Abstract
Aims/Introduction
Although male diabetic patients have an increased risk of fracture, there is little information about this in the literature. The association between heel bone stiffness and the lifestyle of male patients with diabetes was evaluated.
Materials and Methods
The study included 108 participants with type 2 diabetes mellitus patients and 168 age-adjusted, healthy male volunteers. None of the participants had a history of osteoporosis or other severe diseases. Heel bone stiffness was examined by quantitative ultrasound, and each participant completed a health interview survey questionnaire. Bone stiffness was taken as an indicator of bone strength. Stepwise regression analysis was used to investigate associations between bone stiffness and lifestyle-related factors, such as sunlight exposure, intake of milk or small fish, regular exercise, cigarette smoking, consumption of alcohol, and number of remaining teeth.
Results
Bone stiffness showed a significant negative association with cigarette smoking [standardized coefficient (SC) = −0.297, F-value (F) = 10.059] and age (SC = −0.207, F = 7.565) in diabetic patients. Bone stiffness showed a significant negative association with age (SC = −0.371, F = 12.076) and height (SC = −0.193, F = 7.898), as well as a significant positive association with sunlight exposure (SC = 0.182, F = 9.589) and intake of small fish (SC = 0.170, F = 7.393) in controls.
Conclusions
These findings suggest that cigarette smoking and age are negatively associated with bone stiffness in Okinawan male patients with type 2 diabetes mellitus.
Keywords: Heel bone stiffness, Male, Type 2 diabetes mellitus
Introduction
The prevention of bone fractures is an important goal in a society with increasing longevity. It is recognized that patients with type 2 diabetes mellitus are increasing worldwide, and a meta-analysis has shown that diabetic patients have a higher hip fracture risk than people without diabetes1–3. Hip fractures are related to chronic pain and disability, loss of independence, decreased quality of life, and increased mortality.
Although osteoporosis is often thought to be a disease of women, studies show that osteoporotic fractures also result in substantial morbidity, mortality and costs in men4–6. It has been reported that mortality during the first 3 months after hip fracture is higher in men than in women, and more than one-third of men who developed a hip fracture died within 1 year7,8.
However, as the increased fracture risk in men is not sufficiently well known, bone density examinations are infrequent in outpatient clinics. Limited data are available on the relationship between lifestyle and bone status in male patients with type 2 diabetes mellitus1,6. Evidence for the benefit of preventive interventions, such as health education on diabetic patients' quality of life, on relieving the burden on caregivers and on decreasing the costs of fractures, is lacking.
Methods
Data Selection
Heel bone stiffness was measured in 154 male patients with type 2 diabetes mellitus aged 30–83 years who visited the outpatient clinic of the Ryukyu University Hospital. Patients with type 1 diabetes, impaired glucose tolerance (IGT) and slowly progressive insulin-dependent diabetes mellitus (SPIDDM) were excluded. Patients with conditions that could affect bone metabolism, such as osteomalacia, compression fracture, rheumatoid arthritis, thyroidectomy, gastrectomy, duodenectomy, kidney disease, nephrectomy and adrenalectomy, as well as those on medications that affect bone metabolism, were also excluded from the analysis.
The background characteristics of the type 2 diabetes mellitus patients, including blood pressure, retinopathy and nephropathy, were assessed. Nephropathy was classified according to the kidney disease improving global outcomes 2012 criteria9. Patients with nephropathy grades 3b, 4 and 5 who have an estimated glomerular filtration rate (e-GFR) of <44 mL/min/1.73 m2 were also excluded from the analysis. Thus, 108 male patients with type 2 diabetes mellitus were ultimately selected for the present study.
Heel bone stiffness was also examined in 185 age-adjusted healthy volunteers who underwent the local resident medical health check-up; 168 male participants aged 30–83 years satisfied the selection criteria (Figure1).
Figure 1.
