Abstract
Stable carbon isotope composition (δ13C) usually shows a negative relationship with precipitation at a large scale. We hypothesized that sampling method affects foliar δ13C and its response pattern to precipitation. We selected 11 sites along a precipitation gradient in Inner Mongolia and collected leaves of Leymus chinensis with five or six replications repeatedly in each site from 2009 to 2011. Additionally, we collected leaves of L. chinensis separately from two types of grassland (grazed and fenced) in 2011. Foliar δ13C values of all samples were measured. We compared the patterns that foliar δ13C to precipitation among different years or different sample sizes, the differences of foliar δ13C between grazed and fenced grassland. Whether actual annual precipitation (AAP) or mean annual precipitation (MAP), it was strongly correlated with foliar δ13C every year. Significant difference was found between the slopes of foliar δ13C to AAP and MAP every year, among the slopes of foliar δ13C to AAP from 2009 to 2011. The more samples used at each site the lower and convergent P-values of the linear regression test between foliar δ13C and precipitation. Furthermore, there was significant lower foliar δ13C value in presence of grazed type than fenced type grassland. These findings provide evidence that there is significant effect of sampling method to foliar δ13C and its response pattern to precipitation of L. chinensis. Our results have valuable implications in methodology for future field sampling studies.
Keywords: Environmental variability, field experiment, sampling error, temperate steppes, transect
Introduction
Stable carbon isotope composition (δ13C) of the leaves of C3 plants is largely related to the temporally averaged ratio of the concentration of intercellular to atmospheric CO2, ci/ca, which is the result of the balance between stomatal conductance and photosynthesis (Farquhar et al. 1982, 1989). Factors that affect either stomatal conductance or photosynthesis also have effects on foliar δ13C. A close relationship exists between ci/ca and plant water use efficiency (WUE), which means that foliar δ13C can provide an estimate of the integrated long-term WUE of a plant (Ehleringer and Cooper 1988; Farquhar et al. 1989; Bert et al. 1997; Silim et al. 2001; Michelot et al. 2011). Foliar δ13C is thus a complex trait involved in acclimation, adaptive processes.
Foliar δ13C values of C3 plants are known to be affected by environmental factors, with water availability in particular showing a strong negative relationship with foliar δ13C (Stewart et al. 1995; Swap et al. 2004; Liu et al. 2005; Liu, Tian et al. 2013; Liu, Xu et al. 2013; Liu et al. 2014). Many studies have reported a trend higher foliar δ13C values in drier environment (Hausmann et al. 2005; Schulze et al. 2006; Luo et al. 2009; Diefendorf et al. 2010; Prentice et al. 2011; Wang et al. 2013). Many transect studies also showed that foliar δ13C decreases with increasing precipitation (Swap et al. 2004; Guo and Xie 2006; Zheng and Shangguan 2006; Song et al. 2008; Prentice et al. 2011; Liu et al. 2013,b2013). However, very few studies have considered the effect of sampling method on foliar δ13C and its response pattern to precipitation.
Water availability is an important factor that influences plant growth (McConnaughay and Coleman 1999; Poorter and Nagel 2000), and variation in water availability drives variation in vegetation types. This is particularly pronounced in the temperate steppes of Inner Mongolia, China (Gong et al. 2011; Li et al. 2011; Liu et al. 2013,b2013). The central Eastern regions in the Inner Mongolia with undulating terrains are mainly divided into medium and low mountainous regions, and hills and peneplain regions (Fig.1). There is large spatial heterogeneity of water availability also among nearly microhabitats of this area, although they are exposed to similar climatic conditions. Some researchers found different foliar δ13C response patterns to precipitation for the same species (Su et al. 2000; Prentice et al. 2011), and the reason may be that they only collected one sample per site along a precipitation gradient and did not consider the heterogeneity of water availability among microhabitats.
Figure 1.
