.50 (1.02–1.81) |
| Spinal cord | 0.08 (0.07–0.12) | 0.45 (0.24–0.57) | 1.18 (0.64–1.54) |
| Heart | 0.12 (0.10–0.14) | 0.55 (0.39–0.92) | 1.70 (1.47–1.93) |
MV-CT, megavoltage computed tomography; MV-EPI, megavoltage electronic portal image; MV-CBCT, megavoltage cone-beam computed tomography.
Figure 2.
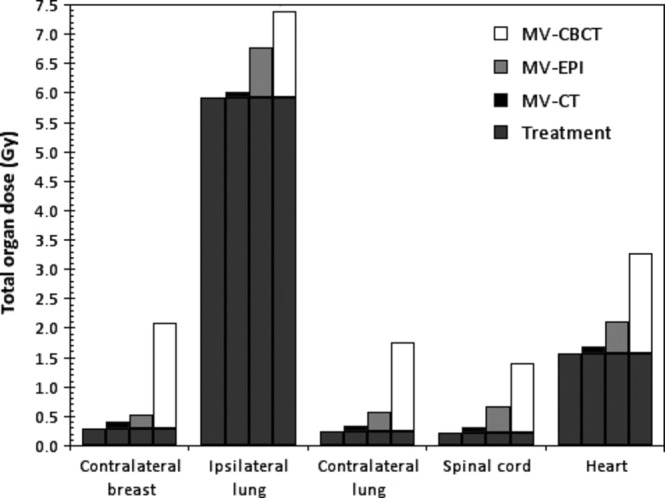
Cumulative (mean) organs at risk dose from treatment alone, and treatment plus daily imaging from megavoltage computed tomography (MV-CT), megavoltage electronic portal image (MV-EPI) and megavoltage cone-beam computed tomography (MV-CBCT).
Primary breast volume and BMI
A positive Pearson correlation (r) was established for primary breast volume and BMI, namely, r = 0.608. The primary breast volume inversely correlated with the contralateral breast dose for all three imaging modalities with r values of −0.399, −0.747 and −0.655 for the MV-CT, MV-EPI and MV-CBCT, respectively, indicating that the absorbed dose to the contralateral breast decreases as the breast volume increases. Pearson's r value is a descriptor of the degree of linear association between primary breast volume and dose. When the value is approaching zero, there is no correlation, but as it approaches −1 or +1 there is a strong negative or positive relationship between primary breast volume and dose. The correlation for MV-CT was weak but statistically significant at the 0.05 level (Fig.3). The Pearson correlation value for primary breast volume versus OAR dose for the three imaging modalities is displayed in Table2. There is strong correlation between primary breast volume and all OAR doses for MV-CBCT, and all but heart for MV-EPI. Only contralateral breast and heart doses correlated with primary breast volume for MV-CT. The correlation between BMI and OAR dose is outlined in Table3 with similar results.
Figure 3.
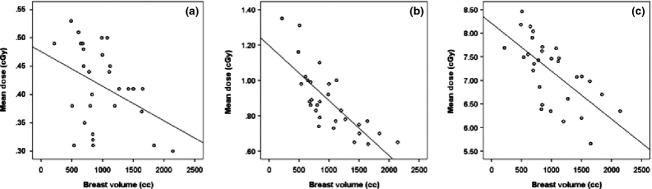
Scatter-plot correlation of primary breast volume versus contralateral breast dose from (A) megavoltage CT, (B) megavoltage electronic portal image and (C) megavoltage cone-beam CT.
Table 2.
Pearson correlation (R) value and the corresponding P value for primary breast volume and organs at risk doses for the three imaging modalities
| MV-CT | MV-EPI | MV-CBCT | ||||
|---|---|---|---|---|---|---|
| Organ at risk | R | P value | R | P value | R | P value |
| Contralateral breast | −0.399 | 0.029* | −0.747 | 0.000** | −0.655 | 0.000** |
| Ipsilateral lung | 0.036 | 0.851 | −0.705 | 0.000** | −0.752 | 0.000** |
| Contralateral lung | −0.087 | 0.647 | −0.712 | 0.000** | −0.663 | 0.000** |
| Spinal cord | −0.223 | 0.237 | −0.510 | 0.004** | −0.696 | 0.000** |
| Heart | −0.512 | 0.004** | −0.307 | 0.099 | −0.860 | 0.000** |
MV-CT, megavoltage computed tomography; MV-EPI, megavoltage electronic portal image; MV-CBCT, megavoltage cone-beam computed tomography.
Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two-tailed).
Table 3.
Pearson correlation (R) value and the corresponding P value for body mass index (BMI) and organs at risk doses for the three imaging modalities
| Organ at risk | MV-CT | MV-EPI | MV-CBCT | |||
|---|---|---|---|---|---|---|
| R | P value | R | P value | R | P value | |
| Contralateral breast | −0.262 | 0.161 | −0.674 | 0.000** | −0.598 | 0.000** |
| Ipsilateral lung | −0.089 | 0.639 | −0.639 | 0.000** | −0.657 | 0.000** |
| Contralateral lung | −0.177 | 0.348 | −0.660 | 0.000** | −0.602 | 0.000** |
| Spinal cord | −0.297 | 0.111 | −0.239 | 0.204 | −0.598 | 0.000** |
| Heart | −0.603 | 0.000** | −0.329 | 0.075 | −0.793 | 0.000** |
MV-CT, megavoltage computed tomography; MV-EPI, megavoltage electronic portal image; MV-CBCT, megavoltage cone-beam computed tomography.
Correlation is significant at the 0.01 level (two-tailed).
Secondary cancer risk
The baseline probability of a woman developing breast cancer over her lifetime is 12.7% based on the Surveillance, Epidemiology, and End Results (SEER) breast cancer incidence rates.22 The LAR for the patient with the smallest breast volume (215.3 cm3) for MV-CT, MV-EPI and MV-CBCT was 0.65%, 0.74% and 1.46% respectively. In contrast, the LAR for the patient with the largest breast volume (2144.5 cm3) was 0.36%, 0.48% and 0.66% for MV-CT, MV-EPI and MV-CBCT respectively. The trend line showing the LAR of developing secondary cancer risk in the contralateral breast with respect to primary breast volume is displayed in Figure4. The displayed risk is the treatment plus daily imaging risk for women exposed at the age of 50 years. As the primary breast volume increases, the likelihood of a patient developing secondary cancer to the contralateral breast decreases. The trend line for MV-CBCT suggests that the LAR of developing an induced lesion in the contralateral breast is greater than that for MV-CT and MV-EPI.
Figure 4.
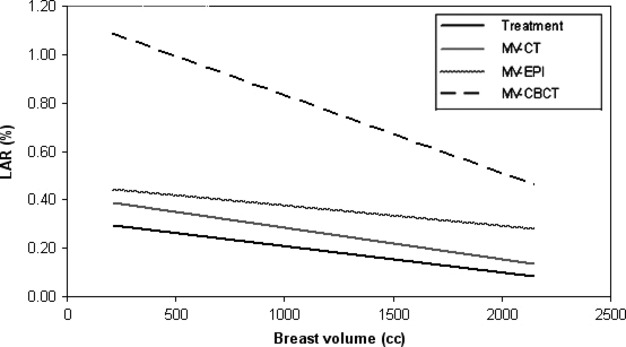
Trendline showing the lifetime attributable risk (LAR) of developing secondary cancer in the contralateral breast relative to breast volume for treatment alone and treatment plus daily imaging with the megavoltage computed tomography (MV-CT), megavoltage electronic portal image (MV-EPI) and megavoltage cone-beam CT (MV-CBCT).
Discussion
Image guidance has previously been shown to reduce setup errors during breast radiotherapy.6–8 The additional dose from MV-CT, MV-EPI and MV-CBCT acquired for breast radiotherapy verification has been presented in this study for patients with a range of breast sizes.
This study found that the highest dose was from MV-CBCT, followed by MV-EPI and MV-CT to all OARs. This is attributed to the MV-CBCT geometry resulting in more scatter and as a consequence increased patient dose in comparison with the MV-CT. Furthermore, MV-CBCT utilises higher beam energy (6 MV) compared to the MV-CT (3.5 MV). MV-CBCT dose was also higher than MV-EPI even though both modalities used 6 MV. This is because the field of view for a single projection of MV-CBCT has a standard field width of 27.4 cm, whereas the field size for MV-EPI was kept to the minimum for each patient resulting in lower dose to OARs.
