Abstract
Aims/Introduction
Recent studies have shown that cell transplantation therapies, such as endothelial precursor cells, bone marrow-derived mononuclear cells (BM-MNCs) and mesenchymal stem cells, are effective on diabetic polyneuropathy through ameliorating impaired nerve blood flow in diabetic rats. Here, we investigated the effects of BM-MNCs transplantation in diabetic polyneuropathy using BM-MNCs derived from adult (16-week-old) diabetic (AD), adult non-diabetic (AN) or young (8-week-old) non-diabetic (YN) rats.
Materials and Methods
BM-MNCs of AD and AN were isolated after an 8-week diabetes duration. The BM-MNCs were characterized using flow cytometry analysis of cell surface markers and reverse transcription polymerase chain reaction of several cytokines. BM-MNCs or saline were injected into hind limb muscles. Four weeks later, the thermal plantar test, nerve conduction velocity, blood flow of the sciatic nerve and capillary-to-muscle fiber ratio were evaluated.
Results
The number of CD29+/CD90+ cells that host mesenchymal stem cells in BM-MNCs decreased in AD compared with AN or YN, and transcript expressions of basic fibroblast growth factor and nerve growth factor in BM-MNCs decreased in AD compared with AN or YN. Impaired thermal sensation, decreased blood flow of the sciatic nerve and delayed nerve conduction velocity in 8-week-diabetic rats were significantly ameliorated by BM-MNCs derived from YN, whereas BM-MNCs from AD or AN rats did not show any beneficial effect in these functional tests.
Conclusions
These results show that cytokine production abilities and the mesenchymal stem cell population of BM-MNCs would be modified by aging and metabolic changes in diabetes, and that these differences could explain the disparity of the therapeutic efficacy of BM-MNCs between young and adult or diabetic and non-diabetic patients in diabetic polyneuropathy.
Keywords: Aging, Diabetic polyneuropathy, Neurotrophic factors
Introduction
Although diabetes is recognized as one of the main non-communicable diseases that should be monitored and prevented utilizing a clear global strategy1, there is no dependable treatment to protect patients with diabetes from complications; for example, coronary artery disease, stroke, diabetic nephropathy, diabetic retinopathy and diabetic neuropathy. Among those complications, diabetic polyneuropathy (DPN) is one of the most prevalent and early-onset complications, and has multipathogenic mechanisms that cause a diversity of physical symptoms: allodynia, hyperalgesia, numbness and cutaneous ulceration2,3. Even patients with impaired glucose tolerance suffer vascular dysfunction and distal nerve fiber loss4, and these impairments in the vascular and nervous system proceed in a synergistic manner, and result in a loss of nerve fibers in the nerve trunk of the lower extremities5. Pathogenetic mechanisms underlying the progressive nerve fiber loss seem to be multifactorial, including activation of polyol pathway, enhanced non-enzymatic glycation, mitochondrial oxidative stress and altered protein kinase C activity6,7.
DPN is pathologically characterized as a distal axonopathy of a dying-back type8, which is accompanied by a failure of axonal regeneration9. Disturbed axonal growth in diabetes has been ascribed in part to the reduction of neurotrophic/angiogenic factors, such as nerve growth factor (NGF), neurotrophin-3 (NT-3), insulin-like growth factor 1, basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), in peripheral nerves and circumambient muscles, and decreased expression of their receptors in dorsal root ganglion neurons10,11. Based on this knowledge, many researchers have tried to evaluate, both experimentally and clinically, the effectiveness of treatment of these trophic factors in DPN12–14. With respect to NGF, although treatment with NGF in diabetic animals was proven to be effective13, a clinical trial failed to show the effectiveness in DPN15, and it has been shown that NGF might cause sensory dysfunctions; for example, hyperalgesia and allodynia12. In a similar manner, VEGF therapy; that is, intramuscular plasmid VEGF gene transfer, has also been proven to be beneficial in an experimental DPN model. However, in a clinical trial15, serious adverse events tended toward an increase in the treated group. The risk that VEGF might trigger a fragile angiogenesis and a hyperpermiability of vessels in diabetes patients has been already pointed out16. Therefore, the clinical application of monotherapy using each growth factor might be difficult, and therapies combined with several growth factors could be one of the future strategies of DPN treatment, as described in other review articles17.
Based on this idea, we have focused on and reported the potential of cell transplantation therapies, because there is a prospect that some immature cells or proper cell mixtures could continue to produce many growth/trophic factors in vivo18,19. First, human endothelial progenitor cells (EPCs), which are a major component of angiogenesis, were used in the treatment of DPN rats, and ameliorated peripheral nerve functions through their function of angiogenesis20. Second, bone marrow-derived mesenchymal stem cells (MSCs), which have multipotent differentiation properties21,22, and secrete several cytokines and growth factors23, were transplanted in the rat model of DPN, and improved nerve functions through their paracrine actions of trophic factors; that is, VEGF and bFGF24. Third, we reported the beneficial effects of bone marrow-derived mononuclear cells (BM-MNCs), which contain both hematopoietic and non-hematopoietic stem/progenitor cells; that is, EPCs, MSCs and hematopoietic stem cells (HSCs), in the early stage of DPN through angiogenesis and paracrine effects25. In contrast, it has been reported that the functions of EPCs and MSCs are impaired under the diabetic condition26–28, and the number of MSCs in BM decreases as age increases29.
Here, we show that transplantation of BM-MNCs derived from young animals ameliorated sensory functions and nerve conduction velocities (NCVs) in DPN model rats. Furthermore, the transplantation of BM-MNCs derived from diabetic or mature animals showed no beneficial effect in developed DPN, which might be caused by the decrease of CD29+/CD90+ cells that host MSCs, and reduction of bFGF and NGF in their bone marrow.
