Abstract
A significant proportion of patients with type 2 diabetes mellitus have a low testosterone level relative to reference ranges based on healthy young men. Only a small number of these patients suffer from classical hypogonadism as a result of recognizable hypothalamic–pituitary–gonadal axis pathology. The cut-off value of the serum testosterone level in men without obvious hypothalamic–pituitary–gonadal axis pathology is controversial. It is unclear to what extent a low serum testosterone level causally leads to type 2 diabetes and/or the metabolic syndrome. From a theoretical standpoint, there can be complex interactions among the hypothalamic–pituitary–gonadal axis, body composition and insulin resistance, which can be further influenced by intrinsic and extrinsic factors to give rise to metabolic syndrome, glucose intolerance, and low-grade inflammation to increase the risk of cardiovascular disease. Although a low serum testosterone level frequently coexists with cardiometabolic risk factors and might serve as a biomarker, more studies are required to clarify the causal, mediating or modifying roles of low serum testosterone level in the development of adverse clinical outcomes. Currently, there are insufficient randomized clinical trial data to evaluate the effects of testosterone replacement therapy on meaningful clinical outcomes. The risk-to-benefit ratio of testosterone therapy in high-risk subjects, such as those with type 2 diabetes, also requires elucidation. The present article aims to review the current evidence on low serum testosterone levels in patients with type 2 diabetes, and its implications on cardiovascular risk factors, metabolic syndrome and adverse clinical outcomes.
Keywords: Metabolic syndrome, Testosterone, Type 2 diabetes
Introduction
Low serum testosterone level have been reported in men with type 2 diabetes mellitus1–3. Inverse relationships between the serum testosterone level and cardiovascular risk factors, such as obesity, hypertension, dyslipidemia and insulin resistance, have been observed4–8. Recent studies have shown that a low serum testosterone level is strongly associated with an increased likelihood of the metabolic syndrome (MES) in both Caucasian and Asian men9–11. In Caucasian and Japanese men, a low serum testosterone level is also related to adverse clinical outcomes including cardiovascular disease (CVD) and premature mortality12–15. In 2010, the high prevalence of a low serum testosterone level in patients with type 2 diabetes was addressed in the Endocrine Society's Clinical Practice Guideline within the context of testosterone replacement therapy in adult men with androgen deficiency syndrome. The latter was defined by consistent symptoms and signs of androgen deficiency, and an unequivocally low serum testosterone level. The Society recommends measurement of morning serum total testosterone in patients with type 2 diabetes who have symptoms of sexual dysfunction, unexplained weight loss, weakness or mobility limitation16. Recent interventional trials examining the effect of testosterone replacement on clinical outcomes have mostly been carried out in men with symptomatic androgen deficiency17,18. To date, the cause of decreased serum testosterone level in patients with type 2 diabetes is not clear. The quest of whether testosterone status should be checked and/or replaced in asymptomatic subjects with type 2 diabetes is ongoing. The aim of the present article was to review the current evidence on low serum testosterone level in patients with type 2 diabetes, and its implications on cardiovascular risk factors, MES and adverse clinical outcomes.
Defining Low Testosterone Level
Defining the lower limit of normal for serum testosterone level poses a challenge for physicians. First, the threshold serum testosterone level below which adverse clinical outcomes occur in the general population is not known16. Second, marked variations in the reference ranges of serum testosterone level among laboratories have been reported19. Furthermore, the reference ranges for testosterone have been derived previously from small convenience samples, or from hospital- or clinic-based patients20. These approaches are limited by their selection bias, as patients seeking medical care are more likely to have diseases than those in the general population. Therefore, currently, most population-based studies use the serum testosterone level corresponding to the lower limit, quoted from 8.7 to 12.7 nmol/L, of the normal range for young Caucasian men as the threshold16,21, although interethnic differences exist as a result of different mechanisms that will be discussed in the present review.
Serum Total, Free and Protein-Bound Testosterone
Testosterone in men is synthesized and secreted into the circulation almost exclusively by the Leydig cells of the testes. It is mostly bound to plasma proteins. Serum total testosterone is composed of 0.5–3.0% of free testosterone unbound to plasma proteins, 30–44% sex hormone binding globulin (SHBG)-bound testosterone and 54–68% albumin-bound testosterone22,23. As the binding of testosterone to albumin is non-specific and therefore not tight, the sum of free and albumin-bound testosterone is named bioavailable testosterone, which reflects the hormone available at the cellular level24. As a significant proportion of circulating serum testosterone level is tightly bound to SHBG, alterations in SHBG concentration might affect total serum testosterone level without altering free or bioavailable testosterone. Conditions that suppress or increase serum SHBG level without affecting circulating free or bioavailable testosterone level are listed in Table116. Researchers tried to examine whether serum total or free testosterone would be a better/more reliable choice when studying the effect of testosterone. The results were mixed. Some reported significant associations of both serum total and free testosterone level with clinical parameters25, whereas others reported that only serum free testosterone26 or only serum total testosterone6 showed significant associations.
Table 1.
Conditions that suppress or increase serum SHBG level without affecting circulating free or bioavailable testosterone level16
| Conditions that suppress serum SHBG level | Moderate obesity, type 2 diabetes, nephrotic syndrome, hypothyroidism, acromegaly, familial SHBG deficiency, and use of glucocorticoids, progestins and androgens |
| Conditions that increase serum SHBG level | Aging, hepatic cirrhosis, hepatitis, hyperthyroidism, infection with human immunodeficiency virus, and use of anticonvulsants and estrogens |
SHBG, sex hormone binding globulin.
Laboratory Assays of Testosterone
Total testosterone assays are readily available in most laboratories, therefore, serum total testosterone remains the recommended initial measurement in the assessment of testosterone level16. Serum total testosterone measured using rapid automated immunoassay analyzers are carried out with proprietary reagents that include analogs of testosterone as standards, and reference ranges are provided by the manufacturer of the analyzers. Although these immunoassays are efficient and economical, many of them have limited published validation data, raising questions about the accuracy of these automated methods27. Furthermore, there is substantial variability in the results from different assays, mostly due to the accreditation of laboratories that are based on the reproducibility of results in comparison with other laboratories using the same kit, rather than on the accuracy of the results21. Some laboratories now measure serum testosterone level by liquid chromatography tandem mass spectrometry (LC-MS) methods with better accuracy than immunoassays, but are more time-consuming and costly. In fact, the United States Centers for Disease Control and Prevention has initiated a program since 2010 to standardize and harmonize testosterone assays using accuracy-based quality control27.