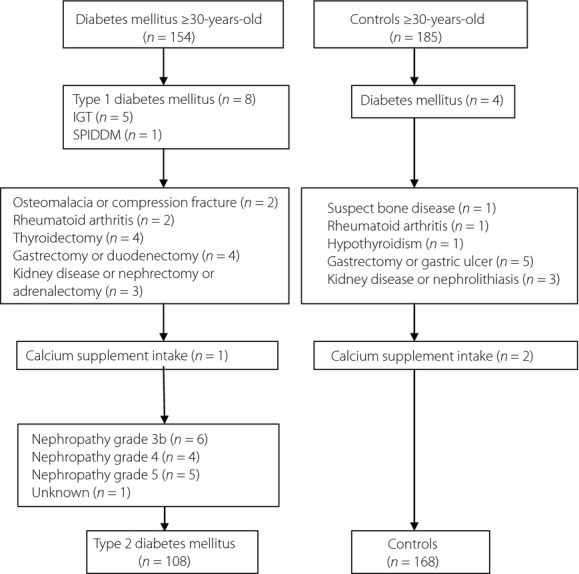
Case-finding protocol. IGT, impaired glucose tolerance; SPPDM, slowly progressive insulin-dependent diabetes mellitus.
Coding Procedures
The face-to-face baseline interviews were carried out using a semistructured questionnaire.
The associations between lifestyle factors and heel bone stiffness were examined. The lifestyle factors were daily dietary calcium intake, habitual exercise, sunlight exposure, cigarette smoking and consumption of alcohol. Dietary calcium intake was estimated by a semiquantitative food frequency questionnaire. Daily dietary intake of a glass of milk or small fish was divided into five frequencies: one to two times a day, once a day, once in 2–3 days, once a week and none at all. Sunlight exposure was divided into three categories: mostly indoors, outdoors for shopping or commuting and mostly working outdoors. Habitual exercise was assessed by regular exercise currently and regular exercise at 20 years-of-age. Cigarette smoking was divided into two categories: currently smoking, smoked in the past or never smoked. Consumption of alcohol was divided into three categories: drink daily, drink sometimes and never drank (Table1).
Table 1.
Screening factors and the 15 factors analyzed
| Questionnaire |
|---|
| I. Check for diseases related to bone status* |
| Thyroid or parathyroid gland diseases, gastrectomy, kidney disease, RA, osteoporosis and prescribed calcium supplement |
| II. Age and physique status |
| 1. Age |
| 2. Height |
| 3. Weight |
| 4. BMI |
| III. Lifestyle-related factors |
| Daily calcium intake from diet |
| 5. Glass of milk intake |
| 1–2 times a day, once a day, once in 2–3 days, once a week, none at all |
| 6. Small fish intake: smelts (semi-dried) or sardines (niboshi) |
| 1–2 times a day, once a day, once in 2–3 days, once a week, none at all |
| Usual lifestyle and exercise |
| 7. Regular exercise currently |
| 8. Regular exercise at 20 years-of-age |
| 9. Sunlight exposure |
| Mostly indoors, outdoors for or shopping or commuting, mostly working outdoors |
| Preferences |
| 10. Cigarette smoking: smoking currently, smoked in the past or never smoked |
| 11. Consumption of alcohol: drink daily, drink sometimes, never drank |
| Remaining no. teeth |
| 12. No. remaining teeth |
| Patients' medical history |
| IV. Diabetes status |
| 13. Presumed diabetes duration |
| 14. Glycated hemoglobin |
| 15. Treatment |
| 16. Blood pressure |
| 17. Retinopathy |
| 18. Nephropathy |
BMI, body mass index; RA, rheumatoid arthritis. These were excluded from the analysis.
Participants' characteristics, which included age, height, weight and body mass index (BMI), were investigated from their medical records. Diabetic status, such as diabetes duration, levels of glycated hemoglobin [HbA1c; National Glycohemoglobin Standardization Program (NGSP)], treatment, blood pressure, retinopathy and nephropathy, were also studied in the type 2 diabetes mellitus group10.
