Abstract
Aims/Introduction
To assess the efficacy and safety of sitagliptin compared with α-glucosidase inhibitors in Japanese patients with type 2 diabetes inadequately controlled by metformin or pioglitazone alone.
Materials and Methods
In the present multicenter, randomized, open-label, parallel-group, active-controlled, non-inferiority trial, 119 patients aged 20–79 years with type 2 diabetes who had glycated hemoglobin 6.9–8.8% on stable metformin (500–1,500 mg/day) or pioglitazone (15–30 mg/day) alone were randomly assigned (1:1) to receive the addition of sitagliptin (50 mg/day) or an α-glucosidase inhibitor (0.6 mg/day voglibose or 150 mg/day miglitol) for 24 weeks. The primary end-point was change in glycated hemoglobin from baseline to week 12. All data were analyzed according to the intention-to-treat principle.
Results
After 12 weeks, reductions in adjusted mean glycated hemoglobin from baseline were −0.70% in sitagliptin and −0.21% in the α-glucosidase inhibitor groups respectively; between-group difference was −0.49% (95% confidence interval −0.66 to −0.32, P < 0.0001), meeting the predefined non-inferiority criterion (0.25%) and showing statistical significance. This statistical significance also continued after 24 weeks. Although sitagliptin did not affect bodyweight, α-glucosidase inhibitors decreased bodyweight significantly from baseline (−0.39 kg; P = 0.0079). Gastrointestinal disorders were significantly lower with sitagliptin than with an α-glucosidase inhibitor (6 [10.3%] patients vs 23 [39.7%]; P = 0.0003). Minor hypoglycemia occurred in two patients (3.5%) in each group.
Conclusions
Sitagliptin showed greater efficacy and better tolerability than an α-glucosidase inhibitor when added to stable doses of metformin or pioglitazone. These findings support the use of sitagliptin in Japanese patients with type 2 diabetes inadequately controlled by insulin-sensitizing agents. This trial was registered with UMIN (no. 000004675).
Keywords: Alpha-glucosidase inhibitor, Combination drug therapy, Sitagliptin
Introduction
Type 2 diabetes accounts for approximately 90% of all cases of diabetes mellitus, and its incidence is rapidly increasing. Currently, approximately 366 million people worldwide have diabetes, and 552 million are expected to have diabetes by 20301. Diabetes can lead to various vascular complications that contribute to mortality, reduced quality of life and excessive medical costs. To prevent diabetic vascular complications, achievement of optimal glycemic control (e.g., glycated hemoglobin [HbA1c] < 7.0%) from an early stage of the disease is recommended2–4. However, hypoglycemia and weight gain associated with tight glycemic control could have potentially negative effects on cardiovascular risk and mortality5.
The American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) recommend metformin as a first-line oral therapy for treatment of type 2 diabetes6. Metformin has a low hypoglycemia risk when used alone, and might reduce cardiovascular events7; thus, metformin is the most widely used drug in the treatment of type 2 diabetes. Pioglitazone, which activates peroxisome proliferator-activated receptor γ (PPARγ)8, also has a low risk of hypoglycemia with a beneficial effect on cardiovascular events, as shown in a large trial involving patients with overt macrovascular disease9. Both of these drugs are insulin-sensitizing agents, contribute to glycemic control and are widely used as an initial drug for treatment of diabetes in Japan. However, because type 2 diabetes is a progressive disease, most people eventually require additional treatment to achieve good glycemic control10, and especially, a choice of the second-line drug is very important to determine subsequent glycemic control.
Sitagliptin is a once-daily dipeptidyl peptidase-4 (DPP-4) inhibitor that improves glycemic control by preventing the rapid degradation of incretin hormones11. Sitagliptin is well-tolerated, and associated with a low risk of hypoglycemia and weight gain when used alone12–14. In Japan, the most frequently used oral hypoglycemic agent had been sulfonylurea until the appearance of DPP-4 inhibitor15. As this relatively new agent is comparatively effective for Japanese patients, it has been becoming widely used in combination therapy as well as in monotherapy. In contrast, α-glucosidase inhibitors, which lower postprandial glucose excursions, have been widely used in Japan as first-line and combined drugs for a long time. Alpha-glucosidase inhibitors have shown clear efficacy and are relatively safe for patients with type 2 diabetes16, and the risk of hypoglycemia and weight gain is minimal, because they reduce oversecretion of insulin and consequently lighten the load of the pancreas. Furthermore, α-glucosidase inhibitors have been shown to reduce cardiovascular events in patients with type 2 diabetes as well as those with impaired glucose tolerance17,18. In fact, in patients with impaired glucose tolerance, α-glucosidase inhibitors help prevent the development of type 2 diabetes19,20. Recently, Iwamoto et al.21 reported that greater efficacy and better tolerability of sitagliptin monotherapy than that of α-glucosidase inhibitor (voglibose) in Japanese patients with type 2 diabetes.
In Western countries, sitagliptin has been shown to be effective in combination with metformin or pioglitazone22–24. However, in Asian patients with diabetes, the glucose-lowering effects, changes in insulin secretion and resistance, frequencies of hypoglycemia, and effects on bodyweight when sitagliptin is used in combination therapy have not been sufficiently documented. Therefore, in the present Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin-1 (SUCCESS-1) trial, focusing on the choice of the second-line drug, we aimed to assess the efficacy and safety of sitagliptin compared with an α-glucosidase inhibitor in Japanese patients with type 2 diabetes who were inadequately controlled by metformin or pioglitazone alone.
