Abstract
Sediment cores were collected from three sites (1000–1200 m water depth) in the northeastern Gulf of Mexico from December 2010 to June 2011 to assess changes in benthic foraminiferal density related to the Deepwater Horizon (DWH) event (April-July 2010, 1500 m water depth). Short-lived radioisotope geochronologies (210Pb, 234Th), organic geochemical assessments, and redox metal concentrations were determined to relate changes in sediment accumulation rate, contamination, and redox conditions with benthic foraminiferal density. Cores collected in December 2010 indicated a decline in density (80–93%). This decline was characterized by a decrease in benthic foraminiferal density and benthic foraminiferal accumulation rate (BFAR) in the surface 10 mm relative to the down-core mean in all benthic foraminifera, including the dominant genera (Bulimina spp., Uvigerina spp., and Cibicidoides spp.). Cores collected in February 2011 documented a site-specific response. There was evidence of a recovery in the benthic foraminiferal density and BFAR at the site closest to the wellhead (45 NM, NE). However, the site farther afield (60 NM, NE) recorded a continued decline in benthic foraminiferal density and BFAR down to near-zero values. This decline in benthic foraminiferal density occurred simultaneously with abrupt increases in sedimentary accumulation rates, polycyclic aromatic hydrocarbon (PAH) concentrations, and changes in redox conditions. Persistent reducing conditions (as many as 10 months after the event) in the surface of these core records were a possible cause of the decline. Another possible cause was the increase (2–3 times background) in PAH’s, which are known to cause benthic foraminifera mortality and inhibit reproduction. Records of benthic foraminiferal density coupled with short-lived radionuclide geochronology and organic geochemistry were effective in quantifying the benthic response and will continue to be a valuable tool in determining the long-term effects of the DWH event on a larger spatial scale.
Introduction
The Deepwater Horizon (DWH) event released over 4.9 million barrels of oil into the Gulf of Mexico from April to July of 2010 [1]. An estimate of 60% of the oil reached the surface where it was subject to skimming, coastal deposition, evaporation, and incorporation into flocculent material [2]. Flocculent material consisting of algae, dispersant, clay particles, and microbes formed at the water surface with the aggregated oil and settled to the sea floor [3]. Subsurface intrusions of natural gas and oil also formed in the water column, with the dominant intrusion occurring from 1000–1300 m predominantly along a northeast to southwest transect [4,5]. Ryerson et al. [6] estimated that only 35% of the oil made it to the water surface and 35% was included in the subsurface intrusion. The estimates from Ryerson et al. [6] and Thibodeaux et al. [2] suggest that as much as 30–40% of the oil unaccounted for was likely deposited on the seafloor.
There are several pathways for oil to be transported to the seafloor. The two primary hypotheses are: (1) the bathtub-ring hypothesis and (2) the flocculent blizzard hypothesis. The bathtub-ring hypothesis refers to the direct contact of microdroplets and dissolved hydrocarbons from the subsurface intrusion [7] at the sediment-water interface on the continental slope. The flocculent blizzard hypothesis refers to the large amount of organic flocculent and hydrocarbon material (large droplet phase) that was deposited during, and following, the event [8,9].
The study of benthic foraminifera provides several strengths in assessing the effects of the Deepwater Horizon event on the benthic environment. There are high densities of benthic foraminifera in the shelf and slope sediments of the Gulf of Mexico [10–12], which allows for a robust assessment of changes in benthic foraminiferal density. The lifespan of benthic foraminifera is on the order of months to years, which readily allows for adaptation to environmental changes [13,14]. This turnover provides an event stratigraphy of benthic foraminiferal density on the order of an event such as the DWH (several months). Finally, benthic foraminifera are sensitive to the introduction of toxins and hydrocarbons [15–21].
Many studies have described the benthic foraminifera assemblages associated with the shelf and slope environments in the Gulf of Mexico [10,11,22–27]. Bernhard et al. [27] documented a dominance of agglutinated benthic foraminifera at all sampling sites from 500–3000 m water depth. Denne and Sen Gupta [24] identified a specific benthic foraminifera assemblage dominated by Cibicidoides wuellerstorfi, Bulimina aculeata and others associated with Caribbean Midwater (CMW), which is dominant between 850 and 1500 m water depth. Culver and Buzas [23] identified the dominant benthic foraminifera species in the outer shelf and slope as Bulimina marginata and Uvigerina peregrina. Osterman [26] also identified Cibicidoides pachyderma (epifaunal), Uvigerina peregrina (shallow infaunal) and Bulimina aculeata (shallow infaunal) as the dominant species in the upper and lower slope sediments. Sen Gupta and Aharon [25] suggested that near hydrocarbon seeps in the northern Gulf of Mexico, several species of benthic foraminifera are possibly facultative anaerobes and can adapt to periods of anoxia and high hydrocarbon concentrations.
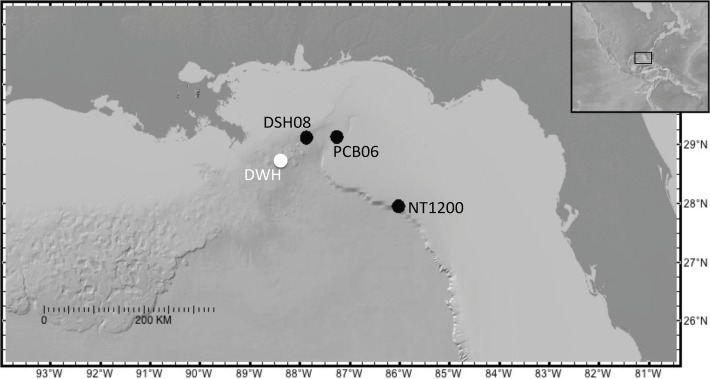
Models suggest that a considerable amount of oil may have been transported into the Desoto Canyon (Northeastern Gulf of Mexico) during and following the DWH event [7]. Other studies have documented a 4–10-fold increase in sediment accumulation rate [9], persistent reducing (anoxic) zones in the surface sediment [28], and a 2–3-fold increase in polycyclic aromatic hydrocarbons (PAH) concentrations [29] in the Desoto Canyon following the DWH event. The primary objective of this study is to characterize the impacts of the Deepwater Horizon event on the benthic foraminiferal density in sediment cores, collected from 1050–1150 m water depth in the Desoto Canyon after the DWH event (Fig. 1). This study aims to report temporal changes in the benthic foraminiferal density and to further propose the most likely factors that caused those changes. The observation of a decline in benthic foraminiferal density synchronous with other sedimentary and geochemical signatures suggests an impact on the benthic environment following the DWH event.
Fig 1. Location of core sampling sites in the northeastern Gulf of Mexico with reference to the Deepwater Horizon.
Methods
Field methods
Sediment cores were collected throughout the northeastern Gulf of Mexico (Fig. 1) using an Ocean Instruments MC-800 multicoring system, which collects eight cores simultaneously. At each site, one of the eight cores collected was utilized for benthic foraminifera analysis and was paired with one core utilized for short-lived radionuclide (SLR) geochronology, one core for organic geochemistry, and one core for redox metal chemistry. Two initial sites [PCB06 (29° 5.99’ N, 87° 15.93 W, 1043 m depth) and DSH08 (29° 7.25’ N, 87° 51.93’ W, 1143 m depth)] were chosen for benthic foraminifera analysis due to preliminary organic geochemistry results suggesting the presence of oil (University of South Florida, College of Marine Science’s baseline survey, 29) and each site was located at a water depth that was within the range of the documented primary hydrocarbon plume (1000–1300 m) [1,6]. Due to the lack of records taken pre-DWH in the area of DSH08 and PCB06, a core was collected at site NT1200 (27° 57.98’ N, 86° 1.38’ W, 1200 m depth) to provide a control record representing an area that was outside of the surface and intrusion (plume) expression of elevated hydrocarbons concentrations related to the DWH event. No specific permissions were required to collect sediments at these sites and did not involve any protected or endangered species. Cores were refrigerated (∼4°C) until sub-sampled by extrusion at 2 mm intervals for the upper 50 mm, with the exception of the December 2010 DSH08 core (2mm to 20mm), and 5 mm intervals for the remainder of the core using a calibrated, threaded-rod extrusion device [30,31].
Benthic foraminifera
Extruded subsamples were freeze-dried, weighed and washed with a sodium hexametaphosphate solution through a 63-μm sieve to disaggregate the clay particles from foraminifera tests. The fraction remaining on the sieve (coarse fraction) was dried, weighed again, and stored at room temperature. All benthic foraminifera were picked from the samples, identified, and counted. Total density is reported, as opposed to living community density for direct comparison of up-core (post-DWH) to down-core (pre-DWH/background) records [26,32,33]. The down-core (pre-DWH) sections were utilized as background samples due to the lack of previous coring efforts at these sites. The use of down-core samples as background samples was based on the assumption that there are statistically negligible numbers of living foraminifera at these depths (e.g. 100 mm) [17,31,34] and that they represent periods of deposition before influence from the DWH event [9]. The total density approach was also appropriate seeing as these records were to be used as reference records when determining any persistent sedimentary (physical, chemical, biological) features related to the DWH event in future sedimentary records on the decadal time-scale [26,32]. Foraminiferal density values were reported in individuals per unit volume (indiv./cm3)[34]. The values were normalized to the known wet volume of each sample based on the diameter of the core tube (10 cm) and the height of each sample (2 or 5 mm). Non-metric multi-dimensional scaling (nMDS) plots (Bray Curtis) were constructed using the PAST paleo-statistics suite to assess the relative control of redox metal concentration and PAH concentration on benthic foraminiferal density.
Short-lived radionuclide geochronology
Extruded subsamples were freeze-dried, weighed, homogenized, and sealed in plastic containers. Short-lived radioisotope geochronology was used to distinguish the pre-DWH and post-DWH intervals. Samples were counted on a Canberra HPGe (high-purity germanium) coaxial planar photon detector to determine 210Pb and 234Th activity. Activities were corrected for counting time, detector efficiency, and self- absorption using the IAEA RGU-1 standard [35, 36]. The constant rate of supply (CRS) model was employed to assign a date-depth relationship, which is appropriate under varying accumulation rates [37, 38].