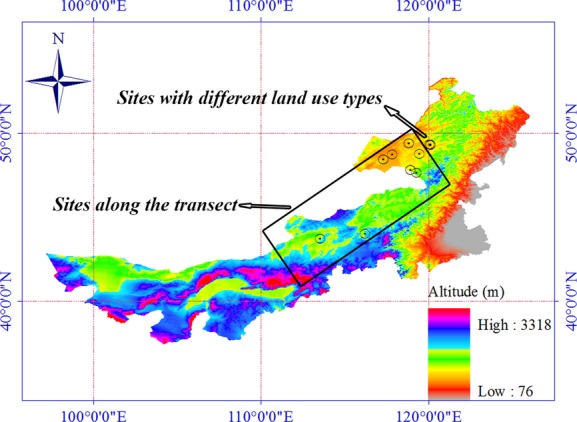
Location of sampling sites on the topographic map of Inner Mongolia, China.
Most of the grasslands are grazed by cattle in this region (Yan et al. 2013), and it is virtually impossible to find undisturbed grassland. However, it is also very common in Inner Mongolia that grassland is fenced in order to prevent its degradation from overgrazing. Few studies considered the impact of land-use types on foliar δ13C when they collected the samples at a large geographical scale. Furthermore, some studies used the mean annual precipitation (MAP) instead of actual annual precipitation (AAP) to test the relationship between foliar δ13C and precipitation at a large scale (Wittmer et al. 2008; Prentice et al. 2011). However, temporal variability of precipitation among years may also affect this relationship.
Herein, we set out to test the hypothesis that sampling design affects foliar δ13C and its response pattern to precipitation, by analyzing leaf samples of the same species distributed along a transect in the central Eastern regions of Inner Mongolia, China. Specifically, the following three questions are addressed: (1) Do the patterns of foliar δ13C values in response to precipitation differ among different years? (2) Do the numbers of samples per site affect the foliar δ13C response pattern to precipitation at a large scale? (3) Does different land use of grassland (grazed and ungrazed (fenced) grasslands) affect foliar δ13C values?
Materials and Methods
Study species
Leymus chinensis (Trin.) Tzvel. (Poaceae), a perennial rhizomatous C3 grass, shows both vegetative reproduction and sexual reproduction (The Integrated Investigation Team in Inner Mongolia and Ningxia, CAS 1985). It occupies large areas as a dominant or codominant species in the eastern parts of the Eurasian steppes and thrives in a diverse range of habitats (The Integrated Investigation Team in Inner Mongolia and Ningxia, CAS 1985; Liu et al. 2007). There are two ecotypes of this species that differ in leaf color: one is yellow-green and the other one is gray-green (Fig. S1). The yellow-green ecotype is only found in meadow steppes, while the gray-green one is distributed across a more extensive range of environmental conditions (Chen and Wang 2009). Furthermore, these two ecotypes also sometimes co-occur in the same region. We only assessed the foliar δ13C values of L. chinensis with gray-green leaf color in our study, because the yellow-green type only occurs in a small region of Inner Mongolia, and because the two ecotypes differ in foliar δ13C (Fig. S2), and we want to unravel the effects of sampling method on foliar δ13C at a large geographical scale.
Large-scale transect study
We selected 11 sites along a west–east precipitation gradient in Inner Mongolia (Table1 and Fig.1). We collected samples from each site in late June 2009, and late August 2010 and 2011. Foliar δ13C values of C3 plants are known to be affected by water availability (Stewart et al. 1995; Liu et al. 2014). We avoided sites located near rivers, because water availability there is not only determined by precipitation. There is spatial heterogeneity (i.e., among microhabitats) within each site mostly due to topography, and therefore, we expected heterogeneity in foliar δ13C values. In order to encompass such heterogeneity and reduce the sampling error by avoiding sampling one specific microhabitat by chance, we first assessed the topography and identified a baseline along the hillside aspect at each site. Then, we systematically collected six samples (five in 2011) from six plots along the baseline. The distance between two adjacent plots along the baseline was 10 m. Foliar δ13C values can vary among leaves within a plant (Yang et al. 2011). Therefore, for each sample, we collected all mature leaves of 5–8 randomly chosen L. chinensis individuals and mixed them. Leaves were microwaved immediately (500 W, 2 min) after collection to make sure that plant enzymes were deactivated and then air-dried. Once back in the laboratory, the samples were further dried in a drying oven at 65°C for 48 h.