Materials and methods
Design
The present multicenter, randomized, open-label, parallel-group, active-controlled, non-inferiority trial was carried out at 37 sites in Japan from January 2011 to September 2012. It included an initial observation period within 8 weeks followed by a 24-week treatment period (sitagliptin or α-glucosidase inhibitor) and, finally, 4 weeks of follow up to record any new adverse events. The present study was approved by the ethics committee at the each center and registered at http://www.umin.ac.jp/ctr/ (UMIN-ID: UMIN 000004675).
Participants
Eligible study participants were men and women aged 20–79 years with type 2 diabetes receiving metformin (500–1,500 mg/day) or pioglitazone (15–30 mg/day) for 2 months or longer, and with a HbA1c level of 6.9–8.8%. The main exclusion criteria were previous treatment with insulin or oral glucose-lowering drugs other than metformin or pioglitazone within the past 2 months; impaired renal function (serum creatinine ≥133 μmol/L in men or ≥115 μmol/L in women); or a diagnosis of stroke, myocardial infarction, or other severe cardiovascular complications requiring hospitalization within the past 6 months. All patients provided written informed consent.
Procedures and Randomization
Patients receiving metformin or pioglitazone entered an initial observation period within 8 weeks. At the end of the observation period, patients were randomly assigned to either 24 weeks of treatment with sitagliptin (50 mg once a day) or an α-glucosidase inhibitor (0.2 mg voglibose or 50 mg miglitol three times a day) added to ongoing metformin or pioglitazone (dose unchanged throughout the study) through a website using a minimization method25 with biased-coin assignment balancing including age (≥65 years vs <65 years), sex and type of premedication (metformin vs pioglitazone).
Study End-Points
The primary efficacy end-point was change in HbA1c from baseline at week 12. Secondary end-points were change in HbA1c from baseline at week 24, proportion of participants reaching HbA1c targets of <7.0%, (as recommended by the ADA)26 at week 12 and 24, changes from baseline at week 12 and 24 in fasting plasma glucose (FPG), glycoalbumin, 1,5-anhydroglucitol (1,5-AG), fasting insulin and C-peptide concentrations, plasma proinsulin/insulin ratio, homoeostasis model assessment of β-cell function (HOMA-β), homoeostasis model assessment of insulin resistance (HOMA-IR), fasting lipid profile, urinary biomarkers, bodyweight, blood pressure, and heart rate. Laboratory analyses were carried out by SRL Corporation (Tokyo, Japan).
Safety and tolerability were assessed throughout the study, and included monitoring for adverse events, physical examinations, vital signs and clinical laboratory measurements. Investigators evaluated each clinical adverse event for intensity, duration, outcome and relationship to study drug. Adverse events of special interest included hypoglycemia and gastrointestinal symptoms. We also assessed medication compliance at the end of the study by asking patients directly. The study was open-label, but data were masked from the statistician until database release.
Statistical Analysis
Based on previous reports21,27,28, the standard deviation was assumed to be 0.6%, and the true difference between the two randomized treatments was zero in HbA1c. We determined that enrolment of 116 patients (58 patients in each group) would provide a power of at least 80% and a significance level of 0.025 (one-sided), to show non-inferiority for sitagliptin, based on the change in HbA1c from baseline to week 12, with a 0.25% HbA1c non-inferiority margin, which we considered to be a clinically acceptable margin24. Additional assumption in sample-size calculations was that 15% of patients would be lost to follow up. Non-inferiority would be established by the upper limit of the two-sided 95% confidence interval (CI) for treatment difference in the adjusted mean change in HbA1c from baseline to end-point of <0.25% (non-inferiority margin).
Data collection and all the statistical analyses were independently carried out at the Chiba University Hospital Clinical Research Center, Chiba, Japan. The analyses of the primary and secondary outcomes were carried out on data from all patients who had undergone randomization, according to the intention-to-treat principle.
For baseline variables, summary statistics were constructed using frequencies and proportions for categorical data, and means and standard deviations for continuous variables. Patient characteristics were compared using Fisher's exact test for categorical outcomes and unpaired t-tests with equal variance for continuous variables, as appropriate.
The primary end-point of change in HbA1c from baseline at week 12 was analyzed by analysis of covariance (ancova) with treatment as fixed effect, sex, premedication and age as covariates. As a sensitivity analysis, for dimensional outcomes, linear mixed-effects models were used to determine mean values at each assessment point (12 and 24 weeks), and to test the study hypotheses with respect to between-group differences at 24 weeks. In the linear mixed-effects model, time and treatment were included as fixed effects, and intercept and linear slope terms as random effects, and a compound symmetry covariance was used to account for within-subject correlation over time.
As with the safety analysis, serious adverse events, anticipated adverse events and adverse events leading to permanent study-drug withdrawal were tabulated according to randomized group assignment, and analyzed by Fisher's exact test.
