Abstract
Background
Remote ischemic conditioning (RIC) is a known manual conditioning to decrease ischemic reperfusion injury (IRI) but not increase ischemic time. Here we tried to establish a rat RIC model of liver transplantation (LT), optimize the applicable protocols and investigate the protective mechanism.
Methods
The RIC model was developed by a standard tourniquet. Sprague-Dawley rats were assigned randomly to the sham operated control (N), standard rat liver transplantation (OLT) and RIC groups. According to the different protocols, RIC group was divided into 3 subgroups (10min×3, n = 6; 5min×3, n = 6; 1min×3, n = 6)respectively. Serum transaminases (ALT, AST), creatine kinase (CK), histopathologic changes, malondialdehyde (MDA), myeloperoxidase (MPO) and expressions of p-Akt were evaluated.
Results
Compared with the OLT group, the grafts subjected to RIC 5min*3 algorithm showed significant reduction of morphological damage and improved the graft function. Also, production of reactive oxygen species (MDA) and neutrophil accumulation (MPO) were markedly depressed and p-Akt was upregulated.
Conclusion
In conclusion, we successfully established a novel model of RIC in rat LT, the optimal RIC 5min*3 algorithm seemed to be more efficient to alleviate IRI of the liver graft in both functional and morphological categories, which due to its antioxidative, anti-inflammation activities and activating PI3K Akt pathway.
Introduction
Nowadays, LT has developed as the universal first line treatment for the end-stage liver disease. Recent years, donation after cardiac death (DCD), domino transplantation and living donor have been increasingly applied in LT [1]. However, insufficient liver donor over the increasing demand has become one of the major existing problems. More about it, ischemic reperfusion injury (IRI) during LT is still major concerning factor in the outcome of LT [2] [3]. As we have demonstrated previously, the ischemic preconditioning (IPC) exerts powerful protective effects on attenuation of hepatic IRI by the reduction of leukocyte infiltration, hepatic enzymatic leakage and apoptotic cells formation [4, 5]. But the wide clinical application of IPC has been limited, mainly because of the unpredictability of the ischemic episode of the donors, direct mechanical trauma to major vascular structures and the potential ethical reasons [6]. Alternatively, the concept of remote ischemic conditioning (RIC) was originally developed by Przyklenk et al in 1993, showing that with the brief ischemia in one organ conferred on protection of distant important organs without direct stress to the target organ [7].The encouraging factor is, the existing research on RIC has shown the ameliorating properties on IRI of lung, intestine, kidney and heart [8–12]. Till the date, only few experimental studies have been carried out to demonstrate in the outcome of RIC on hepatectomy, focusing on brief hindlimb vascular occlusion [6, 13], infrarenal aortic clamping [14] and femoral artery occlusion [15]. Compared to IPC, RIC can be applied before ischemic event of target organ (remote ischemic preconditioning [R-IPC]), after ischemic event and before perfusion also named during ischemic event (remote ischemic perconditioning perfusion [R-IPER]) or at the onset of reperfusion (remote ischemic postconditioning [R-IPostC]) (Fig. 1) without increasing the total ischemic time. Here we mainly focus on the R-IPER which was first demonstrated effective in reducing myocardial infarction by Schimdt et al[16]. Until now, there were only 3 major studies involving the R-IPER in liver IRI which the protection function were also proved and related mechanism were investigated[17–19]. However, the effects of RIC on the liver grafts have not been reported so far. In this study we tried to establish a LT model of R-IPER, optimize the applicable protocols and investigate the potential protective mechanism in rat.
Fig 1. Classification of RIC according to applied time point.
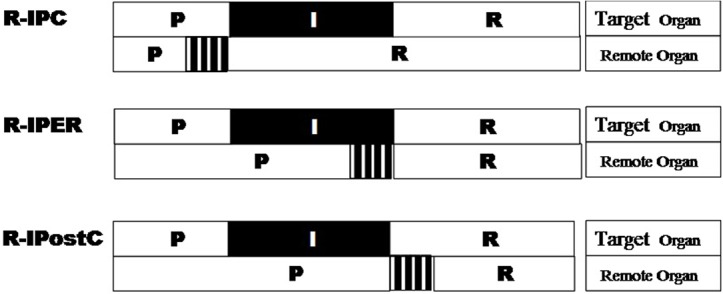
Note, “P” stands for “perfusion”, “I” means “ischemic”, “R” means “reperfusion”.
Materials and Methods
Sprague Dawley rats (adult male, weight 250 300 g) were used in the experiment. The rats were kept in a temperature controlled environment (25 to 30°C) and supplied the standard diet water ad libitum. All procedures in this study were approved by the Ethics Committee for the Use of Experimental Animals in Zhejiang University and were carried out in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines (http://www.nc3rs.org/ARRIVE).
Experimental design and groups
54 Rats (24 donors) were assigned randomly to the different groups as follows, sham operated group (N, n = 6), standard rat orthotopic liver transplantation group (OLT, n = 6); RIC group (OLT with hindlimb ischemic and perfusion for different time intervals starting at the point when recipient anhepatic phase begins). According to the protocol, the RIC group was then divided into 3 subgroups (10min×3, n = 6; 5min×3, n = 6; 1min×3, n = 6)respectively (Fig. 2A).
Fig 2. Study design and RIC model of the study.