Benthic foraminiferal mass accumulation rates (BFAR)
Considering the substantial increase in sedimentation documented in 2010 and 2011 in these cores, the benthic foraminiferal density alone did not account for compaction or dilution [9]. To account for compaction and dilution, a benthic foraminiferal accumulation rate (BFAR) approach was taken to determine changes from the down-core section to the upper section of each record [39,40]. BFAR were reported as the number of foraminifera per unit area over time (fcm−2yr−1).
Redox Metal Concentrations
Redox metal concentration methods and data can be found in Hastings et al. (2014).
Organic Geochemistry
EPA methods (8270D, 8015C) [41,42] and QA/QC protocols were followed for the analysis of hydrocarbons. Freeze-dried samples were extracted under high temperature (100°C) and pressure (1500 psi) with a solvent mixture 9:1v:v dichloromethane: methanol (MeOH) using an Accelerated Solvent Extraction system (ASE 2000, Dionex). Two extraction blanks were included with each set of samples (15–20 samples). The aromatic fraction was separated using solid-phase extraction (SiO2/C3-CN, 1 g/0.5 g, 6 mL) and hexane/dichloromethylene (3:1, v:v) as the solvent. PAHs were quantified using a gas chromatograph/mass spectrometric detector (GC/MS) in full scan mode (m/z 50–550) and splitless injections of 1μL. Oven temperature was 60°C for 8 min, increased to 290°C at a rate of 6°C/min and held for 4 min, then increased to 340°C at a rate of 14°C/min, and held at the upper temperature for 5 min. Concentrations of PAHs were calculated using response factors by comparison with a known standard mixture (16-unsubstituited EPA priority and selected isomers: Ultrascientific US-106N PAH mix, NIST 1491a) and were corrected for the recovery of the surrogate standard (d10-acenaphthene, d10-phenanthrene, d10-fluoranthene, d12-benz(a)anthracene, d12-benzo(a)pyrene, d14-dibenz(ah)anthracene, d14-benzo(ai)perylene). Recoveries from spiked samples were generally within 60–120%.
Prior to analysis of TOC, pre-weighed subsamples were acidified (80% 1.0N HCl) to remove inorganic carbon. Dried subsamples were placed in silver capsules and analyzed using a CarloeErba 2500 Series 2 Elemental Analyzer coupled to a Thermo Finnigan Delta XL. All samples were analyzed in duplicate and data reported as the average (<1% difference between duplicates). Detailed methods can be found in Romero et al. 2014 [29].
Results
Pre-DWH and post-DWH sections of each sedimentary record were established through short-lived radionuclide (210Pb and 234Th) geochronologies (Table 1)[9].
Table 1. Short-lived radioisotope (210Pb, 234Th) activities, constant rate of supply age model, total organic carbon (TOC) percentages and TOC accumulation rates with depth for each core [29].
| Depth | Excess | 210Pb | Excess | 234Th | CRS | CRS | TOC | TOC Acc. |
|---|---|---|---|---|---|---|---|---|
| 210 Pb | Error | 234 Th | Error | Date | Error | Rate | ||
| (mm) | (dpmg −1) | (1σ) | (dpmg −1) | (1σ) | (year) | (1σ) | (%) | (gcm −2 yr −1) |
| December 2010 DSH08 | ||||||||
| 2 | 71.8 | 2.1 | 10.2 | 1 | 2010.9 | 1.3 | 2 | 135 |
| 4 | 71.8 | 1.7 | 9.1 | 0.8 | 2010.9 | 1.3 | 1.9 | 131 |
| 6 | 69.9 | 1.6 | 6.7 | 0.7 | 2010.8 | 1.3 | 2 | 136 |
| 8 | 70.3 | 1.4 | 5.6 | 0.6 | 2010.8 | 1.3 | 2 | 135 |
| 10 | 69.7 | 1.3 | 5.1 | 0.5 | 2010.7 | 1.3 | 2 | |
| 12 | 61.4 | 1.8 | 4.3 | 0.8 | 2009.6 | 1.3 | 2 | Pre-2010 |
| 14 | 56.5 | 1.1 | 4.6 | 0.5 | 2008.5 | 1.3 | 2 | 11.4–12.6 |
| 16 | 63.3 | 1.2 | 4.2 | 0.5 | 2007.5 | 1.3 | 2 | |
| 18 | 51.6 | 1.1 | 3.8 | 0.5 | 2006.5 | 1.3 | 2 | |
| 20 | 51.7 | 1.1 | 3.9 | 0.5 | 2005.6 | 1.3 | 1.9 | |
| 30 | 44.3 | 1 | 4.3 | 0.2 | 2000.7 | 1.4 | 2 | |
| 35 | 38.3 | 0.9 | 3.9 | 0.4 | 1997.2 | 1.4 | 1.8 | |
| 40 | 41.6 | 0.9 | 4.1 | 0.4 | 1996 | 1.4 | 1.9 | |
| 45 | 39.1 | 0.9 | 3.7 | 0.4 | 1993.1 | 1.4 | 1.9 | |
| 50 | 35.2 | 0.8 | 3.8 | 0.4 | 1990.1 | 1.5 | 1.8 | |
| 55 | 38.8 | 0.9 | 3.3 | 0.4 | 1987 | 1.5 | 1.8 | |
| 60 | 32.6 | 0.8 | 3.6 | 0.4 | 1985.3 | 1.5 | 1.8 | |
| 70 | 26.7 | 0.4 | 2.7 | 0.2 | 1983.3 | 1.6 | ||
| 80 | 17.3 | 0.4 | 2.4 | 0.2 | 1965.8 | 2 | ||
| 90 | 10.3 | 0.3 | 2.7 | 0.2 | 1945.9 | 2.8 | ||
| 110 | 5.4 | 0.2 | 3.1 | 0.2 | 1923.3 | 4.2 | ||
| 130 | 2.2 | 0.2 | 3.3 | 0.2 | 1899.1 | 6.4 | ||
| 140 | 1.7 | 0.2 | 2.9 | 0.2 | 1888.5 | 7.3 | ||
| 150 | 1 | 0.3 | 2.5 | 0.3 | ||||
| 160 | 1.1 | 0.2 | 3.1 | 0.2 | ||||
| February 2011 DSH08 | ||||||||
| 2 | 71.3 | 2.3 | 11.3 | 0.6 | 2011.1 | 1.6 | 1.9 | 61.9 |
| 4 | 62.8 | 1.8 | 6.6 | 0.3 | 2011 | 1.6 | 1.6 | 7.4 |
| 6 | 65.8 | 2.1 | 7.1 | 0.4 | 2010.9 | 1.6 | 2 | 135 |
| 8 | 59.9 | 1.8 | 5.9 | 0.3 | 2010.4 | 1.6 | 1.9 | 131 |
| 14 | 57.4 | 1.6 | 5.5 | 0.3 | 2008.9 | 1.7 | 2 | Pre-2010 |
| 18 | 63.2 | 1.7 | 6.3 | 0.3 | 2007.1 | 1.7 | 2 | 11.4–12.6 |
| 24 | 57.2 | 1.6 | 4.7 | 0.2 | 2005.2 | 1.7 | ||
| 30 | 51.3 | 0.3 | 4.7 | 0 | 2003 | 1.8 | 1.9 | |
| 34 | 50.7 | 1.2 | 4.1 | 0.2 | 2000.4 | 1.8 | 1.9 | |
| 36 | 47.3 | 1.3 | 4.3 | 0.2 | 1997.4 | 1.8 | ||
| 40 | 42.9 | 1.1 | 4.2 | 0.2 | 1994 | 1.9 | 1.8 | |
| 50 | 22.2 | 0.6 | 4 | 0.2 | 1990.5 | 1.9 | 1.9 | |
| 70 | 20 | 0.5 | 2.4 | 0.1 | 1986.3 | 2.1 | 1.9 | |
| 90 | 13 | 0.5 | 2 | 0.1 | 1981.5 | 2.3 | ||
| 110 | 9.3 | 0.5 | 2 | 0.1 | 1975.6 | 2.8 | 1.8 | |
| 130 | 5.9 | 0.4 | 2.1 | 0.1 | 1966.9 | 3.6 | 1.8 | |
| 150 | 3.3 | 0.5 | 2.1 | 0.1 | 1954.5 | 4.9 | 1.8 | |
| 170 | 1.3 | 0.3 | 2.2 | 0.1 | 1939.8 | 7.3 | ||
| 180 | 0.8 | 0.5 | 1.9 | 0.1 | 1922.9 | 11.8 | ||
