Abstract
Aims/Introduction
This was a subanalysis of Japanese patients included in the glucagon-like peptide-1 receptor agonist AVE0010 in patients with type 2 diabetes mellitus for glycemic control and safety evaluation (GetGoal-S) study – a 24-week, randomized, placebo-controlled study of lixisenatide in patients with type 2 diabetes mellitus inadequately controlled by sulfonylurea with or without metformin.
Materials and Methods
In GetGoal-S, 127 Japanese patients received the once-daily prandial glucagon-like peptide-1 receptor agonist lixisenatide 20 μg/day or a matching placebo. The primary outcome was change in glycated hemoglobin.
Results
At week 24, lixisenatide significantly reduced mean glycated hemoglobin (least squares mean difference vs the placebo −1.1% [12 mmol/mol, P < 0.0001]), and significantly more lixisenatide patients reached glycated hemoglobin targets of <7% (53 mmol/mol) and ≤6.5% (48 mmol/mol) vs the placebo. Lixisenatide produced statistically significant reductions in 2-h postprandial plasma glucose (least squares mean difference vs the placebo −8.51 mmol/L, P < 0.0001) and glucose excursion vs the placebo, and significantly reduced fasting plasma glucose (least squares mean difference vs the placebo −0.65 mmol/L, P = 0.0454). Bodyweight decreased with both lixisenatide and the placebo (least squares mean change −1.12 kg for lixisenatide, −1.02 kg for placebo). The overall incidence of adverse events was similar for lixisenatide and the placebo (84.2 and 82.4%, respectively), the most frequent being gastrointestinal disorders (52.6% for lixisenatide vs 29.4% for placebo). The incidence of symptomatic hypoglycemia was higher with lixisenatide vs the placebo (17.1 and 9.8%, respectively), with no cases of severe symptomatic hypoglycemia in either group.
Conclusions
In the Japanese subpopulation of the GetGoal-S study, lixisenatide produced a significant and clinically relevant improvement in glycated hemoglobin, with a pronounced improvement in postprandial plasma glucose, and a good safety and tolerability profile.
Keywords: Glucagon-like peptide-1 receptor, Japanese, Lixisenatide
Introduction
Following a similar trend to Western countries, the prevalence of type 2 diabetes mellitus is increasing in Japan, and this nation is now one of the most affected by the worldwide diabetes epidemic1. In 2013, an estimated 7.6% of the population of Japan had type 2 diabetes mellitus and 12.6% had impaired glucose tolerance2,3. This high prevalence of type 2 diabetes mellitus is associated with a significant economic burden, with diabetes accounting for 8% of the total Japanese national healthcare budget in 20104.
For many years, sulfonylureas (SUs) have been the most widely prescribed first-line therapy for type 2 diabetes mellitus in Japan; the use of new drugs, combination therapies and insulin is, however, increasing5. Both SUs and insulin are associated with the risk of weight gain and hypoglycemia. Glucagon-like peptide-1 (GLP-1) receptor agonists are a relatively recent addition to the treatment options for type 2 diabetes mellitus, and are highly effective in reducing glycated hemoglobin (HbA1c), with low risks of hypoglycemia and bodyweight gain6.
Lixisenatide is a once-daily short-acting or prandial GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus7. Lixisenatide has been evaluated in a series of phase III, randomized, placebo-controlled trials known as the GetGoal program (GLP-1 receptor agonist AVE0010 in patients with type 2 diabetes mellitus for glycemic control and safety evaluation) as monotherapy8, in combination with commonly used oral antidiabetic drugs9–13, and as an add-on to basal insulin14–16 in a total of more than 5,000 patients with type 2 diabetes mellitus globally. One of these trials, GetGoal-S, was a randomized, placebo-controlled, multinational study that evaluated the use of lixisenatide in patients with type 2 diabetes mellitus inadequately controlled by SU with or without metformin11. Lixisenatide is approved in several countries, including Japan, for the treatment of adults with type 2 diabetes mellitus, and could have particular efficacy in Japanese patients owing to potential differences in the pathophysiology of diabetes in this population14,17,18. Here, we report the results from an analysis assessing the efficacy and safety of lixisenatide in patients with type 2 diabetes mellitus inadequately controlled by SU with or without metformin that was carried out in a population of Japanese patients from the GetGoal-S study.
Materials and methods
Study Design
GetGoal-S (NCT00713830) was a phase III, randomized, double-blind, placebo-controlled, two-arm, parallel-group, multicenter trial carried out in 859 patients (127 of whom were Japanese) with type 2 diabetes mellitus insufficiently controlled by SU with or without metformin. The study consisted of up to 2 weeks’ screening and a 1-week, single-blind, run-in period followed by a 24-week main treatment period, plus a variable controlled extension period of at least 52 weeks, mainly for safety purposes (not reported here). The study was approved by the local institutional review boards or ethics committees, and was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects provided written informed consent to participate in the study. An independent data monitoring committee supervised the conduct of the study, and any possible allergic events were adjudicated by the external allergic reaction assessment committee.