All comparisons were planned, and all P-values were two-sided. A P-value of <0.05 was considered statistically significant. All statistical analyses were carried out using SAS software version 9.3 (SAS Institute, Cary, NC, USA).
Results
Demographics and Baseline Characteristics
A total of 119 patients were initially screened and randomly assigned to either sitagliptin (n = 60) or α-glucosidase inhibitor (n = 59) in addition to metformin or pioglitazone. A total of 116 (97.5%) received at least one dose of the study drug, and 105 (88.2%) completed the study. Seven patients dropped out from each treatment group during the trial because of withdrawal of consent, adverse events and lost to follow up (Figure1). Characteristics of patients were well balanced between treatment groups (Table1).
Figure 1.
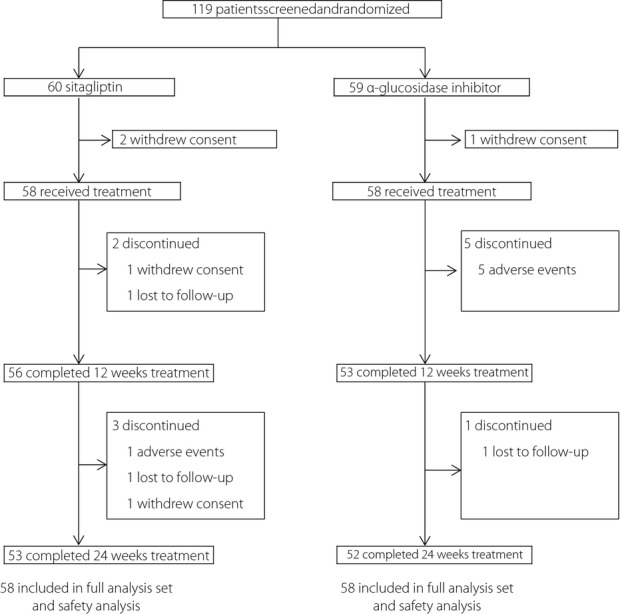
Flow chart of study participants throughout the trial. Data are number of study participants.
Table 1.
Baseline demographic and clinical characteristics
| Parameter | Sitagliptin (n = 58) | α-Glucosidase inhibitor (n = 58) | P-value* |
|---|---|---|---|
| Age (years) | 57.6 ± 12.9 | 59.3 ± 11.3 | 0.45 |
| Sex | |||
| Male | 38 (65.5) | 36 (62.1) | 0.70 |
| Female | 20 (34.5) | 22 (37.9) | 0.70 |
| Height (m) | 1.64 ± 0.10 | 1.62 ± 0.091 | 0.26 |
| Weight (kg) | 70.2 ± 14.2 | 69.8 ± 16.3 | 0.87 |
| BMI (kg/m2) | 25.9 ± 4.3 | 26.3 ± 4.6 | 0.66 |
| Systolic blood pressure (mmHg) | 132.0 ± 13.7 | 132.9 ± 14.9 | 0.73 |
| Diastolic blood pressure (mmHg) | 79.9 ± 10.6 | 79.5 ± 11.0 | 0.81 |
| Duration of diabetes (years) | 6.7 ± 6.4 | 6.8 ± 5.5 | 0.97 |
| HbA1c (%) | 7.6 ± 0.70 | 7.6 ± 0.74 | 0.60 |
| Fasting plasma glucose (mmol/L) | 7.7 ± 1.7 | 7.4 ± 1.7 | 0.33 |
| Fasting C-peptide (mmol/L) | 0.61 ± 0.36 | 0.67 ± 0.34 | 0.40 |
| Fasting insulin (pmol/L) | 51.6 ± 44.2 | 61.5 ± 50.7 | 0.27 |
| HOMA-β | 36.2 ± 26.6 | 52.6 ± 54.9 | 0.045 |
| HOMA-IR | 2.7 ± 3.7 | 2.8 ± 2.4 | 0.88 |
| Fasting lipid profiles | |||
| Total cholesterol (mmol/L) | 5.0 ± 0.88 | 4.9 ± 0.88 | 0.49 |
| LDL cholesterol (mmol/L) | 2.8 ± 0.68 | 2.8 ± 0.71 | 0.69 |
| HDL cholesterol (mmol/L) | 1.4 ± 0.41 | 1.4 ± 0.34 | 0.94 |
| Triglycerides (mmol/L) | 1.7 ± 1.2 | 1.6 ± 1.0 | 0.59 |
| Non-HDL cholesterol (mmol/L) | 3.7 ± 0.85 | 3.5 ± 0.86 | 0.40 |
| Prior antidiabetic treatment | |||
| Metformin | 47 (81.0) | 46 (79.3) | 0.82 |
| Pioglitazone | 11 (19.0) | 12 (20.7) | 0.82 |
| Concomitant treatments | |||
| Antihypertensive drugs | 29 (50.0) | 27 (46.6) | 0.71 |
| Antihyperlipemic drugs | 28 (48.3) | 26 (44.8) | 0.71 |
Data are presented as mean ± standard deviation values, or n (%). *P-values for differences between the sitagliptin and α-glucosidase inhibitor groups. BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-β, homoeostasis model assessment of β-cell function; HOMA-IR, homoeostasis model assessment of insulin resistance.
