Abstract
TRPM4 is a Ca2+-activated non-selective cation channel expressed in a wide range of human tissues. TRPM4 participates in a variety of physiological processes such as T cell activation, myogenic vasoconstriction and allergic reactions. TRPM4 Ca2+ sensitivity is enhanced by calmodulin (CaM) and phosphathydilinositol 4, 5-biphosphate (PI(4,5)P2) binding, as well as, under certain conditions, PKC activation. However, information as to the mechanisms of modulation of this channel remain unknown, including direct identification of phosphorylation sites on TRPM4 and their role in channel features. Here, we use mass-spectrometric-based proteomic approaches (immunoprecipitation and tandem mass spectrometry), to unambiguously identify S839 as a phosphorylation site present on human TRPM4 expressed in a human cell line. Site-directed mutagenesis employing a serine to alanine mutation to eliminate phosphorylation, and a phospho-mimetic aspartate mutation, as well as biochemical and immunocytochemical experiments, revealed a role for S839 phosphorylation in the basolateral expression of TRPM4 channels in epithelial cells. Moreover, we demonstrated that casein kinase 1 (CK1) phosphorylates S839 and is responsible for the basolateral localization of TRPM4.
Keywords: TRP channels, LC-MS/MS, basolateral, phosphorylation, casein kinase
Introduction
TRPM4 is a Ca2+-activated non-selective cation channel (Ca2+-NSCC), expressed in a wide range of human tissues, where it regulates a variety of physiological and pathophysiological processes, including cellular death and proliferation, insulin secretion, T-cell differentiation, and dendritic and mast cell migration [23,2,6,8,13-16,20,1]. Although TRPM4 is intrinsically Ca2+ impermeant, in non-excitable cells that lack voltage-gated Ca2+ channels it plays an important effect in regulating intracellular Ca2+ oscillations by providing membrane depolarization necessary for Ca2+ influx via store operated channels (SOCs) [22]. Physiologically, it has been shown that TRPM4 knockdown alters Ca2+ oscillations during T cell activation, leading to a failure in interleukin-2 secretion [22], and TRPM4 knockout mice exhibit a notable loss in dendritic cell migration and diminished mast cell IgE secretion [2,49]. TRPM4 has also been linked to cerebral blood flow and insulin secretion from pancreatic β cells [6,14,26,30,35]. Pathophysiological roles of TRPM4 have also been recently elucidated. A direct role of this channel has been shown in hypertension and cardiac diseases [20,43,24,25] and its participation in LPS and H2O2-induced cell death have also been described [41,3]
In spite of the important role of TRPM4 in a variety of cellular processes, little is known about the physiological regulation of this channel. Previous studies have shown that binding of calmodulin (CaM) and PI(4,5)P2 enhance the Ca2+ sensitivity of TRPM4 [31,55]. Phorbol ester treatment led to enhanced Ca2+ sensitivity of recombinant human TRPM4 expressed in human HEK293 cells. This treatment affected wild-type TRPM4 but not of two mutants (S1145A or S1152A) at putative PKC phosphorylation sites located on the TRPM4 cytoplasmic C-terminus [32]. In addition, phorbol ester treatment also affected trafficking and surface expression of GFP-tagged mouse TRPM4 expressed in smooth muscle cells [12]. Phosphorylation is a major mechanism in cellular homeostasis and the most common post-translational modification [47], and human TRPM4 contains ~130 serine and threonine and ~27 tyrosine residues in its sequence that can be potentially phosphorylated by different kinases. Here, we performed a mass spectrometric-based proteomics analysis to identify sites chemically modified with phosphate on immunopurified human TRPM4 channels. Using liquid chromatography tandem mass spectrometry (LC-MS/MS) we unambiguously identified S839 as a novel phosphorylation site on the human TRPM4 protein. We provide the first immunochemical evidence for the basolateral localization of the TRPM4 channels in polarized epithelial cells, and that phosphorylation at S839 is crucial for TRPM4 basolateral localization via CK1-dependent phosphorylation.
Materials and methods
Cell culture and plasmids
HEK293 cells were cultured in DMEM low glucose/F12 medium (1:1), supplemented with 5% fetal bovine serum. COS-1 cells were cultured in DMEM high glucose medium, supplemented with 10% bovine calf serum. TREx-TRPM4 cells were generated by stable transfection of the pcDNA4TO/TRPM4 plasmid (kindly provided by Dr P. Launay) into TREx293 cells (Invitrogen, Carlsbad, CA) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) followed by selection with 5 μg/mL blasticidin. These cells were subsequently grown in the same medium used for HEK293 cells. A single positive clone (clone 11) was selected for phosphorylation residue identification using LC-MS/MS. MDCK cells were cultured in DMEM high glucose medium supplemented with 10% fetal bovine serum. Twenty-four hours before transfection, cells were grown in Ca2+-free medium and then were plated to 50% confluence on Transwell Permeable Support (0.4 μm polycarbonate membrane; Costar, Corning, NY) and transfected 12 h later with 500 ng plasmid DNA/well. Cells were transferred to Ca2+-containing medium and grown for an additional 4 days after transfection to establish a confluent monolayer. All cell lines were grown at 37 °C and 5% CO2.
Antibodies
For immunofluorescence, immunoblot and immunoprecipitation experiments, we used the following antibodies: rabbit polyclonal (pAb) anti-FLAG antibody (Sigma-Aldrich, St Louis, MO), rabbit pAb anti-phosphoserine (pS) (ab9332, Abcam), mouse monoclonal (mAb) IgG1 anti-Grp75 (N52A/42, UC Davis/NIH NeuroMab Facility, Davis, CA), mouse mAb IgG1 anti-E-cadherin (RR1, DSHB, Iowa City, IA), and mouse mAb IgG2b 12CA5 anti-HA (BACO, Berkeley, CA).