Epidemiology of Low Serum Testosterone Level
The prevalence of low serum testosterone level in the general population across different age groups has been examined in several large-scale epidemiological studies. In the Baltimore Longitudinal Study of Aging (BLSA) cohort made up of 3,565 middle-class, mostly Caucasian men from the USA, the incidence of low serum total testosterone increased from approximately 20% of men aged over 60 years, 30% over 70 years, to 50% over 80 years-of-age. A significant, independent and longitudinal effect of age on testosterone has been observed with an average change of −0.124 nmol/L/year in serum total testosterone28. The same trend has been shown in Europe and Australia29,30.
A similar trend for men with chronic diseases has been shown in the Massachusetts Male Aging Study (MMAS) cohort in which 415 healthy men and 1,294 men with chronic diseases (cancer, coronary heart, hypertension, diabetes and ulcer) from the USA aged 39–70 years were studied. The reduction of total testosterone was 0.4% per year in both groups. The level of total testosterone followed a parallel course in both groups, and subjects with chronic diseases consistently had a 10–15% lower level compared with age-matched healthy subjects31.
In Caucasians, the mean serum total testosterone level for men in large epidemiological studies has been reported to range from 15.1 to 16.6 nmol/L28,29. In Asians, higher values, ranging from 18.1 to 19.1 nmol/L, were seen in Korea and Japan32,33. A study on a cohort of Hong Kong (HK) Chinese middle-aged men reported a similar mean serum testosterone level of 17.1 nmol/L in 179 men who had a family history of type 2 diabetes and 17.8 nmol/L in 128 men who had no family history of type 2 diabetes11. In a more recent study in HK involving a cohort of 1,489 community-dwelling men with a mean age of 72 years, a mean serum total testosterone of 19.0 nmol/L was reported34. Important geographical and racial differences in the concentrations of serum sex steroids has been reported. Asian men residing in HK and Japan, but not those living in the USA, had 20% higher serum total testosterone than in Caucasians living in the USA, as shown in a large multinational observational prospective cohort of the Osteoporotic Fractures in Men Study. In that study, fasting morning serum samples from these cohorts were sent to a central laboratory in Canada for analysis using the same assay. Group differences in body mass index (BMI) did not explain the geographical differences35. These differences might be explained by environmental and/or genetic factors influencing the testosterone metabolism36.
Testosterone and Type 2 Diabetes Mellitus
Epidemiological Surveys
There is now accumulating evidence that low serum testosterone level is associated with type 2 diabetes with the data summarized in Table2.
Table 2.
Epidemiological surveys comparing serum total testosterone levels in men with and without type 2 diabetes
| Source | Site | Year of publication | Study design | No. participants | Age (years) | Findings |
|---|---|---|---|---|---|---|
| Barrett-Connor et al.1 | USA | 1992 | Cross-sectional | 132 | 53–88 | Low serum total testosterone (defined by < 15.9 nmol/L) was significantly more frequent and lower in men with T2DM (21%, 14.7 ± 5.79 nmol/L) as compared with men without (13%, 17.4 ± 4.74 nmol/L) |
| T2DM 44 | ||||||
| No T2DM 88 | ||||||
| Oh JY et al.124 | USA | 2002 | Prospective | 294 | 55–89 | Odds for new T2DM was 2.7 (95% CI 1.1–6.6) for men in the lowest quartile of total testosterone |
| 8 years | Incident T2DM 26 | |||||
| No T2DM 268 | ||||||
| Ding EL et al.2 | Worldwide | 2006 | Meta-analysis of cross-sectional and prospective studies | 7,100 | 44–80 | Serum total testosterone was significantly lower in men with T2DM in cross-sectional studies (−2.66 nmol/L, 95% CI −3.45 to −1.86) and in prospective studies (−2.48 nmol/L, 95% CI −4.4 to −0.93) |
| Cross-sectional T2DM 964 | ||||||
| No T2DM 2,918 | ||||||
| Prospective T2DM 391 | ||||||
| No T2DM 2,827 | ||||||
| Cao J et al.125 | China | 2011 | Cross-sectional | 492 | 71–73 | Serum total testosterone was significantly lower in subjects with DM (13.8 ± 4.7 nmol/L) than those without (17.1 ± 6.1 nmol/L; P < 0.01) |
| T2DM 129 | ||||||
| No T2DM 363 |
CI, confidence interval; DM, diabetes mellitus; T2DM, type 2 diabetes mellitus.
Effects of Testosterone on Body Composition and Intermediary Metabolism
There are several mechanisms for the association of low serum testosterone level and type 2 diabetes with insulin resistance and obesity as central features. In experimental studies, androgen receptor knockout mice developed significant insulin resistance rapidly37. In mouse models, testosterone promoted differentiation of pluripotent stem cells to the myogenic lineage, but inhibited their commitment into adipocytes through an androgen receptor-mediated pathway. These findings explained the well-known effects of testosterone therapy on body composition in men including increment in muscle mass and reduction in fat mass, both of which were expected to decrease insulin resistance38. In addition to stem cell effects, testosterone decreased insulin resistance by enhancing catecholamine induced lipolysis in vitro, and reducing lipoprotein lipase activity and triglyceride uptake in human abdominal tissue in vivo39,40. In both in vitro and in vivo studies, hypertriglyceridemia led to increased free fatty acids and reduced insulin clearance, which contributed to insulin resistance and hyperinsulinemia (Figure1). Thus, by promoting lipolysis and myogenesis, testosterone might lead to improved insulin resistance13. Besides, microarray studies in mice showed that testosterone regulated skeletal muscle genes involved in glucose metabolism that led to decreased systemic insulin resistance41. In the liver, hepatic androgen receptor signaling inhibited development of insulin resistance in mice42. This is consistent with the independent and inverse association of testosterone with hepatic steatosis shown in a cross-sectional study carried out in humans43. Most recently, a higher level of testosterone was shown to independently predict a reduced risk of type 2 diabetes in elderly men44, whereas a lower level was an independent risk factor for high fasting glucose45. In the Testosterone Replacement in Hypogonadal Men with Either Metabolic Syndrome or Type 2 Diabetes Study, treatment with transdermal testosterone in 136 men with type 2 diabetes improved insulin sensitivity as measured by homeostasis model of assessment-insulin resistance, further supporting the importance of insulin resistance as a mediating factor for the association between low serum testosterone level and type 2 diabetes17. In short, androgen improves insulin resistance by changing body composition and reducing body fat.