Materials and Methods
Animal Procedures
Male Sprague–Dawley (SD) and GFP-expressing SD-Tg (CAG-EGFP) rats were obtained from Japan SLC, Inc. (Shizuoka, Japan) at 6 weeks-of-age. All rats were housed in individual cages under controlled temperature (24 ± 1.0°C) and on a 12-h light/dark cycle, and were given standard laboratory rat chow with water ad libitum. Diabetes was induced by a single intraperitoneal injection of freshly dissolved streptozotocin (STZ; Sigma Chemical Co., St. Louis, MO, USA; 60 mg/kg bodyweight in 0.9% sterile saline) to rats after an overnight fast. Diabetes was identified by polydipsia, polyuria and by measuring the non-fasting serum glucose concentration 1 week after the injection of STZ. Rats with a blood glucose level above 16 mmol/L were considered to be diabetic and were used in the experiments. Age-matched male SD rats were used as control animals. All experimental protocols were carried out according to the Regulations for Animal Experiments in Nagoya University, and were approved by the Institutional Animal Care and Use Committees of Nagoya University.
Isolation of BM-MNCs
Bone marrow was taken from the femoral and tibial bones of young non-diabetic (YN; 8-week-old), adult non-diabetic (AN; 16-week-old), or adult diabetic (AD; 16-week-old) male SD rats or GFP expressing rats, and was suspended in phosphate buffered saline (PBS). BM-MNCs were isolated using the Histopaque-density centrifugation method, as previously described30. The MNC layer was collected, washed twice with PBS and suspended in 0.9% saline with 0.5% bovine serum albumin.
Real-Time Reverse Transcription Polymerase Chain Reaction in BM-MNCs
Total ribonucleic acid (RNA) was extracted from BM-MNCs or the frozen samples of soleus muscles using ISOGEN Reagent (Nippon Gene, Toyama, Japan), according to the manufacturer's instructions. Starting from 1 μg RNA, complementary deoxyribonucleic acid was synthesized using ReverTraAce (Toyobo, Osaka, Japan), according to the manufacturer's instructions. Primers and probes for VEGF, NGF, bFGF, NT-3 and 18S ribosomal RNA for the endogenous control were purchased from Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). Real-time quantitative polymerase chain reaction was carried out and monitored using the Mx3000P QPCR System (Stratagene, La Jolla, CA, USA). Relative quantity was calculated by the 2(-Delta DeltaCt) method31.
Fluorescence-Activated Cell Sorter Analysis
The BM-MNCs were reacted using one or a combination of fluorescent dye conjugated antibodies: peridinin chlorophyll protein (PerCP)-conjugated mouse monoclonal antibodies against rat CD90 (Becton Dickinson, Franklin Lakes, NJ, USA), fluorescein isothiocyanate (FITC)-conjugated hamster anti-rat CD29 antibody (Becton Dickinson), phycoerythrin (PE)-conjugated mouse monoclonal antibodies against rat CD34 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and CD45 (Becton Dickinson). These labeled cells were analyzed utilizing fluorescence-activated cell sorter (BD FACS Canto; Becton Dickinson). Isotype-identical antibodies served as controls.
Transplantation of BM-MNCs
Eight weeks after the STZ injection, the diabetic rats were anesthetized with pentobarbital (50 mg/kg bodyweight, i.p.), and were transplanted with BM-MNCs into the hind limb skeletal muscles. The BM-MNC suspension (0.5 mL in total, 1 × 108 cells) was injected into 10 points in the unilateral femoral quadriceps, femoral biceps and soleus muscles using a 26-G needle. Saline (0.5 mL in total) was also injected into the contralateral hind limb skeletal muscles. Saline was also injected in groups of control rats. With the purpose of tracking the transplanted cell, two rats of each group received the BM-MNCs derived from GFP-expressing rats.
Thermal Plantar Test
Within a 3-day range before and 4 weeks after the BM-MNC transplantations, hind paw withdrawal response against thermal stimuli of radiant heat was measured using a device (Plantar test, 7370; Ugo Basile, Comerio, Italy). Radiant heat was beamed onto the plantar surface of the hind paw. The paw withdrawal latencies were measured five times per session, separated by a minimum interval of 10 min. Paw withdrawals as a result of locomotion or weight shifting were not counted. Data are expressed as paw withdrawal latency in seconds.
NCVs
Within a 3-day range before and 4 weeks after the BM-MNC transplantations, nerve conduction velocities were measured in hind limbs. Rats anesthetized with pentobarbital were placed on a heating pad in a room maintained at 25°C to ensure a constant rectal temperature of 37°C. Motor NCV (MNCV) between the ankle and sciatic notch, and sensory NCV (SNCV) in the sural nerve between the ankle and knee were measured using a non-invasive procedure. MNCV and SNCV were determined with a Neuropak NEM-3102 instrument (Nihon-Koden, Osaka, Japan), as previously described17.
Sciatic Nerve Blood Flow
Sciatic nerve blood flow (SNBF) was measured using a hydrogen clearance technique with an analog recorder (BW-4; Biochemical Science, Kanazawa, Japan) and an electrolysis tissue blood flow meter (RBA-2; Biochemical Science), as previously described14,32, and calculated with the equation used by Koshu et al.33 During this measurement, rats were placed on a heated pad in a room maintained at 25°C to ensure a constant rectal temperature of 37°C.
Tissue Collection
Four weeks after the transplantation, rats were perfused with 50 mL of 4% paraformaldehyde fixative. After perfusion, the soleus muscles from both sides were immersed in 4% paraformaldehyde overnight, embedded in paraffin or Optimal Cutting Temperature compound (Sakura Finetechnical, Tokyo, Japan) after cryoprotection, and cut into 5-μm sections to evaluate the capillary number or the engraftment of GFP-expressing cells.
Capillary Number-to-Muscle Fiber Ratio
The sections of soleus muscles fixed with paraformaldehyde were used for hematoxylin–eosin staining and immunostaining. These stainings were carried out as previously described in detail25. The vascular capillaries were stained by anti-von Willebrand factor polyclonal antibody (1:600; Dako, Tokyo, Japan) and were imaged using the BIOREVO BZ-9000 system (Keyence Japan, Osaka, Japan). The capillary number-to-muscle fiber ratio was determined by using image analysis software, Dynamic cell count BZ-HIC software (Keyence Japan). Five fields from each section were randomly selected for the quantification.