Immunoprecipitation and immunoblotting
TRPM4 protein expression in TREx-TRPM4 cells was induced by adding 1 μg/mL tetracycline to the medium and incubating for 24 h. The cells were then lysed in lysis buffer containing 1% v/v Triton X-100, 150 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl (pH 7.4), 1 mM sodium orthovanadate, 5 mM NaF, 1 mM PMSF, aprotinin (1.5 μg/mL), antipain (10 μg/mL), leupeptin (10 μg/mL), benzamidine (100 μg/mL). The lysates were centrifuged at 12,000 g at 4 °C for 10 min. The supernatants were incubated with anti-FLAG column (Sigma-Aldrich, St Louis, MO) overnight at 4 °C. After incubation, immunoprecipitation reaction products were washed six times in wash buffer (150 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl, pH 7.4) and immunocomplexes were eluted with reducing sample buffer (62.5 mM Tris-HCl, 2% w/v SDS, 10% v/v glycerol, 1% v/v β-mercaptoethanol) and size-fractionated on 6-12% gradient SDS–PAGE, according to the Laemmli method [21].
In gel digestion
The TRPM4 band (~140 kDa) was directly excised from Colloidal Coomassie blue stained gels [33], destained with 50% v/v acetonitrile in 25 mM ammonium bicarbonate and dried in a speed vacuum concentrator. Dried gel pieces were reswollen with 25 mM ammonium bicarbonate (pH 8.0) containing 50 ng/μL trypsin (Promega, Valencia, CA) and incubated at 37°C for 16-24 h. Supernatant peptide mixtures were extracted with 50% v/v acetonitrile in 5% v/v formic acid and dried in a speed vacuum concentrator.
Mass spectrometry analysis
An HPLC system (Paradigm MG4, Michrom Bio Resources, Auburn, CA) directly coupled with an ion trap mass spectrometer (LTQ-FT, Finnigan, San Jose, CA) was used for microcapillary LC-MS/MS data acquisition. A homemade fritless reverse phase (RP) microcapillary column (0.1 mm × 180 mm) was packed with C18 (Phenomenex, Aqua: 5 μm 300 Å). Tryptic peptide mixtures were desalted and concentrated using the RP trap column (0.15 mm × 30 mm) before chromatographic separation by the microcapillary RP column with a flow rate of 300 nL/min. Eluted peptides were then directly sprayed into the mass spectrometer. MS/MS spectra were acquired for the most intense peptide ion from the previous MS spectra with dynamic exclusion for 3 min. The two buffers used for the RP chromatography were 5% v/v acetonitrile /0.1% v/v formic acid (buffer A) and 80% v/v acetonitrile/0.1% v/v formic acid (buffer B). A 2.5 h long gradient (0-10% B for 10 min, 10-45% B for 110 min, 45-100% B for 20 min, 100% B for 10 min) was used for the maximum separation of the peptides. Mascot searches for LC-MS/MS data were performed for the MS/MS data set against the human TRPM4 protein sequence with differential phosphorylation on Tyr, Thr, and Ser and oxidation on Met. Peptide sequences from the search result were filtered out using DTASelect software (5) with a slightly lower criteria than typically used for protein identification as to not miss any possible phosphopeptides: correlation value (Xcorr) > 1.5, 2.0, and 3.0 for singly, doubly, and triply charged tryptic peptide ions, respectively and ΔCn (the difference in correlation with the next highest Xcorr) higher than 0.08. Each filtered MS/MS spectra exhibiting possible phosphorylation was manually checked and validated. Existence of a 98 dalton mass loss (−H2PO4: phosphopeptide specific CID neutral loss) and any ambiguity of phosphorylation sites were carefully examined.
Site-directed mutagenesis and transient transfection
Mutagenesis of recombinant human TRPM4 cDNA in the pcDNA4TO vector was performed using the Quick-change site-directed mutagenesis kit (Stratagene, La Jolla, CA) as per the manufacturer’s instructions. All mutants were checked by DNA sequencing. HEK293 cells were plated in 35 mm plastic tissue culture dishes and transfected with wild-type or mutant pcDNA4TO/TRPM4 plasmid (0.1 μg) using Lipofectamine 2000 reagent (Invitrogen). All experiments were performed 48 h post transfection.
Immunofluorescence
For labeling of the apical membrane surface, MDCK cells were incubated for 30 min at 4°C with 20 μg/mL FITC-WGA in PBS (10 mM phosphate buffer, pH 7.4, 150 mM NaCl, containing 1 mM MgCl2 and 1 mM CaCl2). Cells were washed 3 times and then were fixed for 15 min at room temperature in fixing solution, permeabilized and incubated in blocking solution for 45 min at room temperature and inmunostained with anti-FLAG and mouse monoclonal anti-E-cadherin diluted in blocking solution. Primary antibodies were detected with Alexa 488 goat anti-mouse IgG1 and Alexa 594 goat anti-rabbit or with Alexa 594 goat anti-mouse IgG1 and Alexa 350 goat anti-rabbit secondary antibodies (Invitrogen, Carlsbad, CA) and nuclei were stained with Hoechst 33258 (200 ng/mL). Images were acquired with a CCD camera installed on Axiovert 200M microscope (Zeiss, Oberkochen, Germany) with 100X (for MDCK cells), 1.3 numerical aperture objectives and an ApoTome coupled to Axiovision software (Zeiss, Oberkochen, Germany).
Surface biotinylation
HEK293 cells were grown to 80-90% confluence on poly-L-lysine (200 μg/mL) treated 35 mm tissue culture dishes. Cells were then washed twice with ice-cold DPBS (pH 8.0) and incubated with 500 μg/mL EZ-link sulfo-NHS-LC-biotin (Thermo, Rockford, IL) disolved in DPBS (pH 8.0) for 30 min at room temperature. The reaction was terminated with blocking solution (50 mM Tris-HCl, 154 mM NaCl, pH 8.0) and the cells washed twice with ice-cold PBS. Cells labeled with sulfo-NHS-LC-biotin were lysed as described above, and lysates were incubated with neutravidin-agarose overnight at 4 °C. The precipitated proteins were eluted with reducing sample buffer and resolved in SDS-PAGE for further immunoblot analysis.