| 200 | 0 | 0.4 | 2 | 0.1 | 1902.2 | 19.8 | ||
| December 2010 PCB06 | ||||||||
| 2 | 67.6 | 2.3 | 11.6 | 1.2 | 2010.9 | 1.6 | 1.3 | 95.8 |
| 4 | 66.9 | 1.5 | 5 | 0.6 | 2010.9 | 1.6 | 1.2 | 90.1 |
| 6 | 67.1 | 1.3 | 5.7 | 0.6 | 2010.7 | 1.6 | 1.2 | 6.4 |
| 10 | 57.5 | 0.8 | 2.6 | 0.3 | 2009.3 | 1.7 | 1.2 | Pre-2010 |
| 12 | 60.2 | 1.1 | 3.5 | 0.4 | 2005.9 | 1.7 | 1.2 | 6.3–8.6 |
| 14 | 55.6 | 1.1 | 3.4 | 0.5 | 2003.8 | 1.7 | 1.2 | |
| 16 | 56 | 1.1 | 2.7 | 0.5 | 2002 | 1.8 | 1.2 | |
| 18 | 49.1 | 1 | 3.9 | 0.5 | 2000.1 | 1.8 | 1.3 | |
| 20 | 46.1 | 0.9 | 3.5 | 0.4 | 1998.3 | 1.8 | 1.3 | |
| 30 | 34.4 | 0.8 | 4.1 | 0.4 | 1990.7 | 2 | 1.4 | |
| 38 | 30.2 | 0.8 | 3.1 | 0.4 | 1983.3 | 2.2 | 1.5 | |
| 42 | 28.4 | 0.8 | 3.7 | 0.4 | 1979.9 | 2.3 | 1.5 | |
| 52 | 20.3 | 0.8 | 3.5 | 0.4 | 1970.5 | 2.6 | 1.4 | |
| 62 | 15.8 | 0.5 | 2.8 | 0.3 | 1962.1 | 2.9 | 1.3 | |
| 72 | 12.3 | 0.6 | 3.1 | 0.3 | 1951.8 | 3.4 | 1.3 | |
| 82 | 10 | 0.7 | 3.9 | 0.4 | 1940.8 | 4 | 1.4 | |
| 92 | 6.9 | 0.5 | 3.3 | 0.4 | 1929 | 4.7 | 1.5 | |
| 105 | 2.2 | 0.3 | 1.8 | 0.2 | 1920.2 | 5.2 | 1.4 | |
| 115 | 2.3 | 0.3 | 1.8 | 0.2 | 1915.3 | 5.4 | 1.7 | |
| 135 | 2 | 0.2 | 2.1 | 0.2 | 1902.9 | 6.2 | 1.5 | |
| 155 | 1.3 | 0.2 | 2 | 0.2 | 1887.2 | 7.4 | ||
| February 2011 PCB06 | ||||||||
| 2 | 69.2 | 1.2 | 9.6 | 0.5 | 2011.1 | 1 | 1.9 | 12.1 |
| 6 | 55.3 | 1 | 4.6 | 0.3 | 2009.8 | 1 | 1.3 | 95.8 |
| 10 | 51.1 | 1 | 5.4 | 0.3 | 2008.3 | 1 | 1.2 | 90.1 |
| 14 | 54.9 | 0.9 | 4.1 | 0.2 | 2006.8 | 1 | 1.2 | 6.9 |
| 18 | 78.4 | 1.1 | 3.5 | 0.2 | 2004.7 | 1 | 1.2 | Pre-2010 |
| 26 | 65.2 | 1 | 3.4 | 0.2 | 1999.2 | 1 | 1.2 | 6.3–8.6 |
| 34 | 63.8 | 1 | 4.4 | 0.3 | 1993.5 | 1.1 | 1.2 | |
| 50 | 47.8 | 0.9 | 4 | 0.2 | 1978.9 | 1.2 | 1.2 | |
| 66 | 30.2 | 0.7 | 4 | 0.2 | 1960.8 | 1.4 | 1.3 | |
| 75 | 15.9 | 0.3 | 2.5 | 0.1 | 1954.1 | 1.6 | 1.3 | |
| 95 | 6.6 | 0.2 | 2.3 | 0.1 | 1936.7 | 2 | 1.4 | |
| 125 | 3.3 | 0.2 | 2.8 | 0.1 | 1912.8 | 2.6 | 1.5 | |
| 155 | 2 | 0.2 | 3 | 0.1 | 1885 | 3.5 | 1.5 | |
Benthic foraminiferal density with depth
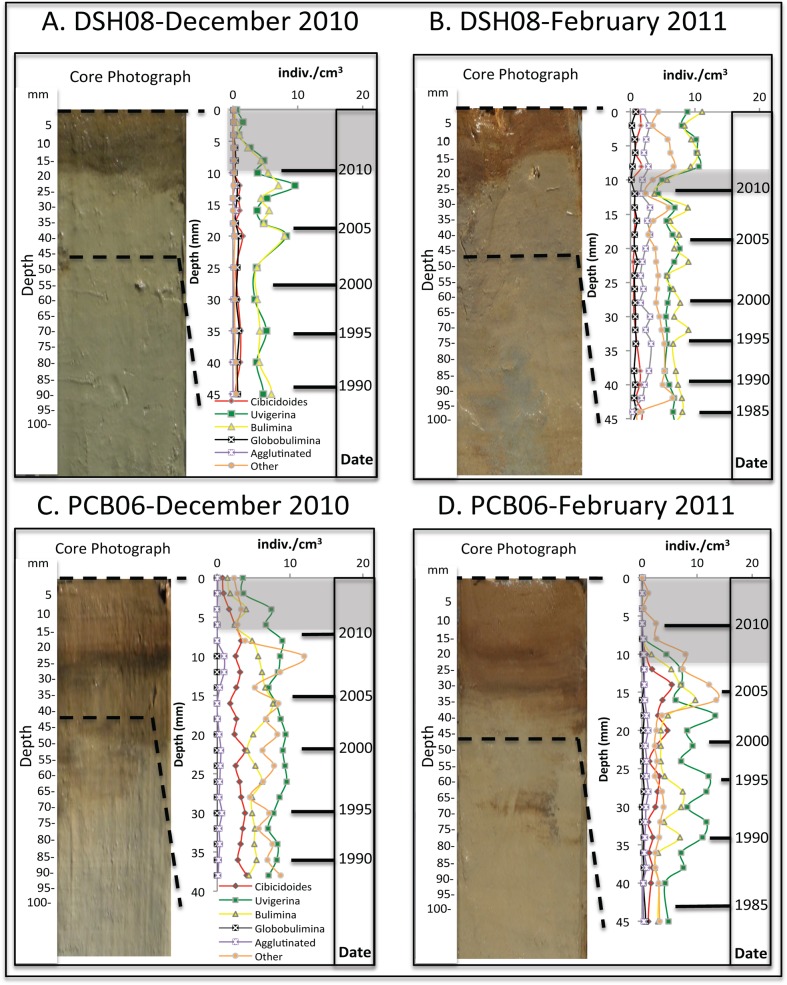
The dominant genera throughout the December 2010 and the February 2011 records from DSH08 were Bulimina spp. and Uvigerina spp. (Fig. 2 A-B). The mean density in the December 2010 and February 2011 records of Bulimina spp. (4.1 and 6.8 indiv./cm3, respectively) and Uvigerina spp. (4.0 and 6.1 indiv./cm3, respectively) were much higher than any of the other genera. In the December 2010 record, the relative abundance of each genus remained the same throughout the lower section from 25–45 mm (pre-DWH). In the surface section (0–12 mm, post-DWH) of the core, there was a sharp decrease in all of the genera with the most pronounced decrease in Bulimina spp., Uvigerina spp. and Cibicidoides spp. Bulimina spp. density decreased from 5.4 indiv./cm3 at 10 mm to 0.2 indiv./cm3 at the surface, Uvigerina spp. density decreased from 3.8 indiv./cm3 at 10 mm to 0.5 indiv./cm3 at the surface, and Cibicidoides spp. decreased from 0.14 indiv./cm3 at 10 mm to 0.07 indiv./cm3 at the surface. The down-core (pre-DWH) section of the February 2011 record (15–45 mm) resembled the December 2010 record with very little variation in the relative abundance of each genus. The most apparent trend in the February 2011 record from DSH08 was the decrease in all genera densities at 10–12 mm (2010 CE), especially Bulimina spp. (3.7 indiv./cm3) and Uvigerina spp. (4.3 indiv./cm3), which was similar to the decrease in the surface (post-DWH) of the December 2010 record. This noticeable decrease was followed by an increase towards the surface (0–8 mm) to densities greater than the down-core record (15–45 mm). The surface (0–8 mm, post-DWH) was very similar to the down-core (15–45 mm, pre-DWH) section except for the increase in Bolivina spp. (“other” category) relative to the dominant Bulimina spp. and Uvigerina spp.
Fig 2. Core photographs and densities (indiv./cm3) of the benthic foraminifera genera throughout the surface sections of each core site including gray areas representing the decline in benthic foraminifera density in each record and the corresponding date.
In contrast to the DSH08 records, the dominant genus throughout the entire December 2010 and February 2011 PCB06 records was Uvigerina spp. (mean-7.7 and 6.1 indiv./cm3, respectively) (Fig. 2 C-D). With the exception of increases in Brizalina spp. (“other”) at 10 mm (11.9 indiv./cm3) and in Bulimina spp. (7.6 indiv./cm3) at 16 mm, there was very little variation in the density of each genus up-core from 38 mm to 8 mm (pre-DWH) in the December 2010 record. From 8 mm to the surface of the record (post-DWH), there was an overall decrease in all of the genera (e.g. Uvigerina spp. 8.9 indiv./cm3 at 8 mm and 3.5 indiv./cm3 at the surface). This decrease was also evident and continued in the February 2011 record from 15 mm to the surface (post-DWH), where the density of every genus, with the exception of Cibicidoides spp. decreased to zero.
Uvigerina spp. was the dominant genus from 35 to 10 mm in the NT1200 record (Table 2). In the surface section of the core (0–10 mm), Uvigerina spp., Cibicidoides spp., and Bulimina spp. all increased in density. Despite these relative increases in density in the surface section of this core, the total density only increases slightly from the down-core section (25.2 indiv./cm3, 10–50 mm) to the surface section (28.9 indiv./cm3, 0–10 mm).