Bone Parameters
Heel bone stiffness was measured using quantitative ultrasound (QUS; AchillesA-1000 PLUS; Lunar Corp., Madison, WI, USA). QUS is recommended for infants and pregnant women, because it does not involve X-rays. Its low cost and portability could make QUS an especially valuable osteoporosis detection tool wherever cost or instrument inaccessibility renders dual-energy X-ray absorptiometry (DXA) difficult or impossible11. The theoretical foundation of QUS is based on the variation in the speed of the ultrasound wave (SOS), in units of m/s, and its attenuation along its transmission path, at frequencies of 0.4–1.0 MHz. Ultrasound waves pass faster in bone with higher density. In addition, as ultrasound passes through bone, it undergoes attenuation, with a consequent loss of transmitted acoustic energy. The slope of attenuation as a function of frequency [the broadband ultrasound attenuation (BUA), in units of dB/MHz] is lower in more porous and less microstructurally intact bone. Aside from SOS and BUA, the most common derived variable is ‘bone stiffness,’ a linear combination of SOS and BUA12. The investigation was carried out from 1999 to 2000.
Statistical Analysis
In the descriptive analysis of baseline characteristics, the data are expressed as means ± SD. The Pearson product-moment correlation coefficient and regression analysis were used to compare aging and bone stiffness in both groups. Levine's test, Student's t-test and the chi square-test were used to compare both groups. One-way analysis of variance (anova) was carried out to compare classification factors and bone stiffness in the type 2 diabetes group. Stepwise regression analysis was carried out to compare lifestyle factors and bone stiffness. All statistical analyses were carried out using SPSS version 17 (SPSS, Chicago, IL, USA).
Ethical Considerations
Informed consent was obtained from all participants. It was explained that no additional fee would be charged for the heel QUS, and that being enrolled in the investigation would not affect their medical care. After the heel QUS was measured, the participants were immediately given an explanation of the measured value, with appropriate health advice.
Results
Participants' Characteristics
Overall, 108 male patients with type 2 diabetes mellitus and 168 male healthy controls were selected for the present study. The average age was 59.7 ± 10.0 years (range 30–80 years) in type 2 diabetes mellitus patients and 60.5 ± 11.7 years (range 30–83 years) in controls. The average height was 162.1 ± 5.5 cm (range 152–178 cm) in type 2 diabetes mellitus patients and 160.6 ± 6.2 cm (range 147–177 cm) in controls. Weight was 65.3 ± 8.8 kg (range 46–88 kg) and 63.8 ± 9.0 kg (range 45–94 kg), and BMI was 24.8 ± 2.8 kg/m2 (range 19–33 kg/m2) and 24.7 ± 2.8 kg/m2 (range 18–34 kg/m2), respectively. These parameters, except for height, were almost identical in the two groups.
The mean duration of illness of type 2 diabetes mellitus patients was 12.7 ± 8.2 years (range 0.8–32 years), and the mean glycated hemoglobin (HbA1c; NGSP) was 8.0 ± 1.8% (5.6–13.8%)10. They were treated with diet (n = 32), insulin injections (n = 23) or oral hypoglycemic agents (n = 53), with patients taking several antidiabetic agents, such as sulfonylureas (n = 44), α-glycosidase inhibitors (n = 14), biguanides (n = 12) or others (n = 1).
The average bone stiffness was 87.8 ± 14.8% in type 2 diabetes mellitus patients and 87.9 ± 14.7% in controls. In normal weight (BMI <25 kg/m2) participants, the average bone stiffness was 83.7 ± 16.3% in type 2 diabetes mellitus patients and 88.6 ± 14.9% in controls, whereas in overweight (BMI ≥25 kg/m2) participants, it was 90.2 ± 13.8% in type 2 diabetes mellitus patients and 87.2 ± 14.4% in controls. Fractures occurred in 10 (9.3%) type 2 diabetes mellitus patients and 12 (7.1%) controls (Table2).
Table 2.