Results
Identification of a novel TRPM4 phosphorylation site
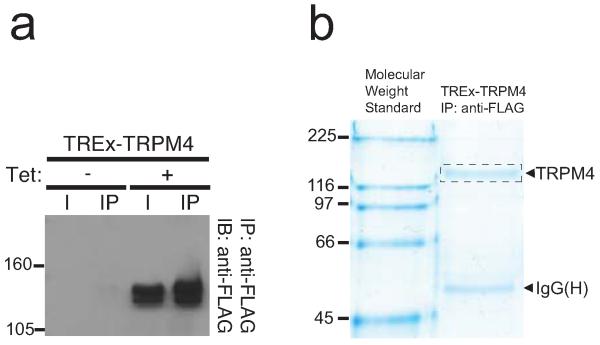
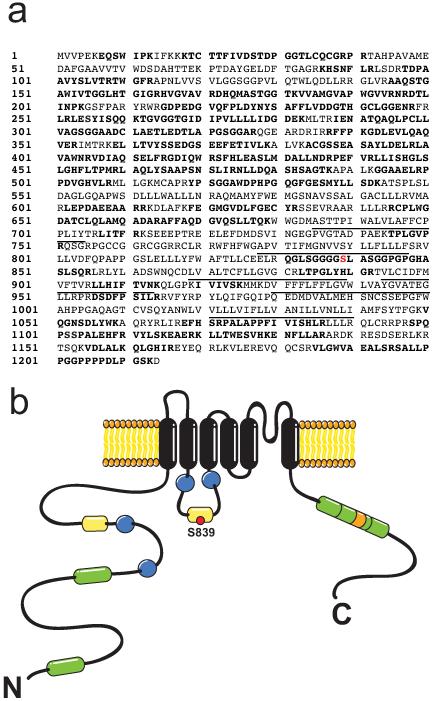
To identify phosphorylation sites on TRPM4, we used a previously characterized stable cell line expressing a FLAG-tagged human TRPM4 under control of a tetracycline-inducible operator (TREx-TRPM4) [41]. Stimulation of these cells with 1 μg/mL tetracycline for 16 h induces high levels of TRPM4 protein expression (Fig. 1a), in contrast to non-stimulated cells, in which no expression of TRPM4 was detected. In order to identify sites chemically modified with phosphate, we purified the recombinant human TRPM4 protein from induced TREx-TRPM4 cells by immunoaffinity chromatography using an anti-FLAG antibody. The purified protein was then size fractionated by SDS-PAGE (Fig. 1b), and the Colloidal Coomassie blue stained TRPM4 band excised. Following in gel trypsin digestion, we analyzed these samples by LC-MS/MS. The TRPM4 peptides were identified using a Mascot and Transproteomic Pipeline database search, resulting in 349 independent spectra that matched human TRPM4, resulting in 63.8% overall coverage of the TRPM4 protein, and 70.7% coverage of the cytoplasmic regions (76.5% of the 684 a.a. of the cytoplasmic N-terminal segment and 69.0% of the 174 a.a. C-terminal segment). Using this approach, we identified phosphorylated residue pS839, located on a tryptic peptide (831QGLSGGGGpSLASGGPGPGHASLSQR855) derived from the S2-S3 cytoplasmic linker of TRPM4 (Fig. 2a-b).
Fig. 1. Immunopurification of hTRPM4 from a plasma membrane-enriched protein fraction from tetracycline-induced (+) and non-induced (−) TREx-TRPM4 cells.
a. SDS-PAGE separation of eluates from immunoprecipitation of TRPM4 with anti-FLAG antibody. Immunoblot of Input (I) and immunoprecipitation products (IP) of TRPM4 from TREx-TRPM4 cells after SDS-PAGE separation. b. Coomassie brilliant blue-stained SDS-PAGE of a large-scale immunopurification showing the yield of the FLAG-TRPM4 channels obtained with the anti-FLAG immunopurification from tetracycline-induced TREx-TRPM4 cells. The left lane corresponds to the molecular weight standards in kDa.
Fig. 2. Identification of phosphosites on recombinant hTRPM4 expressed in HEK293 cells using LC-MS/MS.
a. Deduced amino acid sequence of human TRPM4. The single phosphoresidue identified by LC-MS/MS is in red. The sequence coverage is indicated in bold. Underlined regions correspond to the predicted transmembrane segments of the channel. c. Cartoon indicating the localization of the identified phosphosite at S839. CaM binding motifs (green), ABC transporter motifs (yellow), Walker B motifs (blue) and PH domain (orange) are shown. N and C denote amino- and carboxyl-terminal regions.
Phosphorylation at S839 does not regulate cell surface expression of TRPM4 channels
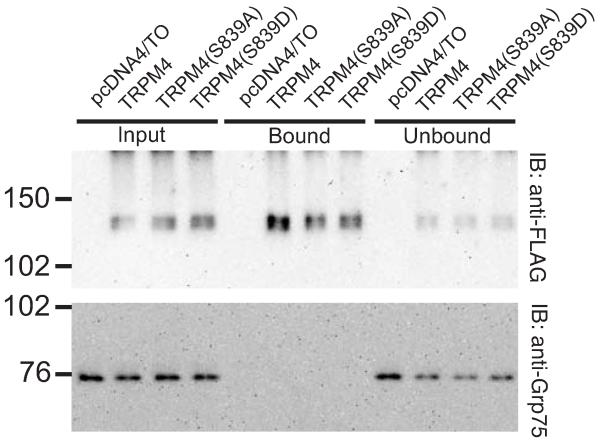
To determine the possible role of S839 phosphorylation on the expression of TRPM4 at the plasma membrane, we transiently transfected HEK293 cells with wild-type TRPM4, the phosphorylation disrupting mutant TRPM4 S839A, and the phosphomimetic mutant TRPM4 S839D, and performed cell surface biotinylation labeling assays. In these experiments, we observed that both mutant TRPM4 proteins (S839A and S839D) are expressed in the plasma membrane at a level similar to wild-type TRPM4 protein (Fig. 3). Therefore, we concluded that the phosphorylation on S839 is not related to a diminished expression on the plasma membrane.
Fig. 3. Loss of phosphorylation in the S839 residue does not affect TRPM4 expression in the plasma membrane.
HEK293 cells were transiently transfected with TRPM4, TRPM4 S839A or TRPM4 S839D and surface-labeled with 500 μg/mL EZ-link sulfo-NHS-LC-biotin. The biotinylated proteins purified by neutravidin-agarose, eluted with 1X RSB, resolved in SDS-PAGE and analyzed by immunoblot using anti-FLAG antibody for TRPM4 detection. The immunoblot for the intracellular protein Grp75 was included as cytosolic control.
