Abstract
Aims/Introduction
Canagliflozin is a sodium–glucose cotransporter 2 inhibitor under development for the treatment of type 2 diabetes. Our aim was to examine its efficacy and safety as monotherapy or in combination with commonly used oral antihyperglycemic drugs in Japanese patients with type 2 diabetes.
Materials and Methods
Patients on diet/exercise alone or diet/exercise plus an oral antihyperglycemic drug (sulfonylurea, glinide, α-glucosidase inhibitor, biguanide, thiazolidinedione or dipeptidyl peptidase-4 inhibitor) were randomized to either 100 or 200 mg canagliflozin while continuing prior therapy. Patients were treated for 52 weeks in an open-label manner.
Results
Canagliflozin significantly reduced hemoglobin A1c, fasting plasma glucose and bodyweight in all the study groups. Improvements were apparent by 4 weeks of treatment, and were maintained for 52 weeks. The reduction in hemoglobin A1c ranged from −0.80 to −1.06%, and from −0.93 to −1.26% in the 100 and 200 mg canagliflozin groups, respectively. Drug-related adverse events occurred in approximately one-third of patients, and included hypoglycemia/asymptomatic hypoglycemia and pollakiuria. Hypoglycemia/asymptomatic hypoglycemia was most common in patients treated with a sulfonylurea. Most adverse events were classified as mild or moderate in severity.
Conclusions
The results of the present study confirmed that treatment with canagliflozin resulted in significant reductions in glycemic control and bodyweight that were maintained for 52 weeks of treatment irrespective of whether it was administered as monotherapy or in combination with another oral antihyperglycemic drug. Canagliflozin was well tolerated, with a low incidence of drug-related adverse events. This trial was registered with ClinicalTrials.gov (no. NCT01387737).
Keywords: Canagliflozin, Sodium–glucose cotransporter 2 inhibitor, Type 2 diabetes mellitus
Introduction
Sodium–glucose cotransporter 2 (SGLT2) inhibitors are a novel class of drugs under development for the treatment of type 2 diabetes that work by blocking glucose reabsorption in the kidney. Inhibition of SGLT2 and blocking glucose reabsorption lowers the renal threshold for glucose, which enhances urinary glucose excretion (UGE) and ultimately lowers PG1–4.
Canagliflozin (TA-7284 and JNJ-28431754; Mitsubishi Tanabe Pharma Corporation/Janssen Research & Development, LLC, Tokyo, Japan; Raritan, NJ, USA) is a SGLT2 inhibitor that was recently approved by the United States Food and Drug Administration for the treatment of type 2 diabetes5. Studies in Western populations confirmed that canagliflozin significantly reduced hemoglobin A1c (HbA1c) when used as monotherapy (with diet and exercise)6, in combination with metformin7,8 and in combination with metformin plus a sulfonylurea9.
In Japanese patients, results of 12-week10 and 24-week11 studies have confirmed that 100 or 200 mg canagliflozin significantly reduced HbA1c, fasting plasma glucose (FPG) and 2-h plasma glucose after a meal or glucose load compared with placebo, and reduced bodyweight when used as monotherapy.
According to the Japan Diabetes Society's Treatment Guide for Diabetes, all classes of antihyperglycemic drugs can be used as monotherapy in Japan12. However, many patients continue to have inadequate glycemic control with diet/exercise alone or with an antihyperglycemic drug as monotherapy. Therefore, it is essential to consider the effects of adding novel oral agents, including SGLT2 inhibitors, to ongoing therapies if insulin therapy can be avoided.
Accordingly, the aim of the present study was to determine the long-term efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes and poor glycemic control (elevated HbA1c) on diet/exercise therapy alone or diet/exercise in combination with an existing oral antihyperglycemic drug. The present study was carried out in accordance with the Japanese Guideline for the Clinical Evaluation of Oral Hypoglycemic Agents13, which states that the efficacy and safety of oral antihyperglycemic agents should be examined in long-term, open-label studies.