Characteristics of type 2 diabetes mellitus patients and controls
| Type 2 diabetes mellitus patients (n = 108) | Controls (n = 168) | P-value | |
|---|---|---|---|
| Age, years (range) | 59.7 ± 10.0 (30–80) | 60.5 ± 11.7 (30–83) | 0.553 |
| Height, cm (range) | 162.1 ± 5.5 (152–178) | 160.6 ± 6.2 (147–177) | 0.044* |
| Weight, kg (range) | 65.3 ± 8.8 (46–88) | 63.8 ± 9.0 (45–94) | 0.161 |
| BMI, kg/m2 (range) | 24.8 ± 2.8 (19–33) | 24.7 ± 2.8 (18–34) | 0.704 |
| Duration of diabetes, years (range) | 12.7 ± 8.2 (0.8–32) | ||
| HbA1c (%) (NGSP) | 8.0 ± 1.8 (5.6–13.8) | ||
| Therapeutic modality | Diet (n = 32) | ||
| Insulin (n = 23) | |||
| OHA† (n = 53) | |||
| Sulfonylurea (n = 44) | |||
| α-Glucosidase inhibitor (n = 14) | |||
| Biguanide (n = 12) | |||
| Others (n = 1) | |||
| Bone stiffness (%) | 87.8 ± 14.8 | 87.9 ± 14.7 | 0.673 |
| Normal weight (BMI <25 kg/m2) | 83.7 ± 16.3 (n = 51) | 88.6 ± 14.9 (n = 88) | 0.078 |
| Overweight (BMI ≥25 kg/m2) | 90.2 ± 13.8 (n = 57) | 87.2 ± 14.4 (n = 80) | 0.227 |
| History of fracture | 10 (9.3%) | 12 (7.1%) | 0.401 |
| Smoking currently | 26 (24.1%) | 42 (25.0%) | 0.157 |
| Smoked in the past or never smoked | 82 (75.9%) | 124 (73.8%) | 0.157 |
| Unknown | – | 2 (1.2%) |
BMI, body mass index; HbA1c, glycated hemoglobin; NGSP, National Glycohemoglobin Standardization Program. Results are expressed as means ± SD (range). Analysis using Levene's test, Student's t-test or the chi square-test.
P < 0.05.
Oral hypoglycemic agents (OHA), some patients took more than one drug. No patients took pioglitazone.
Table3 shows the background characteristics of the type 2 diabetes mellitus patients. Blood pressure was classified according to the standard 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement13. Diabetic retinopathy was graded as no diabetic retinopathy (NDR), simple diabetic retinopathy, preproliferative diabetic retinopathy (pre-PDR) and proliferative diabetic retinopathy (PDR) according to the diagnostic classification by Davis et al.14 Nephropathy was classified based on cause, GFR stage and albuminuria stage according to the Kidney Disease: Improving Global Outcomes 2012 criteria9.
Table 3.
Background characteristics of type 2 diabetes mellitus patients
| Characteristic | Classification | n (%) n = 108 | Stiffness (%) mean ± SD | P-value* |
|---|---|---|---|---|
| Blood pressure | Normal pressure (120–139 mmHg/80–89 mmHg) | 54 (50.0) | 87.2 ± 15.4 | 0.323 |
| Hypertension (≥140 mmHg/90 mmHg or under treatment) | 51 (47.2) | 88.1 ± 15.1 | ||
| Hypotension (<120 mmHg/80 mmHg) | 3 (2.8) | 69.7 ± 7.2 | ||
| Retinopathy | No diabetic retinopathy | 56 (51.9) | 87.6 ± 14.8 | 0.453 |
| Simple diabetic retinopathy | 28 (25.9) | 85.3 ± 16.8 | ||
| Preproliferative diabetic retinopathy | 7 (6.5) | 89.3 ± 10.8 | ||
| Proliferative diabetic retinopathy | 10 (9.3) | 85.0 ± 18.4 | ||
| Unknown | 7 (6.5) | 91.6 ± 14.6 | ||
| Nephropathy | Grade 1 (normal or high: GFR ≥90 mL/min/1.73 m2) | 61 (56.5) | 87.4 ± 15.4 | 0.379 |
| Grade 2 (mildly decreased: GFR 60–89 mL/min/1.73 m2) | 36 (33.3) | 85.1 ± 16.0 | ||
| Grade 3a (mildly or moderately decreased: GFR 45–59 mL/min/1.73 m2) | 11 (10.2) | 93.0 ± 11.9 |
GFR, glomerular filtration rate; SD, statdard deviation. Results are means ± SD. No patients were undergoing dialysis or receiving vitamin D treatment. *P < 0.05, analysis using analysis of variance, with no significant differences.