Table 2. Number of dominant genera with depth for each core and the corresponding BFAR.
| Depth | Cibicidoides | Uvigerina | Bulimina | Globobulimina | Agglut. | Other | Total | BFAR |
|---|---|---|---|---|---|---|---|---|
| (mm) | spp. (#) | spp. (#) | spp. (#) | spp. (#) | (#) | (#) | (#) | (fcm −2 yr −1) |
| December 2010 DSH08 | ||||||||
| 2 | 1 | 7 | 3 | 1 | 3 | 2 | 17 | 1.9 |
| 4 | 3 | 21 | 8 | 1 | 1 | 2 | 36 | 1 |
| 6 | 0 | 12 | 14 | 2 | 2 | 2 | 32 | 0.8 |
| 8 | 1 | 37 | 32 | 5 | 2 | 2 | 79 | 2.2 |
| 10 | 5 | 67 | 60 | 6 | 0 | 3 | 141 | 2.4 |
| 12 | 2 | 53 | 76 | 4 | 0 | 3 | 138 | 4.5 |
| 14 | 15 | 134 | 98 | 9 | 4 | 1 | 261 | 3.7 |
| 16 | 12 | 73 | 61 | 9 | 0 | 0 | 155 | 2.5 |
| 18 | 15 | 52 | 78 | 6 | 6 | 0 | 157 | 3.1 |
| 20 | 6 | 66 | 66 | 6 | 0 | 2 | 146 | 3.1 |
| 25 | 21 | 115 | 111 | 12 | 4 | 4 | 267 | |
| 30 | 18 | 130 | 133 | 26 | 4 | 6 | 317 | 1.7 |
| 35 | 28 | 122 | 134 | 19 | 5 | 10 | 318 | 2.1 |
| 40 | 42 | 185 | 150 | 35 | 7 | 11 | 430 | 2.3 |
| 45 | 40 | 132 | 146 | 24 | 0 | 17 | 359 | 2.3 |
| 50 | 14 | 169 | 214 | 27 | 1 | 19 | 444 | 2.5 |
| 55 | 46 | 126 | 115 | 32 | 1 | 12 | 332 | 1.8 |
| 60 | 26 | 129 | 113 | 29 | 1 | 12 | 310 | 2.7 |
| 70 | 23 | 123 | 133 | 33 | 2 | 14 | 328 | 1.6 |
| 75 | 18 | 132 | 172 | 30 | 5 | 24 | 381 | |
| 80 | 32 | 133 | 172 | 20 | 2 | 30 | 389 | 1.8 |
| 85 | 32 | 147 | 153 | 22 | 3 | 15 | 372 | |
| 90 | 25 | 131 | 190 | 18 | 1 | 22 | 387 | 1.4 |
| February 2011 DSH08 | ||||||||
| 2 | 21 | 123 | 156 | 12 | 27 | 60 | 399 | 3.8 |
| 4 | 22 | 111 | 117 | 3 | 40 | 48 | 341 | 6.9 |
| 6 | 14 | 143 | 132 | 9 | 34 | 81 | 413 | 5.8 |
| 8 | 11 | 144 | 146 | 10 | 30 | 87 | 428 | 5 |
| 10 | 24 | 148 | 131 | 5 | 39 | 94 | 441 | 4.7 |
| 12 | 4 | 69 | 79 | 2 | 26 | 48 | 228 | 3.2 |
| 14 | 9 | 60 | 52 | 9 | 24 | 33 | 187 | 2.1 |
| 16 | 9 | 96 | 125 | 9 | 43 | 83 | 365 | |
| 18 | 10 | 78 | 86 | 14 | 37 | 50 | 275 | 2.6 |
| 20 | 8 | 91 | 106 | 9 | 43 | 39 | 296 | |
| 22 | 8 | 107 | 95 | 9 | 33 | 53 | 305 | |
| 24 | 6 | 95 | 126 | 15 | 26 | 56 | 324 | |
| 26 | 7 | 80 | 79 | 9 | 22 | 60 | 257 | 2.4 |
| 28 | 7 | 86 | 92 | 9 | 28 | 58 | 280 | |
| 30 | 7 | 81 | 108 | 10 | 22 | 56 | 284 | 3.1 |
| 32 | 8 | 77 | 95 | 11 | 42 | 63 | 296 | |
| 34 | 11 | 79 | 126 | 11 | 33 | 68 | 328 | 2.5 |
| 36 | 14 | 79 | 93 | 12 | 45 | 73 | 316 | |
| 40 | 21 | 75 | 98 | 8 | 40 | 74 | 316 | |
| 42 | 20 | 84 | 103 | 11 | 31 | 70 | 319 | 2 |
| 44 | 13 | 95 | 112 | 8 | 25 | 91 | 344 | |
| 46 | 25 | 92 | 113 | 12 | 4 | 20 | 266 | |
| 48 | 24 | 94 | 94 | 4 | 3 | 21 | 240 | |
| 50 | 29 | 68 | 81 | 8 | 20 | 61 | 267 | 1.5 |
| 55 | 51 | 192 | 143 | 19 | 43 | 123 | 571 | |
| 60 | 46 | 156 | 168 | 17 | 28 | 61 | 476 | |
| 65 | 64 | 157 | 181 | 19 | 61 | 103 | 585 | |
| 70 | 38 | 125 | 126 | 13 | 96 | 151 | 549 | |
| 75 | 51 | 189 | 213 | 25 | 72 | 108 | 658 | 1.8 |
| 80 | 38 | 161 | 176 | 22 | 46 | 88 | 531 | |
| 85 | 45 | 139 | 190 | 17 | 28 | 43 | 462 | |
| 90 | 32 | 171 | 181 | 13 | 4 | 82 | 483 | |
| 95 | 41 | 178 | 184 | 27 | 5 | 75 | 510 | 1.2 |
| 100 | 40 | 214 | 201 | 24 | 1 | 112 | 592 | |
| December 2010 PCB06 | ||||||||
| 2 | 10 | 49 | 20 | n.d. | 1 | 32 | 112 | 5.1 |
| 4 | 12 | 50 | 25 | n.d. | 1 | 39 | 127 | 6.1 |
| 6 | 23 | 104 | 56 | n.d. | 0 | 46 | 229 | 8.2 |
| 8 | 34 | 93 | 33 | n.d. | 1 | 39 | 200 | 8.9 |
| 10 | 46 | 125 | 67 | n.d. | 0 | 53 | 291 | 12.7 |
| 12 | 36 | 121 | 79 | n.d. | 13 | 167 | 416 | 12.6 |
| 14 | 44 | 117 | 86 | n.d. | 13 | 121 | 381 | 10.8 |
| 16 | 37 | 99 | 92 | n.d. | 5 | 72 | 305 | 8.2 |
| 18 | 25 | 113 | 107 | n.d. | 0 | 118 | 363 | 9.7 |
| 20 | 37 | 122 | 94 | n.d. | 1 | 93 | 347 | 8.4 |
| 22 | 33 | 131 | 69 | n.d. | 4 | 115 | 352 | 8.4 |
| 24 | 53 | 126 | 58 | n.d. | 7 | 87 | 331 | 6.3 |
| 26 | 36 | 130 | 73 | n.d. | 6 | 109 | 354 | 8 |
| 28 | 43 | 133 | 86 | n.d. | 5 | 88 | 355 | 10.8 |
| 30 | 46 | 120 | 67 | n.d. | 4 | 63 | 300 | 7.5 |
| 32 | 53 | 108 | 67 | n.d. | 9 | 100 | 337 | 5.8 |
| 34 | 49 | 98 | 72 | n.d. | 4 | 80 | 303 | 5.8 |
| 36 | 45 | 116 | 71 | n.d. | 5 | 106 | 343 | 6.1 |
| 38 | 40 | 114 | 75 | n.d. | 3 | 97 | 329 | 6.7 |
| 40 | 57 | 99 | 61 | n.d. | 2 | 122 | 341 | 7.3 |
| February 2011 PCB06 | ||||||||
| 0 | 2 | 0 | 0 | 0 | 0 | 2 | 4 | 0.2 |
| 2 | 0 | 1 | 2 | 1 | 2 | 1 | 7 | 0.5 |
| 4 | 0 | 1 | 2 | 0 | 0 | 15 | 18 | 1.6 |
| 6 | 0 | 0 | 1 | 0 | 0 | 4 | 5 | 0.3 |
| 8 | 1 | 2 | 1 | 0 | 1 | 35 | 40 | 1.7 |
| 10 | 1 | 6 | 2 | 0 | 3 | 37 | 49 | 1.8 |
| 12 | 10 | 62 | 24 | 2 | 5 | 111 | 214 | 6.9 |
| 14 | 25 | 99 | 74 | 4 | 6 | 104 | 312 | 10.3 |
| 16 | 75 | 100 | 98 | 5 | 6 | 170 | 454 | 11.7 |
| 18 | 52 | 86 | 135 | 1 | 7 | 189 | 470 | 7.5 |
| 20 | 41 | 186 | 66 | 5 | 12 | 50 | 360 | 6.2 |
| 22 | 64 | 115 | 48 | 3 | 15 | 36 | 281 | 4.5 |
| 24 | 36 | 129 | 48 | 2 | 9 | 32 | 256 | 4.3 |
| 26 | 19 | 99 | 48 | 3 | 12 | 38 | 219 | 3.3 |
| 28 | 43 | 169 | 58 | 3 | 12 | 33 | 318 | 4.7 |
| 30 | 40 | 163 | 103 | 5 | 16 | 50 | 377 | 4.8 |
| 32 | 34 | 115 | 99 | 0 | 10 | 55 | 313 | 3 |
| 34 | 15 | 163 | 56 | 1 | 9 | 46 | 290 | 3.3 |
| 36 | 27 | 154 | 97 | 4 | 7 | 43 | 332 | 3.4 |
| 38 | 18 | 99 | 41 | 3 | 6 | 34 | 201 | 2.3 |
| 40 | 27 | 105 | 32 | 5 | 11 | 34 | 214 | n.d. |
| 45 | 58 | 151 | 109 | 8 | 8 | 109 | 443 | n.d. |
| 50 | 41 | 172 | 104 | 23 | 10 | 115 | 465 | n.d. |
Areas where there are no data available are denoted “n.d.”.