Statistical Analysis
All group values were expressed as means ± standard deviation. Statistical analyses were made by one-way anova and Bonferroni's multiple comparisons test. Differences were considered significant at the P < 0.05 level.
Results
Alterations in Bodyweight Gain and Blood Glucose Concentrations in Diabetic Rats
Diabetic rats presented significant decreases in bodyweight gain and significant increases in blood glucose concentrations compared with non-diabetic rats (Table1). There was no influence of the transplantation of BM-MNCs on the bodyweights and blood glucose concentrations in the diabetic rats.
Table 1.
Bodyweight and blood glucose
| Non-diabetic rats | Diabetic rats | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||||
| Saline | AN-MNC | AD-MNC | YN-MNC | Saline | AN-MNC | AD-MNC | YN-MNC | |||
| n | 13 | 7 | 6 | 6 | 5 | 10 | 7 | 6 | 6 | 5 |
| Blood glucose (mmol/L) | 5.7 ± 0.7 | 5.2 ± 0.2 | 5.3 ± 0.3 | 5.3 ± 0.4 | 5.0 ± 0.5 | 18.5 ± 3.2* | 19.9 ± 1.6* | 23.1 ± 8.0* | 19.3 ± 2.4* | 20.4 ± 7.7* |
| Bodyweight (g) | 457 ± 21 | 508 ± 15 | 514 ± 54 | 505 ± 15 | 492 ± 38 | 237 ± 48* | 249 ± 14* | 217 ± 23* | 268 ± 40* | 240 ± 38* |
P < 0.05 vs the corresponding group of non-diabetic rats. AD-MNC, rats transplanted with mononuclear cells derived from bone marrow of adult diabetic rats; AN-MNC, rats transplanted with mononuclear cells derived from bone marrow of adult non-diabetic rats; Post, post-transplantation; Pre, pretransplantation; YN-MNC, rats transplanted with mononuclear cells derived from bone marrow of young non-diabetic rats.
Beneficial Effect of BM-MNCs on Thermal Perception
In the thermal plantar test, the response against thermal stimuli on the plantar of diabetic rats (D) was delayed significantly compared with that of non-diabetic rats (N) before and after the injections of BM-MNCs (pretreatment non-diabetic rats [preN] 16.9 ± 1.6 s, pretreatment diabetic rats [preD] 19.82 ± 3.0, P < 0.05; limbs injected with saline in non-diabetic rats [N-S] 16.0 ± 1.7, limbs injected with saline in diabetic rats [D-S] 21.7 ± 3.1, P < 0.05; Figure1). Four weeks after the transplantation, the prolonged response times until foot withdrawals in D were shortened significantly in hind limbs planted with YN-derived BM-MNCs (D-YN), but not those planted with AN-derived BM-MNCs (D-AN) or those planted with AD-derived BM-MNCs (D-AD) compared with D-S (D-YN 17.3 ± 2.3, D-AN 21.5 ± 3.2, D-AD 20.4 ± 3.9, P < 0.05 compared between D-YN and D-S). There was no significant change among the transplanted groups of N 4 weeks after the treatment (limbs planted with YN-derived BM-MNCs in N [N-YN] 16.8 ± 2.4, limbs planted with AN-derived BM-MNCs in N [N-AN] 15.0 ± 2.4, limbs planted with AD-derived BM-MNCs in N [N-AD] 18.0 ± 3.1).
Figure 1.
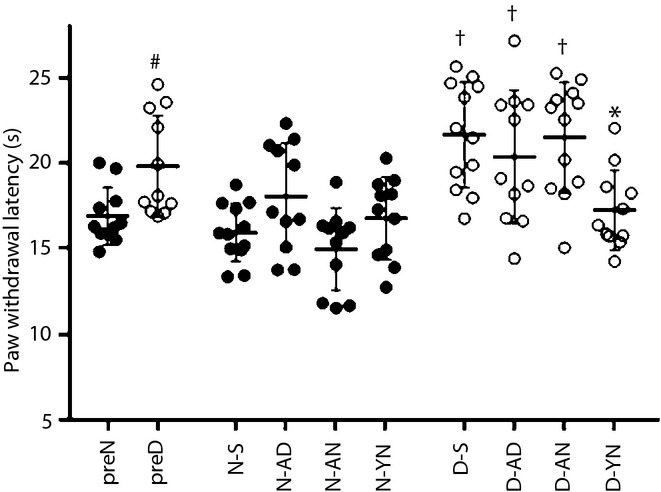
Thermal perception test. After the 8 weeks of diabetes duration, the response times against thermal radiant stimuli were delayed in diabetic rats (preD) compared with non-diabetic rats (preN). The transplantations of bone marrow-derived mononuclear cells (BM-MNCs) ameliorated the response time in limbs of diabetic rats transplanted with BM-MNCs derived from young non-diabetic rats (D-YN), but not limbs of diabetic rats transplanted with BM-MNCs derived from adult non-diabetic rats, (D-AN) or limbs of diabetic rats transplanted with BM-MNCs derived from adult (mature) diabetic rats (D-AD) compared with limbs injected with saline in diabetic rats (D-S). #P < 0.05 vs preN, †P < 0.05 vs limbs injected with saline in non-diabetic rats (N-S), *P < 0.05 vs D-S. N-AD, limbs of non-diabetic rats transplanted with bone marrow-derived mononuclear cells derived from adult diabetic rats; N-AN, limbs of non-diabetic rats transplanted with bone marrow-derived mononuclear cells derived from adult non-diabetic rats; N-YN, limbs of non-diabetic rats transplanted with bone marrow-derived mononuclear cells derived from young non-diabetic rats.