Efficacy
HbA1c was reduced to a significantly greater extent with sitagliptin than with an α-glucosidase inhibitor at weeks 12 and 24 (Figure2). At week 12, adjusted mean decreases in HbA1c from baseline were −0.70% (95% CI −0.83 to −0.58) for sitagliptin and −0.21% (95% CI −0.34 to −0.097) for the α-glucosidase inhibitors in the full analysis set. The least squares mean of treatment difference was −0.49% (−0.66 to −0.32) (P < 0.0001). This result met the predefined non-inferiority criterion of 0.25% and after that confirmed the superiority of sitagliptin over α-glucosidase inhibitors. Additionally, at week 24, adjusted mean decreases in HbA1c from baseline were −0.71% (95% CI −0.88 to −0.54) for sitagliptin and −0.29% (95% CI −0.50 to −0.077) for the α-glucosidase inhibitors (difference −0.43%, 95% CI −0.69 to −0.16, P = 0.0024). Treatment difference for the analyses by a linear mixed model was similar to those derived for the analysis of covariance (P < 0.0001, a mixed-effects model).
Figure 2.
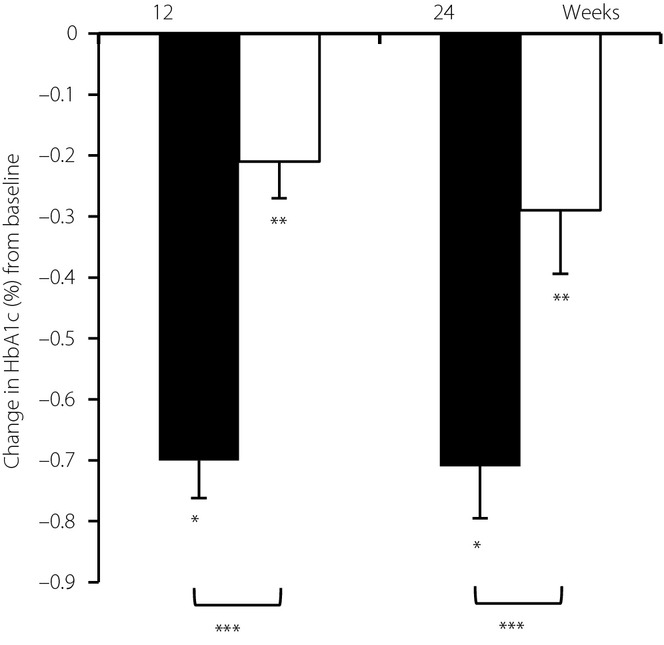
Changes in glycated hemoglobin (HbA1c) from baseline with the sitagliptin group (black bars) and α-glucosidase inhibitors group (white bars) at week 12 and 24. Error bars represent standard error. *P < 0.05 from baseline (sitagliptin group). **P < 0.05 from baseline (α-glucosidase inhibitors group). ***P < 0.05 between sitagliptin and α-glucosidase inhibitors with the same week.
Throughout the study, significantly more participants achieved HbA1c targets <7.0% with sitagliptin combination therapy than with α-glucosidase inhibitor combination therapy. At week 12, 31 (55.3%) of 56 participants receiving sitagliptin combination therapy had a HbA1c <7.0% compared with 17 (32.1%) of 53 receiving α-glucosidase inhibitor combination therapy (P = 0.0089). Similarly, at week 24, 32 (60.0%) of 53 participants on sitagliptin combination therapy had a HbA1c <7.0% compared with 17 (32.7%) of 52 receiving α-glucosidase inhibitor combination therapy (P = 0.0020).
Treatment with sitagliptin also led to a significant decrease in FPG from baseline at week 12 and 24 (Table2). The least squares mean of treatment difference in FPG at week 12 was −0.86 mmol/L (95% CI −1.5 to −0.21) lower with sitagliptin than with α-glucosidase inhibitors (P = 0.010), but no significant difference was noted at week 24. The analyses limited to the patients who had been taking metformin also led to the same results regarding glucose-lowering effects (data not shown).
Table 2.