Age-Related Bone Loss
Heel bone stiffness decreased with age in both groups, but it was significantly associated with age only in controls. The regression slope was flatter in type 2 diabetes mellitus than in controls. Furthermore, from the 30 s to the 40 s, heel bone stiffness was lower in type 2 diabetes mellitus patients than in controls (type 2 diabetes mellitus, stiffness = −0.19 × age + 98.5, r = 0.12, P = 0.20; controls, stiffness = −0.33 × age + 107.9, r = 0.26, P = 0.01; Figure2).
Figure 2.
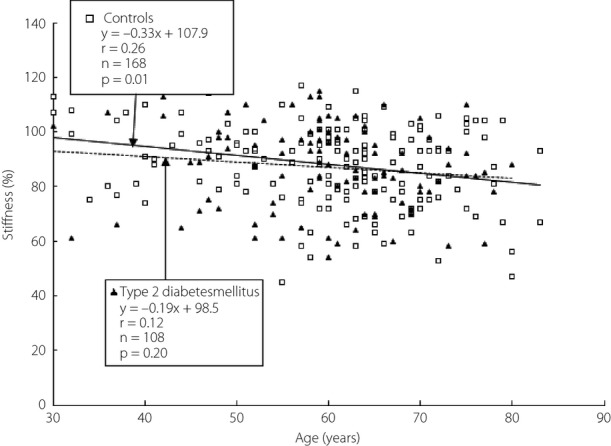
Relationship between age and heel bone stiffness on quantitative ultrasound.
Lifestyle Factor Effects
Table4 shows that 108 type 2 diabetes mellitus patients and 168 control subjects were included in categorical comparisons that included age, height, weight, BMI, intake of a glass of milk or small fish intake as part of the daily diet, regular exercise currently or at 20 years-of-age, sunlight exposure, cigarette smoking, consumption of alcohol and the number of remaining teeth.
Table 4.
Stepwise regression analysis of bone stiffness in type 2 diabetes mellitus patients and controls
| Dependent variable | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bone stiffness (%) | ||||||||
| Type 2 diabetes mellitus | Controls | |||||||
| Independent variables | Standardized coefficient | F-value | P-value | Standardized coefficient | F-value | P-value | ||
| Age (years) | –0.207 | 7.565 | 0.033 | –0.371 | 12.076 | 0.000 | ||
| Height (cm) | –0.193 | 7.898 | 0.018 | |||||
| Sunlight exposure | 0.182 | 9.589 | 0.013 | |||||
| Small fish intake | 0.170 | 7.393 | 0.023 | |||||
| Cigarette smoking | –0.297 | 10.059 | 0.003 | |||||
| n | 108 | 168 | ||||||
| Significance (P) | 0.001 | 0.000 | ||||||
| Adjusted R2 | 0.139 | 0.155 | ||||||
| F-value | 7.565 | 7.393 | ||||||
Controls: eight factors poorly fitting factors (weight, body mass index, milk intake, regular exercise at 20 years-of-age, regular exercise currently, cigarette smoking, consumption of alcohol and number of remaining teeth). Type 2 diabetes mellitus patients: 13 poorly fitting factors (height, weight, body mass index, milk intake, small fish intake, regular exercise at 20 years-of-age, regular exercise currently, sunlight exposure, consumption of alcohol, number of remaining teeth, diabetes duration, glycated hemoglobin and treatments for diabetes).
Weight and BMI had no significant correlations with heel bone stiffness in both groups. However, age had significant negative associations with bone stiffness in both groups [type 2 diabetes mellitus, standardized coefficient (SC) = −0.207, F-value (F) = 7.565; controls, SC = −0.371, F = 12.076]. Furthermore, cigarette smoking was significantly negatively associated with heel bone stiffness in type 2 diabetes mellitus only (SC = −0.297, F = 10.059). In contrast, sunlight exposure and small fish intake were significantly associated with heel bone stiffness in controls only (sunlight exposure, SC = 0.182, F = 9.589; small fish intake, SC = 0.170, F = 7.393).
Duration of diabetes, HbA1c and treatment were not associated with heel bone stiffness.
Discussion
Osteoporosis is defined as a skeletal disorder characterized by compromised bone strength predisposing to an increased fracture risk. Bone strength reflects the integration of two main features: bone density and bone quality. Bone density is expressed as grams of mineral per area or volume, and in any given individual it is determined by peak bone mass and amount of bone loss. Bone quality refers to architecture, turnover, damage accumulation and mineralization. A fracture occurs when a failure-inducing force is applied to osteoporotic bone15.
