Abstract
Purpose
Patients with recurrent medulloblastoma (MB) have a dismal prognosis. There has been a reluctance to use radiation in the salvage therapy regimens for these patients because of concerns about toxicity and unknown efficacy. Comparing survival outcomes and toxicities in relapsed patients treated with and without radiation may help define its role.
Methods and Materials
A retrospective review was conducted that included 38 patients with recurrent MB treated with similar risk-adapted therapy at initial diagnosis; re-irradiation was a component of salvage therapy in 14. Overall survival (OS) and toxicity were evaluated according to the use of radiation, prior risk stratification and other factors.
Results
For relapsed standard risk patients, the use of additional irradiation resulted in a statistically significant improvement in OS from initial diagnosis (p=0.036) where 5- and 10-year OS rates were 55% ± 14% vs. 33% ± 16% and 46% ± 14% vs. 0%, respectively for re-irradiated patients vs. others. A similar improvement was observed in high risk (p=0.003) patients. There was an association between the use of additional irradiation and an increased rate of necrosis as determined by neuroimaging (p=0.0468).
Conclusion
The use of irradiation as a component of salvage therapy for relapsed MB may prolong survival. The benefit appears to be greatest for relapsed standard risk patients.
Keywords: child, recurrent medulloblastoma, re-irradiation, radiotherapy, pediatric brain tumor, necrosis, treatment outcome
Introduction
Medulloblastoma (MB) is an embryonal tumor arising in the posterior fossa and the most common malignant brain tumor in children.1 Current therapy for patients age ≥ 3 years, consists of maximal surgical resection followed by craniospinal irradiation (CSI) with supplemental “boost” treatment of the post-operative tumor bed, followed by platinum-based chemotherapy. This contemporary treatment has resulted in 5-year progression free survival (PFS) of 80% for standard risk (SR) patients, and over 60% for patients with high risk (HR) disease. 2-5 The prognosis remains dismal for patients who experience disease progression. The expected 2-year overall survival (OS) after disease progression is less than 25%.6-8 Management of these patients has been a challenge as there is no standard approach to salvage therapy. 6, 9-12
The present study draws from a cohort of 235 patients ≥3 years of age with MB who received post-operative risk-adapted CSI and post-irradiation chemotherapy on two successive prospective multi-institution studies. Details regarding treatment after relapse were reviewed including the use of additional irradiation. Comparing survival outcomes and toxicities observed in patients with recurrent MB treated with or without additional irradiation may help to define its role in these patients. This represents the largest series reporting outcomes for patients with recurrent MB treated with additional irradiation.
Materials and Methods
Patients
Thirty-eight patients treated at St Jude Children’s Research Hospital who experienced disease progression after treatment for MB were identified among the cohort of 80 patients treated on the SJMB96 protocol (ClinicalTrials.gov:NCT00003211) from October 1996 to August 2003, and 155 patients treated l on the SJMB03 (ClinicalTrials.gov: NCT00085202) protocol from January 2004 to May 2011. Treatment consisted of surgery, with the intent of gross-total resection, and immediate post-operative radiation therapy and post-irradiation chemotherapy. Tumor risk classification and details regarding treatment have been described previously. 2 For purposes of analysis the following information was obtained from the medical record: date of completion of primary therapy, date of tumor progression, location of tumor progression (primary site vs. neuraxis), therapy at time of progression including surgery, parameters associated with the second course of irradiation including toxicity, date of last follow up, disease status and date of death.
Initial Therapy
The SJMB96 and SJMB03 protocols were similar with the exception of clinical target volume for RT and vincristine dose. Until 2003, SR patients received CSI (23.4 Gy), posterior fossa RT (36 Gy), and primary site RT (55.8 Gy) using a 2-cm clinical target volume (CTV) margin. After 2003, SR patients received CSI (23.4 Gy) and primary site RT (55.8 Gy) using a 1-cm CTV. HR patients received CSI (36-39.6 Gy) followed by primary site RT (55.8 Gy) using a 2cm (pre-2003) or 1cm (post-2003) CTV margin.
Following RT, there was a 6-week rest period followed by four cycles of high-dose chemotherapy (cyclophosphamide, cisplatin and vincristine) and stem-cell or bone-marrow rescue. 2 After the patients completed protocol therapy, they underwent disease evaluation every 3 months for the first 18 months, every 6 months for 5 years and then yearly.
Re-irradiation
Of the 38 patients with recurrent disease, 14 were re-irradiated between August 2000 and June 2011. The use of irradiation was not systematic and was based on symptoms, the extent of disease at the time of relapse, the use and response to other treatments administered at the time of relapse including surgery and chemotherapy, prior treatment history and interval from initial treatment, and the goals set by the patient, their family and caregivers.
