Abstract
Introduction
The magnitude of intra- and inter-fractional variation in the set up of breast cancer patients treated with tangential megavoltage photon beams was investigated using an electronic portal imaging device (EPID).
Methods
Daily cine-EPID images were captured during delivery of the tangential fields for ten breast cancer patients treated in the supine position. Measurements collected from each image included the central lung distance (CLD), central flash distance (CFD), superior axial measurement (SAM) and the inferior axial measurement (IAM). The variation of motion within a fraction (intra-fraction) and the variation between fractions (inter-fraction) was analysed to quantify set up variation and motion due to respiration.
Results
Altogether 3775 EPID images were collected from 10 patients. The effect of respiratory motion during treatment was <0.1 cm standard deviation (SD) in the anterior–posterior (AP) direction. The inter-fraction movement caused by variations in daily set up was larger at 0.28 cm SD in the AP direction. Superior–inferior (SI) variation was more difficult to summarise and proved unreliable as the measurements were taken to an ambiguous point on the images. It was difficult to discern true SI movement from that implicated by AP movement.
Conclusion
There is minimal intra-fractional chest wall motion due to respiration during treatment. Inter-fractional variation was larger, however, on average it remained within departmental tolerance (0.5 cm) for set up variations. This review of our current breast technique provides confidence in the feasibility of utilising advanced treatment techniques (field-in-field, intensity modulated radiotherapy or volumetric modulated arc therapy) following a review of the current imaging protocol.
Keywords: Breast cancer, motion, radiotherapy, radiotherapy set up errors
Introduction
Breast cancer has a high profile both within the medical profession and society alike. The Breast Cancer Network of Australia predicts that in 2013 there will be 14,940 women in Australia diagnosed with the disease.1 As a result of high prevalence there have been many public figures willing to raise funds and awareness, particularly by those who have been diagnosed themselves. Further, there have been numerous government-funded randomised studies to investigate ideal treatment regimes. This has lead to the use of whole breast radiation therapy (RT) following surgery as the standard treatment for women with early stage breast cancer due to improvements in local control, relapse-free survival and overall survival.2–5 Combined with surgery, chemotherapy and hormonal therapies of varying regimes, RT is an essential part of the multidisciplinary treatment approach.
The tangential field technique utilised for RT to the breast has traditionally been based on a clinical set up that utilises skin markings to facilitate treatment of the whole breast. The goal of this technique is to include all the breast tissue while limiting the amount of heart and lung within the high dose radiation field. The effects of treating healthy tissues unnecessarily can result in short and long term toxicities and subsequent patient morbidities. Cao et al. state that respiratory motion during treatment can lead to a larger than planned volume of the heart and/or lungs entering the high dose treatment fields. This can lead to late toxicities such as ischaemic heart disease and radiation pneumonitis.6 A recent study by Darby et al. has found that the risk of a major coronary event following RT increases by 7.4% per Gy incidentally delivered to the heart (mean dose).7 A primary goal in planning breast RT should be to minimise the amount of heart and lung irradiated.8,9 Therefore, we have sought to determine the consistency between planning and treatment set up within our department in a cohort of patients with left-sided breast cancer.
Recent trends in RT have seen the implementation of techniques designed to correct for the thoracic motion naturally occurring due to breathing. Specifically, motion due to respiration can potentially be accounted for with devices such as the Varian Real-Time Position Management (RPM) system (Varian, Paolo Alto, CA). Taking this into consideration, an observational study was designed to investigate the impact that respiratory motion has on our institution's current breast RT technique. The intra- and inter-fractional variances in breast and lung position in relation to the treatment field will be analysed from daily treatment imaging data. This information will be used to draw conclusions about the current RT treatment technique and potential areas for improvement including consideration of gated treatment techniques.