Male and female participants with type 2 diabetes mellitus currently receiving SU with or without metformin and with a HbA1c level of 7–10% (53–86 mmol/mol), inclusive, were included in the study. Type 2 diabetes mellitus was diagnosed according to the diagnostic criteria of the World Health Organization, which is in line with those of the Japan Diabetes Society (JDS)19. The main exclusion criteria were: use of oral or injectable glucose-lowering agents other than SU or metformin within 3 months before the time of screening; fasting plasma glucose (FPG) level at screening of >250 mg/dL (13.9 mmol/L); and a history of unexplained pancreatitis, chronic pancreatitis, pancreatectomy, stomach/gastric surgery, inflammatory bowel disease, or end-stage renal disease (defined by serum creatinine clearance of <15 mL/min) and/or dialysis11.
Eligible patients were randomized in a 2:1 ratio to receive lixisenatide once daily or a matching placebo in a two-step dose-increase regimen (10 μg once daily for 1 week, 15 μg once daily for 1 week, then 20 μg once daily). Randomization was stratified by HbA1c at screening (<8.0%, ≥8.0% [<64, ≥64 mmol/mol]) and metformin use at screening (yes/no). As recommended by the JDS, HbA1c values were expressed as the national glycohemoglobin standardization program values20. During the present study, HbA1c was blinded and investigators were alerted when FPG or HbA1c exceeded predefined thresholds for rescue (see Supplementary Table S1).
Treatment
During treatment, lixisenatide or a placebo was given subcutaneously within 1 h before the morning meal. In patients with HbA1c <8.0% (<64 mmol/mol), the SU dose was decreased by 25–50% at the randomization visit to prevent hypoglycemia, then gradually increased to the dose received at screening between weeks 4 and 12, according to fasting self-monitored plasma glucose measurements. Patients with HbA1c ≥8.0% (≥64 mmol/mol) continued on their established doses of SU. If metformin was prescribed, the dose was kept stable throughout the entire study. Rescue therapy was initiated based on routine fasting self-monitored plasma-calibrated glucose and central laboratory alerts set up on FPG (and HbA1c after week 12). Both treatment groups received lifestyle and dietary counseling at screening and then every 3 months thereafter.
Efficacy and Safety Outcomes
The primary efficacy end-point of the GetGoal-S was absolute change in HbA1c from baseline to week 24 for the modified intent-to-treat population, which consisted of all randomized patients who received at least one dose of double-blind investigational product, and had both a baseline and at least one post-baseline assessment of any primary or secondary efficacy parameter. Secondary efficacy measures included the percentage of patients reaching HbA1c <7.0% (53 mmol/mol) or ≤6.5% (48 mmol/mol) at week 24, changes in FPG and bodyweight from baseline to week 24, and the percentage of patients requiring rescue medication during the 24-week treatment period. In addition, at selected sites including those in Japan, all randomized patients underwent a standardized 600-kcal liquid breakfast meal challenge test (400 mL of Ensure Plus® [Abbott Nutrition, Columbus, OH, USA], composed of 53.8% carbohydrate, 16.7% protein and 29.5% fat) 30 min after drug administration at baseline and week 24 for assessment of the secondary efficacy measure of 2-h postprandial plasma glucose (PPG). The 2-h glucose excursion was calculated as 2-h PPG – plasma glucose levels 30 min before the meal test before study drug administration. Changes from baseline to week 24 in 2-h postprandial glucagon, insulin, proinsulin and C-peptide were collected after a standardized meal in a subset of patients in selected centers.
The GetGoal-S safety population comprised all randomized patients exposed to at least one dose of double-blind investigational product. Safety and tolerability were assessed by review of adverse events (AEs) and treatment-emergent AEs, symptomatic and severe hypoglycemia, and clinical laboratory data. Potential allergic or allergic-like reactions were assessed by a blinded allergic reaction assessment committee.
Statistical Analysis
The primary efficacy end-point of the GetGoal-S study was analyzed using an analysis of covariance model, with treatment group, randomization strata and country (for overall population) as fixed effects, and baseline HbA1c as a covariate.
Continuous secondary efficacy variables were also evaluated by analysis of covariance; categorical secondary efficacy variables were analyzed using a Cochran–Mantel–Haenszel method that was stratified on randomization strata. Differences between lixisenatide and the placebo and two-sided 95% confidence intervals (CIs) and P-values were estimated within the framework of analysis of covariance. Statistical analysis was carried out using the Statistical Analysis System software (SAS Institute Inc., Cary, NC, USA) version 9.2. For this subanalysis, no adjustment for multiplicity was carried out.
Results
Demographic and Clinical Characteristics
A total of 127 Japanese patients were randomized in the GetGoal-S study, of whom 114 patients completed the 24-week treatment period (Figure S1). The discontinuation rate was 13.2% (n = 10) in the lixisenatide group and 5.9% (n = 3) in the placebo group. At baseline, demographic and clinical characteristics were generally similar between the two treatment groups (Table1). In the lixisenatide group, measurements of body mass index were slightly lower, and slightly fewer patients were receiving metformin compared with the placebo group (Table1). Baseline HbA1c levels were also slightly lower in the lixisenatide group (8.4% [68 mmol/mol]) compared with the placebo group (8.6% [70 mmol/mol]). The most commonly used SU was glimepiride in both treatment groups.
Table 1.