Phosphorylation at S839 regulates basolateral localization of TRPM4 channels
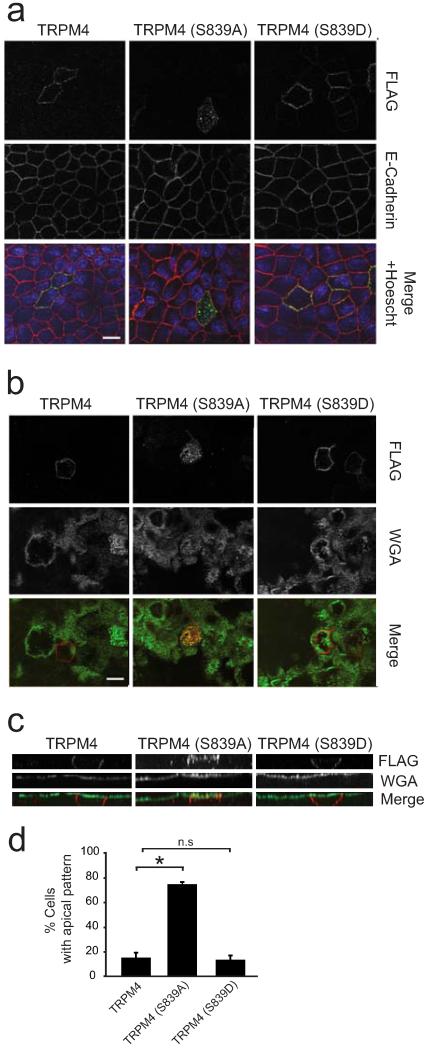
TRPM4 channels are expressed in a polarized manner in kidney [10,17] and bladder [53] epithelial cells. To determine the role of phosphorylation at S839 in polarized expression, we transiently transfected wild-type TRPM4 and the phosphosite mutants (S839A, S839D) in polarized canine kidney epithelial MDCK cells. We then performed immunofluorescence staining and optical sectioning microscopy on polarized confluent MDCK cell monolayers to determine the localization of the exogenous channel proteins. We labeled the apical domain with FITC-WGA before cell permeabilization and immunostaining, and counterstained cells for E-cadherin as a basolateral marker. In these experiments, we observed that wild-type TRPM4 channels exhibited clear basolateral localization in every transfected cell, as showed by its colocalization with E-cadherin (Fig. 4a), and lack of colocalization with WGA (Fig. 4b). In a minor population of cells (15.0 ± 3.9%), apical localization of wild-type TRPM4 was also observed, as demonstrated by clear colocalization with the WGA labeling (Fig. 4). This suggests that polarized expression of TRPM4 in epithelial cells has a component that is variable and/or conditional. Interestingly, the phosphomimetic TRPM4 S839D mutant showed similar basolateral pattern to TRPM4 wild type (Fig. 4). Conversely, the phosphorylation disrupting TRPM4 S839A mutant showed an apical localization, as shown by colocalization with WGA, in the vast majority (74.1 ± 1.9 %) of the cells analyzed. The TRPM4 S839A mutant was also found in the basolateral domain of the same cells (Fig. 4). We conclude that phosphorylation of the S839 residue controls the polarized expression of TRPM4 channels in epithelial cells, but not the biosynthetic trafficking of TRPM4 to the cell surface.
Fig. 4. Phosphorylation at the S839 residue is required for proper TRPM4 basolateral localization.
MDCK cells were grown as confluent polarized monolayers after transfection with TRPM4, TRPM4 S839A or TRPM4 S839D. The apical domain was labeled prior to fixation with WGA. After fixation, cells were immunostained with pAb anti-FLAG (green) and mAb anti-E-cadherin. Nuclei were stained with Hoechst (blue). Scale bar, 10 μm. a. Monolayer of MDCK cells focused at a section midway through the cells, the basolateral domain is labeled for E-cadherin (red). b. Monolayer of transfected MDCK cells focused at the apical domain, as labeled with WGA (red). c. Lateral view of monolayer showing the differences in localization between the different versions of TRPM4. d. Graph shows data on percentage of cells which exhibited apical localization of TRPM4 isoforms. * corresponds to significant differences (ANOVA test, post-Dunnett, p < 0.01) versus wild-type TRPM4. Data are from 3 independent experiments including n=559, 498, and 417 cells for TRPM4, TRPM4 S839A, and TRPM4 S839D, respectively.
Casein kinase 1 (CK1) regulates basolateral localization of TRPM4 channels
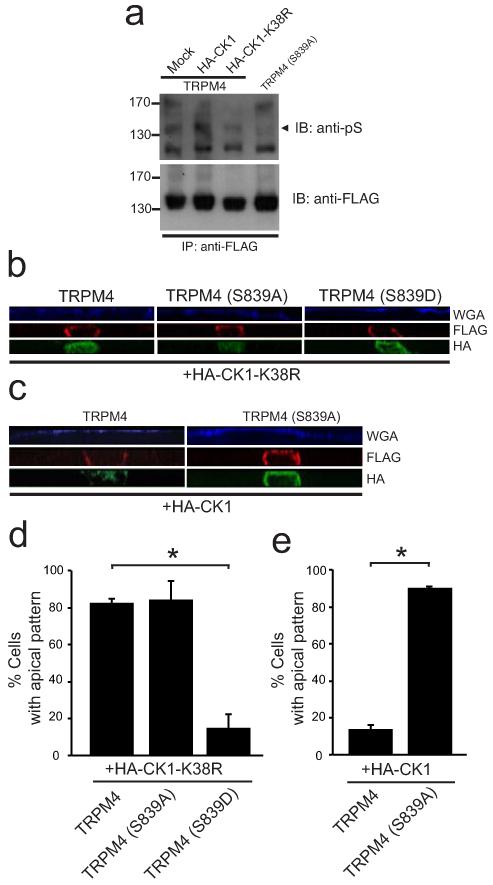
In order to identify the kinase responsible for the phosphorylation of the S839 residue, we performed bioinformatics analyses of the S2-S3 loop sequence, comparing it with the Human Protein Reference database (www.hprd.org). Interestingly, we found that the sequence residues S839-S842 (839SLAS842) and containing pS839, constitutes a putative non-canonical consensus motif for CK1 phosphorylation [27], a highly conserved ubiquitous S/T kinase. Therefore, we hypothesized that this protein kinase might be involved in TRPM4 phosphorylation. In order to explore this possibility, we coexpressed wild-type TRPM4 with wild-type CK1ε (CK1-WT) and a kinase-dead (K38R) dominant-negative version of CK1ε (CK1-DN). Then, we immunopurified FLAG-TRPM4 with an anti-FLAG antibody and serine residues phosphorylation was determined by immunoblot using a phosphoserine antibody (pS). We observed an increase in the immunoreactivity for the pS antibody against TRPM4 present in the CK1 cotransfected cells (Fig. 5a). Conversely, we observed diminished TRPM4 phosphorylation in the presence of the CK1-DN construct, similar to the loss of phosphorylation observed in the S839A mutant (Fig. 5a). Together, these data demonstrate a critical role for CK1 in TRPM4 phosphorylation.