Method
Ethical approval for this study was granted from our institutional Health and Research Ethics Committee. Ten patients with left-sided breast cancer (Stages T1–T3), who were treated between October 2010 and December 2011, were selected for this study. Eligibility criteria included patients to be treated in a supine position and who did not have a large pendulous breast that would present dosimetric challenges. All patients were female with a median age of 59.5 years (44–72). Nine of the ten patients had stage I or II tumours with only one patient having locally advanced breast cancer (stage III). None of the patients had other major co-morbidities (e.g. emphysema) that impacted their respiratory function. However, it was identified that two of the patients did have a previous medical history of asthma.
Simulation of the treatment fields involved a computed tomography (CT) scan of the patient positioned supine on the Civco Breastboard (CIVCO Medical Solutions, Coralville, IA). Departmental protocol stipulated a CT protocol of 3 mm slices from the chin to include all the lungs inferiorly. The board angle was chosen at CT to best represent a chest wall that would be parallel to the treatment field. Both arms were above their head in arm supports to ensure the patients were comfortable and would not interfere with the entrance or exit of the beams. A support was placed under the patient's knees for support. No other stabilisation equipment was used. The medial, lateral, superior and inferior field borders were defined by a radiation oncologist (RO), and marked by radio-opaque markers to enable visualisation of the clinical limits on the CT scan. No motion management techniques were used for simulation.
Pinnacle v9.0 (Philips Medical Systems, Cleveland, OH) treatment planning system (TPS) was used to develop a suitable plan with opposed tangential beams where the field borders were defined by the RO's marks from CT (Fig.1). Each field allowed for 2 cm of overshoot anteriorly to the breast tissue, and used dynamic wedges to provide a homogenous dose distribution. Beam energies of 6 or 10 MV were used depending on breast size. The heart and contra and ipsilateral lungs were outlined as organs at risk, and dose-volume histograms were produced to ensure the toxicity limits were not exceeded. Our protocol stipulates less than 20% of the lung should get 20 Gy, and less than 5% of the heart should get 40 Gy.
Figure 1.

A rendered computed tomography (CT) scan showing the tangential treatment fields in colourwash on the patient's surface.
Patients were treated using Varian Clinac IX linear accelerators (Varian, Palo Alto, CA) equipped with a megavoltage electronic portal imaging device (EPID). During the delivery of each treatment beam, the EPID was used to capture the treatment field image. Twenty images were captured per treatment beam, allowing the creation of a cineloop for each of the medial and lateral treatment beam. Departmental policy required the first three fractions of treatment to include a single radiograph from the medical treatment beam to assess patient set up. This delayed collection of the cineloop image data until fraction four. Some of our breast patients were prescribed a hypo-fractionated treatment regime (42.5 Gy in 16 fractions) which still enabled us to obtain a minimum of ten cineloops.
The data collected to measure motion were recorded from each electronic portal image (EPI) frame in four directions. Central lung distance (CLD), central flash distance (CFD), vertical superior axial measurement from a chosen point along the central axis to breast tissue edge (SAM), and inferior axial measurement from a chosen point along the central axis to breast tissue edge (IAM) (refer to Fig.2 which describes the data points measured). The same automatic histogram equalisation filter was applied to each image before being viewed and measured independently by two radiation therapists in order to limit inter-user variation. The average of both measures was used in the analysis of the data.
Figure 2.