The re-irradiation course followed chemotherapy and/or surgery in all patients. Among the 6 patients (HR=1, SR=5) first treated with surgery, 2 (SR) patients did not receive chemotherapy prior to irradiation. Among the 8 patients (HR=2, SR=6) who did not undergo surgery at the time of relapse, all received chemotherapy prior to irradiation. The second course of irradiation consisted of CSI in 8 (57.2%); spinal only in 3 (21.4%), and primary site only in 3 (21.4%) patients. Craniospinal irradiation was administered using standard beam’s eye-view treatment planning techniques. Boost treatment was administered using 3D-conformal radiation therapy methods. Conventional fractionation (1.5-2.0Gy) was use in all CSI cases. One patient treated with a second course of CSI and focal irradiation did not complete the focal phase of the treatment due to symptomatic cerebellar edema. The median dose for the second treatment was 36 Gy (range 18-54 Gy). The median total maximum cumulative dose was 91.9 Gy (range: 73.8 Gy to 109.8 Gy). The median overall treatment time was 19 days (range, 9 to 44) for the second course of irradiation. By contrast, among the 24 un-irradiated patients, 7 (HR=6, SR=1) were initially treated with surgery; only one (HR) patient did not receive chemotherapy. The remaining 17 un-irradiated patients (HR=12, SR=5) were not treated with surgery and received chemotherapy as their only therapy.
Analysis
Associations between different categorical variables were investigated by Fisher’s exact test. OS was defined between the time of diagnosis and death for patients who failed and between diagnosis and date of last follow-up for patients who were alive. Survival distributions were estimated using the Kaplan-Meier method and compared between two or more groups by the exact log-rank test. Patients were considered to have progressive disease if there were tumor cells detected on examinations of CSF and/or evidence of new lesions, growth of existing lesions or new leptomeningeal disease on MRI. If there was any doubt about whether the lesion represented recurrent MB, surgical biopsy/excision and histologic confirmation of recurrent disease was obtained. If radiation necrosis was suspected, additional imaging was performed to determine whether the region was metabolically active, with hypometabolic FDG PET activity considered consistent with therapy-induced necrosis. Treatment plans were reviewed to determine the association between the imaging changes and the distribution of RT dose. The median follow-up for the 6 patients who are still alive is 12.1 years (range 7.2-14.6).
Results
Study Cohort
A total of 38 patients (29 males and 9 females) with a median age at diagnosis of 8.19 years (range, 3.13-20.10) were included in this study. Initial risk classification was SR in 17 (45%) and HR in 21 (55%). The median time from diagnosis to first progression was 1.26 years (range, 0.18-8.74). Thirteen patients were treated with surgery at the time of recurrence. Extent of resection ranged from biopsy to gross total resection. Six of the 13 surgery patients were irradiated after surgery.
Re-irradiation Cohort
Cohorts of patients that underwent re-RT and those that were treated without re-RT were not substgantially different, other than the HR patients with more disease burden were generally not treated with re-RT. Characteristics of the 14 patients who received a second course of RT and their treatment at the time of initial diagnosis and recurrence are described in Tables 1 and 2. The re-irradiation cohort included 11 SR patients with a median age of 15.4 years (range, 7.35-25.63 years) and 3 HR patients with a median age of 12.32 years (range, 8.5-21.95 years). SR patients were more likely to receive re-irradiation in our cohort (p=0.0022). The median time from first progression to re-irradiation was 3.08 months (range, 0.67-48.33). Nine patients had at least one course of chemotherapy after re-irradiation (Table 1).
Table 1.