Previous treatment planning simulation studies have reported on the dose delivered to the chest region for the MV-EPI,13 MV-CBCT13,15 and MV-CT.16 The organ dose trends reported by Peng et al.13 are in agreement with the results of this study. With the exception of the heart, lower organ dose values for an 8 MU MV-CBCT were reported, 0.8 Gy, 0.82 Gy and 1.68 Gy for the contralateral breast, lungs and heart (25 scans) respectively. Differences are attributed to the variable field lengths utilised in this study and the smaller field width utilised by Peng et al.13 For the same scan parameters, VanAntwerp et al.15 reported comparable mean MV-CBCT organ dose values, 1.65, 1.18 and 1.73 Gy (25 scans) for the lungs, spinal canal and heart respectively. Shah et al.16 reported higher MV-CT organ doses, 0.27, 0.29 and 0.38 Gy for the contralateral breast, lungs and heart respectively. This dose difference is attributed to only one patient image set being assessed by Shah et al.16 The range of organ doses identified in this study indicates that the single mean dose and standard deviation reported in other treatment planning simulation studies are not representative of all patients.
Previous phantom studies have reported lower organ doses in comparison with this study for MV-EPI and MV-CBCT.9,10 However, a phantom MV-CT dosimetry study14 reported higher organ doses compared to this study. Similar to above, this demonstrates that phantom studies are not always a true representative of patient size and doses received by individual patients.
The accuracy of the calculated doses has been investigated by previous studies. Gayou et al.23 investigated patient dose from a MV-CBCT with the XiO treatment planning system as well as with a range of dosimeters in anthropomorphic and cylindrical phantoms. The difference between the calculated and measured doses was found to be less than 5% for the anthropomorphic phantom. Shah et al.15 commissioned and validated the MV-CT model utilised in this study finding the computed doses to be within 5% of doses measured in an anthropomorphic phantom. Joosten et al.24 investigated the peripheral dose calculation accuracy of the XiO treatment planning system and found that at 10 cm from the field edge of a 20 × 20 cm2 open beam, the treatment planning system underestimated the dose by a maximum of 31%.
This study demonstrated that for treatment plus daily imaging protocols the primary breast size does affect OAR dose. The data indicated an inverse linear correlation between primary breast size and OAR doses, indicating that as breast size is increased, OAR dose decreases. A correlation between BMI and OAR dose was significant for the contralateral breast, and lungs for the MV-EPI, all OAR for the MV-CBCT, and only the heart for the MV-CT. A weak relationship between BMI and MV-CBCT dose for 27 thoracic patients was determined by VanAntwerp et al.15; however, no statistical analysis of this relationship was performed. In this study, a more reliable correlation can be suggested between breast size and organ doses, regardless of imaging modality.
The increased likelihood of developing a second cancer in the contralateral breast after breast radiotherapy with daily image verification for three image modalities was estimated. LAR was found to decrease with increasing breast volume and was highest for MV-CBCT imaging. This is attributed to the contralateral breast dose decreasing with increasing breast volume and the contralateral breast dose being greatest for the MV-CBCT. Breast cancer survivors have an 18% higher risk of developing a subsequent cancer.25 Younger patients (less than 45 years) have been found to be at a greater risk of developing a second contralateral breast cancer.26–28 There is conflicting data for women aged 50 years or older.26–30 Five years post treatment, a SEER cancer registry study26 observed that the relative risk of contralateral breast cancer was 1.30, 0.98 and 1.14 for those diagnosed at less than 40, 50–59 years and 60 years and above respectively. However, a meta-analysis of 70 randomised breast radiotherapy treatment studies27 found women diagnosed at the age of 50 years and above had a significant risk of developing contralateral breast cancer, P = 0.002 (ratio of rates 1.25).