Demographic and baseline characteristics (safety population)
| Lixisenatide (n = 76) | Placebo (n = 51) | |
|---|---|---|
| Male (%) | 48 (63.2) | 35 (68.6) |
| Mean age, years (SD) | 59.3 (10.3) | 59.2 (12.1) |
| Mean diabetes duration, years (SD) | 12.0 (7.8) | 12.4 (8.7) |
| Mean weight, kg (SD) | 65.3 (11.5) | 69.9 (16.6) |
| Mean BMI, kg/m2 (SD) | 24.9 (3.2) | 26.2 (4.4) |
| Mean HbA1c, % (SD) [mmol/mol] | 8.4 (0.9) [68] | 8.6 (0.8) [70] |
| Mean FPG, mmol/L (SD) | 9.1 (2.0) | 9.4 (2.2) |
| Mean 2-h PPG, mmol/L (SD) | 17.8 (4.3) | 17.8 (3.6) |
| MET use at screening, n (%) | 47 (61.8) | 36 (70.6) |
| SU use at screening, n (%) | ||
| Glimepiride | 54 (71.1) | 38 (74.5) |
| Glibenclamide | 17 (22.4) | 12 (23.5) |
| Gliclazide LM | 3 (3.9) | 0 (0.0) |
| Gliclazide | 2 (2.6) | 1 (2.0) |
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; LM, libération modifiée (modified release); MET, metformin; PPG, postprandial plasma glucose; SD, standard deviation; SU, sulfonylurea.
Efficacy
At week 24, lixisenatide significantly reduced mean HbA1c (Figure1), with a least squares (LS) mean difference vs the placebo of −1.1% (12 mmol/mol; 95% CI −1.407 to −0.803), P < 0.0001. With lixisenatide treatment, mean (standard error [SE]) HbA1c was reduced from 8.4% (0.12%) at baseline to 7.5% (0.10%) at week 8, 7.3% (0.10%) at week 12, and 7.5% (0.12%) at week 24; mean (SE) HbA1c did not reduce with the placebo, and was 8.6% (0.11%) at baseline, 8.6% (0.13%) at week 8, 8.7% (0.15%) at week 12, and 8.6% (0.17%) at week 24. Significantly more patients in the lixisenatide group reached HbA1c targets of <7.0% (53 mmol/mol) and ≤6.5% (48 mmol/mol) vs those in the placebo group (Figure1). Lixisenatide also led to significant reductions in 2-h PPG and glucose excursion at week 24 (LS mean difference in 2-h PPG vs the placebo of −8.51 mmol/L [95% CI −10.128 to −6.895], P < 0.0001, and LS mean difference in glucose excursion vs the placebo of −7.98 mmol/L [95% CI −9.480 to −6.485], P < 0.0001; Figure2). Lixisenatide provided a significant reduction in FPG from baseline to week 24 compared with the placebo (LS mean difference vs placebo of −0.65 mmol/L [95% CI −1.287 to −0.014], P = 0.0454).
Figure 1.
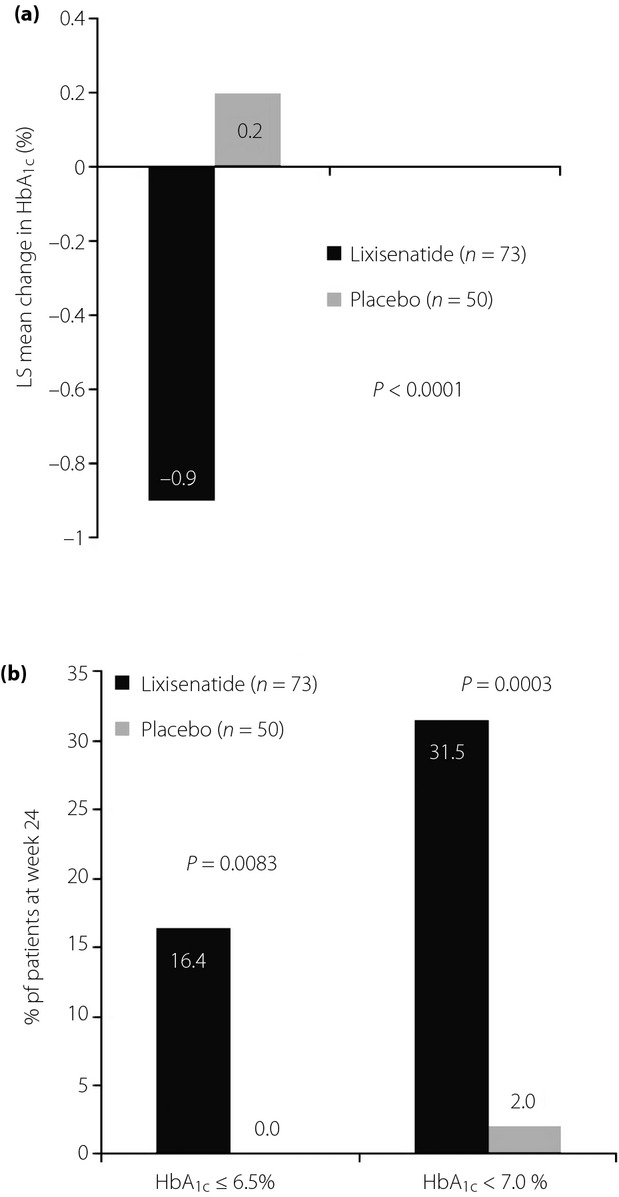
Glycated hemoglobin (HbA1c) at week 24. (a) Least squares (LS) mean change in HbA1c. (b) Proportion of patients achieving HbA1c targets. Data are for the modified intent-to-treat population (last observation carried forward), n = 73 and n = 50 for lixisenatide and the placebo, respectively. HbA1c −0.9% = 10 mmol/mol; HbA1c 0.2% = 2 mmol/mol; HbA1c ≤6.5% = 48 mmol/mol; HbA1c <7% = 53 mmol/mol.