Benthic foraminiferal accumulation rates (BFAR)
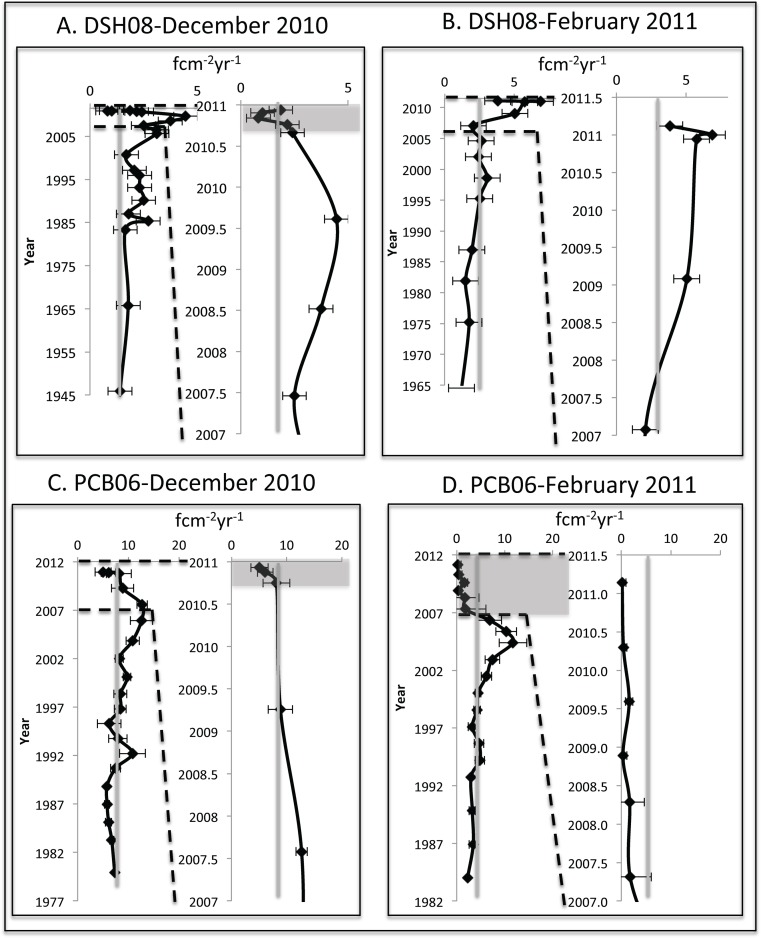
The benthic foraminiferal accumulation rate (BFAR) record from the DSH08 core collected in December 2010 ranged from 1.0–4.5 fcm−2yr−1 (Fig. 3A, Table 2). From 1945 (1.4 fcm−2yr−1) to 2000 (1.7 fcm−2yr−1) the BFAR remained relatively constant. There was an increase from 2005 (3.1 fcm−2yr−1) to 2009 (4.5 fcm−2yr−1). In late 2010, the BFAR (1.0 fcm−2yr−1) decreased below the rate in the bottom section of the core (1945–2000 CE).
Fig 3. Benthic foraminiferal accumulation rate (BFAR) records for each sampling site with the period from 2007–2011 expanded for examination of the period during and after the DWH event.
The gray areas represent the decline in BFAR and the gray lines represent the down-core mean BFAR.
The BFAR from the DSH08 record collected in February 2011 ranged from 1.2 fcm−2yr−1 to 6.9 fcm−2yr−1 (Fig. 3B). There was relatively little variation in BFAR from 1965 (1.2 fcm−2yr−1) to 2007 (2.1 fcm−2yr−1). The BFAR then increased from 2009 (5.0 fcm−2yr-1) to 2011, where the mean BFAR for the two surface samples was 5.4 fcm−2yr−1.
The BFAR record from the PCB06 site in December 2010 ranged from 5.1 fcm−2yr−1 to 12.7 fcm−2yr−1 (Fig. 3C). During late 2010, there was a decrease from 8.5 fcm−2yr−1 to 5.1 fcm−2yr−1 at the surface.
The PCB06 BFAR record from February 2011 ranged from 0.2–11.7 fcm−2yr−1 (Fig. 3D). There was a gradual increase in BFAR (2.2–11.7 fcm−2yr−1) throughout the bottom section of the record (1984–2006). This was followed by a gradual decrease from 2007 (1.7 fcm−2yr−1) to early 2011 (0.2 fcm−2yr−1). The PCB06 dating and accumulation rate records from February 2011 were not coupled with 234Th, and were purely based on 210Pb, which may not have resolved the flocculent pulse in the surface portion of this core and could have affected the dates in the surface 15 mm.
Environmental controls on foraminiferal densities
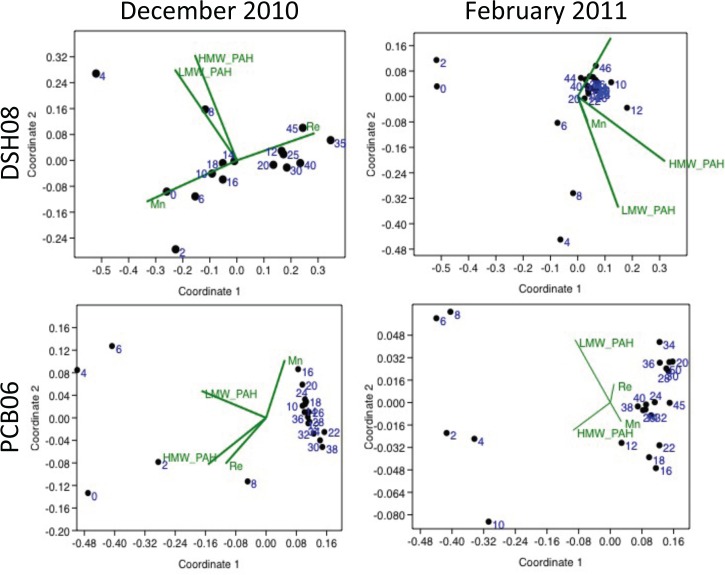
Non-metric multidimensional scaling (nMDS) plots were utilized to distinguish the similarities between foraminiferal densities in each sample increment and the corresponding environmental variable (LMW PAH, HMW PAH, Re, Mn)(Fig. 4). The most notable trend in every core was the separation of the surface interval denisites (∼0–10 mm) from the down-core interval densities (10–50 mm). The separation of the surface interval from the down-core interval in every record was driven by PAH concentration (both HMW and LMW), whereas any variability in foraminiferal density below 10 mm was driven by redox processes (Re,Mn).
Fig 4. Non-metric multidimensional scaling plots for each core, where black dots represent the foraminiferal density at each sample interval (depths labeled in blue) and green vectors represent each environmental variable (HMW PAH, LMW PAH, Re, Mn)[28,29].
The Euclidian distance between each sample depth represents the Bray Curtis similarity and the orientation and length of the green vectors represent the correspondence and intensity of each environmental parameter to the variability between foraminiferal density.
Discussion
General comparison with previous records
Uvigerina spp., Bulimina spp., and Cibicidoides spp. were the dominant genera in the down-core section of PCB06 and DSH08, in upper and lower Gulf of Mexico slope sediments found by Osterman [26], as well as the outer shelf and slope assemblages described by Culver and Buzas [23]. There were also similarities between the CMW assemblage [24] from 850–1500m water depth, and the down-core (below 12 mm, pre-DWH) records from DSH08, which were both dominated by Bulimina spp. and secondarily by Cibicidoides spp. The post-DWH interval from the February 2011 DSH08 record (0–8 mm) was also similar to the CMW assemblage described in Denne and Sen Gupta [24] with the exception of an increase in Bolivina spp. While agglutinated genera were present in every sample at each site, they were certainly not the dominant genera at either of the sampling sites. This disagreement with Bernhard et al. [27] might have been due to the loss of some agglutinated foraminifera in the freeze-drying and wet sieving methods. There were not enough benthic foraminifera in the 10–12 mm (DWH event, 2010 CE) section of the February 2011 DSH08 record to compare with previous studies. The down-core (15–40 mm) PCB06 intervals (pre-DWH) shared two of the dominant species (Bolivina spp. and Uvigerina spp.) with the hydrocarbon seep communities presented in Sen Gupta and Aharon [25]. However, these records differed with respect to the dominance of Bulimina spp. in PCB06 and lack of Bulimina spp. in most of their hydrocarbon seep sites (F1, F12, F15). There were not enough benthic foraminifera, epifaunal or infaunal, in the surface (post-DWH) of the PCB06 cores to compare dominant genera with previous records.
Decline in benthic foraminiferal density
There was no decline in benthic foraminiferal density at the NT1200 control site (Fig. 5, Table 2). However, a decline (i.e. a continuous decrease below down-core mean) was evident in benthic foraminiferal density (all genera, infaunal and epifaunal) and BFAR in the surficial 10 mm at the PCB06 and DSH08 sites in December 2010. The records from February 2011 suggested that a possible recovery from the decline was site specific. At the DSH08 site, there was evidence of the decline from 10–12 mm (2010 CE) and a subsequent increase (apparent recovery) in density and BFAR records in the surface section (0–10 mm, post DWH)). At the PCB06 site, the decline in the surface section (0–8 mm) of the December 2010 record was continued in the 2011 record, where the density reached near-zero values (four individuals) in the surface sample of the core (post-DWH), compared to several hundred (264 individuals) from 10–12mm (pre-DWH). In the PCB06 record from February 2011, it appeared that the decline begins prior to 2010 in the geochronological record. Due to the lack of 234Th dating on this core, the discrepancy may have been due to the inability of the 210Pb geochronology to resolve the flocculent pulse at the surface.
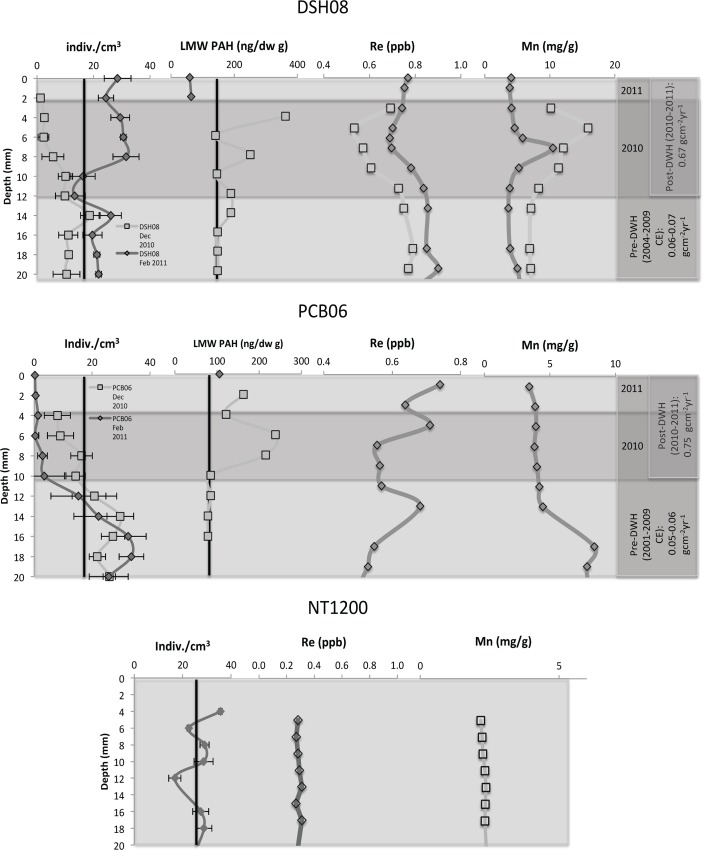
Fig 5. Total benthic foraminiferal density (indiv./cm3), low molecular weight polycyclic aromatic hydrocarbon concentrations (LMW PAH, ng/g dw)[29], and redox sensitive metal (Re, Mn)[28] records stacked using short-lived radioisotope geochronology.