Materials and Methods
Patients
Outpatients aged ≥20 years with type 2 diabetes diagnosed ≥3 months before screening and HbA1c (National Glycohemoglobin Standardization Program values) of ≥7.0 to ≤10.0% (monotherapy) or ≥7.0 to ≤10.6% (combination therapy) were eligible for the present study. Patients were eligible for monotherapy in this study if their diet/exercise therapy had continued unchanged and they had not used an antidiabetic drug for ≥83 days before week 0 (start of the treatment period). Patients who had used a sulfonylurea (glimepiride, gliclazide or glibenclamide), a glinide (nateglinide or mitiglinide), an α-glucosidase inhibitor (α-GI; voglibose, miglitol or acarbose), a biguanide (metformin), a thiazolidinedione (pioglitazone) or a dipeptidyl peptidase-4 (DPP-4) inhibitor (sitagliptin, vildagliptin or alogliptin) for ≥83 days before week 0 were eligible for combination therapy in the present study. Patients who had used another antidiabetic drug were to enter a washout period of ≥83 days after providing informed consent and before starting the treatment period. Major exclusion criteria included contraindications to the study drugs, history or current serious diabetic complication, FPG >270 mg/dL on two consecutive visits between providing informed consent and week 0, indication for insulin, concurrent urinary tract/genital infection, triglyceride level ≥600 mg/dL at screening, systolic/diastolic blood pressure (SBP/DBP) ≥160/≥100 mmHg, history of or current cardiac failure (New York Heart Association Class III or IV), myocardial infarction or cerebrovascular disorder <6 months before week 0, serious liver/kidney disorders, estimated glomerular filtration rate <50 mL/min/1.73 kg/m2, urinary albumin/creatinine ratio ≥300 mg/g creatinine at screening, and participation in another clinical trial or receiving treatment with an investigational product in ≤12 weeks of providing informed consent or prior treatment with canagliflozin.
The study was carried out in accordance with the ethical principles of the Declaration of Helsinki, the Pharmaceutical Affairs Law of Japan, Good Clinical Practice and the approved study protocol. The study was approved by institutional review boards at each participating site. All patients provided written informed consent before enrolment.
Study Design
This was a multicenter, randomized, open-label, 52-week study (Figure1). The trial was registered on ClinicalTrials.gov (no. NCT01387737). After providing informed consent, patients on diet/exercise alone or using one of six classes of oral antihyperglycemic agents underwent screening. A washout period was also included if another antihyperglycemic agent was used. At week 0, eligible patients were randomized to 100 or 200 mg canagliflozin. Diet/exercise therapy and the combination antihyperglycemic drug were to be continued unchanged from 83 days before week 0 until the end of the follow-up period. Visits were scheduled every 4 weeks during the treatment period, and at 2 weeks after study completion or at study withdrawal (if before week 52).
Figure 1.
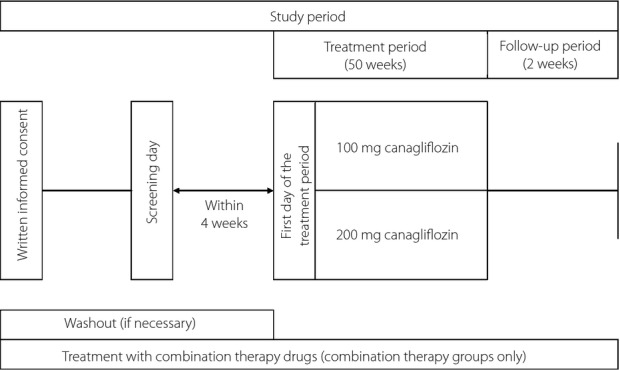
Study design. Visits were scheduled every 4 weeks during the treatment period.
The combination drugs were to be used in accordance with their approved dosage and administration. The doses of canagliflozin and the combination antihyperglycemic drug were to be continued unchanged throughout the study, although the dose of the combination drug could be modified in the follow-up period if the FPG level met the criteria for withdrawal (>270 mg/dL). The doses of sulfonylureas or glinides could be reduced if the investigator considered there was a risk of hypoglycemia.
Patients were randomized by the study enrolment center to either 100 or 200 mg canagliflozin, and the patients were prescribed the allocated dose by the investigator. Randomization was carried out using a variable block design. The doses of canagliflozin were chosen after considering the reductions in HbA1c, FPG and 2-h plasma glucose, and comparable safety profiles of doses of 100–300 mg canagliflozin in an earlier Japanese dose-finding study10.
Continuous systemic corticosteroid treatment for ≥2 weeks, appetite suppressants and other investigational products were prohibited during the study. Patients using glibenclamide were prohibited from using bosentan hydrate.
The investigator verbally assessed compliance with diet/exercise therapy, the combination drug and canagliflozin throughout the study. The investigators were not given specific guidelines for encouraging adherence to diet/exercise therapy, only a guideline that the prior therapy should be continued.