Initial therapy and treatment at the time of progression for re-irradiated patients with medulloblastoma
| Patient | Risk | Extent of Disease at relapse | Surgery prior to reRT | Chemo prior to reRT | reRT Volume | Chemotherapy after reRT | Additional therapy after reRT (type) |
|---|---|---|---|---|---|---|---|
| 1 | Average | PF | Y GTR | Thio, Topo, Carbo | PF | NONE | N |
| 2 | Average | PF, LS | N | Topo, Oxal, VP16 | Spinal | Lonafarnib | N |
| 3 | Average | ventricle | N | VP16 | CSI | NONE | Y, GK |
| 4 | Average | PF | Y GTR | NONE | CSI | GDC-0449 | Y, GK |
| 5 | High | LS | N | Lapatinib | Spinal | NONE | N |
| 6 | Average | PF | Y GTR | NONE | CSI | ICE | N |
| 7 | Average | obex, ventricle | Y STR | CPX, topo, erlotinib | CSI | NONE | N |
| 8 | High | PF | Y STR | GDC-0449 | PF | Tarceva | N |
| 9 | Average | LS | N | ICE | CSI | GDC-0449, Topo, SAHA, isoretinoin | N |
| 10 | Average | LS | N | Erlotinib | Spinal | None | N |
| 11 | Average | PF, SC | Y STR | Vismodegib, Carbo, VP16, CRA | CSI | CRA, Topo, CPX | N |
| 12 | Average | LS | N | Vismodegib, Everolimus, CPX, Topo | CSI | SAHA, CRA, VP-16 | N |
| 13 | Average | LS | N | Topo/CPX/Tarceva | CSI | VP-16 | N |
| 14 | High | Temporal lobe | N | VP16 | Focal | ICE | Y, GK |
Abbreviations: PF: Posterior Fossa, LS: Leptomeningeal Spread, SC: Spinal Cord, STR: sub-total resection, GK: gamma knife, GTR: gross-total resection, Topo: Topotecan, Carbo: Carboplatin, Oxal: Oxaliplatin, VP16: etoposide, CPX:cyclophosphamide, Temo: temozolomide, Thio: Thiotepa, ICE: Ifosfamide, Carboplatin, etoposide, CRA: cis-retinoic acid CSI: craniospinal irradiation, GK: Gamma Knife, GDC-0449: Vismodegib
Table 2.
Parameters of irradiation and outcomes for patients with relapsed medulloblastoma
| Interval between RT courses (mo) | Second Course Dose (Gy) | Cumulative Dose | Current Disease Status | Overall Survival (from date of relapse in years) | Overall Survival (from date of diagnosis in years) | |
|---|---|---|---|---|---|---|
| 1 | 33 | 50.4 | 106.2 | NED | 11.9 | 14.6 |
| 2 | 49 | 24 | 79.8 | DOD | 1.5 | 4.8 |
| 3 | 30 | 18 | 73.8 | NED | 10.6 | 13.1 |
| 4 | 78 | 27 | 82.8 | NED | 6.7 | 13.3 |
| 5 | 47 | 22.5 | 78.3 | DOD | 0.1 | 4.2 |
| 6 | 107 | 54 | 109.8 | NED | 2.5 | 11.2 |
| 7 | 76 | 54 | 109.8 | NED | 4.7 | 11.0 |
| 8 | 95 | 37.5 | 93.3 | DOD | 4.9 | 9.0 |
| 9 | 34 | 50.4 | 106.2 | DOD | 5.4 | 8.3 |
| 10 | 10 | 24 | 79.8 | DOD | 0.3 | 1.2 |
| 11 | 42 | 48 | 103.8 | DOD | 1.1 | 4.7 |
| 12 | 31 | 36 | 91.8 | DOD | 2.0 | 4.0 |
| 13 | 19 | 36 | 91.8 | DOD | 1.2 | 2.6 |
| 14 | 36 | 54 | 109.8 | NED | 6.6 | 7.2 |
NED: no evidence of disease; DOD: died of disease
Among the 11 re-irradiated SR patients, disease progression was observed in 5 within 5 years of diagnosis, and a sixth patient progressed within 10 years of diagnosis. Eight of these 11 patients received CSI and “boost” to the primary site of recurrence, two patients received only spinal re-RT (site of disease recurrence), and one patient received a second course of focal RT to site of recurrence in the posterior fossa. The median OS for 11 SR patients after initial disease progression was 5.39 years (range 0.3-11.9+ years), and for 3 HR patients was 4.94 years (range 0.1-6.6+ years) from first recurrence. There were six SR patients who had tumor progression after receiving re-RT (CSI and boost): Five of these 6 patients had tumor recurrence within the volume of CSI but outside the area of “boost”, 1 patient had disease recurrence both inside and outside of the retreated volume. All patients also received chemotherapy either prior to or immediately after their second course of RT. Two SR patients that experienced tumor progression after re-RT were alive with no evidence of disease at the time of this analysis (Table 2).
Of our 14 patients completing an additional course of RT, there were 3 high-risk patients and none received CSI due to the higher initial dose of RT at the time of diagnosis. Among these HR patients, there was one failure at 5 years, a second failure by 10 years and one patient was alive at the time of this analysis, 7 years post original diagnosis.
