Abstract
In order to assess the effects of climate change in temperate rainforest plants in southern South America in terms of habitat size, representation in protected areas, considering also if the expected impacts are similar for dominant trees and understory plant species, we used niche modeling constrained by species migration on 118 plant species, considering two groups of dominant trees and two groups of understory ferns. Representation in protected areas included Chilean national protected areas, private protected areas, and priority areas planned for future reserves, with two thresholds for minimum representation at the country level: 10% and 17%. With a 10% representation threshold, national protected areas currently represent only 50% of the assessed species. Private reserves are important since they increase up to 66% the species representation level. Besides, 97% of the evaluated species may achieve the minimum representation target only if the proposed priority areas were included. With the climate change scenario representation levels slightly increase to 53%, 69%, and 99%, respectively, to the categories previously mentioned. Thus, the current location of all the representation categories is useful for overcoming climate change by 2050. Climate change impacts on habitat size and representation of dominant trees in protected areas are not applicable to understory plants, highlighting the importance of assessing these effects with a larger number of species. Although climate change will modify the habitat size of plant species in South American temperate rainforests, it will have no significant impact in terms of the number of species adequately represented in Chile, where the implementation of the proposed reserves is vital to accomplish the present and future minimum representation. Our results also show the importance of using migration dispersal constraints to develop more realistic future habitat maps from climate change predictions.
Introduction
In situ conservation of species, communities or ecosystems is widely recognized as the basis for effective biodiversity conservation [1]. The systematic conservation planning (sensu [2]) considers measurable conservation goals in which the representation of biodiversity in reserves is one of the most important issues [3]. Here, representation is understood as the proportion of occurrence of a conservation target within a set of protected areas [4], considering species populations, communities or ecosystems within a geographical context [5].
The Convention on Biological Diversity and other worldwide initiatives recommended a minimum representation level of ecosystems and habitats between 10% and 12% [6,7]. After the Nagoya Summit in 2010, the aim for representation in protected areas (PA) was increased to 17% for terrestrial ecosystems [8,9]. Since the implementation of these policies is a national scale issue, biases and edge artifacts have been found for ecosystems or species distributed in several countries, leading to inefficiencies when establishing protected areas at a continental scale [9]. Furthermore, the long-term persistence of current representation levels of species or ecosystems may be threatened by human-derived global changes, of which climate change is considered one of the most important [10]. As changes become more evident, understanding their potential impact on natural ecosystems will turn to be increasingly important [11], particularly how the representation level of key biodiversity habitats will be affected by projected climate conditions under different conservation schemes.
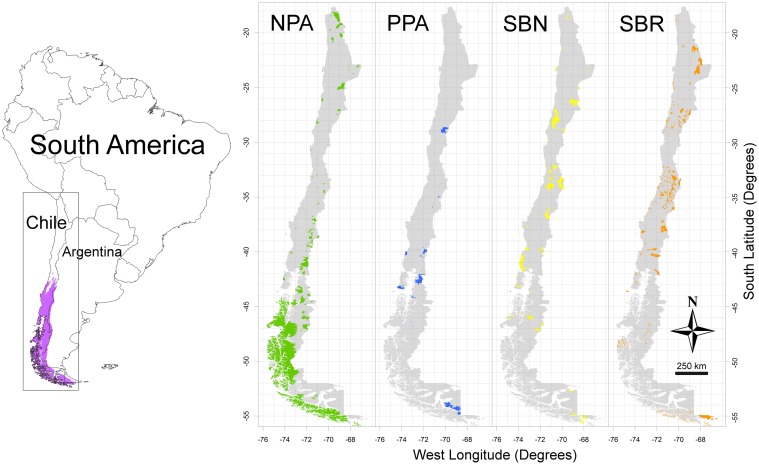
The temperate rainforests of southern South America are located in central and southern Chile with a lesser extent in neighboring areas in southwest Argentina [12], see Fig. 1. They are considered one of the most threatened biodiversity hotspots [13], including different forest communities with high levels of endemism, most of which are dominated by the genus Nothofagus trees [14,15]. Representation assessments have not been carried out for this ecosystem at the plant species level, while proper thresholds for them should consider the national conservation goal of 10% [16] or the Nagoya Summit suggestion of 17% [8].
Fig 1. Location of Nothofagus-dominated temperate rainforests in South America and the geographical distribution of protected areas in Chile: national system of protected areas (NPA), private protected areas (PPA), prioritized sites for biodiversity conservation at national level (SBN) and proposed sites for biodiversity conservation at each Chilean regional administration level (SBR).
Since southern South American temperate rainforests are mainly located in Chile, the Nothofagus-dominated temperate rainforest plants constitute a good case study for assessing the representation of the PA network at a species level, minimizing the biases found by [9] in their representation assessments for species and ecosystems located in several countries. These rainforests have several dominant tree species coexisting with a rich understory including ground and epiphytic plants, allowing comparisons among different plant groups in terms of climate change effects on their habitats and representation in PA as well.
The official Chilean national system of protected areas (NPA) includes national parks and reserves covering more than 18% of the country [17]. However, the NPA shows a strong geographical bias towards the south of the country and high altitudes in the Andes [18] see Fig. 1, and it has been considered insufficient to achieve minimum objectives for conservation at ecosystem or community scales [19] and at the species level for vertebrates [20]. Besides NPA, there are privately owned reserves (PPA), for which a legal regulation is still being developed [21]. Moreover, the Chilean national biodiversity strategy [16] proposed in 2005 a set of prioritized sites for biodiversity conservation in order to strengthen their protection. A new legislation for protected areas and biodiversity issues is currently under debate and from the former set, only a subset is now considered as priority sites for biodiversity conservation at the national level (SBN). The rest of the formerly prioritized sites are now regarded as biodiversity conservation sites at the Chilean regional administration level (SBR) [16,22], see their location in Fig. 1). However, none of the two categories of priority sites have yet been implemented, and there are no comprehensive assessments on how they could help to accomplish conservation goals both currently and in the future. Further, it is known that conclusions about representation levels of protected areas change with the target species or ecosystems [20,23]. Thus, proper evaluations of present and future representation levels should consider assessments with multiple conservation targets (i.e. species).
Niche modeling is the most commonly used method for species distribution projections [11,24,25]. As a set of probabilistic analyses that uses statistical methods linked to geographic information systems, niche modeling depicts the relationships among species spatial distributions and a series of biotic and abiotic variables related to those spatial distributions [26,27,28]. Niche modeling generates species distribution maps, allowing species distributions to be compared at different times if habitat conditions were to change. This has led to a fruitful discussion about the expected spatial shifts in species distribution due to climate change. Several studies in plants have found that species habitats will move towards higher latitudes or altitudes as a result of climate change [24,29]. Interestingly, this has started to be corroborated in the field [30,31].
However, most of potential future distribution maps produced through niche modeling methods have included uncertainties regarding the capability of species populations to effectively migrate to new territories and become part of the species habitat in this new timeframe [32–40]. Depending on the species, full-migration scenarios might be unrealistic because of biological limits for propagule dispersal across landscapes with spatial barriers, and the effective availability of new territories due to increasing human land use changes [41,42]. Different tools have been developed to take these migration constraints on species into account, and hence produce more realistic future distributions for expected climate change scenarios [35,40].