Table 1.
Location and characteristics of the sites sampled along the precipitation gradient in Inner Mongolia, China, from 2009 to 2011
| Site | Latitude (N) | Longitude (E) | Altitude (m a.s.l.) | AAP (mm) | MAP (mm) | ||
|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | |||||
| T1 | 43°43.21′ | 113°31.64′ | 1027 | 181.85 | 183.54 | 163.17 | 265.77 |
| T2 | 44°01.31′ | 116°12.43′ | 1051 | 227.53 | 287.85 | 245.86 | 335.80 |
| T3 | 48°27.20′ | 117°18.80′ | 624 | 314.47 | 182.16 | 258.22 | 330.89 |
| T4 | 48°27.20′ | 117°18.80′ | 624 | 314.47 | 182.16 | 258.22 | 330.89 |
| T5 | 48°46.46′ | 117°49.67′ | 550 | 329.64 | 201.92 | 275.01 | 348.28 |
| T6 | 49°25.96′ | 118°48.21′ | 616 | 394.49 | 260.71 | 333.20 | 381.97 |
| T7 | 49°25.96′ | 118°48.21′ | 616 | 394.49 | 260.71 | 333.20 | 381.97 |
| T8 | 47°50.54′ | 118°54.99′ | 757 | 361.79 | 295.97 | 348.90 | 387.33 |
| T9 | 47°50.54′ | 118°54.99′ | 757 | 361.79 | 295.97 | 348.90 | 387.33 |
| T10 | 47°39.52′ | 119°17.44′ | 871 | 371.58 | 312.46 | 367.57 | 387.01 |
| T11 | 48°46.79′ | 119°27.83′ | 680 | 407.34 | 306.82 | 373.12 | 397.19 |
APP, actual annual precipitation; MAP, mean annual precipitation.
For this study, we used climatic data recorded by 54 weather stations distributed along our transect. Based on these data, we used Kriging interpolation to determine the actual annual precipitation (AAP) at each sample location for 2009, 2010, and 2011. The mean annual precipitation (MAP) across the last 15 years was also calculated. Kriging interpolation was implemented in ArcGIS 10.0.
Small-scale regional study
Grazed and fenced grasslands are two common land-use types in Inner Mongolia. In order to investigate how these two land-use types influence foliar δ13C, we conducted a small-scale regional study in early September 2011. We selected three sites in the Hulunbuir meadow steppe of Inner Mongolia, China (Table2 and Fig.1). Plant communities in these three sites are dominated by L. chinensis,Stipa baicalensis, and mesophytic forbs. Each site had both land-use types; one part was grazed by cattle animals, and another part was enclosed by a fence (Fig. S3). All fenced grasslands had been enclosed since 2006. For each land-use type at the three sites, we randomly placed three quadrats (1 m × 1 m), in which we collected all mature leaves of L. chinensis and mixed them as one sample. All samples were dried in an oven at 65°C for 48 h.
Table 2.