The 10-year OS from initial diagnosis for SR patients treated with irradiation was 45%, compared to 0% for those who were not irradiated. SR patients treated with irradiation had a better OS distribution compared to those who were not (p=0.036) (Figure 1). Similarly, HR patients treated with irradiation had an improved OS compared to those who were not irradiated (p=0.003) (Figure 2). Radiation therapy as a component of salvage therapy appeared to benefit SR and HR patients by extending OS (Figure 1 and Figure 2).
Figure 1. Overall-survival according to the use of irradiation (re-RT) in patients with relapsed standard-risk medulloblastoma.
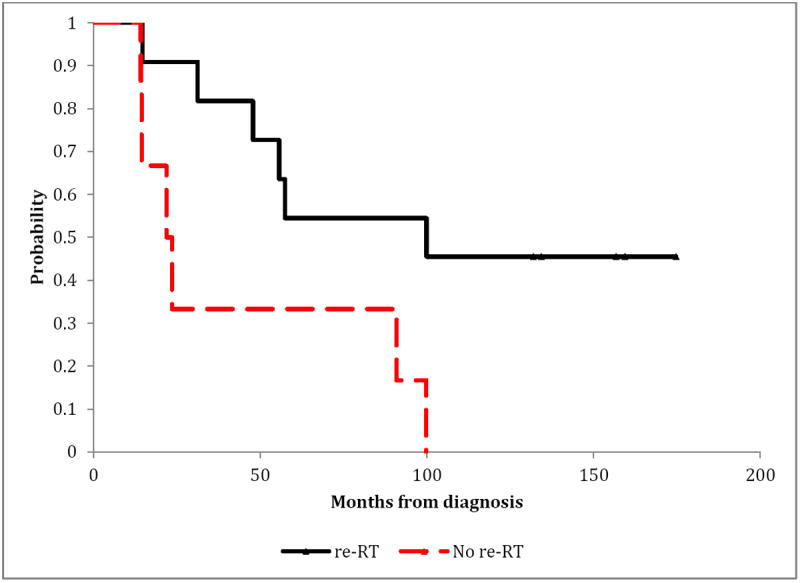
Exact log-rank test: p-value=0.0364
Figure 2. Overall-survival according to the use of irradiation (re-RT) in patients with relapsed high-risk medulloblastoma.
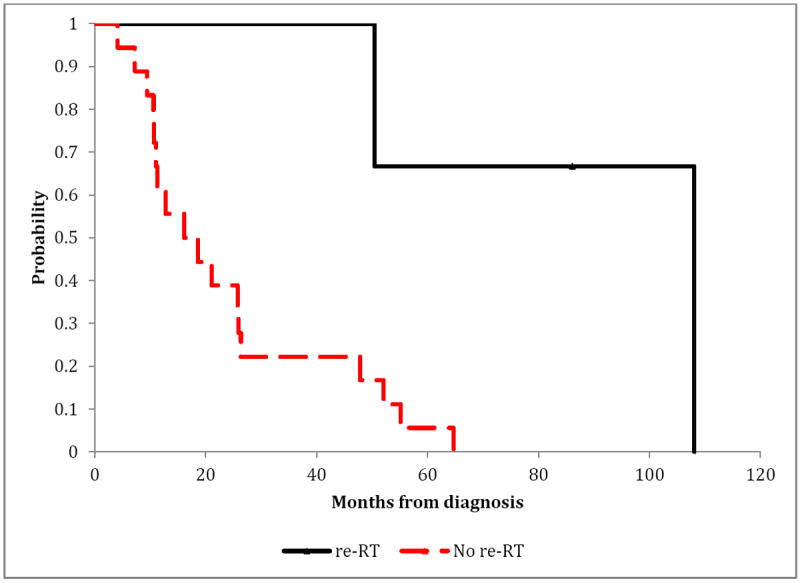
Exact log-rank test: p-value=0.0030
Toxicity
Intratumoral hemorrhage documented by neuroimaging was the most common toxicity observed in this cohort. A total of 24 patients (63%) had asymptomatic neuroimaging evidence of intratumoral hemorrhage (CTCAE version 4.03, Grade 1), 9 were irradiated. The observed rate of hemorrhage was similar (p=1.00) between the irradiated 9/14 (64.3%) and un-irradiated 15/24 (62.5%) patients. There was no difference in hemorrhage rates between 10/17 (59%) SR and 14/21 (67%) HR patients (p=0.7396).
Sixteen patients were noted to have subclinical neuroimaging evidence of necrosis (CTCAE Grade 1 or 2). The proportion of patients with necrosis according to risk classification was 10/17 (59%) for SR patients compared to 6/21 (29%) for HR patients (p=0.0990). The proportion of patients with necrosis was 9/14 (64%) for irradiated patients compared to 7/24 (29%) for patients treated with other modalities (p=0.0468). The patients did not receive additional therapy to treat the subclinical necrosis.