Fig. 5. CK1 activity is required for TRPM4 phosphorylation and proper basolateral localization.
a. HEK293 cells were transiently transfected with wild-type TRPM4 and HA-tagged CK1-DN or CK1, and TRPM4 S839A. FLAG-TRPM4 channels were immunopurified with an anti-FLAG antibody and phosphorylation of Ser residues was determined by immunoblot using a phosphoserine antibody (anti-pS). Arrowhead indicates the phosphorylated form of TRPM4. b-e. MDCK cells were grown as confluent polarized monolayers after cotransfection with TRPM4, TRPM4 S839A or TRPM4 S839D and either HA-tagged CK1-DN or CK1. The apical domain was stained prior to fixation with WGA (blue). After fixation, cells were immunostained with pAb anti-FLAG (red) and mAb anti-HA (green). b. Lateral view of monolayer showing the localization of the different versions of TRPM4 cotransfected with HA-CK1-DN (HA-CK1-K38R). c. Lateral view of MDCK cells monolayer showing the differences of localization between TRPM4 and TRPM4 S839A cotransfected with HA-CK1. d. Graph shows data on percentage of cells which exhibited apical localization of TRPM4 isoforms cotransfected with HA-CK1-DN. * corresponds to significant differences (ANOVA test, post-Dunnett, p < 0.01) versus wild-type TRPM4. Data are from 2 independent experiments including n=68, 46 and 45 cells for TRPM4, TRPM4 S839A, and TRPM4 S839D respectively. e. Graph shows data on percentage of cells which exhibited apical localization of TRPM4 isoforms cotransfected with HA-CK1. * corresponds to significant differences (t-student, p < 0.01) versus wild-type TRPM4. Data are from 2 independent experiments including n=43 and 72 cells for TRPM4 and TRPM4 S839A, respectively.
We next determined whether CK1 activity is required for TRPM4 basolateral localization. We cotransfected MDCK cells with wild-type TRPM4 and CK1-DN, and then performed immunofluorescence staining and optical sectioning microscopy on polarized confluent MDCK cell monolayers. In these experiments, we observed that CK1-DN coexpression disrupts the basolateral localization of wild-type TRPM4, yielding a similar localization to the S839A mutant (Fig. 5b and d). This result suggests that CK1 regulates TRPM4 localization to the basolateral membrane. Because the S839 residue is a putative CK1 substrate, we hypothesized that this residue is required for the CK1-dependent localization. Therefore, we predicted that the TRPM4 S839D phosphomimetic mutant would be resistant to the effect of the CK1-DN. As shown in Fig. 5b, we observed that, unlike wild-type TRPM4, the S839D mutant still localizes to the basolateral membrane in cells coexpressing CK1-DN. In line with this finding, we hypothesized that coexpression of CK1-WT would not rescue the loss of localization of the S839A mutant. We then cotransfected CK1-WT with the S839A mutant, and found that CK1-WT coexpression does not rescue the loss of basolateral localization of this mutant (Fig. 5c and e). In summary, these data suggest that CK1 regulates the basolateral localization of TRPM4 through phosphorylation of TRPM4 at S839.
Discussion
Phosphorylation modulates numerous aspects of ion channel activity, such as voltage-dependence of activation, ligand binding and subcellular trafficking and subcellular localization [7]. Proteomic approaches have been used to identify phosphorylation sites in several members of different families of ion channels, such as Nav, Kv and TRP channels [5,33,34,38,52,45,51,19]. Here we used mass spectrometric-based proteomic approaches to identify a novel phosphorylated serine residue, S839, on recombinant human TRPM4 channel protein immunopurified from the human kidney HEK293 cell line. A previous study using HEK293 cells expressing recombinant human TRPM4 showed that phorbol ester stimulation of altered the Ca2+ sensitivity of TRPM4 channel activation [32]. More recently, high-throughput proteomics studies revealed phosphorylation of mouse TRPM4 at T524, S527, S538 and Y577 [9,37,18,36]. We did not identify phosphorylation on analogous residues in human TRPM4, suggesting species- or tissue-specific differences in TRPM4 phosphorylation. We also did not find evidence for phosphorylation at the sites suggested to mediate the phorbol ester response, perhaps suggesting a requirement for phorbol stimulation [32]. Whether the lack of detectable phosphorylation at these sites in our study is due to differences in sample preparation and/or analysis, or to biological differences (e.g., highly dynamic and and/or tissue-specific phosphorylation at these sites) is not known.
The S839 phosphorylation site is located in the S2-S3 linker (Fig. 2c) in the second ABC signature motif of TRPM4 (833LSGGGGpSLASGGP845). Interestingly, this motif also contains the G844 residue that is mutated to aspartate (i.e. G844D) as the major mutation leading to increased TRPM4 currents in Cardiac Conduction Disease [25]. That the G844D mutation results in a neutral (G) to negatively charged (D) amino acid substitution suggests that alterations in the electrostatic environment of that region is important for determining the level of activity of TRPM4. That phosphorylation at S839, which would also contribute negative charges to this region, impacts the polarized localization of TRPM4 in epithelial cells shows the multifaceted nature of regulation mediated by the S2-S3 linker domain.