QUS methods have been introduced in recent years for the assessment of skeletal status in osteoporosis. Current evidence supports the use of QUS techniques for the assessment of fracture risk in elderly women. This has been best established for water-based calcaneal QUS systems. The rules of physics describe the relationships among mechanical preperties, 3-D bone architecture and velocity or attenuation of transmitted ultrasonic waves. QUS parameters could allow one to assess the mechanical properties of cortical and trabecular bone, which in turn are important determinants of whole bone stiffness and fracture risk16.
A meta-analysis has suggested that low QUS values are associated with overall fracture risk, low-trauma fractures, and with hip and other fractures in older women; the association is similar to that seen with DXA17. BUA is associated with an increased risk for hip fracture. Intertrochanteric fractures in particular are strongly associated with a low BUA measurement18. These indices indirectly describe bone micro-architectural features, such as trabecular spacing, orientation and connectivity, as well as bone density10. Additionally, the heel bone is weight-bearing and contains approximately 90% trabecular bone, which has a high metabolic turnover rate and a pattern of bone loss similar to the spine. Bone stiffness might be thought to be almost the same as ‘bone strength’, which is a combined indicator of ‘bone density’ and ‘bone quality.’
Previous studies reported that diabetic patients had higher bone mineral density (BMD; g/cm2) than those without diabetes, despite an increased fracture risk1–3. This phenomenon might be explained by poor bone quality rather than BMD. Recent studies have suggested that collagen cross-linking, a low level of serum vitamin B6, and advanced glycation end-products play an important role in bone quality19,20. Based on the present definition, both BMD and bone quality, which encompass the structural and material properties of bone, are important factors in the determination of ‘bone strength’15.
In the present study, aging was shown to reduce heel bone stiffness in both groups. The regression slope was flatter in type 2 diabetes mellitus patients than in controls, and from the 30 s to the 40 s, heel bone stiffness was lower in type 2 diabetes mellitus patients than in controls.
These estimates in men were remarkably similar to those in women with type 2 diabetes mellitus21. The metabolic effects of poor glycemic control could lead to bone loss in the initial stage of type 2 diabetes mellitus. Based on a previous study, in patients with diabetes, a low bone formation rate retards bone accumulation during growth, and low bone turnover retards age-related bone loss. In the elderly with a long duration of type 2 diabetes mellitus, the low bone turnover delays bone loss, and might eventually result in bone density that exceeds the value expected for age22.
The present data suggest that cigarette smoking is a significant risk factor for decreased bone strength in type 2 diabetes mellitus. A meta-analysis showed that cigarette smoking produced the greatest increases in hip fracture risk (40%) in men23. There is growing evidence that cigarette smoking is a risk factor for the development of type 2 diabetes24,25. Cigarette smoking is associated with insulin resistance, and affects calcium and vitamin D metabolism26,27. Furthermore, smokers with diabetes have an increased rate of microvascular complications, cardiovascular disease (CVD) and premature death. Therefore, the American Diabetes Association recommends that all patients not smoke24.
Okinawans have approximately 20% fewer hip fractures than mainland Japanese28. It has been found that diabetic osteopenia was higher in northern Japan, Hokkaido and Tohoku regions, than in southern Japan, Kyushu and Chugoku-Shikoku regions29. Total mean monthly sunlight exposure in 2000 was 32,235 MJ/m2 in Okinawa and 27,818 MJ/m2 in northern areas (Sapporo)30. Okinawa is the southernmost point in Japan, and the amount of sunlight exposure per unit is higher than that received in northern areas; therefore, spending time outside allows Okinawan men to have optimal vitamin D levels year round.