Other toxicities included hypopituitarism in 16 patients, 8 of whom received additional RT, and hypothyroidism in 14 patients, 7 of whom received re-RT. Thus 8/14 (57%) patients in irradiated cohort experienced hypopituitarism compared to 8/24 (33%) patients in the cohort that did not receive a second course of RT (p-value=0.187); and 7/14 (50%) patients in the irradiated cohort experienced patients with hypothyroidism compared to 7/24 (29%) patients in the un-irradiated cohort (p-value=0.298). While our data did not suggest an increased incidence of pituitary or thyroid dysfunction in the patients treated with re-RT compared to those treated with other salvage therapies (Table 3), the lack of significance may be attributable to the relatively small sample size. As part of the analysis of the prevalence of these four toxicities, we assessed whether the patients that had received a second course of RT had a larger number of toxicities based upon a trend test. There appears to be some evidence suggesting that patients who received additional RT were more likely to experience a larger number of these toxicities (p-value=0.0124).
Table 3.
Major toxicities observed in relapsed medulloblastoma patients according to the use of irradiation
| Toxicity | Non-irradiated Patients and Toxicity (%) | Irradiated Patients and Toxicity (%) | P-Value | |
|---|---|---|---|---|
| Hemorrhage (Grade 1) | 15/24 (62.5%) | 9/14 (64.3%) | 1.00 | |
| Hypopituitarism | 8/24 (33.3%) | 8/14 (57.1%) | 0.187 | |
| Necrosis (Grade 1, 2) | 7/24 (29.2%) | 9/14 (64.3%) | 0.047 | |
| Hypothyroidism | 7/24 (29.2%) | 7/14 (50.0%) | 0.298 |
Discussion
There is no standard approach to the management of children with recurrent medulloblastoma. The prognosis for these patients remains dismal and there are few long-term survivors. Treatment options include surgery, different methods of irradiation and a variety of chemotherapy regimens 6, 11, 13, 14. While radiation therapy is an essential component of multimodality therapy in the treatment of newly diagnosed MB, there has been much debate about the use of irradiation at the time of progression because of the potential for toxicity and uncertainty about its ability to improve overall survival.10, 15-19 The purpose of our study was to evaluate the impact of irradiation on overall survival as a component of salvage therapy for recurrent MB. Our secondary aim was to assess the toxicity of re-irradiation. The RT volume in our cohort ranged from focal irradiation of the primary site to regional irradiation of the spine to neuroaxis irradiation including CSI (Table 1).
The use of irradiation in our series was not systematic and was based mainly on clinical factors, including symptomatology, and the risk for potential side effects. Clearly patients with asymptomatic progression at the primary site have more options than those with symptomatic neuraxis metastases and similarly those initially treated for SR medulloblastoma have more options than those treated for HR medulloblastoma. Based on the results presented here, the use of radiation therapy at the time of first failure should be considered as a component of therapy and comprehensive irradiation in terms of dose and volume should be presented as an option to those with limited disease burden and limited prior treatment history. The effectiveness of irradiation must be balanced against the risk of potential side effects, most notably the risk of CNS necrosis which may impact quality of life or lead to fatal complications. Fortunately in our small series CNS necrosis was a subclinical observation and transient; however, with the goal of intensifying therapy in these patients to improve outcomes, the additive effects of aggressive surgery, radiation therapy and intensified chemotherapy could lead to clinically significant complications and should be carefully monitored.