Pre-DWH and post-DWH periods are denoted with their respective mass accumulation rates in the gray-shaded areas and the down-core means for benthic foraminiferal density and LMW PAH are represented by black lines.
Sen Gupta and Aharon [25] documented from 84–108 individuals and a total benthic foraminiferal density of 3–5.7 indiv./cm3 from core top sediments impacted by natural hydrocarbon seeps in the northern Gulf of Mexico that ranged from 500–700 m water depth (Table 3). Studies conducted previous to the DWH event reported much higher total benthic foraminiferal density (1000–3000 individuals, 13.1–29.1 indiv./cm3) from sites along the continental slope and rise (900–1850 m water depth), that were not associated with natural seeps [26,33]. The down-core mean from the four cores in this study was 19.6 indiv./cm3 and the mean density at the control site (NT1200) was 27.1 indiv./cm3. Both of these records were similar to the high density found by Osterman et al. and Rowe and Kennicutt [26,33] and therefore validate their representation of background and control values. The post-DWH mean for the four cores in this study was 7.9 indiv./cm3, similar to the low density found by Sen Gupta and Aharon [25] near the natural hydrocarbon seeps. The similarities between the density in the down-core (pre-DWH) sections of the DSH08 and PCB06 records compared with those from Osterman et al. and Rowe and Kennicutt [26,33] along with the similarities between the density in the post-DWH sections of the DSH08 and PCB06 records compared with those from Sen Gupta and Aharon [25] suggest that the sedimentary environment changed dramatically in the surface section of the DSH08 and PCB06 records.
Table 3. Mean impacted and control benthic foraminiferal densities (indiv./cm− 3) from this study are presented with three previous records of total surface benthic foraminiferal densities (indiv./cm− 3) collected at natural seep sites and along the continental slope of the northern Gulf of Mexico.
| Record | Sample Description | Water depth (m) | Impacted Density (indiv./cm3) | Control Density (indiv./cm3) |
|---|---|---|---|---|
| This Study | DSH08 mean density: | 1143 | 8.85 | 16.5 |
| impacted (0–10mm), | ||||
| control (10–45mm) | ||||
| PCB06 mean density: | 1043 | 6.7 | 22.7 | |
| impacted (0–10mm), | ||||
| control (10–45mm) | ||||
| NT1200 (control site) | 1200 | no impact | 27.1 | |
| mean density (0–50mm) | ||||
| Sen Gupta and Aharon (1994) | core tops impacted from | 500–700 | 4.35 | n.d. |
| natural seeps (1992) | ||||
| Osterman | Unimpacted core tops from continental slope (2000) | 900–1000 | no impact | 13.1 |
| −2002 | ||||
| Rowe and Kenicutt (2009) | Unimpacted core top from | 1850 | no impact | 29.1 |
| DeSoto Canyon site S36 (2000) |
Given the lack of replication at each site and time-stamp, it is necessary to address the possibility that spatial patchiness could have been a factor in the variance in foraminiferal density between each site and time-stamp [43]. It was evident that the densities and relative abundances of the dominant genera were different between sites. This was expected considering the distance between the sites, the different sedimentary settings, and the difference in water depth. However, there was independent continuity at each site between the records collected in December 2010 and those in February 2011. For example, the relative abundance of the dominant genera in the December 2010 cores were very similar to those found in the February 2011 cores at both sites, especially in the down-core (pre-DWH) sections. There was also continuity in both the values and covariance in the total density records from December 2010 to those collected in February 2011 at both sites (Fig. 5). Considering the continuity, in not only the relative abundance of the dominant genera from one time-stamp to the next, but also the similarity in the total density values and covariance of the total density records, it was unlikely that patchiness caused any significant variations in the records from each time-stamp at each site.
Evidence for sudden change in sedimentary environment
Brooks et al. [9] documented a widespread sedimentary pulse in late 2010 throughout the Northeastern Gulf of Mexico that produced a layer from 0.4–1.2 cm that was deposited in 4–5 months. The continuous decay of 234Th and 210Pb activity with depth and the lack of step-wise alterations down-core indicated a lack of bioturbation or vertical mixing throughout each record (Table 1). Brooks et al. [9] provide comprehensive sedimentological evidence of lamination and the lack of vertical mixing in the surface intervals (0–10 mm) of these core records. At the DSH08 site, the total sedimentary mass accumulation rate (MAR) increased from 0.06 gcm−2yr−1 (pre-DWH, 1903–2006 CE) to 0.67 gcm−2yr−1 (post-DWH, 2010–2011 CE) (Fig. 5). At the PCB06 site, the MAR increased from 0.06 gcm−2yr−1 (pre-DWH, 1902–2003 CE) to 0.75 gcm−2yr−1 (post-DWH, 2010–2011 CE). The result of this pulse was a finely laminated layer in the surface section (∼0–10 mm) at both PCB06 and DSH08.
An increased flux of organic carbon to the sediments would be expected to decrease the sedimentary pore-water oxygen concentration as the organic matter is decomposed. Altenbach et al. [44] presented a “high flux” North Atlantic benthic foraminifera assemblage that had a POC deposition rate between 2 x 10–4 gcm−2yr−1 and 2 x 10–3 gcm−2yr−1. Rowe et al., [45] measured a deposition rate of particulate organic carbon (POC) of approximately 5.7 x 10–4 gcm−2yr−1 for the mid-slope of the Gulf of Mexico. POC constituted about 2% of the MAR at the PBC06 and DSH08 sites [9,29]. With a MAR of ∼0.67 gcm−2yr−1 during 2010, the POC deposition rate was 1.3 x 10–2 gcm−2yr−1 (Table 1)[9,29]. In 2010, both sites experienced a high flux of POC associated with the flocculent blizzard.
Hastings et al. [28] determined changes in the redox state of sediments at DSH08 and PCB06 in December 2010 and February 2011 based on concentrations of several redox sensitive metals (Mn and Re)(Fig. 5). The DSH08 redox state records corresponded with the decrease in Bulimina spp. and Uvigerina spp. density at the surface in December (enriched Re) and at ∼12 mm in February (Fig. 5). These decreases in benthic foramiiferal density occurred at the same depth as Mn minima and Re enrichment (0.16 ppb), which indicated reducing conditions [28]. Similar corroboration was found in the PCB06 records. At the surface of PCB06, in both the December 2010 and February 2011 records, there was a significant decrease in the density of benthic foraminifera that corresponded to the Mn minimum and an increase in Re concentration (Dec. 0.07 ppb, Feb. 0.18 ppb Re increase). Hastings et al., [28] also found reducing sediments throughout the surface section of the February 2011 PCB06 record, which suggested that reducing (sub-oxic) conditions persisted at this site for as many as ten months. There was no evidence of reducing sediments in the surface 50 mm at the NT1200 control site (Fig. 5).
Oil droplet models suggested that the subsurface intrusion from 1000–1300 m water depth impinged on the continental slope near the two sites discussed in this study (PCB06 and DSH08) [7]. The droplet and dissolved portions of the intrusion included PAHs [1, 42]. These compounds were likely also present in the pulse of flocculent material that was deposited in late 2010 [3,8]. During the Deepwater Horizon event, the sedimentary low molecular weight PAH concentration increased in 2010 from ∼100 ng/g(OC) (background) to higher than 200 ng/g(OC) at PCB06 and from ∼200 ng/g(OC) (background) to higher than 350 ng/g(OC) at DSH08 (Fig. 5)[29].
Potential mechanisms for benthic foraminiferal decline
Possible mechanisms that may have caused the persistent decline in benthic foraminiferal density in the surface of the PCB06 cores are: (1) increased predation, (2) lateral or vertical foraminifera movement, (3) mortality, (4) inhibition of reproduction, and (5) dilution. The fact that the sedimentological and radioisotope (234Th) records [9] showed a lack of bioturbation in the surface section (laminations from ∼0–10 mm) of these cores, eliminates predation and lateral or vertical movement. Increased predation from detritivorous meiofauna or macrofauna would have produced an increased record of bioturbation throughout the surface section of the core. Considering that the decline affected every genus, vertical or lateral movement of the foraminifera would also have increased bioturbation in the 234Th and sedimentological record [46–49]. Also, considering the widespread and sudden nature of the sedimentation event, it is unlikely that lateral movement would account for a decline in surface density on this scale (m to km) due to the relatively slow movement of foraminifera [46,47]. As previously stated, dilution has been ruled out by using benthic foraminiferal accumulation rates. The only two remaining mechanisms are mortality and inhibition of reproduction, which our methods alone cannot directly constrain (lack of staining).