Location and characteristics of the sites sampled in Hulunbuir meadow steppe of Inner Mongolia, China, in 2011
| Site | Latitude (N) | Longitude (E) | Altitude (m a.s.l.) | Land-use type | Dominant species | |
|---|---|---|---|---|---|---|
| 1 | 49°22.33′ | 120°1.73′ | 633 | Grazing | May to September | Leymus chinensis |
| Fenced | Since 2006 | Leymus chinensis | ||||
| 2 | 49°21.17′ | 120°6.15′ | 658 | Grazing | May to September | Stipa baicalensis |
| Fenced | Since 2006 | Stipa baicalensis | ||||
| 3 | 49°19.33′ | 120°5.87′ | 619 | Grazing | May to September | Mesophytic forbs |
| Fenced | Since 2006 | Mesophytic forbs | ||||
Carbon isotope measurement
All collected plant material was ground to a homogenous powder using a ball mill (MM200; Retsch, Haan, Germany). Aliquots of approximately 2.5 mg of plant material were weighed into tin capsules for foliar δ13C measurement. For samples collected in 2009 and 2010, foliar δ13C was measured using continuous-flow gas isotope ratio mass spectrometry (CF-IRMS) with Vario PYRO Cube (IsoPrime100; Isoprime Ltd., Stockport, U.K.) at the Institute of Environment and Sustainable Development in Agriculture, CAAS, China. Vienna Pee Dee Belemnite (VPDB) was used as the reference standard for C isotopic analyses. Reproducibility was high as the standard deviation of repeated measurements was lower than 0.20‰. For samples collected in 2011, foliar δ13C was measured using CF-IRMS with Flash EA1112 and the interface Conflo III (MAT 253; Finnigan MAT, Bremen, Germany) at the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, China. Pee Dee Belemnite (PDB) was used as the reference standard for C isotopic analyses. Reproducibility was high as the standard deviation of repeated measurements was lower than 0.15‰.
Statistical analyses
All statistical analyses were performed using R 3.0.2 (R Core Team 2013). We tested for relationships between foliar δ13C and AAP in the study year, or MAP across the last 15 years, using linear regression. The slope of the regression line of foliar δ13C against AAP or MAP was the response strength of foliar δ13C, that is, indicating how strongly foliar δ13C responded to precipitation. In order to test whether foliar δ13C response to AAP differs from its response to MAP, the difference in response strength with respect to AAP and MAP was tested using the Standardized Major Axis Tests & Routines (SMATR) R package (Falster et al. 2006). In order to test whether foliar δ13C response to AAP varies among years, the difference in response strength with respect to AAP among years was also tested using SMATR. There were six samples in 2009 and 2010, and five samples in 2011 at each site along the transect. In order to test how strongly foliar δ13C responded to AAP at a large geographical scale when different sample sizes were used, we randomly sampled one, two, three, four, and five samples from each site, respectively. Therefore, we obtained different datasets, as each site along the transect had different samples. For these datasets, the relationship between foliar δ13C ratio and AAP was tested using linear regression. Furthermore, for the small-scale region study, in order to test the effects of sampling site and land-use type on foliar δ13C values, we did a two-way analysis of variance (ANOVA).
Results
Large-scale patterns of foliar δ13C in response to precipitation across years
Foliar δ13C values along the transect significantly decreased with increasing precipitation (whether AAP or MAP) in each year from 2009 to 2011 (Fig.2). However, the response of foliar δ13C to MAP was significantly stronger than the response to AAP (2009, P < 0.001, Fig.2A; 2010, P < 0.001, Fig.2B; 2011, P < 0.001, Fig.2C). Furthermore, the response of foliar δ13C to AAP also significantly differed among the 3 years of sampling (P < 0.001, Fig.2D).
Figure 2.
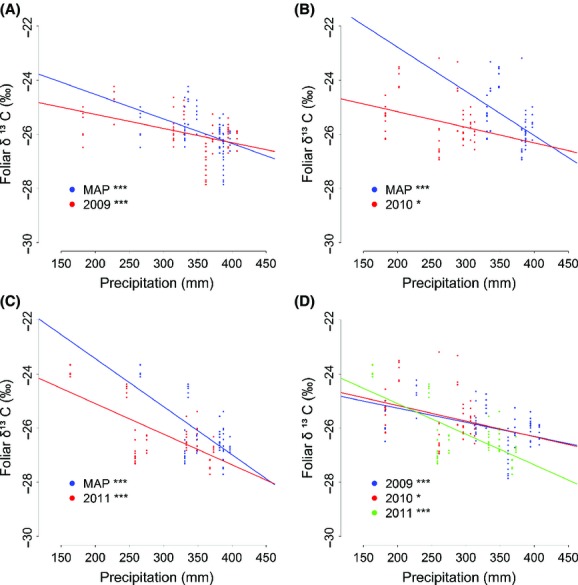
Variation in the response of foliar δ13C to precipitation. Response of foliar δ13C to mean annual precipitation (MAP, blue) and actual annual precipitation (AAP, red) in 2009 (A), 2010 (B), and 2011 (C). Response of foliar δ13C to AAP in 2009, 2010, and 2011 (D). Level of significance: “***”P < 0.001, “**”P < 0.01, “*”P < 0.05.