Figure 1.
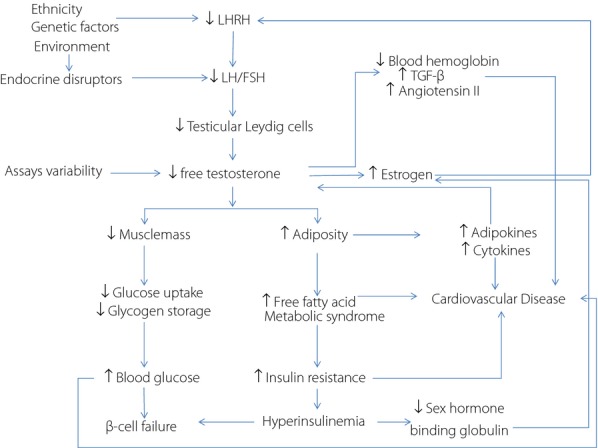
Dysregulation of the hypothalamic-pituitary-gonadal axis due to genetic-environmental interactions can lead to low testosterone causing changes in body composition resulting in insulin resistance and low grade inflammation to increase cardiovascular risk. This abnormal metabolic milieu can alter level of sex hormone binding globulin to reduce the bioavailability of testosterone. Reduced erythropoiesis due to low testosterone may be associated with activation of cell cycle signaling pathways such as angiotensin II to further increase cardiovascular risk. On the other hand, variability in assay standards can confound the accuracy and interpretation of free and total testosterone level. FSH, follicular stimulating hormone; LH, luteinizing hormone; LHRH, luteinizing-hormone-releasing hormone; TGF-β, transforming growth factor-beta.
Although a low serum testosterone level could contribute to the development of obesity and type 2 diabetes through changes in body composition, obesity might also alter the metabolism of testosterone. In obese men, the peripheral conversion from testosterone to estrogen could attenuate the amplitude of luteinizing hormone pulses and centrally inhibit testosterone production46 to exacerbate the vicious cycle of obesity and low serum testosterone level. Adipose tissue, as an endocrine organ, might regulate Leydig cell function47. To this end, leptin, an adipokine, has been shown to be inversely correlated with serum testosterone level in men48,49. In the testes of rodents, Leydig cells expressed leptin receptors and leptin has been shown to inhibit testosterone secretion, suggesting a role of obesity and leptin in the pathogenesis of low testosterone50.
Low testosterone can be perpetuated through defects in the hypothalamic–pituitary–gonadal (HPG) axis. In Caucasian men with type 2 diabetes, 25–40% had hypogonadotropic hypogonadism51–54. The normal response to gonadotrophin-releasing hormone of luteinizing hormone suggested the level of the HPG dysfunction exists at the hypothalamus level. Yet, in a small sample consisting of 34 hypogonadal men with diabetes, ten randomly selected patients had magnetic resonance imaging that showed no abnormalities in the hypothalamus or pituitary gland53. Other researchers suggested that pro-inflammatory factors, such as tumor necrosis factor-α in the testes, could locally inhibit testosterone biosynthesis in Leydig cells47, and testosterone treatment in men was shown to reduce the level of tumor necrosis factor-α55 (Figure1).
In Japanese men with type 2 diabetes, a negative association between testosterone and glycated albumin-to-hemoglobin A1c (HbA1c) ratio has been reported, possibly through the reduced stimulatory effect of testosterone on erythropoiesis, leading to falsely low HbA1c. This finding has raised the possibility that HbA1c might be underestimated in hypogonadal men with diabetes56.
In brief, multiple lines of evidence from animal experiments, observational studies and randomized controlled trials in humans all appeared to implicate the possible causal role of low serum testosterone level in type 2 diabetes and obesity, although larger-scale randomized clinical studies are required.
Genetic Determinants of Testosterone Metabolism
Genetic factors, such as racial differences in steroid metabolism, might play a role in explaining the heterogeneity of serum testosterone level and risk associations. In an exploratory analysis of a local clinic-based cohort of Chinese men with type 2 diabetes, 15% of the patients had low serum total testosterone (defined by < 2.6 ng/mL), which was lower than the prevalence reported in Caucasian men with type 2 diabetes (21–40%; unpublished data), although the differences might be due to geographical, environmental or ethnicity factors1,51–54. Another study reported that total testosterone, but not free testosterone or bioavailable testosterone, were lower in young healthy Chinese men than USA men57. In particular, it was suggested that genetic factors, such as racial differences in steroid metabolism, might play a role. In Asians, a genetic deletion polymorphism of uridine diphosphate-glucuronosyltransferase UGT2B17 was associated with reduced androgen glucuronidation. This resulted in higher level of active androgen in Asians as compared to Caucasians, as Caucasians' androgen would be glucuronidated into inactive forms faster. Compared with Caucasians, the frequency of this deletion polymorphism of UGT2B17 was 22-fold higher in Asian subjects58–60. Other researchers have suggested that environmental, but not genetic, factors influenced serum total testosterone. Here, Chinese men residing in the USA had higher total testosterone than Chinese men living in Beijing, but comparable levels with Caucasians living in the USA61. In contrast, other studies had shown that Asian men living in HK and Japan, but not those living in the USA, have total testosterone approximately 20% higher than in Caucasian men living in the USA, and this difference was not explained by the group differences in BMI and age35. The variation within the same ethnic group has been attributed to geographical and environmental influences including diet, environmental chemicals, climate, physical activity and social status62. In this era of globalization, the interplay between genetic and environmental factors, and their associations with serum testosterone level deserves further investigation, which will throw insight into the pathogenesis, diagnosis and management hypogonadism in different parts of the world for patients with different races and ethnicities.
Testosterone effects are also modified by the genetically determined polymorphism of the androgen receptor (AR) gene63. The basal and ligand-induced activity of the AR is inversely associated with the length of the CAG repeat chain64. Significant ethnic differences had been observed in the length of this CAG repeat. The mean length of CAG repeat for Asians, Caucasians and Africans were 22–23, 21–22, and 18–20, respectively64,36. In the European Male Aging Study, increased estrogen/androgen ratio in association with longer AR CAG repeat was observed29. Furthermore, a smaller number of AR CAG repeat had been shown to be associated with benign prostate hypertrophy and faster prostate growth during testosterone treatment65,66. The length of the CAG repeat also varied among individuals of the same ethnic group. In India, men with CAG ≤19 had increased risk of prostate cancer67. In Caucasians, the odds of having a short CAG repeat (≤17) were substantially higher in patients with lymph node-positive prostate cancer than in those with lymph node-negative disease or in the general population68. Therefore, assessing the polymorphism at the AR level could be a potential tool towards individualized assessment and treatment of hypogonadism.