Ameliorations by BM-MNC Therapy on Impaired NCVs in Diabetic Rats
Eight weeks after the STZ and saline injections, changes of lower limb NCVs in preD and preN were measured. MNCV and SNCV in preD were 41.8 ± 3.7 m/s and 42.0 ± 6.1 m/s, respectively, which were significantly reduced compared with those in the preN (MNCV 53.9 ± 4.9 m/s, SNCV 54.5 ± 3.7 m/s, P < 0.05 for both; Figure2a,b). In diabetic rats, both MNCV and SNCV in D-YN were significantly ameliorated compared with D-S (MNCV: D-S 41.9 ± 3.3, D-YN 50.7 ± 5.1, P < 0.05; SNCV: D-S 46.4 ± 4.1, D-YN 52.7 ± 6.3, P < 0.05), but not in D-AN or D-AD (D-AN: MNCV 47.5 ± 3.6, SNCV 47.0 ± 5.1; D-AD: MNCV 45.7 ± 3.4, SNCV 42.9 ± 5.3). The transplantation of BM-MNCs in non-diabetic rats did not affect the sciatic nerve conduction velocities (MNCV: N-S 56.4 ± 3.8, N-YN 53.4 ± 7.0, N-AN 55.8 ± 2.6, N-AD 55.6 ± 1.3; SNCV: N-S 58.3 ± 5.8, N-YN 55.6 ± 5.8, N-AN 56.5 ± 3.7, N-AD 54.6 ± 5.4).
Figure 2.
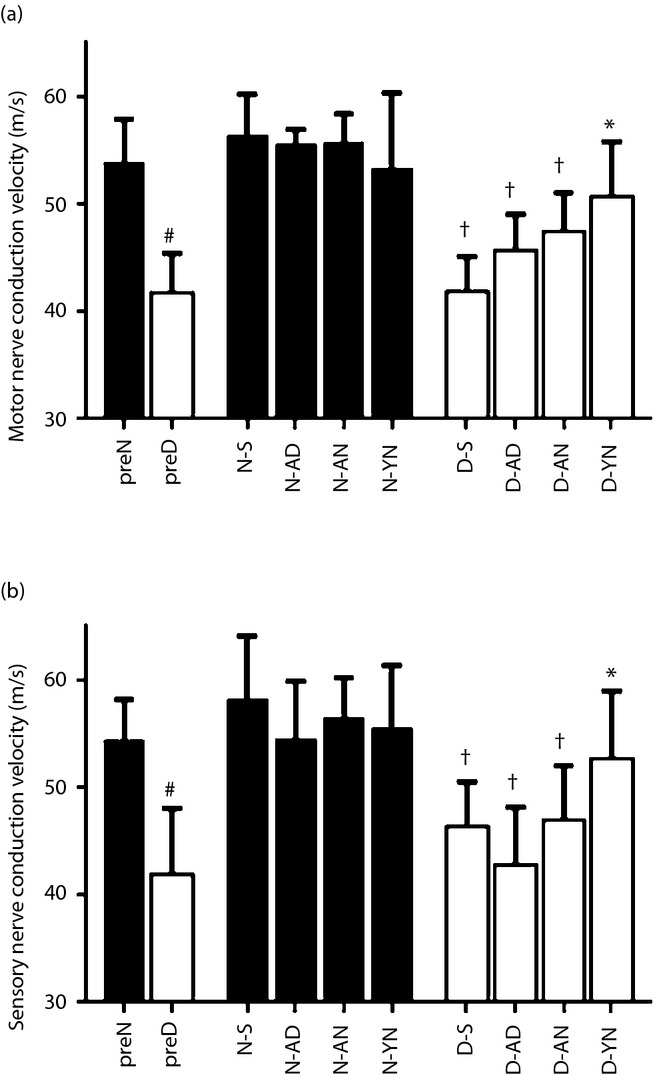
Nerve conduction velocities. After the 8 weeks of diabetes duration, the (a) motor nerve conduction velocities and (b) sensory nerve conduction velocities decreased in diabetic rats (preD) compared with non-diabetic rats (preN). The transplantations of bone marrow-derived mononuclear cells (BM-MNCs) improved the decrease in limbs of diabetic rats transplanted with BM-MNCs derived from young non-diabetic rats (D-YN), but not limbs of diabetic rats transplanted BM-MNCs derived from adult non-diabetic rats (D-AN) or limbs of diabetic rats transplanted with BM-MNCs derived from adult diabetic rats (D-AD) compared with limbs treated with saline (D-S). #P < 0.05 vs preN, †P < 0.05 vs limbs treated with saline (N-S), *P < 0.05 vs D-S. N-AD, limbs of non-diabetic rats transplanted with bone marrow-derived mononuclear cells derived from adult diabetic rats; N-AN, limbs of non-diabetic rats transplanted with bone marrow-derived mononuclear cells derived from adult non-diabetic rats; N-YN, limbs of non-diabetic rats transplanted with bone marrow-derived mononuclear cells derived from young non-diabetic rats.
Augmentation of SNBF in Diabetic Rats Treated With BM-MNCs
SNBF in D or N was also measured 8 weeks after the STZ or saline injections. As shown in Figure3a, SNBF decreased significantly in preD (8.0 ± 2.5 mL/min/100 g) compared with preN (17.2 ± 3.9, P < 0.05). Among the groups of diabetic rats 4 weeks after the treatment, SNBF in D-YN (13.4 ± 4.1) was significantly ameliorated compared with that in D-S (8.9 ± 2.8, P < 0.05). Improvement tendencies, but no statistical significance, were indicated in other diabetic limbs (D-AN 10.1 ± 3.4, D-AD 11.7 ± 3.0). The transplantations of BM-MNCs in non-diabetic rats did not show significant changes of SNBF (N-S 17.3 ± 3.3, N-YN 14.8 ± 3.0, N-AN 15.9 ± 3.1, N-AD 18.1 ± 6.7).
Figure 3.