Change in secondary endpoints from baseline to weeks 12 and 24
| Mean change from baseline (week 12) | Mean change from baseline (week 24) | |||||
|---|---|---|---|---|---|---|
| Sitagliptin | α-Glucosidase inhibitor | P-value* | Sitagliptin | α-Glucosidase inhibitor | P-value* | |
| Glycoalbumin (%) | −2.4 (−2.9 to −2.0) | −0.74 (−1.2 to −0.29) | <0.0001 | −2.5 (−3.0 to −1.9) | −1.1 (−1.8 to −0.31) | 0.0026 |
| 1,5-AG (μmol/L) | 21.6 (16.7 to 26.5) | 28.9 (21.9 to 35.9) | 0.089 | 23.2 (16.8 to 29.6) | 32.6 (24.5 to 40.8) | 0.072 |
| Fasting plasma glucose (mmol/L) | −0.60 (−1.1 to −0.092) | 0.26 (−0.16 to 0.68) | 0.010 | −0.54 (−1.0 to −0.066) | −0.15 (−0.60 to 0.29) | 0.24 |
| Fasting insulin (pmol/L) | 6.7 (−5.7 to 18.9) | 5.6 (−9.4 to 20.6) | 0.91 | −0.0088 (−10.6 to 10.6) | −3.4 (−15.0 to 8.3) | 0.67 |
| Fasting C-peptide (nmol/L) | 0.061 (−0.033 to 0.15) | 0.032 (−0.061 to 0.13) | 0.66 | 0.041 (−0.042 to 0.12) | −0.010 (−0.082 to 0.61) | 0.35 |
| Fasting proinsulin-to-insulin ratio | −0.0057 (−0.068 to 0.056) | −0.031 (−0.086 to 0.025) | 0.55 | −0.041 (−0.11 to 0.027) | 0.028 (−0.043 to 0.10) | 0.16 |
| HOMA-β | 10.8 (6.0 to 15.6) | −2.4 (−16.2 to 11.4) | 0.072 | 8.9 (2.7 to 15.2) | −0.60 (−9.2 to 8.0) | 0.078 |
| HOMA-IR | −0.0094 (−1.0 to 1.0) | 0.57 (−0.32 to 1.5) | 0.39 | −0.38 (−1.4 to 0.64) | −0.12 (−0.91 to 0.67) | 0.68 |
| Bodyweight (kg) | 0.25 (−0.12 to 0.62) | −0.39 (−0.68 to −0.093) | 0.0079 | 0.47 (−0.041 to 0.99) | −0.60 (−1.0 to −0.16) | 0.0021 |
| Systolic blood pressure (mmHg) | 1.4 (−2.2 to 5.1) | 1.0 (−1.8 to 3.9) | 0.87 | 1.6 (−1.9 to 5.0) | 0.059 (−3.7 to 3.8) | 0.55 |
| Diastolic blood pressure (mmHg) | −0.47 (−2.6 to 1.7) | 0.67 (−1.9 to 3.2) | 0.49 | −0.38 (−2.2 to 1.4) | 0.80 (−2.8 to 4.4) | 0.55 |
| Heart rate (beats per min) | 1.5 (−1.2 to 4.3) | −1.9 (−4.0 to 0.30) | 0.054 | 0.67 (−2.0 to 3.3) | −2.4 (−5.3 to 0.56) | 0.12 |
| Total cholesterol (mmol/L) | −0.13 (−0.35 to 0.087) | 0.21 (0.065 to 0.36) | 0.010 | −0.11 (−0.34 to 0.11) | 0.18 (0.044 to 0.32) | 0.025 |
| LDL cholesterol (mmol/L) | 0.0066 (−0.15 to 0.16) | 0.21 (0.077 to 0.35) | 0.047 | −0.013 (−0.19 to 0.16) | 0.19 (0.060 to 0.31) | 0.065 |
| HDL cholesterol (mmol/L) | −0.0024 (−0.044 to 0.039) | −0.012 (−0.058 to 0.034) | 0.76 | 0.025 (−0.33 to 0.084) | −0.0025 (−0.047 to 0.042) | 0.45 |
| Triglycerides (mmol/L) | −0.050 (−0.28 to 0.18) | 0.010 (−0.18 to 0.20) | 0.69 | −0.11 (−0.37 to 0.15) | −0.17 (−0.34 to −0.0076) | 0.68 |
| Non-HDL cholesterol (mmol/L) | −0.13 (−0.34 to 0.078) | 0.22 (0.090 to 0.36) | 0.0050 | −0.13 (−0.35 to 0.093) | 0.18 (0.051 to 0.32) | 0.015 |
Changes from baseline to week 12 or week 24 are expressed as least-squares mean change (95% confidence interval).
P-value was calculated by comparing the difference from baseline between the sitagliptin and α-glucosidase inhibitor groups. HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-β, homoeostasis model assessment of β-cell function; HOMA-IR, homoeostasis model assessment of insulin resistance.
Changes in other secondary end-points are summarized in Table2 and Table S1. In addition to HbA1c, glycoalbumin was reduced to a significantly greater extent with sitagliptin than with α-glucosidase inhibitors throughout the study. 1,5-AG levels significantly increased from baseline in both groups, but differences between groups were not significant. Sitagliptin significantly improved HOMA-β values from baseline, but no significant differences were observed between groups. For other glycemic efficacy parameters, no notable differences were seen in either treatment group.
While sitagliptin did not affect bodyweight, α-glucosidase inhibitors decreased bodyweight significantly from baseline (Table2). The adjusted mean differences were 0.64 kg (95% CI 0.17–1.1, P = 0.0079) at week 12, and 1.1 kg (0.40–1.7; P = 0.0021) at week 24 between two groups. As with the glucose-lowering effects, the analyses limited to the patients who had been taking metformin also showed the same significant differences in bodyweight (data not shown).
No clinically meaningful changes in blood pressure and heart rate were observed in either group. Sitagliptin also had no effects on serum lipid parameters, whereas α-glucosidase inhibitors increased the levels of total cholesterol, low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol and apolipoprotein B (Table S1), which led to the significant differences between the two groups.