Ionizing radiation induces more damage to the immature or developing brain, and is associated with histopathologic changes 20 and cognitive decline.21-24 Age of the patient, use of chemotherapy, volume of brain irradiated, fraction size and total dose are the key factors when determining tolerance of the CNS to irradiation. 25-28 Exposure of brain parenchyma to therapeutic doses of RT is associated with areas of demyelination and vasculopathy, which contribute to thrombosis and may eventually lead to therapy-induced necrosis. 20, 22, 29-31 When using conventional fractionation radiotherapy, a 5% tolerance dose for radiation necrosis is estimated between 45 and 60 Gy delivered in 2-Gy daily fractions. 25, 28, 32-34 Characteristics common to those patients doing well clinically after re-RT include response to initial RT, length between radiotherapies and minimal residual disease.10, 17 Lawrence et al. found that with partial brain RT, a biologically effective dose (BED) of 150 Gy will result in a 10% chance of developing symptomatic radiation necrosis. They also concluded that the brain is sensitive to fraction sizes >2 Gy as well as twice-daily RT. 26 Another study found that after a normalized total dose (NTD) of 100 Gy, brain necrosis tends to occur. When looking at spinal cord second course of RT, Nieder et al. concluded that if each RT course has a BED of less than 98 Gy with an interval of no less than 6 months between the two RT courses, the cumulative BED can reach up to 120 Gy without toxicity in the spinal cord. 35-37 Bakst et al. reports on a cohort of thirteen recurrent MB patients that underwent at least one course of re-RT (intensity modulated radiation therapy was used in 54% of cases) following resection and chemotherapy. The median RT dose and cumulative maximal doses of second course of RT in this study were of 30 and 84, respectively. Six of these patients had no evidence of disease (NED) 5 years after their initial recurrence. Most patients in this study (12 of 13 patients) were standard risk at initial diagnosis, and underwent surgical resection of gross residual disease and 69% also received initial chemotherapy, prior to additional RT. 10 Of note, the majority of these patients had focal recurrences and did not have M+ disease or leptomeningeal spread.
High-dose chemotherapy with peripheral blood stem cell support is not a reliable curative option for patients that have received optimal prior therapy of RT and platinum-containing chemotherapy. Massamino et al. had 10 patients who all underwent induction chemotherapy followed by myeloablative therapy and finally additional RT. Seven patients had an additional RT dose of 20.2 Gy to the entire CNS whereas 3 patients had local additional RT exceeding 50 Gy. Three-year event free survival (EFS) and overall survival (OS) were 19% and 56% respectively. One patient, however, was alive at 93 months (median survival: 41 months). Dunkel et al. administered carboplatin, thiotepa and etoposide to 23 patients who had recurrent MB and seven patients maintained EFS for a median of 54 months post autologous stem cell rescue. The EFS and OS were 34% and 46%, respectively.38 However, this study included younger patients originally treated only with chemotherapy and did not receive RT at time of initial treatment. An expanded follow-up series including only those recurrent MB patients who were irradiated as part of their initial therapy, reported patients who received additional RT as a part of their retrieval therapy had a trend toward better EFS. 39
Another study had administered high-dose cyclophosphamide to seven recurrent MB patients who had all received prior RT. All 7 patients had a response during the treatment, but following completion, relapses occurred (median: 11.5 months) in all except for two patients. They concluded that although cyclophosphamide can induce a high response rate, it cannot achieve long-term control, and MB patients who had both RT and chemotherapy had a better survival period than chemotherapy alone. 40 A third study treated 49 patients with recurrent or poor-prognosis CNS malignancies with high-dose chemotherapy regimens followed by autologous stem-cell rescue. Nineteen patients had MB, four of which remain disease free. It’s important to note that those four patients all had focal recurrences. All patients with widespread recurrence suffered disease progression. 41
Generally, the patients with single site of recurrence and minimal residual disease appear to have the greatest survival benefit after retreatment, irrespective of the modality. 6, 10, 11 Our study suggests that average risk patients who received a second course of RT may have better OS than their counterparts who were treated otherwise. However, this observation is based upon a single variable analysis which does not take into consideration other factors such as other salvage therapies, pathology and molecular subtype, which are known to affect survival. 42 Five SR patients are currently alive and all have NED. Although the OS was improved in our cohort of recurrent high-risk MB patients treated with second course of RT, there was only 3 patients who received re-RT and only 1/3 was alive at 7 years post diagnosis. It is not possible to determine whether CSI contributed to longer survival in this cohort as all but one of the average risk patients, and none of the HR patients, received CSI. There were limitations in providing a second course of CSI to high-risk patients as they had already had near the maximal tolerated dose of radiation at the time of diagnosis.
There were no differences in the incidence of hemorrhage, pituitary or thyroid dysfunction between patients who did or did not receive second course of RT, however our data suggested an association between RT and higher rate of necrosis (p=0.047). We also observed larger cumulative numbers of these toxicities in patients who were treated with RT.
Our data suggest that undergoing second course of RT may have contributed to better OS in patients with recurrent MB. Based upon our relatively small cohort of patients with medulloblastoma who received similar initial treatment, a second course of RT may be considered a reasonable salvage treatment option in select patients who have minimal residual disease at the time of recurrence.
Acknowledgments
Supported, in part, by US National Institutes of Health Cancer Center Support (CORE) Grant P30 CA21765, the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC) and the America Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Disclosures of potential conflicts of interest: None.