Foliar δ13C values significantly decreased with increasing precipitation (AAP) for L. chinensis in any year from 2009 to 2011 (2009: R2 = 0.201, P < 0.001; 2010: R2 = 0.095, P < 0.05; 2011: R2 = 0.449, P < 0.001; Fig.2D and Table S1), when all samples at each site along the transect were analyzed. However, we found that with increasing sample size from the same site, P-values of the linear regression test for the slope if foliar δ13C against AAP decreased (Fig.3 and Table S1). Overall, five samples would be needed as a minimum in order to detest a significant relationship between precipitation and δ13C in this study.
Figure 3.
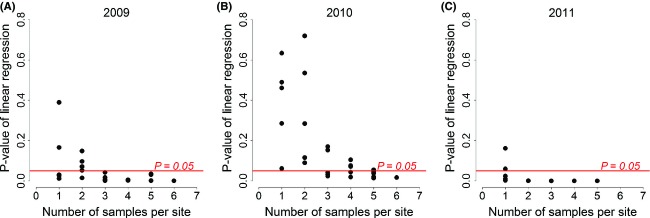
Effect of sample size (number of samples per site) on the P-values of the linear regression between foliar δ13C and precipitation. Samples collected in 2009 (A), 2010 (B), and 2011 (C).
Influence of land-use type on foliar δ13C
Foliar δ13C significantly differed between grazed and fenced areas (P < 0.05), with lower values in area with grazing (−27.28 ‰ ± 0.112‰) than in areas without grazing (−26.92‰ ± 0.055‰; Fig.4).
Figure 4.
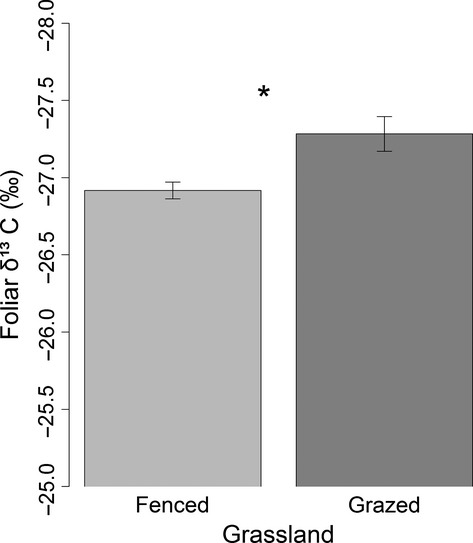
Difference in foliar δ13C under two different land-use types (fenced and grazing). Error bars represent standard errors of the means. Level of significance: “*”P < 0.05.
Discussion
This study represents the comprehensive examination of the effect of sampling methods on foliar δ13C values. We focused on the effect of different sampling methods in a typical microhabitat and avoided obvious factors affecting foliar δ13C values, such as the sites near a river, the different ecotypes of L. chinensis (Figs. S1 and S2), and the leaf position (Yang et al. 2011). Our findings, obtained under optimum field sampling conditions in some microhabitats (50–60 m) along a precipitation gradient, provide evidence that there is significant effect of sampling methods on foliar δ13C: (1) the foliar δ13C response patterns were significantly different between to MAP and to AAP, whereas the response of foliar δ13C to AAP was also significantly different among 3 years from 2009 to 2011; (2) sample size from microhabitats strongly affects the patterns of foliar δ13C in relation to precipitation at a large geographical scale; (3) sampling from same sites with different land-use types (grazed and fenced) affects foliar δ13C values significantly.