Efficacy Outcomes
The efficacy variables included the changes in HbA1c (and proportion of patients with HbA1c <7.0%), FPG, bodyweight (absolute and percent change; and proportion of patients with a decrease in bodyweight of ≥5%), waist circumference, lipids (absolute and percent changes in triglyceride, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C] and non-HDL-C; change in LDL-C/HDL-C ratio), SBP/DBP, proinsulin/C-peptide ratio and homeostatic model assessment (HOMA)2-%B. The time-courses of changes in HbA1c, FPG and bodyweight were also assessed. The efficacy outcomes were generally measured at each visit during the study, including at the time of providing informed consent, at screening and at treatment withdrawal.
All laboratory tests were carried out at SRL Inc. (Tokyo, Japan). HbA1c was measured in Japan Diabetes Society units and converted to National Glycohemoglobin Standardization Program units14 using the certified formula: HbA1c (NGSP [%]) = HbA1c (JDS [%]) × 1.02 + 0.25.
Safety
Adverse events (AEs) and safety assessments, including symptoms or signs of hypoglycemia, vital signs, 12-lead electrocardiography and clinical laboratory tests were to be recorded throughout the study. Hypoglycemia was classified as hypoglycemia (typical hypoglycemic symptoms were present irrespective of the blood glucose level) and asymptomatic hypoglycemia (typical hypoglycemic symptoms were absent, but the blood glucose level was low; ≤70 mg/dL).
During the treatment period, clinical laboratory tests were generally carried out at each visit. Urinary albumin/creatinine ratio was assessed at weeks 0, 4, 8, 12, 24, 36 and 52. Bone markers were assessed, and 12-lead electrocardiography was carried out at weeks 0, 12, 24, 36 and 52. The following equation15 was used to calculate estimated glomerular filtration rate (eGFR): eGFR (mL/min/1.73 m2) = 194 × serum creatinine−1.094 × age−0.287 (women: ×0.739).
AEs were classified according to system organ class and preferred term using MedDRA/J version 15.1 (Japanese Maintenance Organization, Tokyo, Japan). Their potential relationships with the study drug (no causal relationship or possible causal relationship) and their severity (mild, moderate or severe) were also assessed.
Patients were instructed to measure blood glucose levels on ≥3 days per week using a glucose meter as early in the morning as possible in the fasting state throughout the study. Data were to be recorded in a patient diary. The patients were also instructed to measure glucose levels, if possible, in the event of symptoms suggestive of hypoglycemia.
Statistical Analysis
The rationale for the sample size is described in the Supporting Information.
Efficacy variables were analyzed in the full analysis set, excluding patients without type 2 diabetes, who did not receive the study drug or who lacked efficacy data after starting the study drug. The safety analysis set included all patients who started the treatment period except those who did not receive the study drug or who lacked safety data after starting treatment with the study drug.
Efficacy variables were analyzed descriptively for changes over time as means ± standard deviation with 95% confidence intervals, or as the number and percentage of patients. The last observation was carried forward in the event of missing data for the end of treatment. Safety variables were analyzed descriptively as the number and percentage of patients, or as means ± standard deviation. No statistical comparisons were made between the two doses of canagliflozin or the treatment regimens (i.e., monotherapy or combinations of drugs).
Results
Patients
The disposition of patients is summarized in Table1. The most common reasons for patient withdrawals in the treatment phase were patient request or the occurrence of an AE. Table S1 presents the characteristics of patients in each of the study groups. Within each treatment regimen, the characteristics of patients allocated to 100 or 200 mg canagliflozin were generally similar. However, there were some differences among the seven treatment regimens, especially HbA1c and duration of diabetes (Table S1). The median compliance rate ranged from 99.44 to 100.0% among the 14 study groups.
Table 1.
Patient disposition
| Monotherapy | Sulfonylurea | Glinide | α-GI | Biguanide | TZD | DPP-4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg | 200 mg | 100 mg | 200 mg | 100 mg | 200 mg | 100 mg | 200 mg | 100 mg | 200 mg | 100 mg | 200 mg | 100 mg | 200 mg | |
| Patients randomized | 127 | 253 | 124 | 125 | 65 | 64 | 62 | 61 | 72 | 76 | 63 | 62 | 71 | 74 |
| Patients who discontinued | 11 | 26 | 15 | 14 | 5 | 8 | 4 | 5 | 7 | 7 | 5 | 5 | 5 | 9 |
| Reasons for discontinuation† | ||||||||||||||
| Patient request | 6 | 15 | 10 | 1 | 2 | 3 | 1 | 0 | 2 | 3 | 3 | 4 | 3 | 5 |
| Ineligible for the study | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse event | 5 | 10 | 2 | 8 | 2 | 4 | 3 | 3 | 5 | 3 | 1 | 1 | 2 | 1 |
| Worsening of diabetes | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other reason‡ | 0 | 1 | 2 | 3 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 3 |
| Completed treatment | 116 | 227 | 109 | 111 | 60 | 56 | 58 | 56 | 65 | 69 | 58 | 57 | 66 | 65 |
| Full analysis set | 127 | 252 | 124 | 125 | 65 | 64 | 62 | 60 | 72 | 76 | 63 | 62 | 71 | 74 |
| Safety analysis set | 127 | 253 | 124 | 125 | 65 | 64 | 62 | 61 | 72 | 76 | 63 | 62 | 71 | 74 |
Values are the number of patients.