Here we took advantage of modern tools for species distribution models and assessed the representation potential of Chilean protected areas (NPA and PPA) and proposed sites (SBN and SBR), for plant species in southern South American temperate rainforests comparing two groups of dominant trees and two groups of understory plants, using niche modeling tools and considering climate change impact under a future migration constrained scenario.
Materials and Methods
Species selection and data sources
We selected 118 South American temperate rainforest plant species in four groups: a) Nothofagus tree species that dominate most of the South American temperate rainforest (n = 9); b) co-dominant tree and woody species (n = 27) from vegetation communities along with Nothofagus, according to [43]; c) ground ferns, considered as understory species which share their distribution with Nothofagus (n = 55) and d) epiphytic fern species which grow in Nothofagus forests (n = 27). The nomenclatural lists are based on [14] for trees and shrubs, and [44] for ferns. Species localities for trees were taken from the Chilean national forest inventory [45], and the Universidad de Concepción Herbarium (CONC), the most complete collection of Chilean plants. The list of species and the number of valid occurrences for each species is shown in S1 Table.
Selection of climate variables and data sources
Eight variables with the lowest correlations among them were selected from the WorldClim global climate database [46] corresponding to the present climate conditions with a 30 arc-second resolution. Four of them were related to energy constraints: a) mean diurnal temperature range; b) temperature seasonality; c) maximum temperature in the warmest month and d) minimum temperature in the coldest month. The other four variables were directly related to water availability: e) annual precipitation; f) precipitation seasonality; g) precipitation in the warmest quarter and h) precipitation in the coldest quarter.
Modeling methods and present climate models
Plant species distributions were modeled using eight techniques available through the BIOMOD R-package [47–49]: ANN for Artificial Neural Networks, CTA for Classification Tree Analysis, FDA for Flexible Discriminant Analysis, GAM for Generalized Additive Models, GBM for Generalized Boosting Models, GLM for Generalized Linear Models, MARS for Multivariate Adaptive Regression Splines, and RF for Random Forest, details of which are explained in [47] and [48]. The best models according to AUC performance values, Kappa and True skill statistics were selected for each species. We chose the model indicated as the best by a majority of the three criteria. In the few cases where they fully disagreed, we opted for AUC as the selecting criteria (see S2 Table). A current distribution map was produced considering BIOMOD cut-off thresholds to project the best specific niche model. Areas that currently correspond to human land use within these distribution maps were determined by overlaying the models produced with [45] digital information.
Future climate data
We used future climate scenarios available from [50]. Six future scenarios were initially tested for the year 2050: cccma cgcm2 B2a, csiro mk2 B2a, hccpr hadcm3 B2a, cccma cgcm31 a1b, csiro mk30 a1b and ukmo hadcm3 a1b. The most conservative scenario was then chosen according to their least change on Nothofagus species distributions, which was csiro mk2 B2a. The future distribution for each species was projected using BIOMOD from the specific best niche model and the selected future climate data set.
Migration constraints
Once future distribution maps were drawn, migration for each species was modeled using the MIGCLIM R-package [35,49]. MIGCLIM specific parameters to include dispersal kernels, potential propagule production, short-distance dispersal capacity (SDD) and probability for long-distance dispersal (LDD) were developed considering the literature available for each species [51–65] related with propagule dispersal syndromes, probable initial and optimal maturity ages and relative abundance of their populations: see S3 Table for this data. As SDD and LDD are inferred values, a sensitivity analysis of these parameters was carried out to assess how changes in their values affected the predicted habitat size for each species. For this purpose, we performed new runs of every model for each species using new SDD values accounting for 25%, 50% and 200% of the initially inferred value. For LDD, new models were run using 10%, 25%, 50%, 200%, 400% and 1000%. We compared all the MIGCLIM outputs among them and against an unrealistic scenario with no restrictions to migration, observing that the changes included in SDD and LDD values did not generate important changes in habitat sizes, whereas the full migration scenario clearly exaggerated the habitat sizes (See S4 Table and S1 Fig. for further details). Even though native forest substitution in central-south Chile reached an important magnitude during the decades of 1980s and 1990s [66–68], current assessments of land change made by the Chilean Secretary of Forests [69,70] show that this process is declining. In absence of spatial predictions for future land conversion in the whole distribution of these ecosystems, we utilized the current land use maps [44] as a conservative scenario to set the spatial barriers for future dispersion with MIGCLIM.
Protected areas
Both present and migration constrained future distribution outputs of studied species were overlaid on maps corresponding to the following categories of Chilean protected areas: a) the national system of protected wild areas (NPA) managed by the Chilean government; b) private protected areas (PPA) managed by private owners, c) prioritized sites for biodiversity conservation at national level (SBN) as a proposal for new protected areas according to [16], and d) prioritized sites for biodiversity conservation at each Chilean regional administration level (SBR), according to [16]. Maps for the four categories were received from [22,69] and non-governmental organizations such as Así Conserva Chile and World Wildlife Fund Chile, as shown in Fig.1.
Climate change effect assessment
Geographic information system (GIS) processes were performed using the raster R-based package [49,71]. Current and future distribution areas were compared by means of paired Student’s t-test and fitting linear models, both of which utilized the stats R-based package [49]. For assessing the climate change effect on the representation of every species habitats in protected areas, we compared the current and future representation in terms of the percentage of the habitat distribution for each time scenario. Achieving the minimum representation for each species was established according to two thresholds: 10% according to the Chilean national ecosystem representation goal for the year 2015 [16] and 17% in line with the Nagoya summit representation goal for the year 2025 [8].
Results
Expected effects of climate change on habitat size
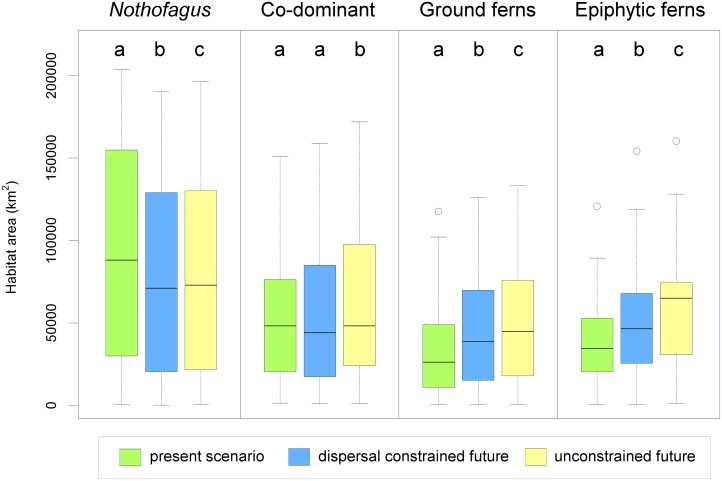
Comparisons between present and the dispersal constrained future scenario for all the studied species showed that Nothofagus species significantly decreased (p = 0.0047) their mean habitat size from an area of 98,553 ± 77,633 km2 to 87,621 ± 72,320 km2 in the future case (Fig. 2, and S5 and S6 Tables). For the co-dominant species group, the mean habitat area (60,130 ± 47,082 km2) showed no significant changes in their evaluation for the future scenario (60,095 ± 47,459 km2). In contrast, ground ferns habitat size (34,619 ± 28,760 km2) would increase in the future (45,423 ± 35,707 km2, p<0.0001), whereas the epiphytic ferns showed in the present scenario a mean habitat size (39,034 ± 28,910 km2) smaller (p<0.0001) than in the future (53,206 ± 37,833 km2). Thus, the future climate change scenario predicted habitat size modifications for the studied plants, with expected increases for 73.7% of the species and shrinkage for 26.3% of them (Table 1). When both unconstrained and dispersal constrained future scenarios were compared, they differed in all the species groups (p = 0.0305 in Nothofagus and p<0.001 in the rest of the groups, see Fig. 2 and S6 Table). Full migration scenario was significantly higher in habitat size (18.7% on average) than migration constrained models, which led us to use only the latter ones, since they consider biological bases for propagule dispersal capacity and life cycle delays, and they were very robust as indicated by the sensitivity analyses (See S4 Table).