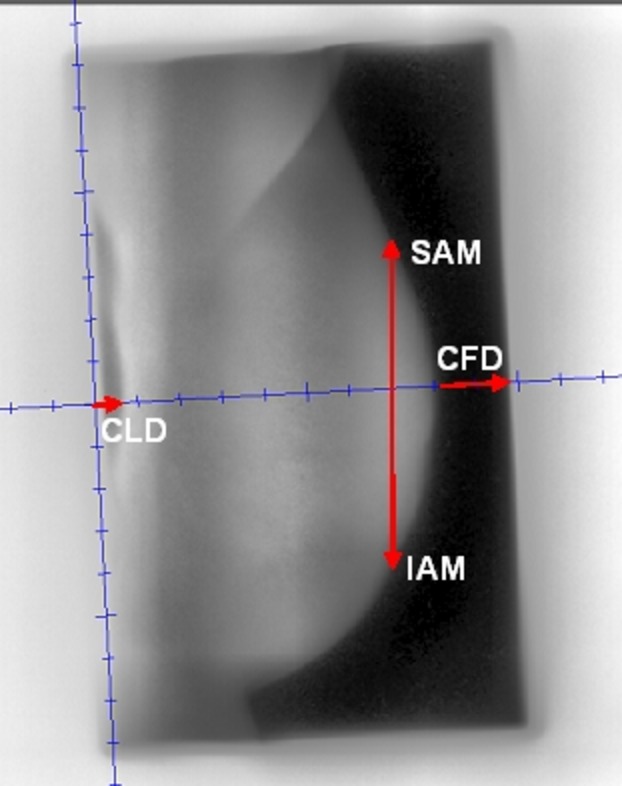
The four data points measured from each cine-electronic portal imaging device (EPID) image. CLD, central lung distance; CFD, central flash distance; SAM, superior axial measurement; IAM, inferior axial measurement.
Statistical methodology
The results were defined by one of two categories; the measurement difference between images within a fraction (intra-fraction motion); and the measurement difference of images between fractions (inter-fraction motion). The latter of these categories also included a comparison with the digitally reconstructed radiograph (DRR) produced from each patient's planning CT scan.
To represent the variation in our measurements (SAM, CLD, IAM and CFD), the SD of the measurements was determined using the methods described by Fein et al.10 Additionally, the maximum and mean ranges were calculated for intra-fractional motion and the maximum and mean difference from CT was tabulated for the inter-fraction motion.
The intra- and inter-fractional results for the four parameters are expressed in terms of 1 standard deviation (SD). Simulation to the treatment set up errors were calculated by measuring the difference between the mean of the intra-fractional measurements for the four parameters and their simulation counterparts. SDs for each patient were then pooled to give a population SD for each of the four parameters. All analyses and calculations were performed using Stata 10.1 (StataCorp, College Station, TX) and MS Excel (Microsoft Corporation, Redmond, WA).
Results
Altogether 14,143 measurements were recorded for all ten patients. Intra-fraction motion was best assessed using the CLD and CFD values from both of the treatment tangents as they accounted for the anterior to posterior motion during respiration. From the first set of images, it became evident that measuring the amount of heart within a field would not be possible as it was difficult to visualise on the EPID images. Equipment breakdown and user error of not scheduling the daily cineloop images resulted in 225 images not being captured (5.6% of the 4000 image target).
The approach for recording the superior to inferior movement was modified due to the obliquity (caused by a rotated treatment collimator) of the image on the EPID. Therefore, a straight vertical measurement was used instead of a line that runs parallel to the treatment field to remove any error associated with inconsistent measurements. However, it must be noted that movement in the anterior–posterior (AP) direction directly altered the superior–inferior (SI) measurement, due to the convex contour of the breast.
Table1 shows the maximum and mean range of intra-fractional motion across the patient cohort. It was determined from our current breast imaging protocol that clinically significant motion in our department was ≥0.5 cm. From these data, it can be seen that on average no patient had a range that exceeded this amount with 5.4% of all fields treated recording motion ≥0.5 cm.
Table 1.
Intra-fraction motion in the anterior–posterior direction
| Patient number | CLD | CFD | ||
|---|---|---|---|---|
| Max. range (cm) | Mean range (cm) | Max. range (cm) | Mean range (cm) | |
| 1 | 0.35 | 0.12 | 0.27 | 0.13 |
| 2 | 0.35 | 0.17 | 0.18 | 0.11 |
| 3 | 0.74 | 0.33 | 0.50 | 0.30 |
| 4 | 0.39 | 0.14 | 0.42 | 0.16 |
| 5 | 0.52 | 0.24 | 0.49 | 0.24 |
| 6 | 1.06 | 0.41 | 1.32 | 0.41 |
| 7 | 0.47 | 0.25 | 0.37 | 0.16 |
| 8 | 0.24 | 0.16 | 0.33 | 0.20 |
| 9 | – | – | 0.33 | 0.19 |
| 10 | 0.51 | 0.15 | 0.20 | 0.13 |
CLD, central lung distance; CFD, central flash distance.