Our results showed the common tendency that foliar δ13C values decreases with increasing precipitation (whether AAP or MAP) (Swap et al. 2004; Song et al. 2008; Prentice et al. 2011); however, the response sensitivity of foliar δ13C values to precipitation was significantly different when we used MAP instead of AAP to analyze the data (Fig.2A–C). The response sensitivity also differed even when only foliar δ13C to AAP among different years was compared (Fig.2D). The most likely explanation for these differences over time is that species growing among different habitats in the temperate steppes with significantly different response sensitivity of foliar δ13C values to precipitation (Liu et al. 2014), and inter-annual variation in precipitation makes foliar δ13C not on same level of fluctuation among different sites. Therefore, temporal variability of foliar δ13C in a microhabitat can affect its response patterns to precipitation at a large scale. Wittmer et al. (2008) reported that Δ13C among Stipa in Central Asian grassland increased with MAP in both 2 years and that the slope of this relationship (Δ13C to MAP) was very similar between 2 years. Contrast to our results of this study, it will be interesting to see how their results hold up when they test the homogeneity between two slopes again using AAP instead of MAP. Furthermore, although foliar δ13C values respond to aridity in a similar way for different C3 species and life forms (Prentice et al. 2011), the patterns might be different over times according to our finding.
Our results have valuable implications in methodology for future field sampling studies. Spatial variability of foliar δ13C in microhabitats can also affect its response patterns to precipitation at a large scale (Fig.3 and Table S1). Therefore, multiple sampling along the direction of the maximum environmental variance in each sampling site can reduce sampling error caused by microhabitat differences. Two transect studies in Inner Mongolia indicated that some species showed no linear regression in foliar δ13C values with aridity gradient (Su et al. 2000; Prentice et al. 2011). The reason for that result could be that the replication in each sampling site was low, or the replications did not represent the maximum environmental variance of the microhabitat surveyed. Alternatively, as grasslands had been increasingly grazed, the most common landscape is a scenario where grazed type and fenced type alternately occupy the temperate steppe of Inner Mongolia. This study also showed that there was lower foliar δ13C value in presence of grazed type than fenced type. It suggests that different land-use types (grazed or fenced) significantly affect foliar δ13C values of species. Hence, when we do field sampling along a transect in Inner Mongolia, all sampling sites are best occupied by visually homogeneous grassland disturbed by land-use types (grazed or fenced). Finally, we suggest for future investigations of the use of AAP in sampling year instead of MAP. It will yield more reliable results of the foliar δ13C in relation to precipitation along transect at a large geographical scales.
Acknowledgments
We thank Mark van Kleunen, Noëlie Maurel, Ayub Oduor, Marcel Dorken, Wayne Dawson, Marc Stift, Janosch Sedlacek, Mialy Razanajatovo, Lei Ning, and Samuel Carleial for valuable comments on previous drafts of the manuscript. This work was supported by the Bureau of Science and Technology for Resources and Environment, Chinese Academy of Sciences (KZCX2-EW-QN604), the National Natural Science Foundation of China (40871032), and a Scholarship form the China Scholarship Council.
Conflict of Interest
None declared.
Supporting Information
Figure S1. Two ecotypes of Leymus chinensis co-occurring in the field in Inner Mongolia.
Figure S2. Difference in foliar δ13C of two ecotypes of Leymus chinensis in Inner Mongolia in 2011.
Figure S3. One sampling sites in the Hulunbuir meadow steppe of Inner Mongolia. China.
Table S1. Results of the linear regression between foliar δ13C and actual annual precipitation using different samples size at each site along the transect of Inner.