Effects of Age on Serum Testosterone Level
Aging is well known to result in a decline in sex hormone level, and is likely a combination of testicular and pituitary/hypothalamic defects. In elderly men, there was reduced testicular response to gonadotropins with suppressed and altered pulsatility of the hypothalamic pulse generator69. In several large cohorts, a significant, independent and longitudinal effect of age on serum total testosterone level had been observed28,30,31,69. A significant graded inverse association between serum testosterone level and insulin levels independent of age has also been reported in Caucasian men70.
Testosterone and the MES
Low testosterone is commonly associated with a high prevalence of MES71–73. MES is a clustering of metabolic risk factors including insulin resistance, hypertension, dyslipidemia and obesity (particularly central adiposity), in part driven by modernization and changing lifestyle. There is now strong epidemiological data supporting the predictive value of MES for further development of type 2 diabetes and/or CVD, although there is ongoing debate whether this is as a result of additive or multiplicative effects of these components. More recent data suggested linkage of this syndrome with other diseases including chronic kidney disease74, non-alcoholic fatty liver disease75, rheumatoid arthritis76 and even cancer77.
Apart from its associations with glycemia and obesity, low serum testosterone level was also associated with high blood pressure78. In a single-blind randomized study of hypogonadal men with MES and newly diagnosed type 2 diabetes, testosterone treatment reduced blood pressure more effectively than diet and exercise alone79. In patients who received androgen deprivation therapy with gonadotrophin-releasing hormone-agonists for prostate cancer, there was stiffening of large arteries associated with a drastic decrease in serum testosterone level80. Testosterone has also been shown to dilate coronary vessels in animals and men, suggesting that it might be an important regulator of vasculature compliance and modifier of blood pressure81–83.
In the Telecom study, low testosterone was associated with higher total serum cholesterol, triglycerides and low-density lipoprotein cholesterol, and lower high-density lipoprotein cholesterol78. A similar lipid pattern had been reported in men with profoundly low testosterone secondary to prostate cancer treatment84. In experimental studies, testosterone had immunomodulating effects with reduced expression of pro-inflammatory cytokines (tumor necrosis factor-α85, interleukin-6 and interleukin-1086) and increased expression of anti-inflammatory cytokine interleukin-1085,87. Heufelder et al.79 first reported the favorable effects of administration of testosterone on lipid profile in men with MES and newly diagnosed type 2 diabetes. In a subsequent randomized, single-blind, placebo-controlled cross-over study involving 27 Caucasian men (mean age 62 years) with symptomatic testosterone deficiency, testosterone treatment shifted cytokine balance to a state of reduced inflammation and improved lipid profile (Figure1)55.
Laaksonen et al.25 reported associations of low serum testosterone level with MES and its components independent of BMI. In the National Health and Nutrition Examination Survey, which involved 1,226 men, serum total testosterone was inversely associated with MES after adjustment for confounders including age, race/ethnicity, use of tobacco and alcohol, physical activity level, low-density lipoprotein cholesterol, C-reactive protein, and insulin resistance as measured by the homeostasis model of assessment-insulin resistance9. Meta-analysis and systemic reviews quantified that serum total testosterone was 2.17–2.64 nmol/L lower in men with MES when compared with those without72,73. Three large cross-sectional studies, each involving more than 1,000 men, showed that the inverse association of serum testosterone level with individual components of MES was strongest for central obesity9,71,88.
An important question is whether low testosterone is a cause or consequence of MES. Although most studies showed that changes in serum testosterone level led to changes in body composition, insulin resistance and the presence of MES, the reverse might also be possible. In a prospective observational study that followed 651 middle-aged Finnish men for 11 years, MES predicted a 2.6-fold increased risk of development of low serum testosterone level independent of age, smoking and other potential confounders. The association, although attenuated, remained after adjustment for BMI with an odds ratio of 2.0 (95% confidence interval [CI] 1.1–3.8)89. Other prospective studies have shown that development of MES accelerated the age-related decline in serum testosterone level90,91. In men with type 2 diabetes, changes in serum testosterone level over time correlated inversely with changes in insulin resistance, raising the possibility that lifestyle and pharmacological management of diabetes that improve insulin resistance might also contribute to increased serum testosterone level. Consistent with this, weight loss by either diet control or bariatric surgery led to a substantial increase in total testosterone, especially in morbidly obese men, and the rise in serum testosterone level was proportional to the amount of weight lost92–98.
Testosterone, Cardiovascular Disease and Mortality
Cardiovascular disease is an important cause of morbidity and mortality in men with type 2 diabetes. In the population-based Osteoporotic Fractures in Men Study cohort from Sweden, men in the highest quartile of serum testosterone level had the lowest risk of cardiovascular events compared with men in the other three quartiles (hazard ratio [HR] 0.70, 95% CI 0.56–0.88, P = 0.002). When men with known CVD at baseline were excluded, the hazard remained (HR 0.71, 95% CI 0.53–0.95, P = 0.029)99. Similar observations had been reported in Asians. In a prospective cohort of 171 middle-aged Japanese men with coronary risk factors without a previous history of CVD, low serum total testosterone was associated with a significant fourfold higher risk of cardiovascular events when comparing men from the lowest testosterone tertile with those in the highest tertile (P < 0.01), independent of coronary risk factors and endothelial dysfunction14.
Mortality is another outcome affected by serum testosterone level. Shores et al. were the first to report that low serum testosterone level, including both serum total and free testosterone, was associated with increased mortality after adjustment for age, medical comorbidity and other clinical covariates in 858 male veterans (HR 1.88, 95% CI 1.34–2.63, P < 0.001)15. In particular, low serum total testosterone predicted increased risk of cardiovascular mortality with a HR of 1.38 (95% CI 1.02–1.85) in the Bernardo Study100. A recent systemic review and meta-analysis of 21 studies involving more than 20,000 men living in communities being followed up for a mean duration of 9.7 years concluded that low serum total testosterone increased all-cause (HR 1.35, 95% CI 1.13–1.62, P < 0.001) and cardiovascular mortality (HR 1.25, 95% CI 0.97–1.60, P = 0.06)101. Preliminary results from a Swedish population-based observational study presented at the European Association for the Study of Diabetes 2013 suggested there was an inverse relationship between serum testosterone level and acute myocardial infarction. Diabetic men in the highest quartile of serum total testosterone had a significantly reduced risk of acute MI when compared with those in the lower quartiles, even after adjustment for confounding variables, such as age (HR 0.75, P = 0.006). Yet, another recent study that divided a cohort of elderly, community-dwelling subjects into four groups based on their serum testosterone level found that having serum total testosterone level in the middle two quartiles at baseline predicted reduced incidence of death compared with having the highest and lowest levels102, challenging the findings from most of the prior studies that suggested that there was a linear progression for testosterone, with lower level associated with worse clinical outcomes.