It has been demonstrated that many foraminifera genera can survive anoxic conditions [50–55]. Risgaard-Petersen et al., [51] documented cases of Globobulimina pseudospinescens surviving for over a month in anoxic conditions by denitrifying stores of nitrate. Piña-Ochoa et al., [52] described several genera (Bulimina spp., Uvigerina spp., and Bolivina spp.) as facultative anaerobes, where cell maintenance and food gathering was possible under anoxic conditions. However, Piña-Ochoa et al., [52] found that oxygen respiration rates were much higher (3–13 times) than denitrification rates, which suggests that oxygen may be necessary for reproduction and growth. Furthermore, the evidence that foraminifera in anoxic conditions for long periods of time (weeks to months) must migrate vertically (not simply extend pseudopodia) to access nitrate [52], along with the lack of bioturbation in the surface of these cores [9], suggests an increase in mortality. Langlet et al. [55] found that a significant portion (∼25–30%) of the original living foraminifera (oxic) could survive up to ten months in anoxic conditions. However, prolonged anoxia caused a decline in the original density by ∼70–75%, which suggested that prolonged anoxia could cause a significant decrease in benthic foraminiferal density [55]. The fact that the decline in density and records of reducing conditions [28] persisted in the surface section (∼10 mm) of the February 2011 record (10 months after the DWH event), it is possible that reducing conditions contributed to the decline by inhibiting reproduction or causing mortality.
Montagna et al. [59] documented severe reduction in abundance of all benthic fauna related to DWH impacts. Benthic foraminiferal exposure to PAH’s has been shown to increase mortality rates and decrease reproduction [15,56,57]. Prolonged exposure (weeks) of benthic foraminifera to PAHs at high concentrations (HMW PAH- 4.9 mg/g, LMW PAH-0.1 mg/g) has been related to cases of complete mortality [56]. The PAH concentrations in the surficial interval (0–10 mm) at DSH08 (145–362 ng/g) and PCB06 (131–238 ng/g) in December 2010 and February 2011 were well below the concentrations reported by Ernst et al. (2006)[29,56]. However, the PAH concentrations in the surface interval (0–10 mm) still increased 2–3 fold relative to baseline (down-core) concentrations and increased PAH concentrations occurred at the same depth as the decline in benthic foraminifera for as many as ten months after the DWH event. Mojtahid et al. (2006) [17] reported declines in density and dominance of opportunistic taxa such as Bulimina spp. and Bolivina spp. at drill cutting disposal sites with total petroleum hydrocarbon (TPH) concentrations ranging from 16–111 mg/g. The baseline (down-core) TPH concentrations at PCB06 and DSH08 in December 2010 and February 2011 ranged from 0.5–1.3 mg/g and increased to 17 mg/g in the surface interval (0–10 mm) [29]. The nMDS results also suggested that PAH concentrations were the dominant driver of variability in foraminiferal density in the surface interval (0–10 mm) of every core (Fig. 4). Considering the documented toxicity of PAHs [15], their effects on reproduction in benthic foraminifera [52] and the sudden nature of the DWH event [9], it is possible that the benthic foraminifera at DSH08 and PCB06 were either not able to adapt quickly enough to such a significant increase in PAHs or could not withstand their persistent toxicity [15,56].
Conclusions
The benthic foraminifera in the down-core (pre-DWH) intervals (>10 mm) at DSH08 and PCB06 resembled assemblages from previous GoM studies related to Caribbean Midwater mass [24] and continental slope sediments [23,26].
An 80–93% decline in the density of all genera (infaunal and epifaunal) of benthic foraminifera has been documented in the surface section (∼0–10 mm) at both of the impacted sampling sites in December 2010 in contrast to the control site.
The decline in the density of benthic foraminifera occurred simultaneously with abrupt and widespread increases in sedimentary accumulation rates, PAH concentrations, and changes in redox conditions.
-
The records from February 2011 suggested a site-specific response:
-There was evidence of a decline and a possible, subsequent recovery in the density and accumulation rate of benthic foraminifera at the DSH08 site. Cores collected after February 2011 will provide further evidence of the potential recovery.
-There was evidence of a continued decline with near-zero values at the surface of the February 2011 PCB06 record. Again, cores collected after February 2011 will provide evidence of the longevity of the decline.
Persistent reducing conditions (10 months after DWH event) in the surface of these cores were a possible contributor to the continued decline at PCB06 due to mortality or inhibited reproduction.
Although our methods could not directly constrain a mortality event (lack of staining), it was likely that the decline in density in the surface of these core records was caused the synchronous, significant increase in concentration of low molecular weight PAHs attributed to the sudden and widespread nature of the DWH event seeing as these compounds are known to be toxic to foraminifera [15,56].
The paired analysis of benthic foraminiferal density records with short-lived geochronology, redox sensitive metal concentrations, and organic chemistry is a robust tool in assessing the impact of the Deepwater Horizon event. This analysis allows for a basic understanding of how deep-water petroleum emissions can affect the benthic habitat health. It identifies specific biological impacts related to the physical and chemical changes in the water column and sediments. It also provides an estimate for the time needed for the benthic communities to recover after a deep-water petroleum emission. By continuing to use this analytical approach it will be possible to also document the long-term recovery from and effects of the Deepwater Horizon event.
Acknowledgments
The authors dedicate this work to Benjamin (Ben) P. Flower, who passed away in July 2012. Without Ben’s foresight, scientific acuity, and immense amount of work, this project would not have been possible. The authors also thank the crew of the R/V Weatherbird II for their assistance during the field program. The authors thank two anonymous reviewers for taking the time to provide their input and constructive comments. Finally, the authors thank Bryan O’Malley, Miaya Glabach, Emily Hladky, Lauren Reilly, Lena Wray, Tess Busch, and Haley Ramirez for their assistance in picking and counting the many-thousand benthic foraminifera included in this study.
Data Availability
Data are available from the Gulf of Mexico Research Initiative Information & Data Cooperative: https://data.gulfresearchinitiative.org/. doi:10.7266/N79021PB.
Funding Statement
This research received funding from these sources: The British Petroleum/Florida Institute of Oceanography (BP/FIO)-Gulf Oil Spill Prevention, Response, and Recovery Grants Program provided funding for several of the initial research cruises and laboratory analyses during 2010 and 2011. http://www.fio.usf.edu (PS, IR, GB, DWH, RL, DJH). The Gulf of Mexico Research Initiative (GOMRI), specifically the Center for Integrated Modeling and Analysis of the Gulf Ecosystem (C-IMAGE) and the Deep Sea to Coast Connectivity in the Eastern Gulf of Mexico (Deep-C) consortia, provided funding in support of field, laboratory, and personnel efforts. http://gulfresearchinitiative.org (PS, IR, GB, DWH, RL, DJH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Camilli R, Reddy CM, Yoerger DR, Van Mooy BAS, Jakuba MV, et al. (2010) Tracking hydrocarbon plume transport and biodegradation at Deepwater Horizon. Science, 330, 201–4. 10.1126/science.1195223 [DOI] [PubMed] [Google Scholar]
- 2. Thibodeaux LJ, Valsaraj KT, John VT, Papadopoulos KD, Pratt LR, et al. (2011) Marine oil fate: knowledge gaps, basic research, and development needs; a perspective based on the Deepwater Horizon spill. Environmental Engineering and Science 28, 87–93. [Google Scholar]
- 3. Ziervogel K, Mckay L, Rhodes B, Osburn CL, Dickson-Brown J, et al. (2012) Microbial activities and dissolved organic matter dynamics in oil-contaminated surface seawater from the Deepwater Horizon oil spill site. PLoS One 7(4), e34816 10.1371/journal.pone.0034816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joye SB, MacDonald IR, Leifer I, Asper V (2011) Magnitude and oxidation potential of hydrocarbon gases released from the BP oil well blowout. Nature Geoscience, 4, 160–4. [Google Scholar]
- 5. Kessler JD, Valentine DL, Redmond MC, Du M, Chan EW, et al. (2011) A persistent oxygen anomaly reveals the fate of spilled methane in the deep Gulf of Mexico. Science, 331, 6015, 312–315. 10.1126/science.1199697 [DOI] [PubMed] [Google Scholar]
- 6. Ryerson TB, Camilli R, Kessler JD, Kujawinski EB, Reddy CM, et al. (2011) Chemical data quantify Deepwater horizon hydrocarbon flow rate and environmental distribution. PNAS, 109(50), 20246–20253. 10.1073/pnas.1110564109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paris CB, Le Hénaff M, Aman ZM, Subramaniam A, Helgers J, et al. (2012) Evolution of the Macondo Well Blowout: Simulating the Effects of the Circulation and Synthetic Dispersants on the Subsea Oil Transport. Environmental Science & Technology, 121203084426001 10.1021/es303197h [DOI] [PubMed]
- 8. Passow U, Ziervogel K, Asper V, Diercks A (2012) Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environmental Research Letters 7, 035301. [Google Scholar]
- 9. Brooks GR, Larson RA, Reichart G-J, Chanton JP, Kostka JE (2014) Sedimentation pulse in the NE Gulf of Mexico following the 2010 DWH blowout Proceedings of the Association for the Sciences of Limnology and Oceanography, Honolulu HI, 16828. [Google Scholar]
- 10. Phleger FB, Parker FL (1951) Gulf of Mexico foraminifera, Part 1 and 2. Geological Society of America Memoirs, 46. [Google Scholar]