Safety
Table3 summarizes treatment-emergent adverse events. Adverse events were reported by 19 (32.8%) of 58 participants in the sitagliptin group, and 30 (51.7%) of 58 participants in the α-glucosidase inhibitor group. One (1.7%) serious treatment-emergent adverse event was observed in each group (ileus and cholangitis); no deaths occurred. Two (3.5%) episodes of mild hypoglycemia occurred in each group. The most common adverse events were gastrointestinal symptoms, and the incidence was significantly greater with the α-glucosidase inhibitors (23 [39.7%]) than with sitagliptin (6 [10.3%]). Each of the prespecified gastrointestinal adverse events (diarrhea, flatulence, abdominal distention) was also significantly greater with the α-glucosidase inhibitors than with sitagliptin. Two (3.5%) of 58 participants in the sitagliptin group and five (8.6%) of 58 participants in the α-glucosidase inhibitor group withdrew from the study because of a treatment-emergent adverse event. Withdrawals in the sitagliptin group were as a result of edema and cholangitis, whereas withdrawals in the α-glucosidase inhibitor group were as a result of ileus, diarrhea, flatulence, abdominal distention and ear pain. Other main adverse events included the common cold, dizziness and headache. The incidence of these adverse events was <5%. Laboratory variables did not show any clinically significant findings. Medication compliance was lower with the α-glucosidase inhibitor than with sitagliptin (88.2% vs 98.0%; P < 0.0001).
Table 3.
Adverse events observed
| Parameter | Sitagliptin (n = 58) | α-Glucosidase inhibitor (n = 58) | P-value* |
|---|---|---|---|
| Any adverse event | 19 (32.8) | 30 (51.7) | 0.0039 |
| Serious adverse events | 1 (1.7) | 1 (1.7) | 0.74 |
| Drug-related adverse events | 4 (6.9) | 19 (32.8) | 0.00050 |
| Adverse events leading to discontinuation | 2 (3.5) | 5 (8.6) | 0.24 |
| Deaths | 0 | 0 | 1.0 |
| Adverse events of special interest | |||
| Hypoglycemia | 2 (3.5) | 2 (3.5) | 1.0 |
| Gastrointestinal disorders | 6 (10.3) | 23 (39.7) | 0.00030 |
| Diarrhea | 2 (3.5) | 11 (19.0) | 0.0081 |
| Flatulence | 3 (5.2) | 10 (17.2) | 0.039 |
| Abdominal distention | 1 (1.7) | 10 (17.2) | 0.0043 |
| Nausea | 1 (1.7) | 3 (5.2) | 0.62 |
| Constipation | 0 | 3 (5.2) | 0.24 |
| Abdominal pain | 0 | 3 (5.2) | 0.24 |
| Loss of appetite | 0 | 1 (1.7) | 0.32 |
Data are presented as n (%).
P-values for differences between the sitagliptin and α-glucosidase inhibitor groups after 24 weeks of treatment.
Discussion
The results of the present study showed that in Japanese patients with type 2 diabetes inadequately controlled by metformin or pioglitazone, sitagliptin, a DPP-4 inhibitor, was not only non-inferior, but also superior to the α-glucosidase inhibitor for lowering HbA1c from baseline (on the same level with previous Japanese report compared with placebo27,28). In addition, more than half of the patients receiving sitagliptin achieved the current ADA glycemic goal of HbA1c <7.0%26.
In parallel to the present study, we also carried out a trial in type 2 diabetes to assess the efficacy and safety of sitagliptin compared with α-glucosidase inhibitor in combination with sulfonylurea (SUCCESS-2 study)29. In the SUCCESS-2 study, sitagliptin was not only non-inferior, but also superior to α-glucosidase for reduction of HbA1c at 12 weeks, but remained non-inferior at 24 weeks. The results of SUCCESS-1 and 2 together showed that combination of sitagliptin with insulin-sensitizing agents might be adequate for glucose lowering at least within a 24-week period.
The reasons why the combination of sitagliptin with metformin or pioglitazone was better than with α-glucosidase inhibitor might depend on the levels of incretin hormones. Metformin has been reported to increase the plasma level of glucagon-like peptide-1 (GLP-1) and enhance the expression of the genes encoding the receptors for incretin hormones in mouse islets30. In line with the finding, an addition of sitagliptin to metformin therapy showed a complementary effect on active GLP-1 concentrations31. In contrast, in obese patients, pioglitazone shifts fat distribution from visceral to subcutaneous adipose depots32, and DPP-4 protein expression in visceral fat is higher than subcutaneous fat33. These findings suggest that sitagliptin, in combination with pioglitazone, might synergistically increase incretin levels in patients with type 2 diabetes.
Although metformin and pioglitazone are classified as insulin-sensitizing agents, their action mechanism is different from each other. In the present study, the number of patients receiving pioglitazone monotherapy was too small to evaluate its precise effects. For that reason, we carried out statistical analyses limited to the patients who had been taking metformin, and confirmed that the effects on glucose-lowering and bodyweight were the same as all the participants.
In contrast, α-glucosidase inhibitor also increases active GLP-1, but it decreases gastric inhibitory polypeptide (GIP) and postprandial serum insulin34,35. It is inferred that these changes of incretin and insulin secretion after meals had occurred in the α-glucosidase inhibitor group.