In summary, large numbers of epidemiological studies, mainly carried out in Caucasian population, had confirmed the inverse relationship between serum testosterone level and aging, MES, CVD, CVD-related, and all-cause mortality. Besides, men with type 2 diabetes were also more prone to developing low serum testosterone levels, which appeared to be hypothalamic in origin53. As both type 2 diabetes and low serum testosterone level are predictors for CVD, and given an aging society with 10% of people affected by diabetes in our region, the impacts of low serum testosterone level in a type 2 diabetic population requires further investigation103.
In line with the predictive value of low serum testosterone levels on adverse clinical outcomes, a low serum testosterone level has been shown to be associated with carotid intima-medial thickness independent of BMI, waist-to-hip ratio, hypertension, type 2 diabetes, smoking and serum cholesterol104. Both animal105 and human studies26,106 showed that a low serum testosterone level was directly linked with factors implicated in atheroma formation. For example, in clinical studies, a low serum testosterone level was associated with inflammation, with elevated C-reactive protein that promoted atheroma formation107–109. Similar to its role in lipid profile modification, testosterone shifted the cytokine balance into an anti-inflammatory state, which might prevent or slow the progression of atheromatic plaque and prevent hypercoagulable state55,85–87. Testosterone treatment also improved the angina threshold in patients with coronary heart disease by inducing coronary vasodilatation as measured by prolongation in the time to 1-mm ST-segment depression during an exercise tolerance test110,111.
Apart from coronary heart disease, clinical studies had shown that low testosterone level conferred a poor prognosis and higher mortality in men with congestive heart failure112,113. The presence of testosterone receptors in the myocardium indicated that testosterone might have a direct impact on the cardiac remodeling and renin–angiotensin system contributing to congestive heart failure. Testosterone receptor gene knockout mice showed exacerbation of angiotensin II induced cardiac fibrosis by the enhancement of cardiac transforming growth factor-β gene expression114. Therefore, a chronic low serum testosterone level state might lead to increased angiotensin II activity and overexpression of transforming growth factor-β with persistent stimulation and differentiation of cardiac fibroblasts to cardiac myofibroblasts (Figure1)115. Oxidative stress activation could be another explanation for the association between low serum testosterone level and the severity of congestive heart failure. In the rat prostate, testosterone deprivation increased the pro-oxidant capacity by upregulating nicotinamide adenine dinucleotide phosphate hydrogen oxidase, and also decreased the anti-oxidant capacity by reducing reactive oxygen species detoxifying enzymes including manganese superoxide dismutase, peroxiredoxin, and thioredoxin 1116–119. Furthermore, in male mice, activation of testosterone receptors was shown to counteract reactive oxygen species damage to the heart induced by doxorubicin120.
Testosterone Replacement Therapy
Despite these experimental findings, results from interventional trials examining the effect of testosterone replacement on clinical outcomes in symptomatic hypogonadal men remained inconclusive. In the Testosterone Replacement in Hypogonadal Men with Either Metabolic Syndrome or Type 2 Diabetes Study, which involved 220 men with type 2 diabetes and/or MES with symptomatic androgen deficiency randomized to receive either a 60-mg transdermal testosterone gel or placebo once daily over 12 months, testosterone replacement resulted in decreased cardiovascular risk including reduction in insulin resistance as measured by the homeostasis model of assessment-insulin resistance, total cholesterol and low-density lipoprotein cholesterol. Although an improvement in insulin resistance could be expected to result in better glycemic control, the effects of reduction in HbA1c (a secondary outcome) were not shown at the end of the study, as the majority of subjects had controlled type 2 diabetes (HbA1c <6.5%), and the effect was confounded by the permitted changes in anti-diabetic medications for ethical reasons17. A larger study with HbA1c as a primary outcome in hypogonadal men with uncontrolled type 2 diabetes is required to investigate this further.
In the Testosterone in Older Men trial, 209 men with low testosterone and self-reported limited mobility were randomized to receive 6-month therapy of either a 100-mg transdermal testosterone gel or placebo once daily with an outcome measure of increased strength and ability to climb stairs. However, the study was prematurely halted by a data and safety monitoring board in 2009, just 3 months after enrollment of the last patient, as a result of increased adverse cardiovascular effects in the treatment group18,121. Of the 106 patients in the active treatment group, 23 experienced cardiovascular events including myocardial infarction, arrhythmia and elevated blood pressure, compared with just five of the 103 men randomized to the placebo group18. In 2012, an observational study of a cohort of middle-aged male veterans with low testosterone and comorbidities showed that testosterone treatment was associated with decreased mortality122. Similarly, a study in Europe that involved replacing testosterone in 64 diabetic men with low testosterone for a mean duration of 41 months found that mortality was reduced with this intervention123. Contrary to these, in 2013, an observational study with a cohort of veterans, with an average age of 60 years, of which many had underlying cardiovascular disease, who underwent coronary angiography and had a low serum testosterone level reported that the use of testosterone therapy was associated with a 30% increased risk of adverse outcomes including all-cause mortality, MI and ischemic stroke124. Another observational study reported an increased risk of MI in both older (twofold) and younger men (threefold) with pre-existing heart disease, who were prescribed testosterone therapy within the past 90 days125. The US Food and Drug Administration had therefore decided to reassess the cardiovascular safety of testosterone therapy since January 2014. To further complicate the issue, post-marketing reports of venous blood clots unrelated to polycythemia in testosterone users had prompted the US Food and Drug Administration to require manufacturers to include a general warning on the drug labeling of all approved testosterone products about the risk of blood clots in the veins in June 2014. A recent meta-analysis that included five randomized controlled trials of 351 men with late onset hypogonadism who were given testosterone replacement/placebo with a mean follow-up time of 6.5 months showed that testosterone reduced fasting plasma glucose, fasting serum insulin and triglyceride levels. However, there was no significant difference for CVD126. One of the latest contributions to the literature on testosterone and CVD was an analysis of the USA Medicare data, which reported no increased risk of MI with injections of testosterone, and testosterone even appeared to be protective in patients who are at the greatest risk of a cardiovascular event127. To date, published clinical trials are small, of short duration and often used pharmacological, not physiological, doses of testosterone17,18,79,111. Given the large number of confounders on serum testosterone level and clinical outcomes, careful phenotyping, large sample size and long follow-up duration will be required to assess the risks and benefits of testosterone replacement therapy.