References
- Bert D, Leavitt SW. Dupouey JL. Variations of wood delta C-13 and water-use efficiency of Abies alba during the last century. Ecology. 1997;78:1588–1596. [Google Scholar]
- Chen L. Wang RZ. Anatomical and physiological divergences and compensatory effects in two Leymus chinensis (Poaceae) ecotypes in Northeast China. Agricult. Ecosyst. Environ. 2009;134:46–52. [Google Scholar]
- Diefendorf AF, Mueller KE, Wing SL, Koch PL. Freeman KH. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl Acad. Sci. USA. 2010;107:5738–5743. doi: 10.1073/pnas.0910513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR. Cooper TA. Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia. 1988;76:562–566. doi: 10.1007/BF00397870. [DOI] [PubMed] [Google Scholar]
- Falster DS, Warton DI. Wright IJ. 2006. SMATR: Standardised Major Axis Tests and Routines. Available at www.bio.mq.edu.au/ecology/SMATR/. (accessed 13 February 2014) [DOI] [PubMed]
- Farquhar GD, Oleary MH. Berry JA. On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust. J. Plant Physiol. 1982;9:121–137. [Google Scholar]
- Farquhar GD, Ehleringer JR. Hubick KT. Carbon isotope discrimination and photosynthesis. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:503–537. [Google Scholar]
- Gong XY, Chen Q, Lin S, Brueck H, Dittert K, Taube F, et al. Tradeoffs between nitrogen- and water-use efficiency in dominant species of the semiarid steppe of Inner Mongolia. Plant Soil. 2011;340:227–238. [Google Scholar]
- Guo GM. Xie GD. The relationship between plant stable carbon isotope composition, precipitation and satellite data, Tibet Plateau, China. Quat. Int. 2006;144:68–71. [Google Scholar]
- Hausmann NJ, Juenger TE, Sen S, Stowe KA, Dawson TE. Simms EL. Quantitative trait loci affecting delta C-13 and response to differential water availibility in Arabidopsis thaliana. Evolution. 2005;59:81–96. [PubMed] [Google Scholar]
- Li JZ, Lin S, Taube F, Pan QM. Dittert K. Above and belowground net primary productivity of grassland influenced by supplemental water and nitrogen in Inner Mongolia. Plant Soil. 2011;340:253–264. [Google Scholar]
- Liu WG, Feng XH, Ning YF, Zhang QL, Cao YN. An ZS. delta C-13 variation of C-3 and C-4 plants across an Asian monsoon rainfall gradient in arid northwestern China. Global Change Biol. 2005;11:1094–1100. [Google Scholar]
- Liu ZP, Li XF, Li HJ, Yang QC. Liu GS. The genetic diversity of perennial Leymus chinensis originating from China. Grass Forage Sci. 2007;62:27–34. [Google Scholar]
- Liu H, Tian F, Hu HC, Hu HP. Sivapalan M. Soil moisture controls on patterns of grass green-up in Inner Mongolia: an index based approach. Hydrol. Earth Syst. Sci. 2013;17:805–815. [Google Scholar]
- Liu YJ, Xu XL. Niu HS. Foliar delta C-13 response patterns along a moisture gradient arising from genetic variation and phenotypic plasticity in grassland species of Inner Mongolia. Ecol. Evol. 2013;3:262–267. doi: 10.1002/ece3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang L, Niu H, Sun Y. Xu X. Habitat-specific differences in plasticity of foliar δ13C in temperate steppe grasses. Ecol. Evol. 2014;4:648–655. doi: 10.1002/ece3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo TX, Zhang L, Zhu HZ, Daly C, Li MC. Luo J. Correlations between net primary productivity and foliar carbon isotope ratio across a Tibetan ecosystem transect. Ecography. 2009;32:526–538. [Google Scholar]
- McConnaughay KDM. Coleman JS. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology. 1999;80:2581–2593. [Google Scholar]
- Michelot A, Eglin T, Dufrene E, Lelarge-Trouverie C. Damesin C. Comparison of seasonal variations in water-use efficiency calculated from the carbon isotope composition of tree rings and flux data in a temperate forest. Plant, Cell Environ. 2011;34:230–244. doi: 10.1111/j.1365-3040.2010.02238.x. [DOI] [PubMed] [Google Scholar]