Figure 2.
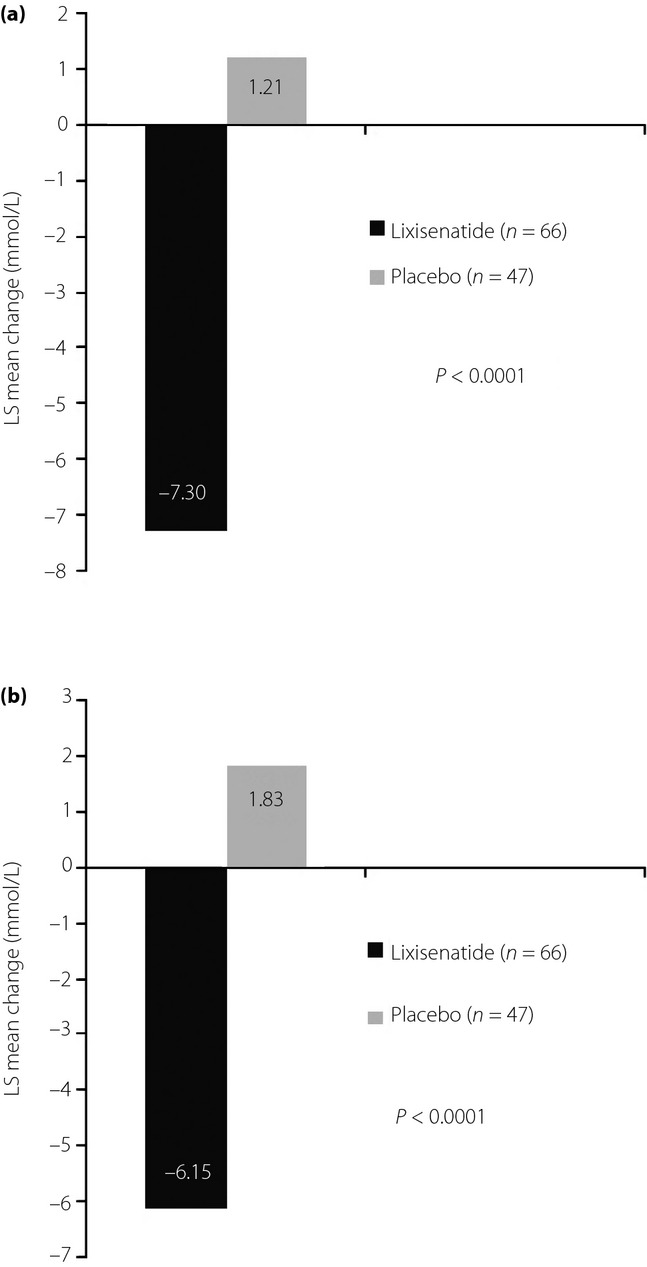
Postprandial plasma glucose at week 24. (a) Least squares (LS) mean change in 2-h postprandial plasma glucose. (b) LS mean change in glucose excursion. *Data are for the modified intent-to-treat population (last observation carried forward), n = 66 and n = 47 for lixisenatide and placebo, respectively. *Based on 127 patients undergoing a standardized breakfast meal test at selected sites. Glucose excursion = 2-h postprandial plasma glucose – plasma glucose 30 min before the meal test before study drug administration.
Improvements in glycemic control with lixisenatide were associated with significant reductions in parameters of postprandial insulin secretion (LS mean difference in 2-h postprandial plasma insulin vs the placebo [n = 113] of −81.50 pmol/L [95% CI −117.934 to −45.072], P < 0.0001, LS mean difference in 2-h postprandial proinsulin vs the placebo [n = 103] of −10.64 pmol/L [95% CI −19.968 to −1.317], P = 0.0257, and LS mean difference in 2-h postprandial C-peptide vs the placebo [n = 112] of −0.44 nmol/L [95% CI −0.671 to −0.203], P = 0.0003). Levels of 2-h postprandial glucagon were also signfiicantly lower with lixisenatide vs the placebo (n = 110; LS mean difference of −17.84 ng/L [95% CI −25.207 to −10.465], P < 0.0001).
Bodyweight decreased with both lixisenatide and the placebo (−1.12 kg for lixisenatide and −1.02 kg for placebo), with a LS mean difference vs the placebo of −0.11 kg (95% CI −0.798 to 0.586, P = 0.7628). The proportion of patients requiring rescue therapy was significantly lower in the lixisenatide group compared with the placebo group (2.6 vs 15.7%, P = 0.0158).