During the treatment period.
None of the patient withdrawals were as a result of the fasting plasma glucose level exceeding 270 mg/dL or the investigator deeming the participant should discontinue the combination drug. α-GI, α-glucosidase inhibitor; DPP-4, dipeptidyl peptidase-4 inhibitor; TZD, thiazolidinedione.
Glycemic Control and β-Cell Function
As shown in Figure S1, HbA1c started to decrease by week 4 of treatment, reaching a plateau after approximately 12 weeks of treatment in all study groups. The changes in HbA1c over time were generally similar for both canagliflozin doses, except in some regimens where the mean change was greater in patients treated with 200 mg canagliflozin when used as monotherapy (−0.80 and −1.00% in the 100 and 200 mg canagliflozin groups, respectively) or in combination with an α-GI (−0.91 and −1.17%) or a DPP-4 inhibitor (−1.04 and −1.26%) (Figure2a; Table S2). The baseline to end-point change in HbA1c was approximately −1.0% in the other study groups. The proportion of patients with HbA1c <7.0% increased between weeks 24 and 52 in each of the study groups, ranging from 31.4 to 50.7% at week 24 and from 38.8 to 59.4% at week 52 (Table S2). In a subgroup analysis, the reduction in HbA1c was greater in patients with high baseline values (≥8.0%) than in those with low baseline values (<8.0%; Table S3).
Figure 2.
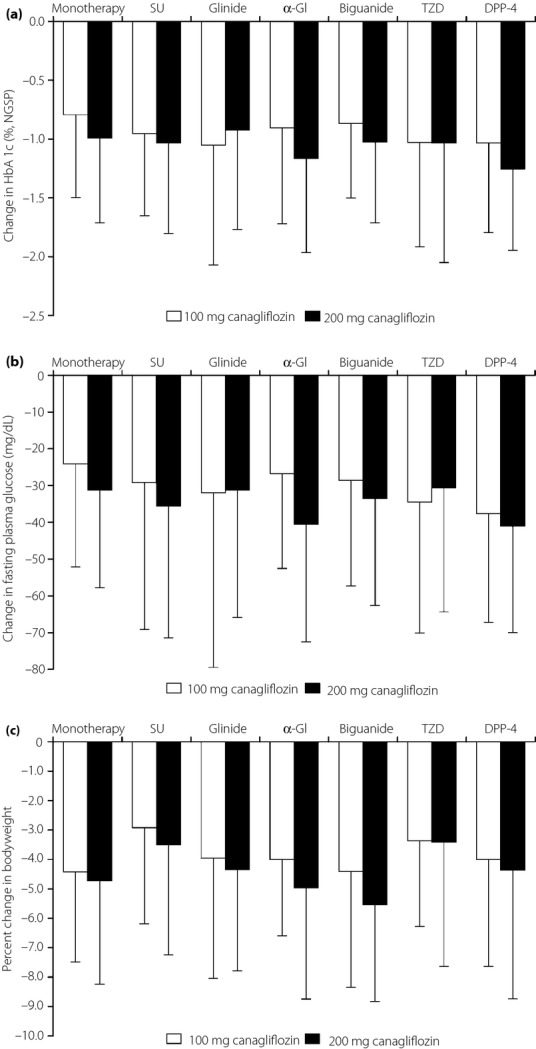
Changes in (a) hemoglobin A1c (HbA1c), (b) fasting plasma glucose and (c) bodyweight from baseline to the end of treatment with last observation carried forward. Light bars, 100 mg canagliflozin; dark bars, 200 mg canagliflozin. Values are means ± standard deviation. α-GI, α-glucosidase inhibitor; DPP-4, dipeptidyl peptidase-4 inhibitor; NGSP, National Glycohemoglobin Standardization Program; SU, sulfonylurea; TZD, thiazolidinedione.