- Poorter H. Nagel O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Funct. Plant Biol. 2000;27:595–607. [Google Scholar]
- Prentice IC, Meng T, Wang H, Harrison SP, Ni J. Wang G. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. New Phytol. 2011;190:169–180. doi: 10.1111/j.1469-8137.2010.03579.x. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. Available at http://www.R-project.org/. (accessed 13 February 2014) [Google Scholar]
- Schulze ED, Turner NC, Nicolle D. Schumacher J. Leaf and wood carbon isotope ratios, specific leaf areas and wood growth of Eucalyptus species across a rainfall gradient in Australia. Tree Physiol. 2006;26:479–492. doi: 10.1093/treephys/26.4.479. [DOI] [PubMed] [Google Scholar]
- Silim SN, Guy RD, Patterson TB. Livingston NJ. Plasticity in water-use efficiency of Picea sitchensis, P-glauca and their natural hybrids. Oecologia. 2001;128:317–325. doi: 10.1007/s004420100659. [DOI] [PubMed] [Google Scholar]
- Song MH, Duan DY, Chen H, Hu QW, Zhang F, Xu XL, et al. Leaf delta C-13 reflects ecosystem patterns and responses of alpine plants to the environments on the Tibetan Plateau. Ecography. 2008;31:499–508. [Google Scholar]
- Stewart GR, Turnbull MH, Schmidt S. Erskine PD. C-13 Natural-abundance in plant-communities along a rainfall gradient – a biological integrator of water availability. Funct. Plant Biol. 1995;22:51–55. [Google Scholar]
- Su B, Han XG, Li LH, Huang JH, Bai YF. Qu CM. Responses of delta 13C value and water use efficiency of plant species to environmental gradients along the grassland zone of Northeast China Transect. Chinese. J. Plant Ecol. 2000;24:648–655. [Google Scholar]
- Swap RJ, Aranibar JN, Dowty PR, Gilhooly WP. Macko SA. Natural abundance of C-13 and N-15 in C-3 and C-4 vegetation of southern Africa: patterns and implications. Global Change Biol. 2004;10:350–358. [Google Scholar]
- The Integrated Investigation Team in Inner Mongolia and Ningxia, CAS. Vegetation of Inner Mongolia. Beijing: Science Press; 1985. [Google Scholar]
- Wang N, Xu SS, Jia X, Gao J, Zhang WP, Qiu YP, et al. Variations in foliar stable carbon isotopes among functional groups and along environmental gradients in China – a meta-analysis. Plant Biology. 2013;15:144–151. doi: 10.1111/j.1438-8677.2012.00605.x. [DOI] [PubMed] [Google Scholar]
- Wittmer MHOM, Auerswald K, Tungalag R, Bai YF, Schaufele R. Schnyder H. Carbon isotope discrimination of C3 vegetation in Central Asian grassland as related to long-term and short-term precipitation patterns. Biogeosciences. 2008;5:913–924. [Google Scholar]
- Yan L, Zhou GS. Zhang F. Effects of different grazing intensities on grassland production in China: a meta-analysis. PLoS One. 2013;8:e81466. doi: 10.1371/journal.pone.0081466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Auerswald K, Bai Y, Wittmer MH. Schnyder H. Variation in carbon isotope discrimination in Cleistogenes squarrosa (Trin.) Keng: patterns and drivers at tiller, local, catchment, and regional scales. J. Exp. Bot. 2011;62:4143–4152. doi: 10.1093/jxb/err102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SX. Shangguan ZP. Spatial patterns of foliar stable carbon isotope compositions of C3 plant species in the Loess Plateau of China. Ecol. Res. 2006;22:342–353. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Two ecotypes of Leymus chinensis co-occurring in the field in Inner Mongolia.
Figure S2. Difference in foliar δ13C of two ecotypes of Leymus chinensis in Inner Mongolia in 2011.
Figure S3. One sampling sites in the Hulunbuir meadow steppe of Inner Mongolia. China.
Table S1. Results of the linear regression between foliar δ13C and actual annual precipitation using different samples size at each site along the transect of Inner.