Conclusion
In conclusion, review of the literature has identified multiple mechanisms supportive of the effects of low serum testosterone level on causing insulin resistance, obesity, vascular dysfunction and inflammation. At this moment, the results from these studies could not support checking testosterone level in asymptomatic men with type 2 diabetes, as an independent predictor effect of low testosterone on adverse clinical outcomes has not been clearly established. Given the high prevalence of type 2 diabetes across the world, future studies with larger cohorts and longer duration of follow up are required to clarify whether low testosterone is merely a reflection of poor cardiovascular risk factors control or is really causing adverse clinical outcomes.
Acknowledgments
This study was partially funded by the Hong Kong Foundation for Research and Development in Diabetes under the Chinese University of Hong Kong. The authors declare no conflict of interest.
References
- Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117:807–811. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Corona G, Monami M, Giulia R, et al. Type 2 diabetes mellitus and testosterone: A meta-analysis study. J Sex Med. 2010;7:421. [Google Scholar]
- Haffner SM, Shaten J, Stern MP, et al. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143:889–897. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- Tsai EC, Matsumoto AM, Fujimoto WY, et al. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, et al. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Kriska A, et al. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol. 1997;146:609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]
- Li C, Ford ES, Li B, et al. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care. 2010;33:1618–1624. doi: 10.2337/dc09-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CH, Huang CY, Li HY, et al. Testosterone and sex hormone-binding globulin have significant association with metabolic syndrome in Taiwanese men. Aging Male. 2012;15:1–6. doi: 10.3109/13685538.2011.597462. [DOI] [PubMed] [Google Scholar]
- Tong PC, Ho CS, Yeung VT, et al. Association of testosterone, insulin-like growth factor-I, and C-reactive protein with metabolic syndrome in Chinese middle-aged men with a family history of type 2 diabetes. J Clin Endocrinol Metab. 2005;90:6418–6423. doi: 10.1210/jc.2005-0228. [DOI] [PubMed] [Google Scholar]
- Traish AM, Saad F, Feeley RJ, et al. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009;30:477–494. doi: 10.2164/jandrol.108.007245. [DOI] [PubMed] [Google Scholar]
- Ma RC, Tong PC. Testosterone levels and cardiovascular disease. Heart. 2010;96:1787–1788. doi: 10.1136/hrt.2010.207068. [DOI] [PubMed] [Google Scholar]
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210:232–236. doi: 10.1016/j.atherosclerosis.2009.10.037. [DOI] [PubMed] [Google Scholar]
- Shores MM, Matsumoto AM, Sloan KL, et al. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–837. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots LR, Potter S, Potter D, et al. Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril. 1998;69:286–292. doi: 10.1016/s0015-0282(97)00464-0. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, et al. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid-hormones – binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human-plasma. J Clin Endocrinol Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. Physiology of the testosterone-binding globulin in man. Ann N Y Acad Sci. 1988;538:103–111. doi: 10.1111/j.1749-6632.1988.tb48855.x. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- Fukui M, Kitagawa Y, Nakamura N, et al. Association between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetes. Diabetes Care. 2003;26:1869–1873. doi: 10.2337/diacare.26.6.1869. [DOI] [PubMed] [Google Scholar]
- Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95:4542–4548. doi: 10.1210/jc.2010-1314. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Tajar A, Forti G, O'Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- Liu PY, Beilin J, Meier C, et al. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab. 2007;92:3599–3603. doi: 10.1210/jc.2007-0862. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Kim YS, Hong D, Lee DJ, et al. Total testosterone may not decline with ageing in Korean men aged 40 years or older. Clin Endocrinol (Oxf) 2012;77:296–301. doi: 10.1111/j.1365-2265.2012.04375.x. [DOI] [PubMed] [Google Scholar]
- Akishita M, Fukai S, Hashimoto M, et al. Association of low testosterone with metabolic syndrome and its components in middle-aged Japanese men. Hypertens Res. 2010;33:587–591. doi: 10.1038/hr.2010.43. [DOI] [PubMed] [Google Scholar]
- Auyeung TW, Lee JSW, Kwok T, et al. Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol. 2011;164:811–817. doi: 10.1530/EJE-10-0952. [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Nielson CM, Labrie F, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95:E151–E160. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houten ME, Gooren LJG. Differences in reproductive endocrinology between Asian men and Caucasian men – a literature review. Asian J Androl. 2000;2:13–20. [PubMed] [Google Scholar]
- Lin HY, Xu Q, Yeh S, et al. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes. 2005;54:1717–1725. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, et al. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- Xu XF, De Pergola G, Bjorntorp P. Testosterone increases lipolysis and the number of beta-adrenoceptors in male rat adipocytes. Endocrinology. 1991;128:379–382. doi: 10.1210/endo-128-1-379. [DOI] [PubMed] [Google Scholar]
- Marin P, Oden B, Bjorntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80:239–243. doi: 10.1210/jcem.80.1.7829619. [DOI] [PubMed] [Google Scholar]
- Haren MT, Siddiqui AM, Armbrecht HJ, et al. Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl. 2011;34:55–68. doi: 10.1111/j.1365-2605.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Lin HY, Yu IC, Wang RS, et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47:1924–1935. doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- Volzke H, Aumann N, Krebs A, et al. Hepatic steatosis is associated with low serum testosterone and high serum DHEAS levels in men. Int J Androl. 2010;33:45–53. doi: 10.1111/j.1365-2605.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- Salminen M, Vahlberg T, Raiha I, et al. Sex hormones and the risk of type 2 diabetes mellitus: A 9-year follow up among elderly men in Finland. Geriatr Gerontol Int. 2014 doi: 10.1111/ggi.12312. doi: 10.1111/ggi.12312. [DOI] [PubMed] [Google Scholar]