This study utilised standard imaging parameters with field length adjusted according to the patient's breast size as it is common in clinical practice. This study demonstrated the resulting contralateral breast dose variation due to the changes in patient anatomy particularly the primary breast size. Although not assessed in this study, it is likely that for standard imaging parameters image quality will also vary with patient anatomical changes.15 The AAPM Task Group 7531 have recommended that strategies for reducing the imaging dose and volume of exposed anatomy be pursued wherever possible, in line with the ALARA (as low as reasonably achievable) principle. Thus, variation of standard imaging parameters on a patient by patient basis could be considered; however, it should also be noted that dose variation between imaging modalities is greater than dose variation between individual patients imaged on single imaging modality.
Conclusions
The range of imaging doses to surrounding OAR for breast radiotherapy patients with different breast sizes has been presented in this study for MV-CT, MV-EPI and MV-CBCT. Imaging dose to the contralateral breast was inversely correlated with primary breast volume for all three imaging modalities. This study showed that for breast patients, imaging dose to OAR depends on imaging modality and treated breast size. When considering the use of daily imaging during breast radiotherapy treatment, the patient's breast size should be taken into account. The clinical benefit of daily imaging should be weighed against the additional risk, as adoption of daily imaging without clinical evidence may have increased risk on patients.
Conflict of Interest
The authors declare no conflict of interest.
References
- Li W, Moseley DJ, Bissonnette JP, Purdie TG, Bezjak A, Jaffray DA. Setup reproducibility for thoracic and upper gastrointestinal radiation therapy: influence of immobilization method and on-line cone-beam ct guidance. Med Dosim. 2011;35:287–96. doi: 10.1016/j.meddos.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Hawkins MA, Brock KK, Eccles C, Moseley D, Jaffray D, Dawson LA. Assessment of residual error in liver position using kV cone-beam computed tomography for liver cancer high-precision radiation therapy. Int J Radiat Oncol Biol Phys. 2006;66:610–9. doi: 10.1016/j.ijrobp.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Sonke JJ, Rossi M, Wolthaus J, Van Herk M, Damen E, Belderbos J. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance. Int J Radiat Oncol Biol Phys. 2009;74:567–74. doi: 10.1016/j.ijrobp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Song WY, Schaly B, Bauman G, Battista JJ, Van Dyk J. Evaluation of image-guided radiation therapy (IGRT) technologies and their impact on the outcomes of hypofractionated prostate cancer treatments: a radiobiologic analysis. Int J Radiat Oncol Biol Phys. 2006;64:289–300. doi: 10.1016/j.ijrobp.2005.08.037. [DOI] [PubMed] [Google Scholar]
- van Haaren PM, Bel A, Hofman P, van Vulpen M, Kotte AN, van der Heide UA. Influence of daily setup measurements and corrections on the estimated delivered dose during IMRT treatment of prostate cancer patients. Radiother Oncol. 2009;90:291–8. doi: 10.1016/j.radonc.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Fatunase T, Wang Z, Yoo S, et al. Assessment of the residual error in sort tissue setup in patients undergoing partial breast irradiation: results of a prospective study using cone-beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;70:1025–34. doi: 10.1016/j.ijrobp.2007.07.2344. [DOI] [PubMed] [Google Scholar]
- White EA, Cho J, Vallis KA, et al. Cone beam computed tomography guidance for setup of patients receiving accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2007;68:547–54. doi: 10.1016/j.ijrobp.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Topolnjak R, Sonke J-J, Nijkamp J, et al. Breast patient setup error assessment: comparison of electronic portal image devices and cone-beam computed tomography matching results. Int J Radiat Oncol Biol Phys. 2010;78:1235–43. doi: 10.1016/j.ijrobp.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Wilkinson M, Rawlings DJ, Moore M. Doses to critical organs following radiotherapy and concomitant imaging of the larynx and breast. Br J Radiol. 2007;80:989–95. doi: 10.1259/bjr/32814323. [DOI] [PubMed] [Google Scholar]