Epithelial tissue is a highly organized structure. Mechanisms for controlling the polarized expression of basolateral and apical protein populations represent a key process for determining epithelial function and homeostasis. Therefore, the rupture of epithelial polarity due to the altered subcellular localization of these protein populations can cause diverse pathologies, such as chronic inflammation and cancer [42]. Previously, the presence of TRPM4-like currents in the basolateral domain of mouse renal tubules has been demonstrated [17,10]. Moreover, there are several reports regarding basolateral localization of nonselective calcium-activated cationic channels in different epithelia [39,46,48]. Here, we provide the first immunocytochemical evidence that demonstrates the specific basolateral localization of TRPM4 channels in polarized epithelial cells. By being located at the basolateral membrane, these channels may contribute to renal homeostasis. Since MDCK cells most probably correspond to renal absorptive tubular epithelium, a basolateral localization of TRPM4 may, upon channel activation, provide increased cation flux that initially stimulates the Na+/K+ pump, thereby triggering apical Na+ reabsorption.
Posttranslational modifications of proteins, such as phosphorylation, constitute a major mechanism in the overall regulation of protein function, activity, trafficking and localization [7]. It is no surprise that the activity and localization of many different ion channels are greatly influenced by phosphorylation-dependent mechanisms [7]. The Src-family protein tyrosine kinase (SFK) increases basolateral K+ channels activity via angiotensin II stimulation of SFK activity in the thick ascending limb of the rat kidney [50], and SFK stimulates basolateral K+ channel activity in mouse initial distal convoluted tubule by phosphorylating the Y9 residue on KCNJ10 channel [54]. Conversely, PKA-dependent phosphorylation of the S256 residue of aquaporin 2 (AQP2) is critical for its apical localization. [11]. In addition, casein kinases have been shown as key regulators of the polarized expression of several proteins [28,40,29,44]. For instance, CK1ε activity is required for proper localization of occludin in basolateral domains at tight junctions [29]. Moreover, CK2 activity is important in the basolateral localization of furin, mannose 6-phosphate receptor and UT-A3 urea transporter [28,40,44]. Here, we show that the CK-mediated phosphorylation at TRPM4 S839 is required for proper polarized localization of TRPM4 channels on the basolateral plasma membrane of epithelial cells. These data provide an additional function for this important protein kinase family.
TRPM4 has been shown to regulate several functions of the immune system, such as the secretion of interleukins by human T lymphocytes, the anaphylactic response of mast cells and cell migration of dendritic cells [2,22,49]. The fundamental mechanism for this regulation is through changes in the concentration of intracellular calcium. It has been suggested that TRPM4 regulates calcium influx by depolarizing the membrane, favoring voltage-gated Ca2+ channel opening. In this study, we identified the S839 phosphorylation site as crucial to modulating the polarized localization of the TRPM4 channel. Therefore, TRPM4 phosphorylation may constitute a regulatory mechanism upstream of local changes in intracellular Ca2+ levels. Future work on the tissue specific phosphorylation and the signaling pathways involved in regulation of TRPM4 phosphorylation will be necessary to determine the physiological and pathophysiological regulation of TRPM4 localization and its role in determining intracellular Ca2+ levels.
Despite the lack of canonical motifs for basolateral localization in the primary sequence of TRPM4, it is clear that phosphorylation in the S839 residue regulates this process. Interestingly, the motif in which S839 residue is localized (837GGpSLASGGP845), resembles the AASLLAP motif of the NPP1 pyrophosphatase, which determines the basolateral localization but not endocytic regulation of this protein [4]. Our data demonstrate that S839 phosphorylation is similarly required for basolateral localization, but does not regulates its surface expression. We also demonstrated that CK1 regulates the TRPM4 basolateral localization in a S839-dependent manner. Therefore, we propose that this region of TRPM4 constitutes a non-canonical basolateral targeting motif whose function is regulated by CK-dependent phosphorylation. Future insights from the study of phosphorylation-dependent protein interactions will shed light on the mechanisms underlying dynamic regulation of TRPM4 channel localization and its physiological function.
Acknowledgments
We thank Dr. Pierre Launay for providing the pcDNA4/TO-hTRPM4. We also thank Dr. David Virshup for providing the 4HA-CKIε and 4HA-CKIε (K38R) plasmids (via Addgene, plasmids 13724 and 13725, respectively). We are grateful to Dr. Jon Sack and Ms. Ashleigh Evans for discussion and critical comments to the manuscript. We also thank to Dr. Ricardo Armisén for constructive discussions and Ms. Heidi Pérez, Mr. Nicanor Villarroel and Mr. Francisco Alfaro for technical support. Mass spectrometry was performed at the University of California Davis Proteomics Facility. FONDECYT 11121239 to O.C., National Institutes of Health Grant NS42225 to J.S.T and FONDAP 15010006 to A.S. funded this research. FONDECYT 3120041 Postdoctoral Grant supported M.C. MECESUP UCH0301 Doctoral Fellowship supported E.L.S. and Conicyt Doctoral Fellowship funded A.R.
Abbreviation used in this paper
- TRPM4
transient receptor potential melastatin-like 4
- PI(4,5)P2
phosphatidylinositide (4, 5) biphosphate
- PKC
protein kinase C
- LC-MS/MS
Liquid Chromatography coupled-Tandem Mass Spectrometry
Footnotes
Author’s contributions
O.C., J.S.T. and A.S. designed research. O.C., M.C., K-S.P, E.L-S., and A.R. performed research. O.C., M.C., K-S.P, E.L-S, D.V., J.S.T and A.S. analyzed data. O.C., J.S.T. and A.S. wrote the manuscript.
The authors declare no conflict of interests.