References
- 1.von Hoff K, Rutkowski S. Medulloblastoma. Curr Treat Options Neurol. 2012;14(4):416–26. doi: 10.1007/s11940-012-0183-8. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–20. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Wang MH, Haida T, et al. Medulloblastoma: long-term results for patients treated with definitive radiation therapy during the computed tomography era. Int J Radiat Oncol Biol Phys. 1996;36(1):29–35. doi: 10.1016/s0360-3016(96)00274-x. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–8. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 5.Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031) J Clin Oncol. 2013;31(23):2936–41. doi: 10.1200/JCO.2012.43.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowers DC, Gargan L, Weprin BE, et al. Impact of site of tumor recurrence upon survival for children with recurrent or progressive medulloblastoma. J Neurosurg. 2007;107(1 Suppl):5–10. doi: 10.3171/PED-07/07/005. [DOI] [PubMed] [Google Scholar]
- 7.Grodman H, Wolfe L, Kretschmar C. Outcome of patients with recurrent medulloblastoma or central nervous system germinoma treated with low dose continuous intravenous etoposide along with dose-intensive chemotherapy followed by autologous hematopoietic stem cell rescue. Pediatr Blood Cancer. 2009;53(1):33–6. doi: 10.1002/pbc.21985. [DOI] [PubMed] [Google Scholar]
- 8.Lefkowitz IB, Packer RJ, Siegel KR, Sutton LN, Schut L, Evans AE. Results of treatment of children with recurrent medulloblastoma/primitive neuroectodermal tumors with lomustine, cisplatin, and vincristine. Cancer. 1990;65(3):412–7. doi: 10.1002/1097-0142(19900201)65:3<412::aid-cncr2820650306>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Aguilera DG, Goldman S, Fangusaro J. Bevacizumab and irinotecan in the treatment of children with recurrent/refractory medulloblastoma. Pediatr Blood Cancer. 2011;56(3):491–4. doi: 10.1002/pbc.22868. [DOI] [PubMed] [Google Scholar]
- 10.Bakst RL, Dunkel IJ, Gilheeney S, et al. Reirradiation for recurrent medulloblastoma. Cancer. 2011;117(21):4977–82. doi: 10.1002/cncr.26148. [DOI] [PubMed] [Google Scholar]
- 11.Bouffet E, Doz F, Demaille MC, et al. Improving survival in recurrent medulloblastoma: earlier detection, better treatment or still an impasse? Br J Cancer. 1998;77(8):1321–6. doi: 10.1038/bjc.1998.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broniscer A, Nicolaides TP, Dunkel IJ, et al. High-dose chemotherapy with autologous stem-cell rescue in the treatment of patients with recurrent non-cerebellar primitive neuroectodermal tumors. Pediatr Blood Cancer. 2004;42(3):261–7. doi: 10.1002/pbc.10369. [DOI] [PubMed] [Google Scholar]
- 13.Belza MG, Donaldson SS, Steinberg GK, Cox RS, Cogen PH. Medulloblastoma: freedom from relapse longer than 8 years--a therapeutic cure? J Neurosurg. 1991;75(4):575–82. doi: 10.3171/jns.1991.75.4.0575. [DOI] [PubMed] [Google Scholar]
- 14.Torres CF, Rebsamen S, Silber JH, et al. Surveillance scanning of children with medulloblastoma. N Engl J Med. 1994;330(13):892–5. doi: 10.1056/NEJM199403313301303. [DOI] [PubMed] [Google Scholar]
- 15.Bauman GS, Sneed PK, Wara WM, et al. Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys. 1996;36(2):433–41. doi: 10.1016/s0360-3016(96)00315-x. [DOI] [PubMed] [Google Scholar]
- 16.Chojnacka M, Skowronska-Gardas A. Medulloblastoma in childhood: Impact of radiation technique upon the outcome of treatment. Pediatr Blood Cancer. 2004;42(2):155–60. doi: 10.1002/pbc.10401. [DOI] [PubMed] [Google Scholar]
- 17.Massimino M, Gandola L, Spreafico F, et al. No salvage using high-dose chemotherapy plus/minus reirradiation for relapsing previously irradiated medulloblastoma. Int J Radiat Oncol Biol Phys. 2009;73(5):1358–63. doi: 10.1016/j.ijrobp.2008.06.1930. [DOI] [PubMed] [Google Scholar]
- 18.Padovani L, Andre N, Gentet JC, et al. Reirradiation and concomitant metronomic temozolomide: an efficient combination for local control in medulloblastoma disease? J Pediatr Hematol Oncol. 2011;33(8):600–4. doi: 10.1097/MPH.0b013e3182331eaf. [DOI] [PubMed] [Google Scholar]