Fig 2. Habitat size for each species group and modeled scenario.
Top letters indicate significant differences within each species group according to paired t-tests in S6 Table.
Table 1. Frequencies of expected effects after climate-change scenario (%) for each species group.
Features in parenthesis indicate number of species.
| Effect in: | Nothofagus | Co-dominant | Ground ferns | Epiphytic ferns | Total species | |
|---|---|---|---|---|---|---|
| Habitat size | Representation | |||||
| Decrease | Increase | 100% (9) | 51.9% (14) | 7.3% (4) | 11.1% (3) | 25.4% (30) |
| Increase | Increase | - | 48.1% (13) | 56.4% (31) | 33.3% (9) | 44.9% (53) |
| Increase | Decrease | - | - | 34.5% (19) | 55.6% (15) | 28.8% (34) |
| Decrease | Decrease | - | - | 1.8% (1) | - | 0.8% (1) |
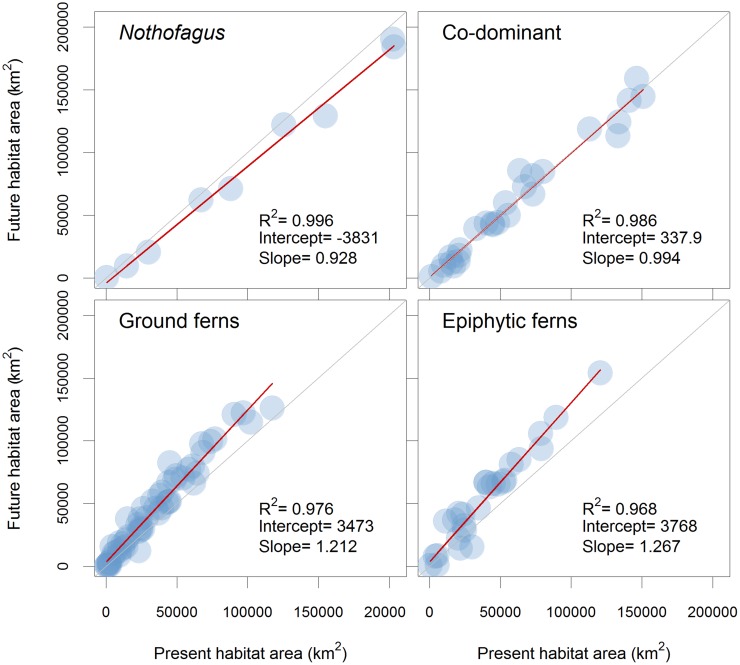
To assess if changes in habitat size were equivalent among species irrespective of their current habitat size, we performed linear general models (LGM) between present and future habitat size for all the species groups (Fig. 3). For Nothofagus, the regression model slope was different (p = 0.003) and lower than 1 (0.928); furthermore, its intercept value was negative, indicating decreases in habitat size where major reductions were observed in species with larger habitats. Co-dominant species proved to maintain their habitat size, or experience only insignificant reductions, since the slope (0.994) did not differ significantly from 1 (p = 0.260). Both species groups, ground ferns and epiphytic ferns, showed linear regression slopes significantly (p<0.001) higher than 1 (1.212 and 1.267, respectively), accompanied by a positive intercept, indicating greater changes for those species with larger current habitats.
Fig 3. Expected changes in habitat area for all the species groups.
Gray diagonal lines represent no change in habitat size; circles below the lines represent species with expected habitat size shrinkages, while circles above the lines represent species with expected habitat size increases. Red lines represent fitted linear models for each species group with their correlation, intercept and slope data.
Expected changes in species representation in protected areas
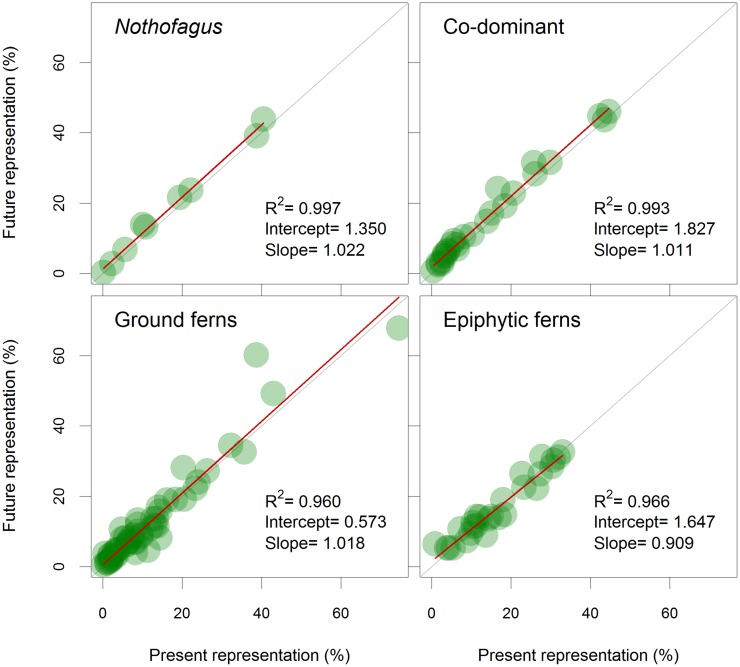
For NPA areas, most of the species showed an increase in representation (70.3%) with climate change; only 29.7% of the studied species showed a decrease in their representation in NPA (Table 1). Considering species groups (Fig. 4), even though all Nothofagus species decreased their habitat size, their habitat representation in NPA increased. This is also seen in the fitted linear model shown in Fig. 4, where we found a positive intercept and a slope significantly (p = 0.0128) greater than 1. Likewise, even though 51.9% of the co-dominant species decreased their habitat size, all of them increased their representation in NPA, as shown by the fitted linear model with a positive intercept and a slope larger than 1 (p<0.001). On the contrary, the habitat size of most ground ferns (90.9%) and epiphytic ferns (88.9%) showed increases in the future scenario. However, future representation in NPA increased by only 63.6% for ground ferns (fitted linear model showing a positive intercept and a slope higher than 1, p = 0.035), versus a decreased future representation of 55.6% for epiphytic fern species, where the slope of the fitted linear model did not differ from 1 (p = 0.270).
Fig 4. Expected changes for all the species group representation.