Both sitagliptin and α-glucosidase inhibitor are known to lower postprandial blood glucose levels; we found that these drugs also significantly increased 1,5-AG levels from baseline, a marker reflecting postprandial blood glucose levels36. In contrast, only sitagliptin significantly decreased FPG levels from baseline with no changes in fasting insulin concentrations or HOMA-IR. These results might show that sitagliptin combined with insulin sensitizer improved not only postprandial hyperglycemia through glucose-dependent insulin secretion, but also inhibiting hepatic glucose production37. Sitagliptin is known to suppress paradoxical glucagon secretion in patients with type 2 diabetes as well38. In the present study, there were no differences in the fasting plasma glucagon levels in both groups. This result leads to our presumption that sitagliptin decreased the postprandial plasma glucagon level more than α-glucosidase inhibitor did.
It is reported that DPP-4 inhibitors have a potential to protect pancreatic islet cells39,40, and are expected to decrease cardiovascular risk in type 2 diabetes41. These findings also give us a rationale and further insights to use sitagliptin in combination with metformin or pioglitazone.
In contrast, α-glucosidase inhibitors significantly decreased bodyweight in the present study. This could have been as a result of a reduction of postprandial insulin secretion, changes in incretin hormone profiles (e.g., increase in active GLP-1 and decrease in GIP)34,35, enhancing sensations of satiety42 and energy expenditure43. These effects of α-glucosidase inhibitor-related incretin secretion seem to be prominent in voglibose and miglitol rather than acarbose44, and in not elderly patients45. The present results show a potential benefit of α-glucosidase inhibitors in treating obese and middle-aged patients with type 2 diabetes.
Although some studies22,24,46 have shown that blood pressure or lipid profiles (total cholesterol, high-density lipoprotein cholesterol and triglycerides) are improved with sitagliptin, no significant changes in these parameters were seen in the present study. When the present study started, nearly half of the patients had already been taking antihypertensive or antihyperlipidemic drugs, and their blood pressure and lipid profiles had been in relatively good control (Table1). So in our study, the effects on blood pressure and lipid profiles by sitagliptin might have been attenuated. The reasons why α-glucosidase inhibitor increased the levels of lipid parameters in this study remains to be elucidated.
As reported previously12–14,21–24,27,28, sitagliptin was well tolerated, with a small increase in the risk of gastrointestinal disorders and hypoglycemia. In contrast, we observed a higher frequency of gastrointestinal disorders and lower medication compliance during treatment with an α-glucosidase inhibitor.
There were some potential limitations to the present study. First, the number of participants enrolled was small, and the treatment period of 24 weeks might have been too short. Second, this study included possible selection bias. Also, the present study was open-label trial, but the primary end-point HbA1c was a hard end-point and other measures were put in place in order to minimize the potential for bias. Third, we only examined fasting parameters. Further studies are required with a large number, longer follow-up period and taking into account postprandial parameters.
In conclusion, we showed that sitagliptin, compared with α-glucosidase inhibitors, provided greater glycemic control and better tolerability with significantly lower gastrointestinal symptoms and higher medication compliance when used in combination with metformin or pioglitazone in Japanese patients with type 2 diabetes. Our findings support the use of sitagliptin in combination with metformin or pioglitazone in Japanese patients with type 2 diabetes.
Acknowledgments
This study was designed and led by a steering committee of the SUCCESS study group, funded by the Waksman Foundation of Japan Inc., Tokyo, the Grant-in-Aid from the Japanese Ministry of Health, Labor and Welfare, and the Ministry of Education, Culture, Sports, Science and Technology. KY has received research endowment for his Department from MSD (Merck). The sponsors played no role in the design and management of the study, collection and analysis of the data, interpretation of the results, or the writing of the report. The authors declare no conflict of interest. The authors thank the SUCCESS investigators for participating in this study (see the list of investigators in Appendix 1) and the staff of the Chiba University Hospital Clinical Research Center for their assistance in the performance of this study: Junpei Kamimoto, Ami Kuninobu, Ayumi Miura, Yumiko Imai, Asako Kohno, Nanae Tanemura, Asuka Nemoto, Nobuko Yamaguchi and Yasuhisa Fujii.
Appendix 1
List of investigators
Tokuyoshi An, Hirotake Tokuyama, Hideaki Bujyo, Harukiyo Kawamura, Takahisa Shibata, Ichiro Tatsuno, Takayuki Ishibashi, Susumu Nakamura, Keiji Mikami, Kazuo Yamamoto, Kenichi Yamada, Yusuke Hirota, Naoko Tadokoro, Takahiko Tokuyama, Ryoichi Ishibashi, Shin Nakamura, Jun Tashiro, Kyohei Yamamoto, Toshiaki Ban, Rie Sano, Hiroko Ito, Tomoaki Tanaka, Sawako Nishimura, Keiko Saito, Motonobu Nishimura, Masami Fuse, Masahiro Mimura, Sawako Suzuki, Kaori Tachibana, Masahiko Yamada, Takahiro Ishikawa, Miwako Nakamura, Tsuyoshi Oji, Masaki Fujimoto, Kazuki Kobayashi, Hidetaka Yokoh, Yasunori Sato, Minoru Takemoto, Daigaku Uchida, Azuma Kanatsuka, Nobuichi Kuribayashi, Takashi Terano, Naotake Hashimoto, Kenichi Sakurai, Hideki Hanaoka, Ko Ishikawa, Shunichiro Onishi and Koutaro Yokote.