Calcium is an essential nutrient for critical biological functions, such as nerve conduction, muscle contraction and structural support of the skeleton. Studies show that, among Japanese women, plants and fish contributed 46.7% of total dietary calcium, whereas 32.4% was derived from milk31. Japanese people are accustomed to traditional dishes, such as fish, especially small fish eaten with soft and edible bones, like sardines and smelts. One hundred grams of milk, smelts (semi-dried) and sardines (niboshi) contain 110, 380, and 2200 mg of calcium per 100 g edible portion, respectively32. Smelts are not a common food in Okinawa, but sardines (niboshi) are very commonly eaten. Regarding the data concerning calcium intake in men, the total mean intake was 516 mg/day in Japan and 444 mg/day in Okinawa. As for dried small fish intake in men, total mean intake was 16 g/day in Japan and 5 g/day in Okinawa33,34. Therefore, the calcium intake was lower in Okinawan men than in mainland Japanese men.
The positive relationships between small fish intake and sunlight exposure and bone stiffness, which were seen in controls, disappeared in patients with type 2 diabetes mellitus. Patients with diabetes require more calcium intake and sunlight exposure than those without diabetes, because of increased calcium loss from urine with glycosuria or exacerbating diabetic complications, such as kidney failure.
Bone loss appears to be progressive in diabetic patients with chronic kidney disease (CKD). A previous study has noted that patients with initial eGFR 43.8 ± 3.6 mL/min/1.73 m2 were not on dialysis after 2-year follow up35. Another study has reported that parathyroid hormone (PTH) was found to be elevated in more than 20% of CKD grade 3 patients36. PTH stimulates the bone metabolism and rotation. PTH increases the phosphate excretion in urine by acting on a proximal renal tubule, PTH also increases reabsorption of calcium with stimulating production of activated vitamin D (1α, 25[OH]2 D3). Therefore, complicating factors are involved in calcium homeostasis and bone metabolism in type 2 diabetes patients with renal failure. Consequently, patients with nephropathy grades 3b, 4 and 5 with an eGFR of <44 mL/min/1.73 m2 were excluded from the analysis in the present study.
It has also been reported that diabetic retinopathy is associated with fracture risk37. The effects of atherosclerosis, or increased advanced glycation end-products, which are often seen in diabetic patients with retinopathy and/or nephropathy, might be involved. The metabolic effects of diabetes on the skeleton are complex. Further study is required to determine whether intervention could prevent the reduction of bone strength and hip fractures in diabetic patients.
Although there are several life factors, cigarette smoking was strongly associated with bone strength in patients with diabetes mellitus; therefore, all patients with diabetes should not smoke.
Acknowledgments
The authors thank the doctors of the Second Department of the University of the Ryukyus Hospital. This work was supported by the staff in the Outpatient Department and in the Okinawa General Health Service Association. No financial support was provided.
References
- Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 Diabetes. JAMA. 2011;305:2184–2192. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaseem A, Snow V, Shekelle P, et al. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2008;148:680–684. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- Liu H, Paige NM, Goldzweig CL, et al. Screening for osteoporosis in men: a systematic review for an American College of Physicians guideline. Ann Int Med. 2008;148:685–701. doi: 10.7326/0003-4819-148-9-200805060-00009. [DOI] [PubMed] [Google Scholar]
- Brauer CA, Coca-Perraillon M, Cutler DM, et al. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haentjens P, Magaziner J, Colon-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Int Med. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. Preventive Services Task Force recommendation statement. Ann Int Med. 2011;154:356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- KDIGO. 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program Values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier H, Marc-Antoine K. Quantitative ultrasound for the detection and management of osteoporosis. Salud Publica Mex. 2009;51:525–537. doi: 10.1590/s0036-36342009000700006. [DOI] [PubMed] [Google Scholar]
- Tan BK, Price R. Quantitative Ultrasound (QUS) Aust J Physiother. 2007;53:290. doi: 10.1016/s0004-9514(07)70016-8. [DOI] [PubMed] [Google Scholar]
- World Health Organization, International Society of Hypertension Writing Group. 2013 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Davis MD, Meyers FL, Bresnick GH. Natural evolution. In: L'Esperance FA Jr, et al., editors. Current diagnosis and Management of Chorioretinal Diseases. St Louis, MO: C.V. Mosby; 1977. pp. 179–184. [Google Scholar]
- Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online 2000 March 27-29; 17: 1-36 [accessed on 2012, March 17]. NIH Consensus Statement Web site. Available at: http://consensus.nih.gov/2000/2000Osteoporosis111html.htm.