Figure3 displays the CLD measurements from the two patients with the greatest range variation (patient 3 and 6). The frequency of intra-fractional motion range ≥0.5 cm was 30% for patient 3 and 10% for patient 6. From the graph there was no visual pattern detectable in the occurrence of these excursions. Despite patient 6 having a maximum range more than double the acceptable clinical value of 0.5 cm, the mean indicates that the frequency was low and had a minimal impact.
Figure 3.

Comparison of the range frequency of the measured central lung distance (CLD) value for patients 3 and 6. RAO, right anterior oblique; LPO, left posterior oblique.
No CLD data were able to be collected from patient 9 as the images did not provide visualisation of the chest wall. Given that the CLD measurement from this patient's DRR was 0.3 cm then it can be deduced that the isocentre was regularly set up too anteriorly by ≥0.3 cm. It cannot be determined, however, whether the AP set up variation was ≥0.5 cm.
Table2 was produced using the pooled SD formula10 as displayed in equation 1,
 |
1 |
where SD12 … SDk2 are the variances in the parameter determined for treatment field and ni is the number of treatment fields imaged during the ith fraction. This formula provides an insight into the overall intra-fractional SD of the patient cohort. The differences listed compare the measurements with the original CT DRR value. Both the intra- and inter-fractional tables display the same differences as they are both calculated using the mean measurements. It can be seen that intra-fractional motion was minimal in the AP direction and was the greatest in magnitude for the SI measurements. The AP values had a SD of 0.08 cm meaning that 95% of measurements were within 0.16 cm of the mean. The SAM, however, had a much larger SD of 0.37 cm showing greater variation.
Table 2.
Intra-fraction variations for the four data points (cm)
| Data point | Pooled SD | Difference |
|---|---|---|
| CLD | 0.08 | 0.25 |
| CFD | 0.08 | 0.35 |
| SAM | 0.37 | 1.47 |
| IAM | 0.09 | 0.68 |
CLD, central lung distance; CFD, central flash distance; SAM, superior axial measurement; IAM, inferior axial measurement.
Table3 summarises the inter-fractional variation in CLD and CFD. Seven of the ten patients had a maximum set up difference ≥0.5 cm for the CLD measurements. None of these patients, however, had a mean CLD value ≥0.5 cm which indicates fractions with a large set up variation were infrequent. The CFD measurements on the other hand showed all 10 patients to have a maximum difference from CT greater than clinical significance. This translated to a mean ≥0.5 cm in 40% of patients. The population SD as calculated by equation 210,
 |
2 |
is used to calculate inter-fractional motion, where j represents twice the number of patients because each of the tangents are treated as independent. The individual SD's are, therefore, the inter-fractional SD value for any given patient and this is reflected in Table4.
Table 3.
Inter-fraction motion in the anterior–posterior direction
| Patient number | CLD | CFD | ||
|---|---|---|---|---|
| Max. difference from CT (cm) | Mean difference from CT (cm) | Max. difference from CT (cm) | Mean difference from CT (cm) | |
| 1 | 0.49 | 0.25 | 0.78 | 0.22 |
| 2 | 0.36 | 0.15 | 1.8 | 0.26 |
| 3 | 0.9 | 0.35 | 0.68 | 0.26 |
| 4 | 0.87 | 0.21 | 2.26 | 0.54 |
| 5 | 1.64 | 0.28 | 2.06 | 0.63 |
| 6 | 1.17 | 0.3 | 1.55 | 0.31 |
| 7 | 0.93 | 0.25 | 2.46 | 0.49 |
| 8 | 0.81 | 0.34 | 2.85 | 0.58 |
| 9 | – | – | 2.4 | 1.09 |
| 10 | 1.0 | 0.32 | 1.84 | 0.35 |
CLD, central lung distance; CFD, central flash distance.