Safety and Tolerability
The overall incidence of AEs during the 24-week treatment period was similar in the lixisenatide (84.2%) and placebo treatment groups (82.4%; Table2); however, fewer serious AEs were reported for lixisenatide than for the placebo (n = 3 [3.9%] vs n = 4 [7.8%], respectively). AEs leading to discontinuation were more frequent in the lixisenatide group (n = 10, 13.2%) compared with the placebo group (n = 2, 3.9%; Table2). The most frequent AEs were gastrointestinal disorders (52.6 vs 29.4% in the lixisenatide and placebo groups, respectively), with the most frequent being nausea (25.0 vs 2.0% for lixisenatide and placebo, respectively; Table2), which was mostly reported during the initial weeks of treatment, was generally mild-to-moderate and transient in nature.
Table 2.
Adverse events over the 24-week main treatment period (safety population)
| Lixisenatide (n = 76) | Placebo (n = 51) | |
|---|---|---|
| Any AE | 64 (84.2) | 42 (82.4) |
| Serious AE | 3 (3.9) | 4 (7.8) |
| Any AE leading to discontinuation | 10 (13.2) | 2 (3.9) |
| GI disorders (all) | 40 (52.6) | 15 (29.4) |
| Nausea | 19 (25.0) | 1 (2.0) |
| Nausea leading to discontinuation | 6 (7.9) | 0 (0.0) |
| Vomiting | 4 (5.3) | 2 (3.9) |
| Diarrhea | 9 (11.8) | 4 (7.8) |
| Symptomatic hypoglycemia* | 13 (17.1) | 5 (9.8) |
| Severe symptomatic hypoglycemia† | 0 (0.0) | 0 (0.0) |
| Lipase increased | 0 (0.0) | 1 (2.0) |
Data are n (%).
Symptomatic hypoglycemia is an event with clinical symptoms considered to result from a hypoglycemic episode (e.g., sweating, palpitations, hunger, restlessness, anxiety, fatigue, irritability, headache, loss of concentration, somnolence, psychiatric or visual disorders, transient sensory or motor defects, confusion, convulsions, or coma), with an accompanying plasma glucose <60 mg/dL (3.3 mmol/L) or associated with prompt recovery after oral carbohydrate administration, intravenous glucose, or glucagon injection if no plasma glucose measurement was available.
Severe symptomatic hypoglycemia is symptomatic hypoglycemia requiring the assistance of another person, because the patient could not treat him/herself because of acute neurological impairment, and which was associated either with a plasma glucose level <36 mg/dL (2.0 mmol/L) or, if no plasma glucose measurement was available, a prompt recovery with carbohydrate, intravenous glucose or glucagon administration. AE, adverse event; GI, gastrointestinal.
The incidence of symptomatic hypoglycemia in the lixisenatide group was 17.1% compared with 9.8% in the placebo group, with no cases of severe symptomatic hypoglycemia in either group (Table2).
No allergic reactions were reported during the 24-week, main double-blind treatment period. As per specified protocol recommendations, events potentially qualifying as pancreatitis were reported separately. One patient (2.0%) in the placebo group reported an event (lipase increase) compared with none in the lixisenatide group for the whole period.
Discussion
In the present analysis of Japanese patients with type 2 diabetes mellitus inadequately controlled by SU with or without metformin, lixisenatide 20 μg once daily significantly reduced HbA1c, mostly through a significant reduction in PPG and glucose excursions. The current analysis showed a major improvement in glycemic control, as assessed by change in HbA1c (LS mean difference vs placebo −1.1% [12 mmol/mol]; 95% CI −1.407 to −0.803, P < 0.0001), PPG parameters (2-h PPG [LS mean difference vs placebo −8.51 mmol/L, P < 0.0001] and glucose excursion [LS mean difference vs placebo −7.98 mmol/L, P < 0.0001]) and FPG (LS mean difference vs placebo −0.65 mmol/L, P = 0.0454), in line with the overall study results11. Additionally, the reductions in mean HbA1c levels from baseline to week 8, 12 and 24 showed the quick and durable efficacy of lixisenatide compared with the placebo.
In a 24-week study of short-acting exenatide added to SU-based oral therapy in Japanese patients21, twice-daily exenatide 10 μg was associated with a significant reduction in HbA1c vs the placebo (−1.62 vs −0.28%, P < 0.001) and FPG (−1.61 vs 0.42 mmol/L, P = 0.0002 vs placebo) with a treatment difference comparable with that achieved in the present study with lixisenatide21. The long-acting GLP-1 receptor agonist liraglutide 0.9 mg/day also showed substantial improvements in HbA1c in Japanese patients when added to SU therapy, with a treatment difference over the placebo of −1.3% (P < 0.0001 vs placebo) over 24 weeks22. The reduction in FPG with liraglutide vs placebo (−1.80 mmol/L, P < 0.0001)22 was greater than the smaller, though significant, reduction in FPG with lixisenatide in the current study, or with exenatide as previously reported21. This is despite similar reductions in HbA1c in the three studies, and suggests that there are different mechanisms underlying glycemic control with the long- vs short-acting agents.