FPG decreased over time in each of the study groups (Figure S2), and decreased significantly from baseline to the end of treatment in each group (Figure2b; Table S2). Canagliflozin also decreased the proinsulin/C-peptide ratio, and increased HOMA2-%B, indicating an improvement in β-cell function (Table S2).
Other Efficacy Variables
Table S2 presents the changes in other clinically relevant efficacy variables, including the changes in bodyweight, waist circumference, SBP/DBP and lipid levels, from baseline to the end of treatment.
Reductions in bodyweight were apparent within 4 weeks of starting treatment in each of the study groups (Figure S3). The magnitude of the bodyweight reduction between baseline and the end of treatment was generally comparable between both canagliflozin doses, although it varied among the seven treatment regimens, being greater in the monotherapy group, and in the biguanide, glinide and α-GI combination groups than in the sulfonylurea and thiazolidinedione combination groups (Figure2c; Table S2). The proportions of patients with a reduction in bodyweight of ≥5% increased between weeks 24 (range 13.7–39.5%) and 52 (range 25.4–55.3%). Reductions in waist circumference were also observed during the study.
Reductions in SBP and DBP were observed in each of the groups; the mean change from baseline to the end of treatment among the study groups ranged from −2.84 to −7.78 mmHg for SBP and from −1.90 to −4.44 mmHg for DBP (Table S2).
HDL-C and LDL-C levels increased, whereas triglyceride levels and the LDL-C/HDL-C ratio decreased in each of the study groups (Table S2).
Safety
Although AEs occurred in the majority of patients (≥72.6%), serious AEs (SAEs) were reported in 2.6–9.2% of patients in each of the study groups (Table S4). Five deaths occurred in patients treated with the antihyperglycemic agents (2 patients in the 100 mg group, 3 patients in the 200 mg group). Drug-related AEs occurred in approximately one-third of patients in each of the study groups (Table S4). Between 1.4 and 6.9% of patients in each group discontinued because of AEs. The most common study drug-related AEs included hypoglycemia/asymptomatic hypoglycemia and pollakiuria.
The sulfonylurea dose was reduced in 5.6% (7/124) and 6.4% (8/125) of patients in the 100 and 200 mg canagliflozin dose groups, respectively. The glinide dose was reduced in 1.5% (1/65) and 1.6% of (1/64) patients in the 100 and 200 mg dose groups, respectively.
As expected from the properties of the combination drugs, the incidence of hypoglycemia/asymptomatic hypoglycemia was highest in patients treated with canagliflozin in combination with a sulfonylurea (Table S4). Among patients treated with a sulfonylurea, hypoglycemia occurred in 17.7% (22/124) and 14.4% (18/125) of patients treated with 100 or 200 mg, respectively, whereas asymptomatic hypoglycemia occurred in 14.5% (18/124) and 12.8% (16/125) of patients treated with 100 or 200 mg, respectively. There were no episodes of severe hypoglycemia (events that made the patient's daily activities impossible to perform) in any group. In patients treated with canagliflozin in combination with a sulfonylurea, the incidence of hypoglycemia/asymptomatic hypoglycemia (events per person-year) decreased from 4.28 before the sulfonylurea dose reduction to 2.24 after the dose reduction in the 100 mg canagliflozin group and from 8.57 to 3.09 in the 200 mg canagliflozin group.
Genital infections mostly occurred in females. Most of the events were mild in severity and the patients recovered after antifungal therapy. Urinary tract infections occurred in ≤6.5% of patients in each group, and none of these events were severe. Volume depletion-related AEs occurred in ≤4.8% of patients in each of the study groups. These events were generally classified as mild; none were severe.
Changes in laboratory values are presented in Table S5. Reductions in liver enzymes (aspartate aminotransferase, alanine aminotransferase and γ-glutamyl transpeptidase) were observed in each of the groups, although the magnitude of reductions was not clearly related to the canagliflozin dose. Hematocrit and blood urea nitrogen levels increased in each of the groups, whereas the urinary albumin/creatinine ratio decreased. Serum creatinine levels increased slightly, whereas eGFR and serum uric acid levels decreased slightly in each of the groups. There were no significant changes in calcium and phosphorus levels, whereas magnesium levels increased slightly in all of the groups.