- Quinn A, Holloway L, Koh E-S, et al. Radiation dose and contralateral breast cancer risk associated with megavoltage cone-beam computed tomographic image verification in breast radiation therapy. Pract Radiat Oncol. 2013;3:93–100. doi: 10.1016/j.prro.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Quinn A, Holloway L, Hardcastle N, Tomé WA, Rosenfeld A, Metcalfe P. Normal tissue dose and second cancer risk due to megavoltage fan-beam CT, static tomotherapy and helical tomotherapy in breast radiotherapy. Radiother Oncol. 2013;108:266–8. doi: 10.1016/j.radonc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Xu XG, Bednarz B, Paganetti H. A review of dosimetry studies on external-beam radiation treatment with respect to second cancer induction. Phys Med Biol. 2008;53:R193–241. doi: 10.1088/0031-9155/53/13/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng LC, Yang CCJ, Sim S, Weiss M, Bielajew A. Dose comparison of megavoltage cone-beam and orthogonal-pair portal images. J Appl Clin Med Phys. 2007;8:10–20. doi: 10.1120/jacmp.v8i1.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Aird E, Shekhdar J. Contribution to normal tissue dose form concomitant radiation for two common kV-CBCT systems and one MVCT system used in radiotherapy. Radiother Oncol. 2012;105:139–44. doi: 10.1016/j.radonc.2012.04.017. [DOI] [PubMed] [Google Scholar]
- VanAntwerp AE, Raymond SM, Addington MC, Gajdos S, Vassil A, Xia P. Dosimetric evaluation between megavoltage cone-beam computed tomography and body mass index for intracranial, thoracic, and pelvic localization. Med Dosim. 2011;36:284–91. doi: 10.1016/j.meddos.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Shah AP, Langen KM, Ruchala KJ, Cox A, Kupelian PA, Meeks SL. Patient dose from megavoltage computed tomography imaging. Int J Radiat Oncol Biol Phys. 2008;70:1579–87. doi: 10.1016/j.ijrobp.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Lirette A, Pouliot J, Aubin M, Larochelle M. The role of electronic portal imaging in tangential breast irradiation: a prospective study. Radiother Oncol. 1995;37:241–5. doi: 10.1016/0167-8140(95)01653-8. [DOI] [PubMed] [Google Scholar]
- Donovan E, James H, Bonora M, Yarnold J, Evans P. Second cancer incidence risk estimates using BEIR VII models for standard and complex external beam radiotherapy for early breast cancer. Med Phys. 2012;39:5814–24. doi: 10.1118/1.4748332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEIR. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- Struikmans H, Warlam-Rodenhuis C, Stam T, et al. Interobserver variability of clinical target volume delineation of glandular breast tissue and of boost volume in tangential breast irradiation. Radiother Oncol. 2005;76:293–9. doi: 10.1016/j.radonc.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–8. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics Review, 1975-2005. (accessed 20 February 2012). Available from: http://seer.cancer.gov/csr/1975; 2005.
- Gayou O, Parda DS, Johnson M, et al. Patient dose and image quality from mega-voltage cone-beam computed tomography imaging. Med Phys. 2007;34:499–506. doi: 10.1118/1.2428407. [DOI] [PubMed] [Google Scholar]
- Joosten A, Bochud F, Baechler S, Levi F, Mirimanoff R, Moeckli R. Variability of a peripheral dose among various linac geometries for second cancer risk assessment. Phys Med Biol. 2011;56:5131. doi: 10.1088/0031-9155/56/16/004. [DOI] [PubMed] [Google Scholar]
- Curtis R, Freedman D, Ron E, et al. 2006. USA National Cancer Institute New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000.
- Stovall M, Smith S, Langholz B, et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72:1021–30. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice J, Jr, Harvey E, Blettner M, et al. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781–5. doi: 10.1056/NEJM199203193261201. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Curtis R, Gilbert E, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102:220–6. doi: 10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- Ndimofor C, Dietrich H, Kay CW, Björn P. Internal scatter, the unavoidable major component of the peripheral dose in photon-beam radiotherapy. Phys Med Biol. 2012;57:1733. doi: 10.1088/0031-9155/57/6/1733. [DOI] [PubMed] [Google Scholar]
- Murphy MJ, Balter J, Balter S, et al. The management of imaging dose during image-guided radiotherapy: report of the AAPM Task Group 75. Med Phys. 2007;34:4041–63. doi: 10.1118/1.2775667. [DOI] [PubMed] [Google Scholar]