Table 4.
Inter-fraction variations for the four data points (cm)
| Data point | Population SD | Difference |
|---|---|---|
| CLD | 0.24 | 0.25 |
| CFD | 0.28 | 0.35 |
| SAM | 1.07 | 1.47 |
| IAM | 0.54 | 0.68 |
CLD, central lung distance; CFD, central flash distance; SAM, superior axial measurement; IAM, inferior axial measurement.
Figure4 graphically displays the frequency of the set up error for the two patients that showed the largest maximum difference and largest mean difference inter-fractionally. Both patient 3 and 5 displayed a set up error beyond tolerance on three separate occasions. The timing of the variations throughout the treatment course was different between patients, and sometimes even between individual fields for a given patient. The nature of these errors was random and variable in magnitude.
Figure 4.
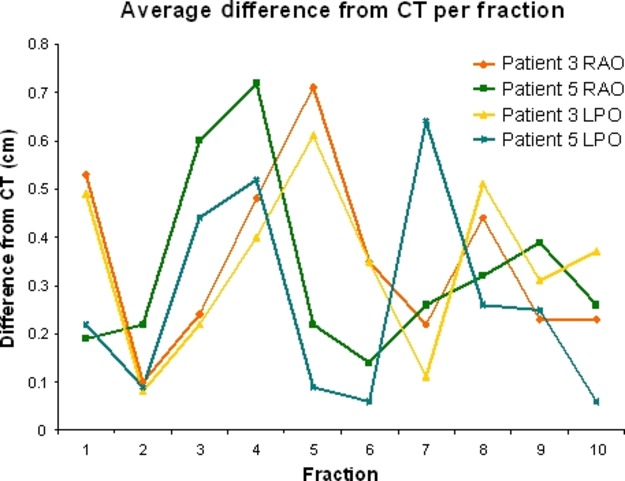
Comparison of the set up difference frequency (difference from computed tomography (CT) digitally reconstructed radiograph (DRR) to treatment electronic portal image (EPI)) for patients 3 and 5. RAO, right anterior oblique; LPO, left posterior oblique.
The separation change variation that occurs over the entire treatment course in the SI direction can be seen in the population SD in Table4 for the SAM and IAM data points. Similarly to the intra-fractional data, this variation appears more pronounced in the SAM, with 95% of recorded measurements falling within 2.14 cm of the mean.
Discussion
This study provided a geometric uncertainty information relating to our equipment and technique as recommended by Saliou et al.11 This knowledge is important for assessing the imaging protocols in place, as well as developing a basis to advance current treatment techniques for breast cancer patients.
As a whole these data provide evidence that set up variation, that is, inter-fractional motion is more frequent and is of a greater magnitude than intra-fractional motion. Only in the case of mean inter-fractional CFD measurements does this exceed clinical significance though, and there are several factors that could contribute to this. Anecdotal evidence suggests that the anterior contour of the breast changes throughout treatment due to hormonal variations and inflammatory responses to treatment. This would obviously impact the CFD measurement taken. Of greatest importance is maintaining ample field coverage of the anterior breast surface. So while CFD is somewhat indicative of AP movement it is also a means of ensuring the whole breast is receiving treatment.
The CLD, however, was able to provide a more accurate description of AP movement occurring as it is measured to a more stable point. In a study by Kong et al. they found that there was a correlation between the CLD and lung dose.12 For cases where the CLD variation was ≤0.5 cm, the formulae by Kong et al. showed a mean lung dose increase of no more than 2 Gy. It would be fair to conclude, therefore, that the majority of patients in this study received far less than 2 Gy extra to their ipsilateral lung. This group further states that a CLD variation of ≤0.5 cm will not increase the volume of ipsilateral lung receiving 20 Gy by any more than 5%. Therefore, in view of our data the delivered lung dose-volume relationship will have varied minimally.