Gray diagonal lines show no change in representation in NPA, circles under the lines represent expected shrinkages in NPA representation, while circles above the lines depict expected increases in representation in NPA. Red lines represent fitted linear models for each species group with their correlation, intercept and slope data.
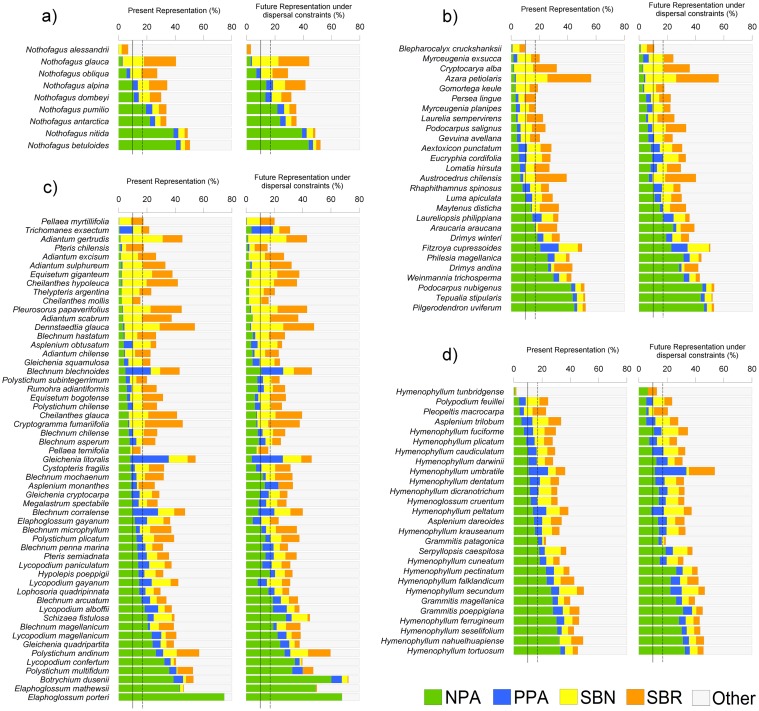
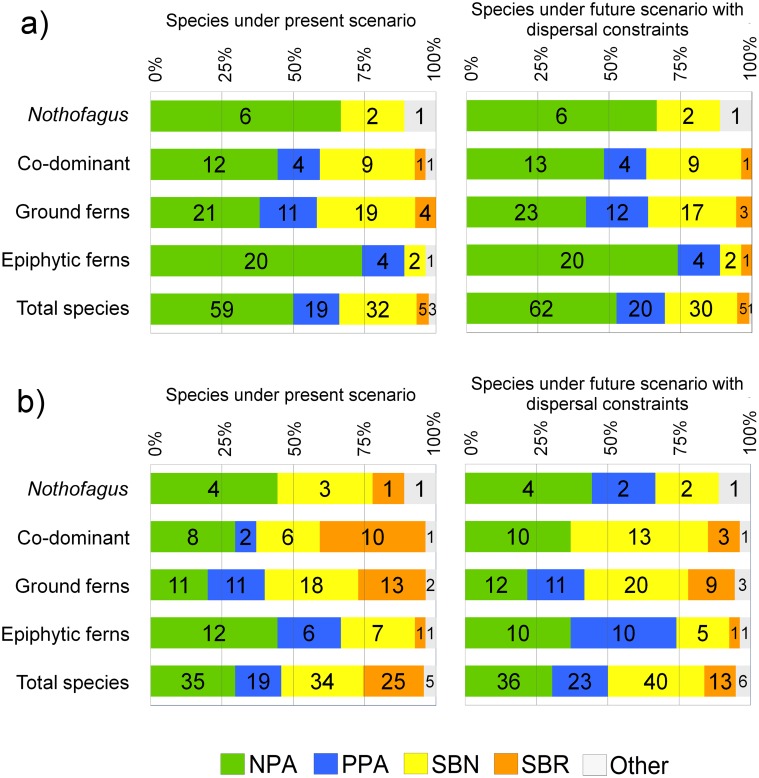
Changes in the representation of all the species in the assessed conservation schemes can be observed in Fig. 5. When a 10% threshold was used as a minimum representation in NPA, only 50% of the total species appeared as adequately protected by the NPA official system. In particular, 67% of Nothofagus species (Fig. 5a), 44% of co-dominant species (Fig. 5b), 38% of ground ferns (Fig. 5c) and 74% of epiphytic ferns (Fig. 5d) are represented in NPA. The NPA representation increased in the climate change scenario for co-dominant species from 44% to 48% of adequately protected species and for ground ferns from 38% to 42%, indicating an increase from 50% to 53%, looking at all the evaluated species (Fig. 6).
Fig 5. Present and future accumulated representation of a) Nothofagus tree species, b) co-dominant plant species, c) ground ferns and d) epiphytic ferns, in the Chilean national protected area system (NPA), private protected areas (PPA), prioritized sites for biodiversity conservation at national level (SBN) and proposed sites for biodiversity conservation at each Chilean regional administration level (SBR).
Species lists in each group are hierarchically ordered by the representation of their present habitats in NPA official system. Continuous vertical lines indicate a 10% representation threshold and dotted lines indicate a 17% representation threshold.
Fig 6. Accumulated proportion of species number with minimum representation in protected areas, considering: a) a 10% threshold as proposed by [16], and b) a 17% threshold as proposed by [8], according to Chilean national protected areas system (NPA), private protected areas (PPA), prioritized sites for biodiversity conservation at national level (SBN) and proposed sites for biodiversity conservation at each Chilean regional administration level (SBR).
Features within strips indicate the aggregated number of species that achieve the minimum conservation target by each protection system.
Considering the 17% threshold, Chilean national protected areas provided protection for only 30% of the total number of studied species: 44% of Nothofagus species, 30% of co-dominant species, 20% of ground ferns and 44% of epiphytic ferns. For the future scenario, 31% of all the studied species may be well represented in the NPA, with the same species number for Nothofagus, but a lower species number for epiphytic ferns (37%), and an increasing representation for co-dominant plants and ground ferns up to 37% and 22%, respectively.
The inclusion of private parks (PPA) significantly affected the minimum representation for some species, such as Nothofagus alpina and Nothofagus dombeyi in the future scenario when considering a 17% threshold, and co-dominant Aextoxicon punctatum and Eucryphia cordifolia with a 10% threshold. At least 11 species of ground ferns proved to be well represented as a result of the inclusion of PPA: Gleichenia litoralis for both thresholds, Blechnum asperum and Megalastrum spectabile for the 10% threshold, and Blechnum penna-marina, Pteris semiadnata and Lycopodium paniculatum for the 17% threshold. The addition of PPA also helped four more epiphytic fern species to be well represented, such as Asplenium trilobum and Hymenophyllum plicatum with the 10% threshold in both present and future scenarios; and for the 17% threshold, 6 and 10 species respectively for each time scenario, including species like Hymenophyllum umbratile and Hymenophyllum dentatum. The inclusion of PPA generated complementary protection to the NPA, aiding 19 more species to be well represented in the present scenario (for both the 10% and 17% thresholds) and 20 more species in the future scenario considering the 10% threshold, and 23 for the 17% threshold.