Of note in this analysis was the pronounced reduction in PPG seen with lixisenatide vs placebo. Indeed, only modest reductions in PPG have been reported in studies of long-acting GLP-1 receptor agonists, which have minimal impact on gastric emptying due to tachyphylaxis with continuous GLP-1 receptor activation23. Conversely, short-acting GLP-1 receptor agonists lead to a substantial and maintained delay in gastric emptying, with the consequence of pronounced PPG reduction, making them an effective prandial therapy24, and a complementary therapy for use with basal insulin, which predominantly lowers FPG. The beneficial efficacy of lixisenatide in reducing PPG compared favorably with a long-acting GLP-1 receptor agonist in a head-to-head study. A recent 28-day, randomized, open-label, parallel-group, multicenter study carried out in seven German centers found that once-daily prandial lixisenatide reduced PPG significantly more than the long-acting GLP-1 receptor agonist liraglutide at the 1.8 mg/day dose (mean change in area under the curve(0.30–4.30 h) −12.6 vs −4.0 h/mmol/L, respectively, P < 0.0001)25. Indeed, the placebo-subtracted PPG reduction in this Japanese population was substantial: −8.51 mmol/L.
The reduction in 2-h PPG levels seen in the present study is consistent with previous studies in mixed populations, in whom lixisenatide has shown profound effects on PPG as monotherapy8, as add-on to oral antidiabetic drugs9,11, and in combination with basal insulin15,16. In an analysis of lixisenatide in a mixed Asian population (including Japanese patients) in combination with basal insulin with or without SU (GetGoal-L-Asia)14, comparable results with our analysis were reported, with significant reductions in both HbA1c vs the placebo (−0.88%) and 2-h PPG vs the placebo (−7.83 mmol/L)14. It has been previously reported that GLP-1 receptor agonists exert a greater effect on glycemic control in Asian patients compared with non-Asian patients26. The improvements in glucose control observed in Japanese patients can be explained by two factors: (i) limited meal-induced enhancement of GLP-1 secretion17,18; and (ii) a pathophysiology of impaired glucose tolerance characterized by impaired early phase insulin secretion and insulin resistance27,28. Lixisenatide has been shown to restore first-phase insulin secretion in patients with type 2 diabetes mellitus, making it pertinent for use in a Japanese population29.
In addition, other possible predictive factors of efficacy with GLP-1 receptor agonists include baseline HbA1c, residual β-cell function and sex30–34. Although not evaluated here, it would be of interest to evaluate these possible predictors in Japanese and Asian patients treated with lixisenatide in the future.
In contrast to the overall GetGoal-S population, in which there was a significant treatment difference in bodyweight of 0.84 kg vs the placebo11, no significant difference in bodyweight reduction was reported in this Japanese subpopulation. The study of liraglutide added to SU in Japanese patients also failed to show a reduction in bodyweight despite significant reductions in other populations22. This could be attributed to the fact that Asian populations typically have lower bodyweight than Western patients and, as such, are less likely to see large bodyweight reductions. This is supported by the GetGoal-L-Asia study14, in which a small reduction in bodyweight was reported over time in an Asian population with relatively low mean body mass index14.
In the present Japanese population, lixisenatide was generally well tolerated, with nausea being the most commonly reported gastrointestinal event (25.0%), similar to the incidence with lixisenatide in the overall study population (25.3%) of GetGoal-S11. The incidence of symptomatic hypoglycemia was higher in the lixisenatide group vs the placebo (17.1 vs 9.8%, respectively), and no severe symptomatic hypoglycemia was reported in either group. This is similar to symptomatic hypoglycemia incidence levels in the overall GetGoal-S population, in which only one patient (in the lixisenatide group) experienced a severe hypoglycemic event. As in the present study, the incidence of symptomatic hypoglycemia in the GetGoal-L-Asia study was also higher with lixisenatide than with the placebo; however, in patients not receiving SU, the incidence was similar to the placebo, suggesting possible involvement of SUs in symptomatic hypoglycemia14. In comparison, with exenatide 10 μg twice daily in a similar population of Japanese patients, the rate of any treatment-emergent AE was 94%, the rate of nausea was 36% and the proportion of patients who experienced an AE leading to study withdrawal was 25%21. The rate of symptomatic hypoglycemia regardless of blood glucose levels was also notably higher with exenatide than with the placebo21.
Different to exenatide, lixisenatide is administered once daily. Pharmacological studies have shown that once-daily lixisenatide administration before breakfast is as effective as twice-daily dosing before breakfast and the evening meal in both Japanese and Caucasian patients with type 2 diabetes mellitus35,36. A lixisenatide dose of 20 μg once daily, as used in the current study, showed the best efficacy-to-tolerability ratio reduces the burden of injection for patients compared with twice-daily dosing36. Administering lixisenatide before breakfast controls morning postprandial hyperglycemia, which is typically the largest postmeal excursion of the day because of hepatic glucose production in response to the overnight fast (the dawn phenomenon)37. However, the timing of peaks in postprandial glucose can vary between patients depending on the distribution of carbohydrate between the meals of the day38, which is often highest at the evening meal for Japanese patients. The GetGoal-M study of lixisenatide added to metformin in a global population of patients with type 2 diabetes mellitus has shown comparable improvements in glycemic control with morning or evening administration of lixisenatide9. Finally, a recently published 24-week study showed that once-daily lixisenatide is as effective when administered at patients’ self-reported main meal of the day compared with pre-breakfast administration39.