Measurement variations appear greatest in the SI direction as evidenced by the maximum, mean and SD results tabulated, however, this is not necessarily indicative of poor set up or motion due to respiration. Rather its value relies upon both the concurrent AP movement and contour variability of the skin point being measured, particularly in the superior region where the contour slope is quite steep. Factors such as patient anxiety and set ups being performed by different staffing groups are additional variables that will negatively impact data reliability and limit study conclusions.
The differences obtained in this study between inter- and intra- fractional motion, taking for example, the population SD and pooled SD CLD measurements (0.24 and 0.08 cm respectively), agreed with the previously published data that reported the inter-fractional variation to be of a greater magnitude than intra-fractional motion (0.35 cm (1.34–0.17 cm) and 0.17 cm (0.24–0.07 cm)) respectively).10,13–16 In some cases the magnitude of which this occurs varied between our work and other authors. This is likely due to variations in stabilisation equipment and patient positioning technique. Such alternatives could be the use of contralateral levelling tattoos and vacbags for set up; however, these details were scarcely available in the literature. Having a review of the breast set up specific to a department such as this will prove useful in directing continued improvements in practice.
The overall stability of our technique and minimal impact from respiration in this patient cohort indicates that moving towards techniques such as field-in-field, intensity modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT) would be both feasible and favourable to a patient's treatment.17–19 However, as aforementioned, a large CFD distance is routinely encountered for tangential treatments and would need to be replicated with an anterior flash distance for IMRT and VMAT treatments. The affect of this would be twofold, with the planning system potentially under dosing the anterior breast tissue, and not accounting for motion. These results do not diminish the importance of respiratory gating as a technique for minimising incidental heart and lung dose, but rather the identification of suitable patients may best occur during the planning phase. Gated techniques are becoming more popular in many centres and a method of flagging suitable patients during the planning stage based on the positional relationship of the heart, lungs and treatment fields may allow us to indentify the subset of patients that would benefit most from it. Lastly, hormonal and inflammatory changes may cause fluctuations in breast shape, which will directly impact the intricate dosimetry of IMRT and VMAT.
In pursuing any of these complex treatment techniques it will be important to review our imaging protocol in an attempt to reduce random errors. Ideally, an imaging protocol that involved daily imaging would be the simple answer to this problem as recommended by Lirette et al. and Prabhakar et al.,13,16 however, the implications on time, cost and increased patient dose may restrict this as an option.
There were several limitations in this study that resulted in a lengthy patient accrual and data analysis process. Out of the four machines available within the department, only two of them were equipped with amorphous silicon EPID's required for better soft tissue visualisation. Concurrently, the same machines were taking the entire IMRT workload of our department due to their kilovoltage (kV) imaging capabilities. This made scheduling study patients difficult, and in turn often resulted in only being able to approach patients about the study when we could be certain they would receive treatment on the desired machines.
It was also identified that the sample size chosen would limit the amount of statistical significance which could be drawn from the results. However, the sample size was intentionally restricted due to the aforementioned treatment machine limitation and to make the image data extraction process feasible by limiting the volume of images to assess.
Conclusions
The majority of patients within this small study cohort treated for left-sided breast cancer displayed minimal intra-fraction motion. The inter-fraction motion measured was comparatively greater, however, on average was still within the 5 mm clinical threshold defined in our department imaging protocol. The increase in lung dose due to AP movement was <2 Gy, with <5% variation at the 20 Gy dose level.12
The small amount of variation noticed during treatment can potentially justify the use of more conformal treatments such as field-in-field, IMRT or VMAT. This study does not deny the benefits of gated breast treatments when applied to the correct subset of patients. Rather it confirms confidence in our ability to set up patients accurately for any technique. In looking ahead to the implementation of any of these more advanced techniques (field-in-field, IMRT or VMAT) it would be advantageous to reduce the random error where possible with a review of the imaging protocol.
Conflict of Interest
The authors declare no conflict of interest.
References
- BCNA.org.au. Australia: Breast Cancer Network Australia: About Breast Cancer. c2010. [cited 2013 October 9]. Available from: http://www.bcna.org.au/about-breast-cancer.