Prioritized sites for biodiversity conservation at Chilean national level (SBN) were crucial for achieving a satisfactory representation in most species. If the 10% threshold is considered and SBN were implemented at the present, 110 of the 118 evaluated species (93%) achieved enough representation while 95% of the assessed species would attain the minimum representation in the future scenario. When using 17% threshold, SBN could add up to 75% of the species as suitably represented under the present scenario and the SBN location may help to adequately represent up to 84% of the studied species.
The effect of including SBR after SBN is relevant when considering the 17% threshold, since the former could increase the species under proper representation from 75% up to 96% in the present scenario, and from 84% up to 95% for the future scenario, helping to achieve enough representation for co-dominants and ground ferns. However, when using the 17% threshold, 5 species would be currently underrepresented, and only 6 of the studied species would lack minimum representation in the future scenario. The species that would not be well represented under the 17% scenario, considering NPA and PPA, even if the SBN and SBR were implemented, include the endangered tree Nothofagus alessandrii, co-dominant Blepharocalyx cruckshanksii, ground ferns Pteris chilensis, Cheilanthes mollis and Pellaea ternifolia and epiphytic fern Hymenophyllum tumbridgense.
Discussion
The current level of representation within the Chilean NPA at the plant species level can be considered low compared to other studies: only 50% of the species are minimally represented, compared to 100% in Western Europe and 89% in the South African Cape Region [34]. This may be due to the fact that NPA is strongly southward biased and it is not coincident with the greatest diversity of vascular plants located in the center-south of Chile [18]. Interestingly, our results showed that NPA will maintain or even increase the representation level of temperate rainforest plants in the climate change scenario by mid-century, reaching up to 52.5% of species in Chile. This contrasts with a projected decreasing representation of 94% for Western Europe plant species and 78% for species in the Cape Region, for the same 10% protection threshold [34] and with some vertebrate species in Europe which are also expected to lose representation under climate change scenarios [37,38].
The future scenario for climate change indicated changes in the habitat size of the Nothofagus-dominated temperate rainforest of southern South America. Nevertheless, these changes are not predicted to be similar for each plant group, nor are the changes in habitat size necessarily in line with modifications in the representation level of their habitats in official areas such as NPA. For example, while the distributional habitat sizes for Nothofagus species are expected to decrease in the future, their representation level is projected to increase, suggesting that NPA reserves are located in useful areas for this species group in the long-term. In addition, NPA may include areas prone to be colonized by these species in a short-term future, according to the migration models. The same observation can be made with co-dominants; the species for which habitat sizes are projected to decrease (51.9% of species number) are expected to increase their representation levels in NPA. Furthermore, all of the species for which distribution size is anticipated to expand (48.1%) are expected to increase their representation proportion as well. Examples of this may be seen in species such as N. alpina and N. dombeyi, whose northern distribution and habitats at lower altitudes of the Andes mountain range are expected to be lost due to climate change. At the same time, their future habitats are expected to include present habitats that will persist in addition to new expanding zones, both coincident with the current location of NPA reserves at higher altitudes. The same situation may occur with co-dominant trees endemic to central-southern Chile that require high water availability, like Laurelia sempervirens and Persea lingue. These species are expected to decrease their northern lowland distributions where rainfall during the growing season is projected to decrease and this is coincident with areas where NPA reserves are scarce or non-existent. In addition, these species are expected to maintain their southern distributions or expand into areas close to their southern distributions, where more NPA reserves are located.
Understory ferns are expected to differ from the two groups mentioned above: 90.9% of ground fern species are projected to expand their habitat size, but only 63.6% will most likely increase their representation level in NPA. Nonetheless, the most striking difference is seen in epiphytic ferns; 88.9% of these species are expected to gain distribution area, but only 44.4% are anticipated to expand their representation proportion. This is most likely related to the fact that NPA units are geographically biased towards higher elevations, while a significant proportion of epiphytic fern species are prone to expand to lower altitude forests not included in NPA. The latter implies that the location of NPA reserves will continue to be useful in the future, except for one third (29.7%) of the total studied species for which new colonizable areas will not coincide with NPA or some habitats currently protected by NPA may not be climatically suitable in the future. Among these species we can mention a set of ground ferns distributed in the Mediterranean climate of central Chile, such as Cheilanthes hypoleuca, Dennstaedtia glauca, Pteris chilensis and Thelypteris argentina, whose future representation is expected to decrease because the few NPA units existing in central Chile may not be useful for the conservation of these ferns after climate change. Some other ground fern species with larger habitat distributions like Blechnum magellanicum and Lycopodium magellanicum are expected to have a distribution expansion outside NPA units, resulting in a decrease in their future representation. Other cases like Elaphoglossum porteri or Elaphoglossum gayanum have very small present distribution areas, and the decrease in their representation level can be attributed to the fact that their current distributions are mostly within NPA reserves, and in the future they would expand their populations to other places outside NPA. The ecological niches of both groups ground and epiphytic ferns resulted more heterogeneous compared to those of the dominant trees, with a differential response in habitat size changes also, and hence in their representation levels in the NPA system.
The current location of NPA parks and reserves assures enough future protection to maintain the same number of species of Nothofagus trees and epiphytic ferns. Thus, the current location of NPA reserves is important for current representation and it will continue to be important when temperate rainforest species face the expected climate change effects in the future. This suggests that any alterations to NPA units, such as clipping or area reduction for productive exploitations or changes in their main objectives to aims other than preserving habitat representation, are therefore strongly discouraged from the standpoint of biological resource policies for overcoming climate change. Despite the high proportion of Chilean land dedicated to NPA, representation goals are still not being met when biodiversity levels are considered, such as plant species levels, and future climate change for the region. Moreover, the current role of NPA in long-term biodiversity conservation is not fully assured yet since management plans have not been completed for all their units. In recent years, there has been a debate over redefining the main objectives of the NPA system and the government has included tourism among them, leading to building facilities in formerly fully preserved lands. Restricting grazing for the livestock of nearby communities, alien species control and setting maximum visitor limits inside reserves are among the challenges to be accomplished in order to assure the NPA long-term conservation role.
PPA reserves have not yet been fully formalized under law. However, our results showed that they would have a very positive complementary effect to official NPA, since PPA would add conserved habitats for 97.5% of the evaluated species, increasing additional conservation for 73.7% of the studied species in the future scenario. When minimum representation thresholds were considered, PPA reserves were shown to help 16.1% of the studied species to attain a well-conserved status, for both thresholds in the present scenario. Moreover, they could aid 16.9% of these species to obtain a well-represented future condition under the 10% threshold; this is 19.5% of the species if the 17% threshold were used for the future scenario. The positive effects of including PPA are more important for understory fern species than dominant tree species because the distribution of PPA areas is complementary to NPA in low elevations, where some understory ferns may tend to expand their distribution in the future. Since NPA reserves are located unevenly across the Chilean territory, proportionally more distributed towards southern and higher areas, PPA may spatially fit as a significant complement for NPA areas, in the same direction of those found by other evaluations of private reserves [72,73].