The Japanese population in the current study had been diagnosed with type 2 diabetes mellitus for approximately 12 years, and 60–70% of individuals had received a combination of SU and metformin; as such, this population might be considered as being at a relatively advanced stage of diabetes, with reduced β-cell function40,41. Other studies have shown that liraglutide significantly reduces HbA1c in Japanese patients treated with SU21,42. Recently, however, it has been shown that the glucose-lowering efficacy of some GLP-1 receptor agonists might depend on the remaining β-cell function43,44, and in such a population, lixisenatide could offer a well-tolerated alternative to treatment intensification with basal insulin, and might compensate for reduced β-cell function.
Limitations of the present study include that the analysis was not prespecified, and that there were some differences in baseline characteristics.
This analysis of lixisenatide carried out in a Japanese subpopulation of the GetGoal-S study showed that lixisenatide was well tolerated and provided significant improvement in glycemic control, as assessed by change in HbA1c, with a pronounced improvement in PPG control. The current results showed the efficacy–tolerability profile of lixisenatide once daily in the context of SU-based oral agent failure, and emphasize its potential as an important and beneficial option for the treatment of type 2 diabetes mellitus in the Japanese population.
Acknowledgments
The study was supported by Sanofi. Editorial support was provided by Medicus International London (UK) and Caudex Medical, and was funded by Sanofi. Yukiko Onishi has acted as medical advisor for Sanofi, Novo Nordisk Pharma Ltd and AstraZeneca. Daisuke Yabe has received consulting and/or speaker fees from Eli Lilly, MSD, Sanofi, Novo Nordisk and Takeda. Yutaka Seino has acted as medical advisor for Eli Lilly, Sanofi, Novo Nordisk, GlaxoSmithKline, Taisho Pharmaceuticals, Astellas Pharmaceuticals, Becton, Dickinson & Company, Boerhringer Ingelheim, Johnson & Johnson, Takeda Pharmaceuticals and Otsuka Pharmaceuticals. Elisabeth Niemoeller, Yukio Ikeda and Hiroki Takagi are employees of Sanofi. Sanofi was responsible for the design and implementation of this study. Yukiko Onishi was one of the principle investigators of this study. All authors contributed to the interpretation of the data and provided comments on the report at various stages in its development. Hiroki Takagi undertook statistical analyses. Elisabeth Niemoeller made significant suggestions to analysis and interpretation of data. Yukio Ikeda drafted the manuscript. All authors were involved in developing the manuscript, providing critical review of the content and final approval of the version to be published.
Supporting Information
Table S1| Criteria for initiation of rescue therapy.
Figure S1 | Patient disposition flow.
References
- Whiting D, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas. 6th edn. Brussels, Belgium: International Diabetes Federation; 2013. http://www.idf.org/diabetesatlas. Accessed May 2014. [PubMed] [Google Scholar]
- Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamazaki K, Hirao K, et al. The status of diabetes control an antidiabetic drug therapy in Japan - a cross sectional survey of 17,000 patients with diabetes mellitus (JDDM 1) Diabetes Res Clin Pract. 2006;73:198–204. doi: 10.1016/j.diabres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Garber AJ. Novel GLP-1 receptor agonists for diabetes. Expert Opin Investig Drugs. 2012;21:45–57. doi: 10.1517/13543784.2012.638282. [DOI] [PubMed] [Google Scholar]
- Barnett A. Lixisenatide: evidence for its potential use in the treatment of type 2 diabetes. Core Evid. 2011;6:67–79. doi: 10.2147/CE.S15525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca VA, Alvarado-Ruiz R, Raccah D, et al. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono) Diabetes Care. 2012;35:1225–1231. doi: 10.2337/dc11-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrén B, Dimas L, Miossec P, et al. Efficacy and safety of lixisenatide once-daily morning or evening injections vs placebo in type 2 diabetes inadequately controlled on metformin (GetGoal-M) Diabetes Care. 2013;36:2543–2550. doi: 10.2337/dc12-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli G, Munteanu M, Dotsenko S, et al. Efficacy and safety of lixisenatide once-daily vs placebo in people with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1) Diabet Med. 2014;31:176–184. doi: 10.1111/dme.12328. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Hanefeld M, Shamanna P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S) J Diabetes Complications. 2014;28:386–392. doi: 10.1016/j.jdiacomp.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Pinget M, Goldenberg R, Niemoeller E, et al. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P) Diabetes Obes Metab. 2013;15:1000–1007. doi: 10.1111/dom.12121. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X. Diabetes Care. 2013;36:2945–2951. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Min KW, Niemoeller E, et al. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia) Diabetes Obes Metab. 2012;14:910–917. doi: 10.1111/j.1463-1326.2012.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle M, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36:2489–2496. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36:2497–2503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D, Kuroe A, Lee S, et al. Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. J Diabetes Invest. 2010;1:56–59. doi: 10.1111/j.2040-1124.2010.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D, Watanabe K, Sugawara K, et al. Comparison of incretin immunoassays with or without plasma extraction: incretin secretion in Japanese patients with type 2 diabetes. J Diabetes Invest. 2012;3:70–79. doi: 10.1111/j.2040-1124.2011.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Seino Y, Nanjo K, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National glycohemoglobin standardization program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Mitsuyoshi N, Takeshi I, et al. Improved glycemic control and reduced bodyweight with exenatide: a double-blind, randomized, phase 3 study in Japanese patients with suboptimally controlled type 2 diabetes over 24 weeks. J Diabetes Invest. 2011;2:210–217. doi: 10.1111/j.2040-1124.2010.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku K, Rasmussen MF, Clauson P, et al. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human glucagon-like peptide-1 analogue liraglutide as add-on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:341–347. doi: 10.1111/j.1463-1326.2009.01194.x. [DOI] [PubMed] [Google Scholar]