- Glüer C-C. Quantitative ultrasound techniques for the assessment of osteoporosis: expert agreement on current status. J Bone Miner Res. 1997;12:1280–1288. doi: 10.1359/jbmr.1997.12.8.1280. [DOI] [PubMed] [Google Scholar]
- Marín F, González-Macías J, Díez-Pérez A, et al. Relationship between bone quantitative ultrasound and fractures: a meta-analysis. J Bone Miner Res. 2006;21:1126–1135. doi: 10.1359/jbmr.060417. [DOI] [PubMed] [Google Scholar]
- Bauer DC, Gluer CC, Cauley JA, et al. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Arch Int Med. 1997;157:629–634. [PubMed] [Google Scholar]
- Saito M, Fujii K, Mori Y, et al. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17:1514–1523. doi: 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- Gushiken M, Maeshiro C, Kuniyoshi M, et al. A survey of the bone mineral density in Okinawan female with type 2 diabetes mellitus. Jpn J Health Hum Ecol. 2003;69:57–63. (Japanese) [Google Scholar]
- Krakauer JC, Mckenna MJ, Buderer NF, et al. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68:259–270. doi: 10.1007/bf02390832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2014;37:s14–s80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- Carole W, Patrick B, William AG, et al. Active smoking and the risk of type 2 diabetes. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- Targher G, Alberiche M, Zenere MB, et al. Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82:3619–3624. doi: 10.1210/jcem.82.11.4351. [DOI] [PubMed] [Google Scholar]
- Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53:920–926. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- Ross PD, Norimatsu H, Davis JW, et al. A comparison of hip fracture incidence among native Japanese, Japanese Americans, and American Caucasians. Am J Epidemiol. 1991;133:801–809. doi: 10.1093/oxfordjournals.aje.a115959. [DOI] [PubMed] [Google Scholar]
- Imura H, Seino Y, Nakagawa S, et al. Diabetic osteopenia in Japanese: a geographic study. J Jpn Diabetes Soc. 1987;30:929–934. (Japanese) [Google Scholar]
- The Ministry of Land, Infrastructure, and Transport. Solar and infrared radiation data (Japanese). No date [accessed on 2013, June 21]. The Ministry of Land, Infrastructure, and Transport, Japan Meteorological Agency. Available at: http://www.data.kishou.go.jp/obs-env/radiation/data_rad.html.
- Zhang Y, Ojima T, Murata C. Calcium intake pattern among Japanese women across five stage of health behavior change. J Epidemiol. 2007;17:45–53. doi: 10.2188/jea.17.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Ministry of Education, Culture, Sports, Science, and Technology. Standard tables of food composition in Japan: 2010 (Japanese). 2010 [accessed on 2013, June 25]. The Ministry of Education, Culture, Sports, Science, and Technology. Available at: http://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/houkoku/1298713.htm.
- The Ministry of Health, Labour and Welfare. National Health and Nutrition Survey: 2011 (Japanese). 2013 [accessed on 2013 June 25]. The Ministry of Health, Labour and Welfare. Available at: http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h23-houkoku.pdf.
- The Department of Welfare and Health in Okinawa prefecture. The Okinawa Prefectural Health and Nutrition Survey: 2011 (Japanese). 2013 [accessed on 2013 Jun 25]. The Department of Welfare and Health in Okinawa prefecture. Available at: http://www.kenko-okinawa21.jp/
- Rigalleau V, Lasseur C, Raffaitin C, et al. Bone loss in diabetic patients with chronic kidney disease. Diabet Med. 2007;24:91–93. doi: 10.1111/j.1464-5491.2007.02026.x. [DOI] [PubMed] [Google Scholar]
- Donadio C, Ardini M, Lucchesi A, et al. Parathyroid hormone and large related C-terminal fragments increase at different rates with worsening of renal function in chronic kidney disease patients. A possible indicator of bone turnover status? Clin Nephrol. 2007;67:131–139. doi: 10.5414/cnp67131. [DOI] [PubMed] [Google Scholar]
- Ivers RQ, Mitchell P, Cumming RG, et al. Diabetes and risk of fracture. Diabet Care. 2001;24:1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]