Supporting Information
Table S1 | Changes in secondary end-points from baseline to week 12 and 24.
References
- Whiting DR, Guariguata L, Weil C, et al. IDF Diabetes Atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Turner RC, Cull CA, Frighi V, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:291–298. doi: 10.1016/j.diabres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Kanatsuka A, Kawai K, Hirao K, et al. Research on antihyperglycemic drug therapies in patients with type 2 diabetes mellitus in Japan (1): drug therapies and actual drug use. J Jpn Diabetes Soc. 2006;49:409–915. [Google Scholar]
- van de Laar FA, Lucassen PL, Akkermans RP, et al. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–16. doi: 10.1016/s0195-668x(03)00468-8. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Kawamori R, Tajima N, Iwamoto Y, et al. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607–1614. doi: 10.1016/S0140-6736(09)60222-1. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Tajima N, Kadowaki T, et al. Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind trial. Diabetes Obes Metab. 2010;12:613–622. doi: 10.1111/j.1463-1326.2010.01197.x. [DOI] [PubMed] [Google Scholar]
- Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(Suppl. 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Tajima N, Odawara M, et al. 2008. Proceedings of the 68th ADA Scientific Sessions San Francisco, CA Sitagliptin added to ongoing treatment with metformin improved glycemic control and was well tolerated in Japanese patients with type 2 diabetes. Abstract Number: 2135-PO.
- Kashiwagi A, Tajima N, Kadowaki T, et al. 2008. Proceedings of the 68th ADA Scientific Sessions San Francisco, CA Sitagliptin added to ongoing treatment with pioglitazone improved glycemic control and was well tolerated in Japanese patients with type 2 diabetes. Abstract Number: 2136-PO.
- Kobayashi K, Yokoh H, Sato Y, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin compared with α-glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on sulfonylurea alone (SUCCESS-2): a multicenter, randomized, open-label, non-inferiority trial. Diabetes Obes Metab. 2014;16:761–765. doi: 10.1111/dom.12264. [DOI] [PubMed] [Google Scholar]
- Maida A, Lamont BJ, Cao X, et al. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia. 2011;54:339–349. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin Pharmacol Ther. 2010;88:801–808. doi: 10.1038/clpt.2010.184. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T, Katsuura Y, Sato T, et al. Miglitol induces prolonged and enhanced glucagon-like peptide-1 and reduced gastric inhibitory polypeptide responses after ingestion of a mixed meal in Japanese Type 2 diabetic patients. Diabet Med. 2009;26:187–188. doi: 10.1111/j.1464-5491.2008.02651.x. [DOI] [PubMed] [Google Scholar]
- Narita T, Yokoyama H, Yamashita R, et al. Comparisons of the effects of 12-week administration of miglitol and voglibose on the responses of plasma incretins after a mixed meal in Japanese type 2 diabetic patients. Diabetes Obes Metab. 2012;14:283–287. doi: 10.1111/j.1463-1326.2011.01526.x. [DOI] [PubMed] [Google Scholar]
- Yamanouchi T, Ogata N, Tagaya T, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- Solis-Herrera C, Triplitt C, Garduno-Garcia JeJ, et al. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care. 2013;36:2756–2762. doi: 10.2337/dc12-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–4619. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes. 2003;52:741–750. doi: 10.2337/diabetes.52.3.741. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Winzell MS, Wierup N, et al. DPP-4 inhibition improves glucose tolerance and increases insulin and GLP-1 responses to gastric glucose in association with normalized islet topography in mice with beta-cell-specific overexpression of human islet amyloid polypeptide. Regul Pept. 2007;143:97–103. doi: 10.1016/j.regpep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Patil HR, Al Badarin FJ, Al Shami HA, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol. 2012;110:826–833. doi: 10.1016/j.amjcard.2012.04.061. [DOI] [PubMed] [Google Scholar]
- Lee A, Patrick P, Wishart J, et al. The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab. 2002;4:329–335. doi: 10.1046/j.1463-1326.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shimpuku M, Kitazumi T, et al. Miglitol prevents diet-induced obesity by stimulating brown adipose tissue and energy expenditure independent of preventing the digestion of carbohydrates. Endocr J. 2013;60:1117–1129. doi: 10.1507/endocrj.ej13-0333. [DOI] [PubMed] [Google Scholar]
- Hucking K, Kostic Z, Pox C, et al. Alpha-Glucosidase inhibition (acarbose) fails to enhance secretion of glucagon-like peptide 1 (7-36 amide) and to delay gastric emptying in Type 2 diabetic patients. Diabet Med. 2005;22:470–476. doi: 10.1111/j.1464-5491.2005.01451.x. [DOI] [PubMed] [Google Scholar]
- DeLeon MJ, Chandurkar V, Albert SG, et al. Glucagon-like peptide-1 response to acarbose in elderly type 2 diabetic subjects. Diabetes Res Clin Pract. 2002;56:101–106. doi: 10.1016/s0168-8227(01)00359-x. [DOI] [PubMed] [Google Scholar]
- Horton ES, Silberman C, Davis KL, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759–1765. doi: 10.2337/dc09-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Changes in secondary end-points from baseline to week 12 and 24.