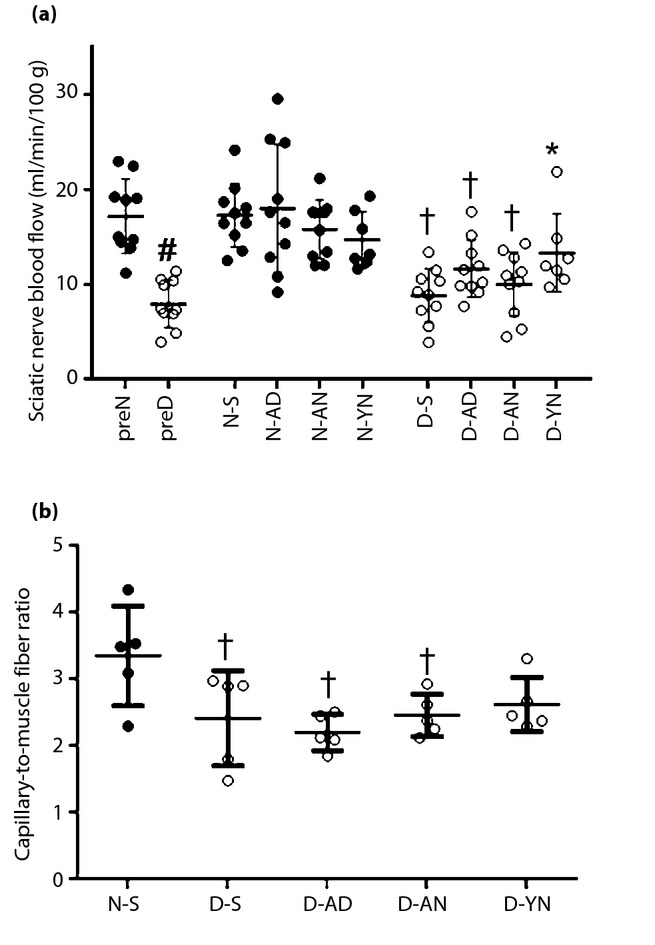
(a) Sciatic nerve blood flow. Blood flow of sciatic nerves decreased in pre-treated diabetic rats (preD) compared with pre-treated non-diabetic rats (preN). The decrease in limbs of diabetic rats transplanted with bone marrow-derived mononuclear cells (BM-MNCs) derived from young non-diabetic rats (D-YN) was improved by the transplantations of BM-MNCs. However, no amelioration was shown in imbs of diabetic rats transplanted with BM-MNCs derived from adult non-diabetic rats (D-AN) or mbs of diabetic rats transplanted with BM-MNCs derived from adult diabetic rats (D-AD). (b) Capillary-to-muscle fiber ratio. The ratio of capillary-to-muscle fiber decreased in diabetic rats compared with non-diabetic rats. No significant improvement has been shown among the transplanted groups of diabetic rats. †P < 0.05 vs limbs treated with saline (N-S), #P < 0.05 vs preN, *P < 0.05 vs limbs treated with saline (D-S).
Capillary Densities in Skeletal Muscles by the BM-MNC Transplantation
Vasculatures in soleus muscles were visualized using immunostaining against von Willebrand factor, a specific marker for endothelial cells. As shown in Figures3b and S1a, fewer capillaries relative to muscle fibers were observed in soleus muscles of D-S. Quantitative analyses showed that the capillary/muscle ratio in D-S was significantly reduced compared with that in N-S (N-S 3.4 ± 0.7, D-S 2.4 ± 0.7, P < 0.05). Among groups of diabetic rats, the transplantations of BM-MNCs tended, but not significantly, to increase the densities in D-YN compared with those in D-S (D-YN 2.6 ± 0.4, D-AN 2.2 ± 0.3, D-AD 2.4 ± 0.3; Figures3b and S1b). There were no GFP-expressing cells in the soleus muscles (data not shown).
Messenger RNA Expression Levels of Cytokines in Skeletal Muscles
Given the possibility that some kinds of growth factors or cytokines were responsible for these ameliorations of functions and microcirculation in the PNS of diabetic rats that received YN, we examined the messenger RNA (mRNA) expression levels of several well-established growth factors or cytokines. The mRNA expression of bFGF tended to decrease in the saline-injected side of the soleus muscles of diabetic rats (n = 3–5). No significant increase of bFGF, VEGF, NT-3 and NGF mRNA expressions was detected in the rats with transplanted BM-MNCs (Figure S2).
Different mRNA Expressions of Cytokines Among BM-MNCs
As detailed in the previous section, the growth factor/cytokine levels in the soleus muscles showed no pronounced difference among these transplantation therapies. Because this lack of significance might be attributable to an abundance of the recipient tissue compared with the transplanted BM-MNCs, we compared the mRNA expression levels among BM-MNCs. The mRNA expression of bFGF was significantly decreased in BM-MNCs derived from AD in comparison with those from YN or AN (Figure4a). The NGF expression was significantly decreased in BM-MNCs derived from AD compared with AN (Figure4b). The expression levels of other cytokines; that is, VEGF (Figure4c) and NT-3 (data not shown), showed no significant difference among these three BM-MNCs.
Figure 4.
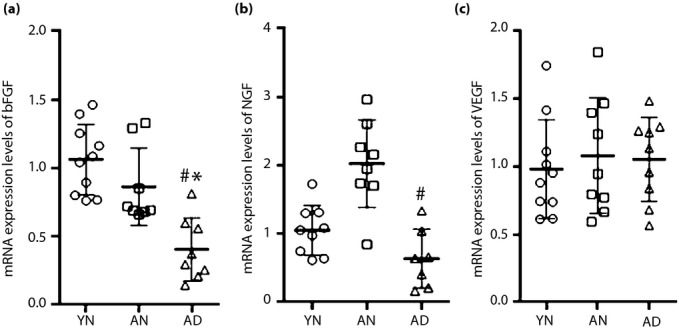
Messenger ribonucleic acid (mRNA) expression levels of cytokines in bone marrow-derived mononuclear cells (BM-MNCs). Real-time reverse transcription polymerase chain reaction analysis showed the differences of cytokine expression levels among BM-MNCs in the young non-diabetic (YN), adult non-diabetic (AN) and adult diabetic rats (AD). (a) The expression levels of basic fibroblast growth factor (bFGF) decreased in BM-MNCs derived from AD compared with those from YN or AN. (b) Nerve growth factor (NGF) levels decreased in BM-MNCs derived from AD compared with AN. (c) There was no significant difference in the expressions of vascular endothelial growth factor (VEGF) among those groups. *P < 0.05 vs YN, #P < 0.05 vs AN.