- 11. Parker FL (1954) Distribution of foraminifera in the Northeastern Gulf of Mexico. Bulletin of the Museum of Comparative Zoology, 111, 453–588. [Google Scholar]
- 12. Buzas MA, Hayek LC, Culver SJ (2007) Community structure of benthic foraminifera in the Gulf of Mexico. Marine Micropaleontology, 65, 43–53. [Google Scholar]
- 13. Corliss BH, Silva KA (1993) Rapid growth of deep-sea benthic foraminifera. Geology, 21, 991–994. [Google Scholar]
- 14. Gooday AJ (2003) Benthic foraminifera (Protista) as tools in deep-water paleoceanography: environmental influences on faunal characteristics. Advances in Marine Biology, 46, 18–31. [DOI] [PubMed] [Google Scholar]
- 15. Yanko V, Arnold JJ, Parker WC (1999) Effects of marine pollution on benthic foraminifera, In: Sen Gupta B. (ed.), Modern Foraminifera, Kluwer, Boston, 217–235. [Google Scholar]
- 16. Alve E (1995) Benthic foraminiferal responses to estuarine pollution: a review. Journal of Foraminiferal Research, 25, 3, 190–203. [Google Scholar]
- 17. Mojtahid M, Jorissen F, Durrieu J, Galgani F, Howa H (2006) Benthic foraminfera as bio-indicators of drill cutting disposal in tropical east Atlantic outer shelf environments. Marine Micropaleontology, 61, 58–75. [Google Scholar]
- 18. Martinez-Colon M, Hallock P, Green-Ruiz C (2009). Strategies for using shallow-water benthic foraminifera as bioindicators of potentially toxic elements: a review. Journal of Foraminiferal Research, 39, 4, 278–299. [Google Scholar]
- 19. Bejarano AC, Michel J (2010) Large-scale risk assessment of polycyclic aromatic hydrocarbons in shoreline sediments from Saudi Arabia: environmental legacy after twelve years of the Gulf War oil spill. Environmental Pollution, 158, 1561–1569. 10.1016/j.envpol.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 20. Denoyelle M, Jorissen FJ, Martin D, Galgani F, Mine J (2010) Comparison of benthic foraminifera and macrofaunal indicators of the impact of oil-based drill mud disposal. Marine Pollution Bulletin, 60, 11, 2007–2021. 10.1016/j.marpolbul.2010.07.024 [DOI] [PubMed] [Google Scholar]
- 21. Eichler PPB, Eichler BB, Sen Gupta BK, Rodrigues AR (2012) Foraminifera as indicators of marine pollutant contamination on the inner continental shelf of southern Brazil. Marine Pollution Bulletin, 64, 22–30. 10.1016/j.marpolbul.2011.10.032 [DOI] [PubMed] [Google Scholar]
- 22. Poag WC (1984) Distribution and ecology of deep-water benthic foraminifera in the Gulf of Mexico. Palaeogeography, Palaeoclimatology, Palaeoecology, 48, 25–37. [Google Scholar]
- 23. Culver SJ, Buzas MA (1983) Recent benthic foraminiferal provinces in the Gulf of Mexico. Journal of Foraminiferal Research, 13, 21–31. [Google Scholar]
- 24. Denne RA, Sen Gupta BK (1991) Association of bathyal foraminifera with water masses in the northwestern Gulf of Mexico. Marine Micropaleontology, 17, 173–193. [Google Scholar]
- 25. Sen Gupta BK, Aharon P (1994) Benthic foraminifera of bathyl hydrocarbon vents of the Gulf of Mexico: initial report on communities and stable isotopes. Geo-Marine Letters, 14, 88–96. [Google Scholar]
- 26. Osterman LE (2003) Benthic foraminifers from the continental shelf and slope of the Gulf of Mexico: an indicator of shelf hypoxia. Estuarine, Coastal and Shelf Science, 58, 17–35. [Google Scholar]
- 27. Bernhard JM, Sen Gupta BK, Baguley JG (2008). Benthic foraminifera living in Gulf of Mexico bathyal and abyssal sediments: Community analysis and comparison to metazoan meiofaunal biomass and density. Deep Sea Research II, 55, 2617–2626 [Google Scholar]
- 28.Hastings DW, Schwing PT, Brooks GR, Larson RA, Morford JL, et al. (2015) Changes in sedimentary redox conditions following the BP DWH blowout event. Deep Sea Research II, 10.1016/j.dsr2.2014.12.009 [DOI]
- 29. Romero IC, Toro-Farmer GA, Larson RA, Schwing PT, Hollander DJ (2014) Hydrocarbon deposition in deep-sediments following the Deepwater Horizon blowout: spatial analysis of organic geochemical signatures Proceedings of the Association for the Sciences of Limnology and Oceanography, Honolulu HI, 16336. [Google Scholar]
- 30. Engstrom DR (1993) A lightweight extruder for accurate sectioning of soft-bottom lake sediment cores in the field. Limnology and Oceanography, 38(8), 1796–1802. [Google Scholar]
- 31. Valsangkar AB (2007) A device for finer-scale sub-sectioning of aqueous sediments. Current Science, 92(4), 5–8. [Google Scholar]
- 32. Scott DB, Medioli FS (1980) Living vs. total foraminiferal populations: their relative usefulness in paleoecology. Journal of Paleontology, 54, 814–831. [Google Scholar]
- 33. Rowe GT, Kennicutt MC II, eds. (2009) Northern Gulf of Mexico continental slope habitats and benthic ecology study: Final report U.S. Dept. of the Interior, Minerals Management. Service, Gulf of Mexico OCS Region, New Orleans, LA: OCS Study MMS; 2009–039. 456 pp. [Google Scholar]
- 34. Sen Gupta BK, Lobegeier MK, Smith LE (2009) Foraminiferal communities of bathyal hydrocarbon seeps, northern Gulf of Mexico: A taxonomic, ecologic, and geologic study U.S. Dept. of the Interior, Minerals Management Service, Gulf of Mexico OCS Region, New Orleans, LA: OCS Study MMS; 2009–013, 385. [Google Scholar]
- 35. Kitto ME (1991) Determination of photon self-absorption corrections for soil samples, 672 Appl. Radiat. Isot. 42, 835–839. [Google Scholar]
- 36.Cutshall NH, Larsen IL, Olsen CR (1983) Direct analysis of 210Pb in sediment samples: self-absorption corrections.
- 37. Appleby PG, Oldfield F (1983) The assessment of 210Pb data from sites with varying sediment accumulation rates. Hydrobiologia 103, 29–35. [Google Scholar]
- 38. Binford MW (1990) Calculation and uncertainty analysis of 210Pb dates for PIRLA project lake sediment cores. J. Paleolimnology 3, 253–67. [Google Scholar]
- 39. Herguera JC, Berger WH, (1991) Paleoproductivity from benthic foraminifera abundance: glacial to post-glacial change in the west-equatorial Pacific. Geology, 19, 1173–1176. [Google Scholar]
- 40. Handwerger DA, Jarrard RD (2003) Neogene changes in Southern Ocean sedimentation based on mass accumulation rates at four continental margins. Paleoceanography 18, 5-1–5-15. [Google Scholar]
- 41.EPA. EPA Method 8015C: Nonhalogenated Organics by Gas Chromatography 2007, 1–68.
- 42.EPA. EPA Method 8270D: Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (Gc/Ms) 2007, 1–72.
- 43. Bernstein BB, Hessler RR, Smith R, Jumars PA (1978) Spatial dispersion of benthic foraminifera in the abyssal central North Pacific. Limnology and Oceanography, 23, 3, 401–416. [Google Scholar]
- 44. Altenbach AV, Pflaumann U, Schiebel R, Thies A, Timm S (1999) Scaling percentages and distributional patterns of benthic foraminifera with flux rates of organic carbon: Journal of Foraminiferal Research, 29, 173–185. [Google Scholar]
- 45. Rowe GT, Wei C, Nunnally C, Haedrich R, Montagna P, et al. (2008) Comparative biomass structure and estimated carbon flow in food webs in the deep Gulf of Mexico. Deep Sea Research II, Topical Studies in Oceanography, 55(24–26), 2699–2711. [Google Scholar]
- 46. Moodley L, van der Zwaan GJ, Rutten GMW, Boom RCE, Kempers AJ (1998) Subsurface activity of benthic foraminifera in relation to porewater oxygen content: laboratory experiments. Marine Micropaleontology, 34, 91–106. 9476230 [Google Scholar]
- 47. Gross O (2000) Sediment interactions of foraminifera: implications for food degradation and bioturbation processes. Journal of Foraminiferal Research, 32, 4, 414–424. [Google Scholar]
- 48. Khare N, Nigam R (2000) Laboratory experiment to record rate of movement of cultured benthic foraminifera. ONGC Bulletin, 37(1), 53–61. [Google Scholar]
- 49. Geslin E, Heinz P, Jorrissen FJ, Hemleben C (2004) Migratory responses of deep sea benthic foraminifera to variable oxygen conditions: laboratory investigations. Marine Micropaleontology, 53, 227–243. [Google Scholar]
- 50. Bernhard JM, Alve E (1996) Survival, ATP pool, and ultrastructural characterization of benthic foraminifera from Drammenfjord (Norway): response to anoxia. Marine Micropaleontology, 28, 5–17. 8698046 [Google Scholar]
- 51. Risgaard-Petersen N, Langezaal AM, Ingvardsen S, Schmid MC, Jetten MSM, et al. (2006) Evidence for complete denitrification in a benthic foraminifer. Nature, 443, doi: 10.1038. [DOI] [PubMed] [Google Scholar]
- 52. Piña-Ochoa E, Hogslund S, Geslin E, Cedhagen T, Revsbech NP, et al. (2010) Widespread occurrence of nitrate storage and denitrification among foraminifera and Gromiida . PNAS, 107, 3, 1148–1153. 10.1073/pnas.0908440107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koho KA, Pina-Ochoa E, Geslin E, Risgaard-Petersen N (2011) Vertical migration, nitrate uptake and denitrification: survival mechanisms of froaminifers (Globobulimina turgida) under low oxygen conditions. FEMS Microbiology Ecology, 75, 273–283. 10.1111/j.1574-6941.2010.01010.x [DOI] [PubMed] [Google Scholar]
- 54. Geslin E, Risgaard-Petersen N, Lombard F, Metzger E, Langlet D, et al. (2011) Oxygen respiration rates of benthic foraminifera as measured with oxygen microsensors. Experimental Marine Biology and Ecology, 396, 108–114. [Google Scholar]
- 55. Langlet D, Geslin E, Baal C, Metzger E, Lejzerowicz F, Reidel B, et al. (2013) Foraminferal survival after long term experimentally induced anoxia. Biogeosciences Discussions, 10, 9243–9284. [Google Scholar]
- 56. Ernst SR, Morvan J, Geslin E, Le Bihan A, Jorissen FJ (2006) Benthic foramineral response to experimentally induced Erika oil pollution. Marine Micropaleontology, 61, 76–93. [Google Scholar]
- 57. Di Leonardo R, Bellanca A, Capotondi L, Cundy A, Neri R (2007) Possible impacts of Hg and PAH contamination on benthic foraminiferal assemblages: an example from the Sicilian coast, central Mediterranean. Science of the Total Environment, 388, 168–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Gulf of Mexico Research Initiative Information & Data Cooperative: https://data.gulfresearchinitiative.org/. doi:10.7266/N79021PB.