- Nauck M, Kemmeries G, Holst JJ, et al. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–1565. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- Kapitza C, Forst T, Coester HV, et al. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15:642–649. doi: 10.1111/dom.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Hahn S, Oh TJ, et al. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:900–909. doi: 10.1111/dom.12293. [DOI] [PubMed] [Google Scholar]
- Onishi Y, Hayashi T, Sato KK, et al. Fasting tests of insulin secretion and sensitivity predict future prediabetes in Japanese with normal glucose tolerance. J Diabetes Invest. 2010;1:191–195. doi: 10.1111/j.2040-1124.2010.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A, Tatsumi Y, Deura K, et al. Impact of impaired insulin secretion and insulin resistance on the incidence of type 2 diabetes mellitus in a Japanese population: the Saku study. Diabetologia. 2013;56:1671–1679. doi: 10.1007/s00125-013-2932-y. [DOI] [PubMed] [Google Scholar]
- Becker RHA, Stechl J, Msihid J, et al. Lixisenatide resensitizes the insulin-secretory response to intravenous glucose challenge in people with type 2 diabetes – A study in both people with type 2 diabetes and health subjects. Diabetes Obes Metab. 2014;16:793–800. doi: 10.1111/dom.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anichini R, Cosimi S, Di CA, et al. Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: a real world experience. Diabetes Metab Syndr Obes. 2013;6:123–129. doi: 10.2147/DMSO.S42729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF, Mannucci E, Ahren B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes Metab. 2012;14:762–767. doi: 10.1111/j.1463-1326.2012.01603.x. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Vilsboll T, Donsmark M, et al. Greater glycaemic control is achieved with the once-daily human GLP-1 analogue liraglutide vs comparators across the continuum of estimated beta cell mass. Diabetologia. 2011;54(Suppl 1):1–542. [Google Scholar]
- Meier JJ, Yabe D, Wang E, et al. Efficacy of lixisenatide in patients with different levels of beta cell function as assessed by C-peptide/glucose ratio. Diabetologia. 2013;56(Suppl 1):S1–S566. (Abstract 896) [Google Scholar]
- Usui R, Yabe D, Kuwata H, et al. Retrospective analysis of safety and efficacy of insulin-to-liraglutide switch in Japanese type 2 diabetes: a caution against inappropriate use in patients with reduced β-cell function. J Diabetes Invest. 2001;4:585–594. doi: 10.1111/jdi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Takami A, Boka G, et al. Pharmacodynamics of the glucagon-like peptide-1 receptor agonist lixisenatide in Japanese and Caucasian patients with type 2 diabetes mellitus poorly controlled on sulphonylureas with/without metformin. Diabetes Obes Metab. 2014;16:739–747. doi: 10.1111/dom.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner RE, Rosenstock J, Boka G. Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med. 2010;27:1024–1032. doi: 10.1111/j.1464-5491.2010.03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier L, Colette C, Dunseath GJ, et al. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263–269. doi: 10.2337/dc06-1612. [DOI] [PubMed] [Google Scholar]
- Pearce KL, Noakes M, Keogh J, et al. Effect of carbohydrate distribution on postprandial glucose peaks with the use of continuous glucose monitoring in type 2 diabetes. Am J Clin Nutr. 2008;87:638–644. doi: 10.1093/ajcn/87.3.638. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Vorokhobina N, Souhami E, et al. Equal improvement in glycaemia with lixisenatide given before breakfast or the main meal of the day. J Diabetes Complications. 2014;28:735–741. doi: 10.1016/j.jdiacomp.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Funakoshi S, Fujimoto S, Hanmasaki A, et al. Analysis of factors influencing pancreatic beta-cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract. 2008;82:353–358. doi: 10.1016/j.diabres.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Funakoshi S, Fujimoto S, Hamasaki A, et al. Analysis of factors influencing postprandial C-peptide levels in Japanese patients with type 2 diabetes: comparison with C-peptide levels after glucagon load. J Diabetes Invest. 2011;2:429–434. doi: 10.1111/j.2040-1124.2011.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Rasmussen MF, Nishida T, et al. Glucagon-like peptide-1 analog liraglutide in combination with sulfonylurea safely improves blood glucose measures vs sulfonylurea monotherapy in Japanese patients with type 2 diabetes: results of a 52-week, randomized, multicenter trial. J Diabetes Invest. 2011;2:280–286. doi: 10.1111/j.2040-1124.2011.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui R, Yabe D, Kuwata H, et al. Retrospective analysis of safety and efficacy of insulin-to-liraglutide switch in Japanese type 2 diabetes: a caution against inappropriate use in patients with reduced β-cell function. J Diabetes Invest. 2013;4:585–594. doi: 10.1111/jdi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozawa J, Inoue K, Iwamoto R, et al. Liraglutide is effective in type 2 diabetic patients with sustained endogenous insulin-secreting capacity. J Diabetes Invest. 2012;3:294–297. doi: 10.1111/j.2040-1124.2011.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1| Criteria for initiation of rescue therapy.
Figure S1 | Patient disposition flow.