- Fisher B, Redmond C, Poisson R, et al. Eight-year results of a randomised clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320:822–8. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- Viani GA, Stefano EJ, Afonso SL, et al. Breast-conserving surgery with or without radiotherapy in women with ductal carcinoma in situ: a meta-analysis of randomized trials. Radiat Oncol. 2007;2:28. doi: 10.1186/1748-717X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, E. B. C. T. C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover S, Bloom E, Patel S. Review of breast conservation therapy: then and now. ISRN Oncol. 2011;2011:617593. doi: 10.5402/2011/617593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Roeske JC, Chmura SJ, et al. Calculation and prediction of the effect of respiratory motion on whole breast radiation therapy dose distributions. Med Dosim. 2009;34:126–32. doi: 10.1016/j.meddos.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- Senkus-Konefka E, Jassem J. Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. 2007;33:578–93. doi: 10.1016/j.ctrv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Demirci S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys. 2009;73:980–7. doi: 10.1016/j.ijrobp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Fein DA, McGee KP, Schultheiss TE, Fowble BL, Hanks GE. Intra- and Interfractional reproducibility of tangential breast fields: a prospective on-line portal image study. Int J Radiat Oncol Biol Phys. 1996;34:733–40. doi: 10.1016/0360-3016(95)02037-3. [DOI] [PubMed] [Google Scholar]
- Saliou MG, Giraud P, Simon L, et al. Radiotherapy for breast cancer: respiratory and set-up uncertainties. Cancer Radiother. 2005;9:414–21. doi: 10.1016/j.canrad.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kong F, Klein EE, Bradley JD, et al. The impact of central lung distance, maximal heart distance, and radiation technique on the volumetric dose of the lung and heart for intact breast radiation. Int J Radiat Oncol Biol Phys. 2002;54:963–71. doi: 10.1016/s0360-3016(02)03741-0. [DOI] [PubMed] [Google Scholar]
- Lirette A, Pouliot J, Aubin M, Larochelle M. The role of electronic portal imaging in tangential breast irradiation: a prospective study. Radiother Oncol. 1995;37:241–5. doi: 10.1016/0167-8140(95)01653-8. [DOI] [PubMed] [Google Scholar]
- Lee C, Kron T, Perera F, Yu E. Evaluation of intra- and inter- fraction motion in breast radiotherapy using electronic portal cine imaging. Technol Cancer Res Treat. 2004;3:443–9. doi: 10.1177/153303460400300505. [DOI] [PubMed] [Google Scholar]
- Smith RP, Bloch P, Harris EE, et al. Analysis of interfraction and intrafraction variation during tangential breast irradiation with an electronic portal imaging device. Int J Radiat Oncol Biol Phys. 2005;62:373–8. doi: 10.1016/j.ijrobp.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Prabhakar R, Rath GK, Julka PK, Ganesh T, Joshi RC. Reproducibility of tangential breast fields using online electronic portal images. Rep Prac Oncol and Radiother. 2007;12:323–8. [Google Scholar]
- Vicini FA, Sharpe M, Kestin L, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1336–44. doi: 10.1016/s0360-3016(02)03746-x. [DOI] [PubMed] [Google Scholar]
- Stillie AL, Kron T, Herschtal A, et al. Does inverse-planned intensity-modulated radiation therapy have a role in the treatment of patients with left-sided breast cancer? J Med Imaging Radiat Oncol. 2011;55:311–9. doi: 10.1111/j.1754-9485.2011.02273.x. [DOI] [PubMed] [Google Scholar]
- Giorgio P, Grimaldi L, Fidanza C, et al. Geometric and dosimetric approach to determine probability of late cardiac mortality in left tangential breast irradiation: comparison between wedged beams and field-in-field technique. Int J Radiat Oncol Biol Phys. 2011;81:894–900. doi: 10.1016/j.ijrobp.2010.12.021. [DOI] [PubMed] [Google Scholar]