References
- 1.Armisén R, Marcelain K, Simon F, Tapia JC, Toro J, Quest AF, Stutzin A. TRPM4 enhances cell proliferation through up-regulation of the β-catenin signaling pathway. J Cell Physiol. 2011;226(1):103–109. doi: 10.1002/jcp.22310. doi:10.1002/jcp.22310. [DOI] [PubMed] [Google Scholar]
- 2.Barbet G, Demion M, Moura IC, Serafini N, Leger T, Vrtovsnik F, Monteiro RC, Guinamard R, Kinet JP, Launay P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol. 2008;9(10):1148–1156. doi: 10.1038/ni.1648. doi:ni.1648 [pii] 10.1038/ni.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becerra A, Echeverría C, Varela D, Sarmiento D, Armisén R, Núñez-Villena F, Montecinos M, Simon F. Transient receptor potential melastatin 4 inhibition prevents lipopolysaccharide-induced endothelial cell death. Cardiovasc Res. 2011;91(4):677–684. doi: 10.1093/cvr/cvr135. doi:10.1093/cvr/cvr135. [DOI] [PubMed] [Google Scholar]
- 4.Bello V, Goding JW, Greengrass V, Sali A, Dubljevic V, Lenoir C, Trugnan G, Maurice M. Characterization of a di-leucine-based signal in the cytoplasmic tail of the nucleotide-pyrophosphatase NPP1 that mediates basolateral targeting but not endocytosis. Mol Biol Cell. 2001;12(10):3004–3015. doi: 10.1091/mbc.12.10.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berendt FJ, Park KS, Trimmer JS. Multisite phosphorylation of voltage-gated sodium channel α subunits from rat brain. J Proteome Res. 2010;9(4):1976–1984. doi: 10.1021/pr901171q. doi:10.1021/pr901171q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol. 2008;35(9):1116–1120. doi: 10.1111/j.1440-1681.2007.04855.x. doi:CEP4855 [pii]10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerda O, Trimmer JS. Analysis and functional implications of phosphorylation of neuronal voltage-gated potassium channels. Neurosci Lett. 2010;486(2):60–67. doi: 10.1016/j.neulet.2010.06.064. doi:10.1016/j.neulet.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic β-cells. Cell Calcium. 2007;41(1):51–61. doi: 10.1016/j.ceca.2006.04.032. doi:S0143-4160(06)00105-9 [pii] 10.1016/j.ceca.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary C, Olsen JV, Brandts C, Cox J, Reddy PN, Bohmer FD, Gerke V, Schmidt-Arras DE, Berdel WE, Muller-Tidow C, Mann M, Serve H. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell. 2009;36(2):326–339. doi: 10.1016/j.molcel.2009.09.019. doi:10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Chraibi A, Van den Abbeele T, Guinamard R, Teulon J. A ubiquitous non-selective cation channel in the mouse renal tubule with variable sensitivity to calcium. Pflug Arch Eur J Phy. 1994;429(1):90–97. doi: 10.1007/BF02584034. [DOI] [PubMed] [Google Scholar]
- 11.Christensen BM, Zelenina M, Aperia A, Nielsen S. Localization and regulation of PKA-phosphorylated AQP2 in response to V(2)-receptor agonist/antagonist treatment. Am J Physiol Renal. 2000;278(1):F29–42. doi: 10.1152/ajprenal.2000.278.1.F29. [DOI] [PubMed] [Google Scholar]
- 12.Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, Jaggar JH, Earley S. Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2010;299(3):C682–94. doi: 10.1152/ajpcell.00101.2010. doi:ajpcell.00101.2010 [pii]10.1152/ajpcell.00101.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res. 2007;73(3):531–538. doi: 10.1016/j.cardiores.2006.11.023. doi:10.1016/j.cardiores.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95(9):922–929. doi: 10.1161/01.RES.0000147311.54833.03. doi:01.RES.0000147311.54833.03 [pii]10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 15.Gerzanich V, Woo SK, Vennekens R, Tsymbalyuk O, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V, Freichel M, Simard JM. De novo expression of TRPM4 initiates secondary hemorrhage in spinal cord injury. Nat Med. 2009;15(2):185–191. doi: 10.1038/nm.1899. doi:nm.1899 [pii]10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinamard R, Demion M, Launay P. Physiological roles of the TRPM4 channel extracted from background currents. Physiology. 2010;25(3):155–164. doi: 10.1152/physiol.00004.2010. doi:25/3/155 [pii]10.1152/physiol.00004.2010. [DOI] [PubMed] [Google Scholar]
- 17.Guinamard R, Paulais M, Lourdel S, Teulon J. A calcium-permeable non-selective cation channel in the thick ascending limb apical membrane of the mouse kidney. Biochim Biophys Acta. 2012;1818(5):1135–1141. doi: 10.1016/j.bbamem.2011.12.024. doi:10.1016/j.bbamem.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. doi:10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TY, Shin SK, Song MY, Lee JE, Park KS. Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem Bioph Res Co. 2012;417(3):1030–1034. doi: 10.1016/j.bbrc.2011.12.085. doi:10.1016/j.bbrc.2011.12.085. [DOI] [PubMed] [Google Scholar]
- 20.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest. 2009;119(9):2737–2744. doi: 10.1172/JCI38292. doi:38292 [pii]10.1172/JCI38292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306(5700):1374–1377. doi: 10.1126/science.1098845. doi:306/5700/1374 [pii]10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 23.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109(3):397–407. doi: 10.1016/s0092-8674(02)00719-5. doi:S0092867402007195 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Chatel S, Simard C, Syam N, Salle L, Probst V, Morel J, Millat G, Lopez M, Abriel H, Schott JJ, Guinamard R, Bouvagnet P. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS One. 2013;8(1):e54131. doi: 10.1371/journal.pone.0054131. doi:10.1371/journal.pone.0054131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet. 2010;3(4):374–385. doi: 10.1161/CIRCGENETICS.109.930867. doi:10.1161/CIRCGENETICS.109.930867. [DOI] [PubMed] [Google Scholar]