As proposed for new protected areas under the Chilean national biodiversity strategy [16], the implementation of the SBN sites virtually completes the supplementary needs for minimum representation for most of the studied species after considering NPA and PPA, both in the present and future scenarios with a 10% threshold. The effect of including the SBR sites resulted important to accomplish satisfactory levels of species representation when applying the most restrictive threshold. This contrasts to the fact that Chilean environmental policy has recently considered most of these SBR areas in a low priority for being implemented as reserves, because they mostly comprised private properties. If the Nagoya summit threshold were applied, the set of species mentioned at the end of Results would still not achieve a minimum representation with NPA, PPA, SBN and SBR combined; most of them have habitats included in a Mediterranean-climate zone with scarce representation under conservation schemes, associated with high replacement due to human land-use. If SBN and SBR were carried out, it would be wise to include the habitat area of those species in the proposed reserves as well, especially for the endangered tree Nothofagus alessandrii, tree Blepharocalyx cruckshanksii and the ground fern Pteris chilensis.
Even though climate change is expected to affect the habitats of the evaluated plant species from these important South American temperate rainforests, the current location of the conservation units determines that there will be no significant change in terms of the number of species with a minimum representation for the evaluated future scenario. Analyzing climate change effects on habitat size and representation in parks, considering only the dominant trees as a basis may not be applicable to other species groups as it was the case for understory plants in this study. The evaluated groups are an example set of plants growing in a temperate rainforest, and their distributions are expected to react differently with climate change. Since their habitat representation may be achieved in a specific manner for every group, it is important to realize that this kind of assessment should consider a diverse and wide spectrum of species groups.
Conclusions
When applying the future scenario, most of the dominant tree species are expected to decrease in habitat size while their representation levels increase, but most of the understory plant species increased their habitat size, as well as their representation level in NPA. Contrary to former worldwide analyses, our study indicated that official Chilean NPA reserves are spatially arranged in a way which corresponds to areas prone to be maintained or expanded as useful habitats for most of the studied species after climate change. Our results highlight the need for strengthening NPA in order to contribute to overcome the climate change in South American temperate rainforest plants. Therefore, it is extremely important not to affect or reduce NPA units due to policy changes or productive purposes.
Private contributions to conservation through PPA reserves help official NPA to achieve minimum representation of most of the studied plant species. This will remain to be true or even increase in importance after climate change for many of the studied species. Implementing the prioritized SBN as formal reserves is also strongly recommended since they virtually complete the minimum representation needs for most of the studied plants in the present as well as the future climate-change scenario if the 10% threshold is observed. Considering an aggregated implementation of proposed SBR would be essential if 17% threshold was taken into account. The evaluated species were chosen because they represent some substantial structural elements of South American temperate rainforests; dominant elements such as Nothofagus tree species and co-dominant woody species are expected to react in their own specific way, different from understory components such as ground ferns and epiphytic ferns. Consequently, climate change assessments must be carried out on a species level and it is important not to assume that expected effects to dominant tree species are necessarily applicable to understory species. Finally, the use of migration dispersal constraints should always be included when more realistic future habitat maps are developed.
Supporting Information
Vertical red line represents 0% change in habitat size considering the parameters set in S3 Table as 100% for both SDD and LDD. A full migration scenario is also included as a comparison for habitat sizes without using MIGCLIM.
(TIF)
Each species is presented according to taxonomic family, species group and endemism to South American temperate rainforests, and the number of valid occurrences used in niche modeling.
(DOC)
The following modeling techniques were included: ANN for Artificial Neural Networks, CTA for Classification Tree Analysis, FDA for Flexible Discriminant Analysis, GAM for Generalized Additive Models, GBM for Generalized Boosting Models, GLM for Generalized Linear Models, MARS for Multivariate Adaptive Regression Splines, and RF for Random Forest.
(DOC)
(DOC)
A full migration scenario is also included as a comparison for habitat sizes without using MIGCLIM.
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank Roberto Rodríguez and Alicia Marticorena from Universidad de Concepción Herbarium (CONC) for their help with species localities, CONAF for information on Chilean forest inventory, Así Conserva Chile and WWF Chile for PA maps.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
DA received funding from the Chilean National Commission for Scientific and Technological Research (http://www.conicyt.cl) as a doctoral grant. DA and LAC received funding from the Institute of Ecology and Biodiversity (IEB, http://www.ieb-chile.cl) funded by projects FICM P05-002 and PFB-023. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
References
- 1. Rodrigues ASL, Andelman SJ, Bakarr MI, Boitani L, Brooks TM, Cowling RM, et al. Effectiveness of the global protected area network in representing species diversity. Nature. 2004;428: 640–643. 10.1038/nature02422 [DOI] [PubMed] [Google Scholar]
- 2. Margules CR, Pressey RL. Systematic conservation planning. Nature. 2000;405: 243–253. [DOI] [PubMed] [Google Scholar]
- 3. Margules CR, Sarkar S. Systematic conservation planning. Cambridge University Press; 2007. [Google Scholar]
- 4. Cabeza M, Moilanen A. Design of reserve networks and the persistence of biodiversity. Trends Ecol Evol. 2001;16: 242–248. [DOI] [PubMed] [Google Scholar]
- 5. Kukkala AS, Moilanen A. Core concepts of spatial prioritisation in systematic conservation planning. Biol Rev Camb Philos Soc. 2013;88: 443–164. 10.1111/brv.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tear TH, Kareiva P, Angermeier PL, Comer P, Czech B, Kautz R, et al. How much is enough? The recurrent problem of setting measurable objectives in conservation. Bioscience. 2005;55: 835–849. [Google Scholar]
- 7. Burgess N, Küper W, Mutke J, Brown J, Westaway S, Turpie S, et al. Major gaps in the distribution of protected areas for threatened and narrow range Afrotropical plants. Biodivers Conserv. 2005;14: 1877–1894. 10.1007/s10531-004-1299-2 [DOI] [Google Scholar]
- 8.UNEP/CBD. Decisions adopted by the Conference of the Parties to the Convention on Biological Diversity at its tenth meeting. Nagoya, Japan. 2010. Available: http://www.cbd.int/doc/decisions/cop-10/full/cop-10-dec-en.pdf.