Decrease of CD29+/CD90+ Subpopulation in BM-MNCs Derived From Mature or Diabetic Rats
Although the decreased expression levels of bFGF and NGF in the BM-MNCs derived from AD might allow speculation that these growth factors contributed to the amelioration of DPN, the ineffectiveness of the transplantation therapy using BM-MNCs derived from AN could not be explained with the alterations of these factors. Therefore, we additionally focused on alternation of the stem/progenitor cell component in BM-MNCs. We evaluated quantitative differences in subpopulations of BM-MNCs among YN, AN and AD using marker sets of stem/progenitor cells: CD29, CD34, CD45 and CD90. CD29, also known as integrin beta 1, which is one of the indispensable MSC markers in cultured bone marrow, is crucial for rolling and adhesion of MSCs to endothelial cells; which means that CD29 has evident functions in the process of homing and engraftment of MSCs into recipient tissue34. Considering that CD29 is also a common antigen of hematopoietic or non-hematopoietic cells in uncultured bone marrow, CD90 – the expression of which is specific to MSCs and HSCs35 – was combined with CD29 for the identification of MSCs from other bone marrow cells. Given this evidence, although no studies to date have verified a set of molecular markers to identify MSCs in tissue, the combination of CD29 and CD90 antigens could feasibly identify a parent population of MSCs in bone marrow. The CD29+/CD90+ subpopulation decreased significantly in BM-MNCs derived from AN (26.8 ± 1.6%) or AD (16.3 ± 1.9%) compared with those from YN (48 ± 1.1%; Figure5a,b). Other subpopulations distinguished by CD34 and CD45, which include hematopoietic progenitor cells and HSCs, showed no difference among those BM-MNCs (Figure S3a,b).
Figure 5.
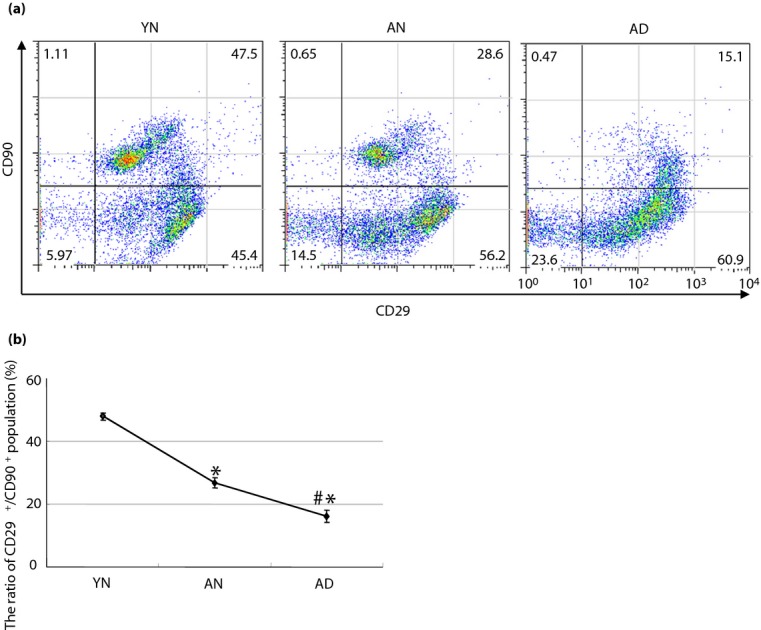
Change of subpopulation in bone marrow-derived mononuclear cells. Fluorescence-activated cell sorter analysis showed a change of subpopulation among bone marrow-derived mononuclear cells derived from the young non-diabetic (YN), adult non-diabetic (AN) and adult diabetic rats (AD). (a) Representative figures of fluorescence-activated cell sorter analysis in each bone marrow-derived mononuclear cell. (b) The CD29+/CD90+ population decreased in AN (26.8 ± 1.6%) or AD (16.3 ± 1.9%) compared with YN (48 ± 1.1%). (a,b) Furthermore, the population decreased significantly in AD compared with AN. *P < 0.05 vs YN, #P < 0.05 vs AN.
Discussion
The present study showed that the intramuscular transplantation of BM-MNCs improved the neuronal functions in DPN rats. However, the beneficial abilities of BM-MNCs were impaired in those derived from mature and/or diabetic rats. Therefore, we have evaluated and shown the difference of the cellular subpopulation and expression levels of cytokines among those BM-MNCs. Although there are some reports about dysfunction or decrease of EPC or MSC in the peripheral blood of diabetic patients26–28, the current study showed the dysfunction and component alteration of BM-MNC, which is the parent population of both EPC and MSC, in matured or diabetic subjects.
It has been proven in several clinical studies that the transplantation of BM-MNCs into skeletal muscles could be an effective treatment for ischemic limbs, and the effectiveness might result from enhancement of post-ischemic neovascularization and increase of vascular blood flow36,37. In addition, the efficacies of the transplantation of BM-MNCs retrieved from young rats in early- or late-stage DPN have been shown in previous studies25,38. Because one of the benefits of this therapy is that BM-MNCs can be acquired with relatively little effort and be transplanted autogenously, we carried out the transplantation of BM-MNCs derived from age-matched diabetic patients with purpose toward a clinical application. However, BM-MNCs acquired from age-matched subjects exercised no beneficial effect in DPN. Therefore, we have investigated the difference between BM-MNCs of young, mature and diabetic rats, with a focus on the alterations of subpopulations and cytokine expressions of these cells.