The mean total ketone body concentration increased in each of the groups, although the magnitude of the increase was not apparently related to the canagliflozin dose or combination treatment. Total ketone bodies (reference range ≤130 μmol/L) increased to ≥3,000 μmol/L in 22 patients and to ≥5,000 μmol/L in six patients. The median total ketone body level increased to approximately 200 μmol/L within 4–8 weeks, but then declined to approximately 150 μmol/L in each of the groups by week 24, and then remained stable thereafter. There was one episode of diabetic ketoacidosis, which was rated as severe, in one patient treated with 100 mg canagliflozin in combination with a sulfonylurea. This event occurred 13 days after discontinuing treatment with the study, and was accompanied by hyperglycemia (≥600 mg/dL), subjective feeling bad and vomiting. The patient's highest total ketone body level was 13,263 μmol/L. The event was preceded by infectious gastroenteritis. Therefore, it was suspected that the episode of diabetic ketoacidosis was a result of the development of fulminant type 1 diabetes mellitus after viral infection. The patient's laboratory data were generally consistent with the Japanese criteria for fulminant type 1 diabetes mellitus16. The patient's diabetic ketoacidosis resolved after treatment with insulin and was not considered related to the study drug. This case of fulminant type 1 diabetes mellitus was reported as a SAE.
Discussion
The present study confirmed that canagliflozin as monotherapy or in combination with another oral antihyperglycemic agent lowered HbA1c and FPG, with effects that were apparent within 4–8 weeks and were sustained for 52 weeks. Furthermore, the proportion of patients with HbA1c <7.0% increased between week 24 and week 52, showing the improvements in HbA1c continued over 52 weeks of treatment. We also observed marked improvements in bodyweight, BP and lipid levels (HDL-C and triglyceride) during the study.
Some antihyperglycemic drugs, including sulfonylureas and thiazolidinediones, are associated with weight gain17–19, especially when used in combination therapy20. Therefore, identifying treatments that have beneficial effects on bodyweight is particularly important in the context of type 2 diabetes care21. In the present study, reductions in mean bodyweight were apparent within 4 weeks of treatment, and continued throughout the 52-week study without an apparent rebound. Bodyweight reductions were also apparent in patients treated with a sulfonylurea or a thiazolidinedione, although the magnitude of the bodyweight reduction tended to be smaller in these groups than in the other groups. It is likely that the reductions in bodyweight in the first 4–8 weeks of the study are partly related to changes in fluid volume, mediated by increases in urine volume. However, the continued reductions in bodyweight over the 52-week study suggest that changes in metabolic activity also contribute to this outcome. This is supported by the changes in lipid levels and total ketone bodies, suggesting that the reduction in circulating glucose is compensated for by increased triglyceride metabolism.
The reductions in BP and changes in lipid levels, especially HDL-C and triglyceride levels, might be of relevance in terms of cardiovascular disease risk, although the study was not designed to address this possibility in terms of study duration or sample size. The mechanism and clinical effects, if any, of the increase in LDL-C levels observed in the present study and in studies of other SGLT2 inhibitors22 also warrant investigation. The ongoing Canagliflozin Cardiovascular Assessment Study should provide insight into the cardiovascular effects of canagliflozin23.
The improvements in glycemic control and bodyweight observed in the present study were consistent with those reported in other clinical studies of canagliflozin in Western6–9 and Japanese patients10.
The progression of type 2 diabetes is invariably associated with progressive β-cell dysfunction and loss of insulin secretion. In the present study, we observed a reduction in the proinsulin/C-peptide ratio and an increase in HOMA2-%B, similar to the results reported by Rosenstock et al.7 and Stenlöf et al.6, suggesting that treatment with canagliflozin led to some improvement in β-cell function. Although the mechanism is unclear, we suspect that the improvement in β-cell function was secondary to a reduction in glucotoxicity24–26 rather than as a direct effect of canagliflozin on β-cells, because β-cells do not express SGLT2. Intriguingly, SGLT2 deletion preserved β-cell function and increased β-cell mass in db/db mice fed a high-fat diet for 4 weeks27, probably by preventing excess hyperglycemia.
Study drug-related AEs occurred in approximately one-third of patients, and the most common were pollakiuria and hypoglycemia/asymptomatic hypoglycemia. However, the incidences of AEs and drug-related AEs were similar in each group, and most were classified as mild or moderate in severity.
The incidence of hypoglycemia/asymptomatic hypoglycemia is at least partly attributed to the combination drug, because it was highest in patients treated with canagliflozin in combination with a sulfonylurea, and lowest in those treated with canagliflozin in combination with an α-GI, which are associated with low rates of hypoglycemia. Nevertheless, there were no episodes of severe hypoglycemia.
Urinary tract infection and genital infection have been reported in patients treated with canagliflozin, and are associated with the increased UGE28,29. However, these events were relatively rare, were generally classified as mild in severity and were manageable.