- Mazur A, Westerman R, Werdecker A, et al. Testosterone and type 2 diabetes in men. Aging Male. 2014;17:18–24. doi: 10.3109/13685538.2013.879113. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Deslypere JP, et al. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–1146. doi: 10.1210/jcem.76.5.8496304. [DOI] [PubMed] [Google Scholar]
- Saez JM. Leydig-cells – endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Luukkaa V, Pesonen U, Huhtaniemi I, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 1998;83:3243–3246. doi: 10.1210/jcem.83.9.5134. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Miettinen H, Karhapaa P, et al. Leptin concentrations, sex hormones, and cortisol in nondiabetic men. J Clin Endocrinol Metab. 1997;82:1807–1809. doi: 10.1210/jcem.82.6.3978. [DOI] [PubMed] [Google Scholar]
- Caprio M, Isidori AM, Carta AR, et al. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology. 1999;140:4939–4947. doi: 10.1210/endo.140.11.7088. [DOI] [PubMed] [Google Scholar]
- Corona G, Mannucci E, Petrone L, et al. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res. 2006;18:190–197. doi: 10.1038/sj.ijir.3901391. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: Correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- Dhindsa S, Prabhakar S, Sethi M, et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–5468. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- Dandona P, Dhindsa S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96:2643–2651. doi: 10.1210/jc.2010-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Fukui M, Tanaka M, Hasegawa G, et al. Association between serum bioavailable testosterone concentration and the ratio of glycated albumin to glycated hemoglobin in men with type 2 diabetes. Diabetes Care. 2008;31:397–401. doi: 10.2337/dc07-1898. [DOI] [PubMed] [Google Scholar]
- Xu L, Au Yeung SL, Kavikondala S, et al. Testosterone concentrations in young healthy us versus Chinese men. Am J Hum Biol. 2014;26:99–102. doi: 10.1002/ajhb.22482. [DOI] [PubMed] [Google Scholar]
- Swanson C, Mellstrom D, Lorentzon M, et al. The uridine diphosphate glucuronosyltransferase 2B15 D85Y and 2B17 deletion polymorphisms predict the glucuronidation pattern of androgens and fat mass in men. J Clin Endocrinol Metab. 2007;92:4878–4882. doi: 10.1210/jc.2007-0359. [DOI] [PubMed] [Google Scholar]
- Crabbe P, Bogaert V, De Bacquer D, et al. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab. 2007;92:3604–3610. doi: 10.1210/jc.2007-0117. [DOI] [PubMed] [Google Scholar]
- Couzin J. Human genetics. In Asians and whites, gene expression varies by race. Science. 2007;315:173–174. doi: 10.1126/science.315.5809.173a. [DOI] [PubMed] [Google Scholar]
- Santner SJ, Albertson B, Zhang GY, et al. Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab. 1998;83:2104–2109. doi: 10.1210/jcem.83.6.4898. [DOI] [PubMed] [Google Scholar]
- Wang C, Christenson P, Swerdloff R. Editorial: Clinical relevance of racial and ethnic differences in sex steroids. J Clin Endocrinol Metab. 2007;92:2433–2435. doi: 10.1210/jc.2007-1085. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Brune M, Kornmann B, et al. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J Clin Endocrinol Metab. 2001;86:4867–4873. doi: 10.1210/jcem.86.10.7889. [DOI] [PubMed] [Google Scholar]
- Francomano D, Greco EA, Lenzi A, et al. CAG repeat testing of androgen receptor polymorphism: is this necessary for the best clinical management of hypogonadism? J Sex Med. 2013;10:2373–2381. doi: 10.1111/jsm.12268. [DOI] [PubMed] [Google Scholar]
- Mitsumori K, Terai A, Oka H, et al. Androgen receptor CAG repeat length polymorphism in benign prostatic hyperplasia (BPH): correlation with adenoma growth. Prostate. 1999;41:253–257. doi: 10.1002/(sici)1097-0045(19991201)41:4<253::aid-pros5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Depenbusch M, Gromoll J, et al. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab. 2003;88:2049–2054. doi: 10.1210/jc.2002-021947. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi K, Thangaraj K, Rajender S, et al. GGN repeat length and GGN/CAG haplotype variations in the androgen receptor gene and prostate cancer risk in south Indian men. J Hum Genet. 2006;51:998–1005. doi: 10.1007/s10038-006-0051-z. [DOI] [PubMed] [Google Scholar]
- Hakimi JM, Schoenberg MP, Rondinelli RH, et al. Androgen receptor variants with short glutamine or glycine repeats may identify unique subpopulations of men with prostate cancer. Clin Cancer Res. 1997;3:1599–1608. [PubMed] [Google Scholar]
- Tajar A, Huhtaniemi IT, O'Neill TW, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS) J Clin Endocrinol Metab. 2012;97:1508–1516. doi: 10.1210/jc.2011-2513. [DOI] [PubMed] [Google Scholar]
- Simon D, Preziosi P, Barrett-Connor E, et al. Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia. 1992;35:173–177. doi: 10.1007/BF00402551. [DOI] [PubMed] [Google Scholar]
- Kupelian V, Hayes FJ, Link CL, et al. Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab. 2008;93:3403–3410. doi: 10.1210/jc.2008-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand JS, van der Tweel I, Grobbee DE, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40:189–207. doi: 10.1093/ije/dyq158. [DOI] [PubMed] [Google Scholar]
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- Jindal A, Brietzke S, Sowers JR. Obesity and the cardiorenal metabolic syndrome: therapeutic modalities and their efficacy in improving cardiovascular and renal risk factors. Cardiorenal Med. 2012;2:314–327. doi: 10.1159/000343803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- da Cunha VR, Brenol CV, Brenol JC, et al. Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scand J Rheumatol. 2012;41:186–191. doi: 10.3109/03009742.2011.626443. [DOI] [PubMed] [Google Scholar]
- Chen W, Lu F, Liu SJ, et al. Cancer risk and key components of metabolic syndrome: a population-based prospective cohort study in Chinese. Chin Med J (Engl) 2012;125:481–485. [PubMed] [Google Scholar]
- Simon D, Charles MA, Nahoul K, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The telecom study. J Clin Endocrinol Metab. 1997;82:682–685. doi: 10.1210/jcem.82.2.3766. [DOI] [PubMed] [Google Scholar]
- Heufelder AE, Saad F, Bunck MC, et al. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–733. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- Chou TM, Sudhir K, Hutchison SJ, et al. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- Yue P, Chatterjee K, Beale C, et al. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995;91:1154–1160. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]
- Webb CM, McNeill JG, Hayward CS, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- Haidar A, Yassin A, Saad F, et al. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007;10:189–196. doi: 10.1080/13685530701653538. [DOI] [PubMed] [Google Scholar]