As mentioned in the Introduction, although NGF and VEGF still failed to become an established clinical treatment of DPN, these are well-studied cytokines as angiogenic/neurotrophic factors. Hence, we have evaluated these two cytokines and other cytokines that have been indicated as important angiogenic/neurotrophic factors: bFGF and NT-339,40. Among these cytokines, bFGF and NGF significantly decreased in BM-MNCs of diabetic rats. The bFGF is known as a potent angiogenic inducer, and might control neovascularization interacting with VEGF or other growth factors40. In our experiment, VEGF or other growth factors did not exert their plausible effects on DPN. Furthermore, it has been validated that bFGF exerted as a neurotrophic factor41, and the administration of bFGF was beneficial in DPN model animals14. Thus, additional well-conceived investigations to clarify the indispensability of bFGF in the prevention of DPN should be taken into consideration in the future.
A series of investigations have justified the existence of subpopulations in bone marrow, which can be exploited for remedies in numerous diseases: peripheral vascular disease42, stroke43 and myocardial infarction44. Nevertheless, most of the unique molecular markers or sets of markers to identify each subpopulation still remain indeterminate. In the current inspection, we assessed the capital sets of rat MSC markers; that is, CD29 and CD9022, and discovered the decrease of the CD29+/CD90+ subpopulation, which contains the most part of MSCs, in diabetic or matured rats. However, there is a perplexing recent report that showed increases of fractions of CD29+ or CD90+ cells in cultures of MSCs derived from the bone marrow of type 2 model rats45. In their research, the authors utilized fluorescence-activated cell sorter analysis against cultured adherent MSCs. In contrast, we utilized the analysis against freshly acquired heterogeneous BM-MNCs in type 1 model rats. As is basically known, profiles of extracellular matrix and cell surface adhesion molecules are modified in diabetic patients46, which might result in different adhesion ability between MSCs of non-diabetic and diabetic rats. Furthermore, the in vitro culture itself might make a biased selection of subpopulation within the culture of heterogeneous MSCs through their proliferation ability or cell integrity.
There are several other limitations and difficulties to explain in the present research. First, a successful engraftment of the BM-MNCs in lower muscles could not be confirmed histologically. It is interesting to examine the distribution and differentiation property of BM-MNCs in vivo, and a previous study has shown the distribution around nerves and vasa nervorum38. The number of transplanted BM-MNCs in the current study was larger than that in the previous study carried out by Kim et al.38, because a series of transplantations using smaller amounts of BM-MNCs could not improve any nerve functions and peripheral blood flows in our DPN model rats (data not shown). The vast amount of transplanted cells might invite an enhancement of acute phase response of focal inflammation and subsequent rejection. Alternatively, a graft rejection might be caused by GFP of the transplanted cells. An improvement of cell tracking methods and long-term observations should be carried out in the future. Second, we have examined the expression levels of just four cytokines in BM-MNCs. In actuality, there are many other cytokines that behave as neuroprotective and regenerative cytokines. We will evaluate other major types of cytokines; for example, GDNF, BDNF, CNTF and PDGF, in our future study. Third, although the number of CD29+/CD90+ cells in diabetic rats decreased significantly compared with that in age-matched non-diabetic rats, there was no convincing difference in the therapeutic efficacies between these two groups in DPN. This might signify the existence of other aging related factors that have a more significant impact on the functions of BM-MNCs. Although we recognize that we should consider an experiment designed with a longer duration in the future, we anticipate that our current work might cause controversy in this field of research.
We showed that the transplantation of BM-MNCs derived from young rats, but not mature or diabetic rats, ameliorated the neuronal function in DPN rats. To our knowledge, this is the first report showing differences in therapeutic efficacy between transplantations of BM-MNCs of young and mature or diabetic rats in diabetic polyneuropathy.
Acknowledgments
The authors thank Michiko Yamada and Keiko Shimamoto for technical assistance, and Minoru Tanaka in the Division for Medical Research Engineering, Nagoya University Graduate School of Medicine for maintenance of the fluorescence-activated cell sorter instrument. The authors declare no conflict of interest.
Supporting Information
Figure S1| The capillary densities in skeletal muscles by bone marrow-derived mononuclear cell (BM-MNC) transplantation.
Figure S2| Change of subpopulation in bone marrow-derived mononuclear cells (BM-MNCs).
Figure S3| Local gene expressions in skeletal muscles.