- 26.Marigo V, Courville K, Hsu WH, Feng JM, Cheng H. TRPM4 impacts on Ca2+ signals during agonist-induced insulin secretion in pancreatic beta-cells. Mol Cell Endocrinol. 2009;299(2):194–203. doi: 10.1016/j.mce.2008.11.011. doi:S0303-7207(08)00547-9 [pii]10.1016/j.mce.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, Allende CC, Pinna LA, Allende JE. A noncanonical sequence phosphorylated by casein kinase 1 in β-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc Natl Acad Sci USA. 2003;100(18):10193–10200. doi: 10.1073/pnas.1733909100. doi:10.1073/pnas.1733909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271(4):2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- 29.McKenzie JA, Riento K, Ridley AJ. Casein kinase I epsilon associates with and phosphorylates the tight junction protein occludin. FEBS Lett. 2006;580(9):2388–2394. doi: 10.1016/j.febslet.2006.03.048. doi:10.1016/j.febslet.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 30.Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, Brayden JE. Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharmacol Sci. 2007;103(4):417–426. doi: 10.1254/jphs.fp0061332. doi:JST.JSTAGE/jphs/FP0061332 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25(3):467–478. doi: 10.1038/sj.emboj.7600963. doi:7600963 [pii]10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280(8):6423–6433. doi: 10.1074/jbc.M411089200. doi:M411089200 [pii]10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 33.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313(5789):976–979. doi: 10.1126/science.1124254. doi:10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 34.Park KS, Mohapatra DP, Trimmer JS. Proteomic analyses of K(v)2.1 channel phosphorylation sites determining cell background specific differences in function. Channels (Austin) 2007;1(2):59–61. doi: 10.4161/chan.4388. [DOI] [PubMed] [Google Scholar]
- 35.Reading SA, Brayden JE. Central role of TRPM4 channels in cerebral blood flow regulation. Stroke. 2007;38(8):2322–2328. doi: 10.1161/STROKEAHA.107.483404. doi:STROKEAHA.107.483404 [pii]10.1161/STROKEAHA.107.483404. [DOI] [PubMed] [Google Scholar]
- 36.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. doi: 10.1016/j.cell.2007.11.025. doi:10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA. 2010;107(8):3882–3887. doi: 10.1073/pnas.0910646107. doi:10.1073/pnas.0910646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seikel E, Trimmer JS. Convergent modulation of Kv4.2 channel α subunits by structurally distinct DPPX and KChIP auxiliary subunits. Biochemistry. 2009;48(24):5721–5730. doi: 10.1021/bi802316m. doi:10.1021/bi802316m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siemer C, Gogelein H. Activation of nonselective cation channels in the basolateral membrane of rat distal colon crypt cells by prostaglandin E2. Pflug Arch Eur J Phy. 1992;420(3-4):319–328. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- 40.Simmen T, Nobile M, Bonifacino JS, Hunziker W. Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol Cell Biol. 1999;19(4):3136–3144. doi: 10.1128/mcb.19.4.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon F, Leiva-Salcedo E, Armisén R, Riveros A, Cerda O, Varela D, Eguiguren AL, Olivero P, Stutzin A. Hydrogen peroxide removes TRPM4 current desensitization conferring increased vulnerability to necrotic cell death. J Biol Chem. 2010;285(48):37150–37158. doi: 10.1074/jbc.M110.155390. doi:10.1074/jbc.M110.155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh B, Bogatcheva G, Washington MK, Coffey RJ. Transformation of polarized epithelial cells by apical mistrafficking of epiregulin. Proc Natl Acad Sci USA. 2013;110(22):8960–8965. doi: 10.1073/pnas.1305508110. doi:10.1073/pnas.1305508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stallmeyer B, Zumhagen S, Denjoy I, Duthoit G, Hebert JL, Ferrer X, Maugenre S, Schmitz W, Kirchhefer U, Schulze-Bahr E, Guicheney P, Schulze-Bahr E. Mutational spectrum in the Ca2+-activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mut. 2012;33(1):109–117. doi: 10.1002/humu.21599. doi:10.1002/humu.21599. [DOI] [PubMed] [Google Scholar]
- 44.Stewart GS, Thistlethwaite A, Lees H, Cooper GJ, Smith C. Vasopressin regulation of the renal UT-A3 urea transporter. Am J Physiol Renal. 2009;296(3):F642–648. doi: 10.1152/ajprenal.90660.2008. doi:10.1152/ajprenal.90660.2008. [DOI] [PubMed] [Google Scholar]
- 45.Surti TS, Huang L, Jan YN, Jan LY, Cooper EC. Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels. Proc Natl Acad Sci USA. 2005;102(49):17828–17833. doi: 10.1073/pnas.0509122102. doi:10.1073/pnas.0509122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teulon J, Paulais M, Bouthier M. A Ca2+-activated cation-selective channel in the basolateral membrane of the cortical thick ascending limb of Henle’s loop of the mouse. Biochim Biophys Acta. 1987;905(1):125–132. doi: 10.1016/0005-2736(87)90016-2. [DOI] [PubMed] [Google Scholar]
- 47.Ubersax JA, Ferrell JE., Jr. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8(7):530–541. doi: 10.1038/nrm2203. doi:nrm2203 [pii]10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 48.Van den Abbeele T, Tran Ba Huy P, Teulon J. A calcium-activated nonselective cationic channel in the basolateral membrane of outer hair cells of the guinea-pig cochlea. Pflug Arch Eur J Phy. 1994;427(1-2):56–63. doi: 10.1007/BF00585942. [DOI] [PubMed] [Google Scholar]
- 49.Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8(3):312–320. doi: 10.1038/ni1441. doi:ni1441 [pii]10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 50.Wang M, Luan H, Wu P, Fan L, Wang L, Duan X, Zhang D, Wang WH, Gu R. Angiotensin II stimulates basolateral 50-pS K channels in the thick ascending limb. Am J Physiol Renal. 2014;306(5):F509–516. doi: 10.1152/ajprenal.00476.2013. doi:10.1152/ajprenal.00476.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, Aldrich RW, Fakler B, Trimmer JS. Profiling the phospho-status of the BKCa channel α subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics. 2008;7(11):2188–2198. doi: 10.1074/mcp.M800063-MCP200. doi:10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci USA. 2007;104(50):20055–20060. doi: 10.1073/pnas.0708574104. doi:0708574104 [pii]10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu W, Hill WG, Apodaca G, Zeidel ML. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am J Physiol Renal. 2011;300(1):F49–59. doi: 10.1152/ajprenal.00349.2010. doi:10.1152/ajprenal.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10 protein. J Biol Chem. 2013;288(36):26135–26146. doi: 10.1074/jbc.M113.478453. doi:10.1074/jbc.M113.478453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 2005;280(47):39185–39192. doi: 10.1074/jbc.M506965200. doi:M506965200 [pii] 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]