- D'Agostino P, Milano S, Barbera C, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y Acad Sci. 1999;876:426–429. doi: 10.1111/j.1749-6632.1999.tb07667.x. [DOI] [PubMed] [Google Scholar]
- Li ZG, Danis VA, Brooks PM. Effect of gonadal steroids on the production of IL-1 and IL-6 by blood mononuclear cells in vitro. Clin Exp Rheumatol. 1993;11:157–162. [PubMed] [Google Scholar]
- Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- Corona G, Mannucci E, Petrone L, et al. NCEP-ATPIII-defined metabolic syndrome, type 2 diabetes mellitus, and prevalence of hypogonadism in male patients with sexual dysfunction. J Sex Med. 2007;4:1038–1045. doi: 10.1111/j.1743-6109.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- Laaksonen DE, Niskanen L, Punnonen K, et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab. 2005;90:712–719. doi: 10.1210/jc.2004-0970. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, Kupelian V, et al. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–555. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- Haring R, Ittermann T, Volzke H, et al. Prevalence, incidence and risk factors of testosterone deficiency in a population-based cohort of men: results from the study of health in Pomerania. Aging Male. 2010;13:247–257. doi: 10.3109/13685538.2010.487553. [DOI] [PubMed] [Google Scholar]
- Stanik S, Dornfeld LP, Maxwell MH, et al. The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab. 1981;53:828–832. doi: 10.1210/jcem-53-4-828. [DOI] [PubMed] [Google Scholar]
- Pritchard J, Despres JP, Gagnon J, et al. Plasma adrenal, gonadal, and conjugated steroids following long-term exercise-induced negative energy balance in identical twins. Metabolism. 1999;48:1120–1127. doi: 10.1016/s0026-0495(99)90125-7. [DOI] [PubMed] [Google Scholar]
- Kaukua J, Pekkarinen T, Sane T, et al. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–694. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- Niskanen L, Laaksonen DE, Punnonen K, et al. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6:208–215. doi: 10.1111/j.1462-8902.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- Khoo J, Piantadosi C, Worthley S, et al. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int J Obes (Lond) 2010;34:1396–1403. doi: 10.1038/ijo.2010.76. [DOI] [PubMed] [Google Scholar]
- Hammoud A, Gibson M, Hunt SC, et al. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab. 2009;94:1329–1332. doi: 10.1210/jc.2008-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omana JJ, Tamler R, Strohmayer E, et al. Sex hormone levels in men undergoing bariatric surgery. J Am Coll Surg. 2009;209:S22–S23. [Google Scholar]
- Ohlsson C, Barrett-Connor E, Bhasin S, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men the mros (osteoporotic fractures in men) study in sweden. J Am Coll Cardiol. 2011;58:1674–1681. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap BB, Alfonso H, Chubb SA, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- Janus ED, Wat NMS, Lam KSL, et al. The prevalence of diabetes, association with cardiovascular risk factors and implications of diagnostic criteria (ADA 1997 and WHO 1998) in a 1996 community-based population study in Hong Kong Chinese. Diabet Med. 2000;17:741–745. doi: 10.1046/j.1464-5491.2000.00376.x. [DOI] [PubMed] [Google Scholar]
- van den Beld AW, Bots ML, Janssen JA, et al. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol. 2003;157:25–31. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- Kelly DM, Sellers DJ, Woodroofe MN, et al. Effect of testosterone on inflammatory markers in the development of early atherogenesis in the testicular-feminised mouse model. Endocr Res. 2013;38:125–138. doi: 10.3109/07435800.2012.735307. [DOI] [PubMed] [Google Scholar]
- Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary-artery disease in men. Arterioscler Thromb. 1994;14:701–706. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Clarke S, Stanworth R, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- Yang YM, Lv XY, Huang WD, et al. Study of androgen and atherosclerosis in old-age male. J Zhejiang Univ Sci B. 2005;6:931–935. doi: 10.1631/jzus.2005.B0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V, Chaudhuri A, Tomar R, et al. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care. 2006;29:2289–2294. doi: 10.2337/dc06-0637. [DOI] [PubMed] [Google Scholar]
- Rosano GM, Leonardo F, Pagnotta P, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- English KM, Steeds RP, Jones TH, et al. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- Guder G, Frantz S, Bauersachs J, et al. Low circulating androgens and mortality risk in heart failure. Heart. 2010;96:504–509. doi: 10.1136/hrt.2009.181065. [DOI] [PubMed] [Google Scholar]
- Jankowska EA, Biel B, Majda J, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Aihara K, Sato T, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280:29661–29666. doi: 10.1074/jbc.M411694200. [DOI] [PubMed] [Google Scholar]
- Evangelou A, Jindal SK, Brown TJ, et al. Down-regulation of transforming growth factor beta receptors by androgen in ovarian cancer cells. Cancer Res. 2000;60:929–935. [PubMed] [Google Scholar]
- Tam NN, Gao Y, Leung YK, et al. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol. 2003;163:2513–2522. doi: 10.1016/S0002-9440(10)63606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ST, Dillner K, Wu X, et al. Gene expression profiling of androgen deficiency predicts a pathway of prostate apoptosis that involves genes related to oxidative stress. Endocrinology. 2002;143:4897–4906. doi: 10.1210/en.2002-220327. [DOI] [PubMed] [Google Scholar]
- Shan W, Zhong W, Zhao R, et al. Thioredoxin 1 as a subcellular biomarker of redox imbalance in human prostate cancer progression. Free Radic Biol Med. 2010;49:2078–2087. doi: 10.1016/j.freeradbiomed.2010.10.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best CJ, Gillespie JW, Yi Y, et al. Molecular alterations in primary prostate cancer after androgen ablation therapy. Clin Cancer Res. 2005;11:6823–6834. doi: 10.1158/1078-0432.CCR-05-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Aihara K, Akaike M, et al. Androgen receptor counteracts doxorubicin-induced cardiotoxicity in male mice. Mol Endocrinol. 2010;24:1338–1348. doi: 10.1210/me.2009-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Testosterone trial halted. JAMA. 2010;304:846. [Google Scholar]
- Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- Vigen R, O'Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Tian Y, Wu T, et al. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl. 2014;16:146–152. doi: 10.4103/1008-682X.122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon J, Urban RJ, Kuo YF, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48:1138–1144. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]