References
- Beaglehole R, Bonita R, Horton R, et al. Measuring progress on NCDs: one goal and five targets. Lancet. 2012;380:1283–1285. doi: 10.1016/S0140-6736(12)61692-4. [DOI] [PubMed] [Google Scholar]
- Malik R, Veves A, Tesfaye S, et al. Small fiber neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev. 2011;27:678–684. doi: 10.1002/dmrr.1222. [DOI] [PubMed] [Google Scholar]
- Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Invest. 2011;2:18–32. doi: 10.1111/j.2040-1124.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaerts BW, Rathmann W, Kowall B, et al. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35:1891–1893. doi: 10.2337/dc11-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NE, Eaton SE, Cotter MA, et al. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Ogasawara S, Mizukami H, et al. Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-gamma-ligand, in insulin-deficient diabetic rats. J Neurochem. 2008;104:491–499. doi: 10.1111/j.1471-4159.2007.05050.x. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Verge VM, Cheng C, et al. Does diabetes target ganglion neurones? Progressive sensory neurone involvement in long-term experimental diabetes. Brain. 2001;124:2319–2334. doi: 10.1093/brain/124.11.2319. [DOI] [PubMed] [Google Scholar]
- Ekstrom AR, Tomlinson DR. Impaired nerve regeneration in streptozotocin-diabetic rats. Effects of treatment with an aldose reductase inhibitor. J Neurol Sci. 1989;93:231–237. doi: 10.1016/0022-510x(89)90193-7. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997;46(Suppl. 2):S43–S49. doi: 10.2337/diab.46.2.s43. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Fernyhough P, Diemel LT, et al. Deficient neurotrophic support in the aetiology of diabetic neuropathy. Diabet Med. 1996;13:679–681. doi: 10.1002/(SICI)1096-9136(199607)13:7<679::AID-DIA4138>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Apfel SC, Schwartz S, Adornato BT, et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: a randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000;284:2215–2221. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- Elias KA, Cronin MJ, Stewart TA, et al. Peripheral neuropathy in transgenic diabetic mice: restoration of C-fiber function with human recombinant nerve growth factor. Diabetes. 1998;47:1637–1642. doi: 10.2337/diabetes.47.10.1637. [DOI] [PubMed] [Google Scholar]
- Nakae M, Kamiya H, Naruse K, et al. Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes. 2006;55:1470–1477. doi: 10.2337/db05-1160. [DOI] [PubMed] [Google Scholar]
- Ropper AH, Gorson KC, Gooch CL, et al. Vascular endothelial growth factor gene transfer for diabetic polyneuropathy: a randomized, double-blinded trial. Ann Neurol. 2009;65:386–393. doi: 10.1002/ana.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahergorabi Z, Khazaei M. Imbalance of angiogenesis in diabetic complications: the mechanisms. Int J Prev Med. 2012;3:827–838. doi: 10.4103/2008-7802.104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4:271–285. doi: 10.1155/EDR.2003.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, et al. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95:354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- Naruse K, Hamada Y, Nakashima E, et al. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes. 2005;54:1823–1828. doi: 10.2337/diabetes.54.6.1823. [DOI] [PubMed] [Google Scholar]
- Pittenger F, Mackay M, Beck C, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 2005;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Zhao M, Nonoguchi N, Ikeda N, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57:3099–3107. doi: 10.2337/db08-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Sato J, Funakubo M, et al. Transplantation of bone marrow-derived mononuclear cells improves mechanical hyperalgesia, cold allodynia and nerve function in diabetic neuropathy. PLoS One. 2011;6:e27458. doi: 10.1371/journal.pone.0027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouza CV, Hamel FG, Bidasee K, et al. Role of inflammation and insulin resistance in endothelial progenitor cell dysfunction. Diabetes. 2011;60:1286–1294. doi: 10.2337/db10-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MF, Iacopino P, Cuzzola M, et al. Type 2 diabetes mellitus impairs the maturation of endothelial progenitor cells and increases the number of circulating endothelial cells in peripheral blood. Cytometry A. 2012;81:856–864. doi: 10.1002/cyto.a.22109. [DOI] [PubMed] [Google Scholar]
- Yan J, Tie G, Wang S, et al. Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J Am Heart Assoc. 2012;1:e002238. doi: 10.1161/JAHA.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stlozing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Parker SJ, Didier DN, Karcher JR, et al. Bone marrow mononuclear cells induce beneficial remodeling and reduce diastolic dysfunction in the left ventricle of hypertensive SS/MCWi rats. Physiol Genomics. 2012;44:925–933. doi: 10.1152/physiolgenomics.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Kato K, Hamada Y, et al. A protein kinase C-beta-selective inhibitor ameliorates neural dysfunction in streptozotocin-induced diabetic rats. Diabetes. 1999;48:2090–2095. doi: 10.2337/diabetes.48.10.2090. [DOI] [PubMed] [Google Scholar]
- Koshu K, Kamiyama K, Oka N, et al. Measurement of regional blood flow using hydrogen gas generated by electrolysis. Stroke. 1982;13:483–487. doi: 10.1161/01.str.13.4.483. [DOI] [PubMed] [Google Scholar]
- Rüster B, Göttig S, Ludwig J, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motukuru V, Suresh KR, Vivekanand V, et al. Therapeutic angiogenesis in Buerger's disease (thromboangiitis obliterans) patients with critical limb ischemia by autologous transplantation of bone marrow mononuclear cells. J Vasc Surg. 2008;48:53S–60S. doi: 10.1016/j.jvs.2008.09.005. discussion 60S. [DOI] [PubMed] [Google Scholar]
- Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;10:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Kim H, Park JS, Choi YJ, et al. Bone marrow mononuclear cells have neurovas-cular tropism and improve diabetic neuropathy. Stem Cells. 2009;27:1686–1696. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofaro B, Stone OA, Caporali A, et al. Neurotrophin-3 is a novel angiogenic factor capable of therapeutic neovascularization in a mouse model of limb ischemia. Arterioscler Thromb Vasc Biol. 2010;30:1143–1150. doi: 10.1161/ATVBAHA.109.205468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Nguyen LT, Zhuang ZW, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz M, Grosheva M, Skouras E, et al. Poor functional recovery and muscle polyinnervation after facial nerve injury in fibroblast growth factor-2-/- mice can be improved by manual stimulation of denervated vibrissal muscles. Neuroscience. 2011;182:241–247. doi: 10.1016/j.neuroscience.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Blum A, Balkan W, Hare JM. Advances in cell-based therapy for peripheral vascular disease. Atherosclerosis. 2012;223:269–277. doi: 10.1016/j.atherosclerosis.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Bakondi B, Shimada IS, Perry A, et al. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–1947. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, From AH, Zhang J, et al. Myocardial regeneration: the role of progenitor cells derived from bone marrow and heart. Prog Mol Biol Transl Sci. 2012;111:195–215. doi: 10.1016/B978-0-12-398459-3.00009-5. [DOI] [PubMed] [Google Scholar]
- Madhira SL, Challa SS, Chalasani M, et al. Promise(s) of mesenchymal stem cells as an in vitro model system to depict pre-diabetic/diabetic milieu in WNIN/GR-Ob mutant rats. PLoS One. 2012;7:e48061. doi: 10.1371/journal.pone.0048061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf M, Hoefer J, et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1| The capillary densities in skeletal muscles by bone marrow-derived mononuclear cell (BM-MNC) transplantation.
Figure S2| Change of subpopulation in bone marrow-derived mononuclear cells (BM-MNCs).
Figure S3| Local gene expressions in skeletal muscles.