- 9. Moilanen A, Anderson BJ, Arponen A, Pouzols FM, Thomas CD. Edge artefacts and lost performance in national versus continental conservation priority areas. Divers Distrib. 2013;19: 171–183. 10.1111/ddi.12000 [DOI] [Google Scholar]
- 10. Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, et al. Global Biodiversity Scenarios for the Year 2100. Science. 2000;287: 1770–1774. 10.1126/science.287.5459.1770 [DOI] [PubMed] [Google Scholar]
- 11. Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc Natl Acad Sci U S A. 2009;106: 19729–19736. 10.1073/pnas.0901639106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donoso C. Bosques templados de Chile y Argentina Variación, estructura y dinámica. Editorial Universitaria, Santiago, Chile; 1993. [Google Scholar]
- 13. Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403: 853–858. [DOI] [PubMed] [Google Scholar]
- 14. Zuloaga FO, Morrone O, Belgrano MJ, Marticorena C, Marchesi E. Catálogo de Plantas Vasculares del Cono Sur. Monogr. Missouri Bot. Garden USA; 2008. [Google Scholar]
- 15. Moreira-Muñoz A. Plant Geography of Chile. Springer; 2011. 10.1007/978-90-481-8748-5 [DOI] [Google Scholar]
- 16. CONAMA. Plan de Acción de País para la Implementación de la Estrategia Nacional de Biodiversidad. Comisión Nacional del Medio Ambiente, Gobierno de Chile; Santiago, Chile; 2005. [Google Scholar]
- 17. Pauchard A, Villarroel P. Protected Areas in Chile: History, Current Status, and Challenges. Nat Areas J. 2002;22: 318–330. [Google Scholar]
- 18. Armesto JJ, Rozzi R, Smith-Ramírez C, Arroyo MTK. Conservation targets in South American temperate forests. Science. 1998;282: 1271–1272. [Google Scholar]
- 19. Pliscoff P, Fuentes-Castillo T. Representativeness of terrestrial ecosystems in Chile's protected area system. Environ Conserv. 2011;38, 303–311. 10.1017/S0376892911000208 [DOI] [Google Scholar]
- 20. Tognelli MF, Ramírez de Arellano PI, Marquet PA. How well do the existing and proposed reserve networks represent vertebrate species in Chile? Divers Distrib. 2008;14: 148–158. 10.1111/j.1472-4642.2007.00437.x [DOI] [Google Scholar]
- 21. Squeo FA, Estévez RA, Stoll A, Gaymer CF, Letelier L, Sierralta L, et al. Towards the creation of an integrated system of protected areas in Chile: achievements and challenges. Plant Ecol Divers. 2012;5: 233–243. 10.1080/17550874.2012.679012 [DOI] [Google Scholar]
- 22.Ministerio del Medio Ambiente de Chile. Infraestructura de datos espaciales. Gobierno de Chile. 2014. Available: http://ide.mma.gob.cl/.
- 23. Andrew ME, Wulder MA, Cardille JA. Protected areas in boreal Canada: a baseline and considerations for the continued development of a representative and effective reserve network. Environ Rev. 2014;22: 135–160. 10.1139/er-2013-0056 [DOI] [Google Scholar]
- 24. Guisan A, Theurillat JP. Equilibrium modeling of alpine plant distribution: how far can we go? Phytocoenologia. 2000;30: 353–384. [Google Scholar]
- 25. Zimmermann NE, Yoccoz NG, Edwards TC, Meier ES, Thuiller W, Guisan A, et al. Climatic extremes improve predictions of spatial patterns of tree species. Proc Natl Acad Sci U S A. 2009;106: 19723–19728. 10.1073/pnas.0901643106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Modell. 2000;135: 147–186. [Google Scholar]
- 27. Anderson RP, Lew D, Peterson AT. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol Modell. 2003;162: 211–232. 12876274 [Google Scholar]
- 28. Guisan A, Lehmann A, Ferrier S, Austin M, Overton JMC, Aspinall R, et al. Making better biogeographical predictions of species' distributions. J Appl Ecol. 2006;43: 386–392. 10.1111/j.1365-2664.2006.01164.x [DOI] [Google Scholar]
- 29. Jump AS, Mátyás C, Peñuelas J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol Evol. 2009;24: 694–701. 10.1016/j.tree.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 30. Walther G. Plants in a warmer world. Perspect Plant Ecol Evol Syst. 2003;6: 169–185. [Google Scholar]
- 31. Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320: 1768–1771. 10.1126/science.1156831 [DOI] [PubMed] [Google Scholar]
- 32. Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proc Natl Acad Sci U S A. 2005;102: 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Araújo MB, Lobo JM, Moreno JC. The effectiveness of Iberian protected areas in conserving terrestrial biodiversity. Conserv Biol. 2007;21: 1423–1432. 10.1111/j.1523-1739.2007.00827.x [DOI] [PubMed] [Google Scholar]
- 34. Hannah L, Midgley G, Andelman S, Araújo M, Hughes G, Martinez-Meyer E, et al. Protected area needs in a changing climate. Front Ecol Environ. 2007;5: 131–138. [Google Scholar]
- 35. Engler R, Hordijk W, Guisan A. The MIGCLIM R package—seamless integration of dispersal constraints into projections of species distribution models. Ecography. 2012;35: 872–878. 10.1111/j.1600-0587.2012.07608.x [DOI] [Google Scholar]
- 36. Araújo MB, Alagador D, Cabeza M, Nogués-Bravo D, Thuiller W. Climate change threatens European conservation areas. Ecol Lett. 2011;14: 484–492. 10.1111/j.1461-0248.2011.01610.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D’Amen M, Bombi P, Pearman PB, Schmatz DR, Zimmermann NE, Bologna MA, et al. Will climate change reduce the efficacy of protected areas for amphibian conservation in Italy? Biol Conserv. 2011;144: 989–997. 10.1016/j.biocon.2010.11.004 [DOI] [Google Scholar]
- 38. Carvalho SB, Brito JC, Crespo EG, Watts ME, Possingham HP. Conservation planning under climate change: Toward accounting for uncertainty in predicted species distributions to increase confidence in conservation investments in space and time. Biol Conserv. 2011;144: 2020–2030. 10.1016/j.biocon.2011.04.024 [DOI] [Google Scholar]
- 39. Kuhlmann M, Guo D, Veldtman R, Donaldson J. Consequences of warming up a hotspot: species range shifts within a centre of bee diversity. Divers Distrib. 2012;18: 885–897. 10.1111/j.1472-4642.2011.00877.x [DOI] [Google Scholar]
- 40. Bateman BL, Murphy HT, Reside AE, Mokany K, VanDerWal J. Appropriateness of full-, partial- and no-dispersal scenarios in climate change impact modelling. Divers Distrib. 2013;19: 1224–1234. 10.1111/ddi.12107 [DOI] [Google Scholar]
- 41. Engler R, Randin CF, Vittoz P, Czáka T, Beniston M, Zimmermann NE, et al. Predicting future distributions of mountain plants under climate change: does dispersal capacity matter? Ecography. 2009;32: 34–45. 10.1111/j.1600-0587.2009.05789.x [DOI] [Google Scholar]
- 42. Summers DM, Bryan BA, Crossman ND, Meyer WS. Species vulnerability to climate change: impacts on spatial conservation priorities and species representation. Glob Chang Biol. 2012;18: 2335–2348. 10.1111/j.1365-2486.2012.02700.x [DOI] [Google Scholar]
- 43. Gajardo R. La vegetación natural de Chile: clasificación y distribución geográfica. Editorial Universitaria, Santiago, Chile; 1994. [Google Scholar]
- 44. Rodríguez R. Pteridophyta In: Marticorena C, Rodríguez R, editors. Flora de Chile: Vol. 1. Pteridophyta—Gymnospermae. Universidad de Concepción; 1995. pp. 119–309. [Google Scholar]