The rise in urinary glucose increases the osmotic pressure of urine, hindering water reabsorption and promoting urine excretion, as evidenced by the high incidence of pollakiuria. High water loss might ultimately lead to osmotic diuresis and a reduction in intravascular volume. However, volume depletion-related AEs occurred in ≤4.8% of patients in each group, and none of these events were severe. Nevertheless, slight changes in plasma volume could explain or contribute to the increase in hematocrit and the reduction in BP. We also noted a slight increase in serum creatinine, which might reflect dehydration over the short term.
There were increases in total ketone bodies in the early phase of the study, which likely reflect the changes in metabolic activity, shifting from predominantly glucose metabolism to triglyceride metabolism. However, the magnitude of the increase was not apparently related to the canagliflozin dose, and the levels gradually decreased over time. Although one patient experienced diabetic ketoacidosis as a severe event, it was not considered related to the study drug. There were no apparent explanations for the increases in ketone bodies in the other patients.
We also observed reductions in liver enzymes, suggestive of improvements in liver function, and in the urinary albumin/creatinine ratio, suggestive of an improvement in kidney function.
Some limitations of the present study warrant mention, including the descriptive nature of the analyses and the absence of placebo or other control groups. However, the inclusion of control groups was deemed unnecessary, because multiple studies have already confirmed the superiority of canagliflozin to placebo and would have exposed a large number of patients to unnecessary harm or high withdrawal rates because of inadequate glycemic control. Although the inclusion of active control groups was possible, this would have increased the complexity of the study. Furthermore, we did not statistically compare the doses or treatment regimens. However, our objective was to document the long-term safety and efficacy of canagliflozin in combination with a variety of oral antihyperglycemic drugs in accordance with Japanese regulatory guidelines, not to compare its effects with those of other drugs or to compare the effects of different doses of canagliflozin.
In conclusion, treatment with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs improved glycemic control, bodyweight, BP and markers of β-cell function in Japanese patients with type 2 diabetes. Canagliflozin was generally well tolerated, with a low incidence of SAEs. AEs of specific interest were generally infrequent and were easily manageable, although the incidence of hypoglycemia/asymptomatic hypoglycemia was slightly higher in patients treated with canagliflozin in combination with a sulfonylurea. The current data support the use of canagliflozin in clinical practice in Japan.
Acknowledgments
Canagliflozin is being developed by Mitsubishi Tanabe Pharma Corporation in collaboration with Janssen Research & Development, LLC. This study was funded by Mitsubishi Tanabe Pharma Corporation. We thank all of the staff at each institution for participating in this study, as listed in the Supporting Information online. We thank Nicholas D Smith, PhD, for providing medical writing assistance. Nobuya Inagaki has received honoraria for lectures, clinical commissioned/joint research grants and scholarship grants from Mitsubishi Tanabe Pharma Corporation. Nobuya Inagaki has also received honoraria for lectures from MSD K.K., Novartis Pharma K.K., Sanofi K.K. and Nippon Boehringer Ingelheim Co., Ltd.; clinical commissioned/joint research grants from MSD K.K. and Eli Lilly Japan K.K.; and scholarship grants from MSD K.K., Daiichi Sankyo Company, Ltd., Astellas Pharma Inc., Sanofi K.K., Nippon Boehringer Ingelheim Co., Ltd., AstraZeneca K.K. and Novartis Pharma K.K. Kazuoki Kondo, Toru Yoshinari and Hideki Kuki are employees of Mitsubishi Tanabe Pharma Corporation. The authors declare that they have no conflict of interest.
Supporting Information
Figure S1 | Change in hemoglobin A1c from baseline to each visit.
Figure S2 | Change in fasting plasma glucose from baseline to each visit.
Figure S3 | Percent change in bodyweight from baseline to each visit.
Table S1 | Baseline characteristics of patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs (full analysis set).
Table S2 | Changes in efficacy variables from baseline (week 0) to the end of treatment in patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs.
Table S3 | Change in hemoglobin A1c from baseline to the end of treatment in subgroups of patients divided by hemoglobin A1c at baseline (<8.0 vs ≥8.0%).
Table S4 | Adverse events in patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs.
Table S5 | Changes in laboratory parameters in patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs.
Data S1 |Rationale for the sample size.
Data S2 | References.
Data S3 | Study investigators.