- 19.Veninga T, Langendijk HA, Slotman BJ, et al. Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol. 2001;59(2):127–37. doi: 10.1016/s0167-8140(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 20.Oi S, Kokunai T, Ijichi A, Matsumoto S, Raimondi AJ. Radiation-induced brain damage in children--histological analysis of sequential tissue changes in 34 autopsy cases. Neurol Med Chir (Tokyo) 1990;30(1):36–42. doi: 10.2176/nmc.30.36. [DOI] [PubMed] [Google Scholar]
- 21.Blomstrand M, Brodin NP, Munck Af Rosenschold P, et al. Estimated clinical benefit of protecting neurogenesis in the developing brain during radiation therapy for pediatric medulloblastoma. Neuro Oncol. 2012;14(7):882–9. doi: 10.1093/neuonc/nos120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffner PK. Risk factors for cognitive decline in children treated for brain tumors. Eur J Paediatr Neurol. 2010;14(2):106–15. doi: 10.1016/j.ejpn.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Merchant TE, Happersett L, Finlay JL, Leibel SA. Preliminary results of conformal radiation therapy for medulloblastoma. Neuro Oncol. 1999;1(3):177–87. doi: 10.1093/neuonc/1.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant TE, Kiehna EN, Li C, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;65(1):210–21. doi: 10.1016/j.ijrobp.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 25.Dritschilo A, Bruckman JE, Cassady JR, Belli JA. Tolerance of brain to multiple courses of radiation therapy. I Clinical experiences. Br J Radiol. 1981;54(645):782–6. doi: 10.1259/0007-1285-54-645-782. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–7. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys. 2008;70(5):1350–60. doi: 10.1016/j.ijrobp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31(5):1093–112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 29.Fouladi M, Langston J, Mulhern R, et al. Silent lacunar lesions detected by magnetic resonance imaging of children with brain tumors: a late sequela of therapy. J Clin Oncol. 2000;18(4):824–31. doi: 10.1200/JCO.2000.18.4.824. [DOI] [PubMed] [Google Scholar]
- 30.Liu AK, Bagrosky B, Fenton LZ, et al. Vascular abnormalities in pediatric craniopharyngioma patients treated with radiation therapy. Pediatr Blood Cancer. 2009;52(2):227–30. doi: 10.1002/pbc.21787. [DOI] [PubMed] [Google Scholar]
- 31.Uh J, Merchant TE, Li Y, et al. Differences in brainstem fiber tract response to radiation: a longitudinal diffusion tensor imaging study. Int J Radiat Oncol Biol Phys. 2013;86(2):292–7. doi: 10.1016/j.ijrobp.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 33.Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7(2):243–52. doi: 10.1016/0360-3016(81)90443-0. [DOI] [PubMed] [Google Scholar]
- 34.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):1215–28. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 35.Nieder C, Andratschke NH, Grosu AL. Increasing frequency of reirradiation studies in radiation oncology: systematic review of highly cited articles. Am J Cancer Res. 2013;3(2):152–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Nieder C, Grosu AL, Andratschke NH, Molls M. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys. 2006;66(5):1446–9. doi: 10.1016/j.ijrobp.2006.07.1383. [DOI] [PubMed] [Google Scholar]
- 37.Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10(3):200–9. doi: 10.1053/srao.2000.6593. [DOI] [PubMed] [Google Scholar]
- 38.Dunkel IJ, Boyett JM, Yates A, et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children’s Cancer Group. J Clin Oncol. 1998;16(1):222–8. doi: 10.1200/JCO.1998.16.1.222. [DOI] [PubMed] [Google Scholar]
- 39.Dunkel IJ, Gardner SL, Garvin JH, Jr, Goldman S, Shi W, Finlay JL. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol. 2010;12(3):297–303. doi: 10.1093/neuonc/nop031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen JC, Helson L. High-dose cyclophosphamide chemotherapy for recurrent CNS tumors in children. J Neurosurg. 1981;55(5):749–56. doi: 10.3171/jns.1981.55.5.0749. [DOI] [PubMed] [Google Scholar]
- 41.Graham ML, Herndon JE, 2nd, Casey JR, et al. High-dose chemotherapy with autologous stem-cell rescue in patients with recurrent and high-risk pediatric brain tumors. J Clin Oncol. 1997;15(5):1814–23. doi: 10.1200/JCO.1997.15.5.1814. [DOI] [PubMed] [Google Scholar]
- 42.Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–7. doi: 10.1016/S1470-2045(13)70449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