- 45. CONAF/CONAMA/BIRF. Catastro y evaluación de recursos vegetacionales nativos de Chile. Corporación Nacional Forestal; Santiago, Chile; 1999. [Google Scholar]
- 46. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25: 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- 47. Thuiller W. BIOMOD—optimizing predictions of species distributions and projecting potential future shifts under global change. Glob Chang Biol. 2003;9: 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thuiller W, Lafourcade B, Engler R, Araújo MB. BIOMOD—a platform for ensemble forecasting of species distributions. Ecography. 2009;32: 369–373. 10.1111/j.1600-0587.2008.05742.x [DOI] [Google Scholar]
- 49. R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2012. Available: http://www.r-project.org/. 10.1002/jcc.22917 [DOI] [Google Scholar]
- 50. Ramirez J, Jarvis A. High resolution statistically downscaled future climate surfaces. International Center for Tropical Agriculture (CIAT), Cali, Colombia; 2008. [Google Scholar]
- 51. Tryon R. Development and evolution of fern floras of Oceanic Islands. Biotropica. 1970;2: 76–84. [Google Scholar]
- 52. Tryon R. The biogeography of species, with special reference to ferns. Bot Rev. 1986;52: 117–156. 10.1007/BF02860999 [DOI] [Google Scholar]
- 53. Armesto JJ, Rozzi R. Seed dispersal syndromes in the rain forest of Chiloé: evidence for the importance of biotic dispersal in a temperate rain forest. J Biogeogr. 1989;16: 219–226. 10.2307/2845258 [DOI] [Google Scholar]
- 54. Donoso C, Lara A. Silvicultura de los bosques nativos de Chile. Santiago: Editorial Universitaria; 1998. [Google Scholar]
- 55. Cuevas J. Tree recruitment at the Nothofagus pumilio alpine timberline in Tierra del Fuego, Chile. J Ecol. 2000;88: 840–855. 10.1046/j.1365-2745.2000.00497.x [DOI] [Google Scholar]
- 56. Loehle C. Strategy space and the disturbance spectrum: a life-history model for tree species coexistence. Am Nat. 2000;156: 14–33. 10.1086/303369 [DOI] [PubMed] [Google Scholar]
- 57. Armesto JJ, Díaz I, Papic C, Willson MF. Seed rain of fleshy and dry propagules in different habitats in the temperate rainforests of Chiloé Island, Chile. Austral Ecol. 2001;26: 311–320. 10.1046/j.1442-9993.2001.01116.x [DOI] [Google Scholar]
- 58. Figueroa J. Seed germination in temperate rain forest species of southern Chile: chilling and gap-dependency germination. Plant Ecol. 2003;166: 227–240. 10.1023/A:1023286521721 [DOI] [Google Scholar]
- 59. Hechenleitner PV, Gardner MF, Thomas PI, Echeverría C, Escobar B, Brownless P, et al. Plantas amenazadas del centro-sur de Chile. Universidad Austral de Chile y Real Jardín Botánico de Edimburgo; 2005. [Google Scholar]
- 60. Donoso C. Las especies arbóreas de los bosques templados de Chile y Argentina Autoecología. Valdivia: Marisa Cuneo Ediciones; 2006. [Google Scholar]
- 61. Smith-Ramírez C, Rovere AE, Núñez-Ávila MC, Armesto JJ. Habitat fragmentation and reproductive ecology of Embothrium coccineum, Eucryphia cordifolia and Aextoxicon punctatum in southern temperate forests In: Newton A, editor. Biodiversity loss and conservation in fragmented forest landscapes: the forests of Montane Mexico and Temperate South America; 2007. pp. 102–119. 10.1079/9781845932619.0102 [DOI] [Google Scholar]
- 62. Rodríguez R, Alarcón D, Espejo J. Helechos nativos del centro y sur de Chile. Guía de Campo; Concepción: Ed. Corporación Chilena de la Madera; 2009. [Google Scholar]
- 63. De Groot GA, During HJ, Ansell SW, Schneider H, Bremer P, Wubs ER, et al. Diverse spore rains and limited local exchange shape fern genetic diversity in a recently created habitat colonized by long-distance dispersal. Ann Bot. 2012;109: 965–978. 10.1093/aob/mcs013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK. Long-distance dispersal: a framework for hypothesis testing. Trends Ecol Evol. 2012;27: 47–56. 10.1016/j.tree.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 65. Núñez-Ávila MC, Uriarte M, Marquet P, Armesto JJ. Decomposing recruitment limitation for an avian-dispersed rain forest tree in an anciently fragmented landscape. J Ecol. 2013;101: 1439–1448. 10.1111/1365-2745.12148 [DOI] [Google Scholar]
- 66. Echeverria C, Coomes D, Salas J, Rey-Benayas JM, Lara A, Newton A. Rapid deforestation and fragmentation of Chilean Temperate Forests. Biol Conserv. 2006;130: 481–494. 10.1016/j.biocon.2006.01.017 [DOI] [Google Scholar]
- 67. Aguayo M, Pauchard A, Azócar G, Parra O. Cambio del uso del suelo en el centro sur de Chile a fines del siglo XX. Entendiendo la dinámica espacial y temporal del paisaje. Rev Chil Hist Nat. 2009;82: 361–374. 10.4067/S0716-078X2009000300004 [DOI] [Google Scholar]
- 68. Echeverría C, Newton A, Nahuelhual L, Coomes D, Rey-Benayase JM. How landscapes change: Integration of spatial patterns and human processes in temperate landscapes of southern Chile. Appl Geogr. 2012;32: 822–831. 10.1016/j.apgeog.2011.08.014 [DOI] [Google Scholar]
- 69.Ministerio de Agricultura de Chile. Sistema de información territorial. Gobierno de Chile. 2014. Available: http://sit.conaf.cl/.
- 70.SINIA. Cubiertas de localización de áreas silvestres protegidas. Sistema Nacional de Información Ambiental de Chile. 2012. Available: http://www.sinia.cl/.
- 71. Hijmans RJ, van Etten J. raster: Geographic analysis and modeling with raster data R package version 1.8–39 2012. Available: http://cran.r-project.org/package=raster. [Google Scholar]
- 72. Jackson SF, Gaston KJ. Incorporating private lands in conservation planning: protected areas in Britain. Ecol Appl. 2008;18: 1050–1060. [DOI] [PubMed] [Google Scholar]
- 73. Gallo JA, Pasquini L, Reyers B, Cowling RM. The role of private conservation areas in biodiversity representation and target achievement within the Little Karoo region, South Africa. Biol Conserv. 2009;142: 446–454. 10.1016/j.biocon.2008.10.025 [DOI] [Google Scholar]
- 74. Vergara R, Gitzendanner MA, Soltis DE, Soltis PS. Population genetic structure, genetic diversity, and natural history of the South American species of Nothofagus subgenus Lophozonia (Nothofagaceae) inferred from nuclear microsatellite data. Ecol Evol. 2014;4: 2450–2471. 10.1002/ece3.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vertical red line represents 0% change in habitat size considering the parameters set in S3 Table as 100% for both SDD and LDD. A full migration scenario is also included as a comparison for habitat sizes without using MIGCLIM.
(TIF)
Each species is presented according to taxonomic family, species group and endemism to South American temperate rainforests, and the number of valid occurrences used in niche modeling.
(DOC)
The following modeling techniques were included: ANN for Artificial Neural Networks, CTA for Classification Tree Analysis, FDA for Flexible Discriminant Analysis, GAM for Generalized Additive Models, GBM for Generalized Boosting Models, GLM for Generalized Linear Models, MARS for Multivariate Adaptive Regression Splines, and RF for Random Forest.
(DOC)
(DOC)
A full migration scenario is also included as a comparison for habitat sizes without using MIGCLIM.
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.