References
- Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95:34–42. doi: 10.1210/jc.2009-0473. [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract. 2008;14:782–790. doi: 10.4158/EP.14.6.782. [DOI] [PubMed] [Google Scholar]
- Chen LH, Leung PS. Inhibition of the sodium glucose co-transporter-2: its beneficial action and potential combination therapy for type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:392–402. doi: 10.1111/dom.12064. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- Canaglifozin (Invokana) for type 2 diabetes. Med Lett Drugs Ther. 2013;55:37–39. [PubMed] [Google Scholar]
- Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15:1136–1145. doi: 10.1111/dom.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled. Phase III study. Expert Opin Pharmacother. 2014;15:1501–1515. doi: 10.1517/14656566.2014.935764. [DOI] [PubMed] [Google Scholar]
- Japan Diabetes Society. Treatment Guide for Diabetes 2012–2013. Tokyo: Bunkodo; 2013. [Google Scholar]
- Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Guideline for Clinical Evaluation of Oral Hypoglycemic Agents. Tokyo: Ministry of Health, Labour and Welfare; 2010. [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:8–10. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus. New diagnostic criteria of fulminant type 1 diabetes mellitus (2012) J Diabetes Invest. 2012;3:536–539. doi: 10.1111/jdi.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CL, Jenkins-Jones S, Evans M, et al. Weight change in people with type 2 diabetes: secular trends and the impact of alternative antihyperglycaemic drugs. Diabetes Obes Metab. 2012;14:424–432. doi: 10.1111/j.1463-1326.2011.01552.x. [DOI] [PubMed] [Google Scholar]
- van Dieren S, Czernichow S, Chalmers J, et al. Weight changes and their predictors amongst 11 140 patients with type 2 diabetes in the ADVANCE trial. Diabetes Obes Metab. 2012;14:464–469. doi: 10.1111/j.1463-1326.2012.01556.x. [DOI] [PubMed] [Google Scholar]
- Campbell IW. Comparing the actions of older and newer therapies on body weight: to what extent should these effects guide the selection of antidiabetic therapy? Int J Clin Pract. 2010;64:791–801. doi: 10.1111/j.1742-1241.2009.02292.x. [DOI] [PubMed] [Google Scholar]
- McIntosh B, Cameron C, Singh SR, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5:e35–e48. [PMC free article] [PubMed] [Google Scholar]
- Meneghini LF, Orozco-Beltran D, Khunti K, et al. Weight beneficial treatments for type 2 diabetes. J Clin Endocrinol Metab. 2011;96:3337–3353. doi: 10.1210/jc.2011-1074. [DOI] [PubMed] [Google Scholar]
- Bristol-Myers Squibb Company/AstraZeneca Pharmaceuticals LP. Full prescribing information: FARXIGA®(dapagliflozin). January 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s000lbl.pdf (last accessed April 30, 2014)
- Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)–a randomized placebo-controlled trial. Am Heart J. 2013;166:217–223. doi: 10.1016/j.ahj.2013.05.007. .e211. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Dotta F, Lauro D, et al. An overview of pancreatic beta-cell defects in human type 2 diabetes: implications for treatment. Regul Pept. 2008;146:4–11. doi: 10.1016/j.regpep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Solomon TP, Knudsen SH, Karstoft K, et al. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J Clin Endocrinol Metab. 2012;97:4682–4691. doi: 10.1210/jc.2012-2097. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- Jurczak MJ, Lee HY, Birkenfeld AL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60:890–898. doi: 10.2337/db10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle LE, Capuano G, Ways K, et al. Effect of canagliflozin, a sodium glucose co-transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12-week, phase 2 study. Curr Med Res Opin. 2012;28:1167–1171. doi: 10.1185/03007995.2012.689956. [DOI] [PubMed] [Google Scholar]
- Nyirjesy P, Zhao Y, Ways K, et al. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin. 2012;28:1173–1178. doi: 10.1185/03007995.2012.697053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Change in hemoglobin A1c from baseline to each visit.
Figure S2 | Change in fasting plasma glucose from baseline to each visit.
Figure S3 | Percent change in bodyweight from baseline to each visit.
Table S1 | Baseline characteristics of patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs (full analysis set).
Table S2 | Changes in efficacy variables from baseline (week 0) to the end of treatment in patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs.
Table S3 | Change in hemoglobin A1c from baseline to the end of treatment in subgroups of patients divided by hemoglobin A1c at baseline (<8.0 vs ≥8.0%).
Table S4 | Adverse events in patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs.
Table S5 | Changes in laboratory parameters in patients treated with canagliflozin as monotherapy or in combination with other oral antihyperglycemic drugs.
Data S1 |Rationale for the sample size.
Data S2 | References.
Data S3 | Study investigators.