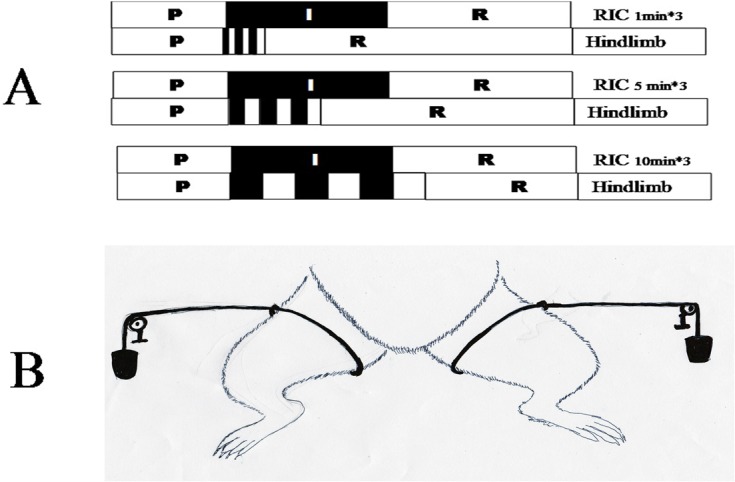
A. Details and schematic representation of RIC protocol in this study. RIC rats were divided into three subgroup (10min×3, n = 6; 5min×3, n = 6; 1min×3, n = 6). B. Schematic diagram of RIC applied in this study. Standard tourniquet was used for ligature of the hindlimb with one 1kg weight in the end.
Rat OLT and RIC models
The OLT was performed by the method of Kamada[20] with some modification as per our research center[21]. In brief, both donor and recipient were anesthetized by intraperitoneal injection of 4% chloral hydrate anesthesia (Shanghai No. 1 Biochemical & Pharmaceutical, China). The donor was anesthetized after 12h-fasting while the recipient was done after the isolation of donor liver, then the graft was perfused through the portal vein with cold saline containing 25U/mL heparin. The graft placed into cold saline (0–4°C) for about 40 minutes before graft being transplanted into the recipient. After the completion of anastomosis of the supra-hepatic inferior vena cava followed by portal vein cuff connection, the liver was reperfused with the hepatic artery being ligated. Then the connection of infra-hepatic inferior vena cava by the cuff method, and the reconstruction of common bile duct by tying the duct over a stent was carried out.1.5 ml. Saline injected through the penile vein of the recipient after the operation. The RIC model was developed by a standard tourniquet which was performed in recipient. RIC protocols were performed by the designed cycles of reperfusion and reocclusion of the hindlimb applied immediately at the onset of recipient anhepatic phase with 1KG weight both sides (Fig. 2B). In the sham operated group, the abdomen was just opened for 75min (the mean total ischemic time during OLT in our center) and then closed under anesthesia. Upon awakening from anesthesia, rats were provided free access to sterilized water and standard rodent chow.
Sample collection
All rats from each group were anesthetized with 4% chloral hydrate at 180 min. after OLT for the collection of samples. In detail, the rats were euthanized 3h after the portal vein of recipient was open. Blood samples were collected from the portal vein for liver function test. The liver tissue was obtained and fixed in 10% neutral formalin for the histological study purpose. The fresh liver tissue was collected and stored in −80°C for further analysis.
Histopathologic examination
Excised liver specimens were fixed in 10% neutral buffered formalin with paraffin embedded and sectioned to 4 mm thick in accordance with our standard protocol. The sections were gradually deparaffinized and hydrated, and examined with haematoxylin and eosin staining. Morphological assessment was performed by well experienced liver pathologist in a blind fashion. A grading scale of 0–4 was used for the histopathological assessment of IRI based on the degree of sinusoidal congestion, and necrosis of parenchymal cells according to the Suzuki classification[22] and our previous studies[23].
Liver function and CK test
Blood samples collected were centrifuged at 3,000×g for 15 minutes in room temperature to collect the serum for liver function test and creatine kinase (CK) analysis by Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan).
Levels of lipid peroxidation and myeloperoxidase activity
Lipid peroxidation in liver tissue was determined by measuring the formation of malondialdehyde (MDA), which is considered as an indirect measurement of oxidative damage induced by ROS[24], by the protocols of manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All assays were performed with Bechman Coulter DU-800 Spectrophotometer (USA). As a marker of polymorphonuclear neutrophil (PMN) infiltration[25], the activity of myeloperoxidase (MPO) in hepatic tissue was measured with MPO assay kit (Nanjing Jiancheng Bioengineering Institute) in accordance with the instructions provided by manufacturer.
Measurement of P-Akt expression in liver graft
Protein was extracted from liver by the method as described previously [26] and quantified with the Bradford assay. Lysate protein was separated by 10% SDS-PAGE gels and then transferred to nitrocellulose membranes (Bio-Rad Laboratories). After blocking with 5% fetal bovine serum in tris-buffered saline with tween (10 mmol/L Tris-HCl, 0.15 mol/L NaCl, and 0.05% Tween 20, pH 7.2), blots were incubated with the rabbit polyclonal anti- P-Akt antibody (1: 1000; Signalway antibody, USA) or mouse monoclonal anti-β-actin antibody (1: 1000; Dawenbioscience, China) for 16 h at 4°C. Blots were washed and incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibodies (1:2000; Dawenbioscience, China) for 1.5 h. Western blots were developed with the enhanced chemiluminescence system (ECL kit; Pierce Biotechnology, Rockford, IL, USA) and captured on light-sensitive imaging film.
Statistical analysis
All experimental data are shown as mean ± SD. Statistical differences were calculated with one-way analysis of variance (ANOVA) followed by the Dunnett’s test for multiple comparisons or by Student t test for the histopathological outcomes. A p<0.05 was considered to be statistically significant.
Results
All operations were successful. During the 3h observation, no death and other complications such as infection and embolism et al were found. All the following outcome points were at 3h after the portal vein of recipient was open.
Optimal RIC algorithm improves the liver histopathological outcomes
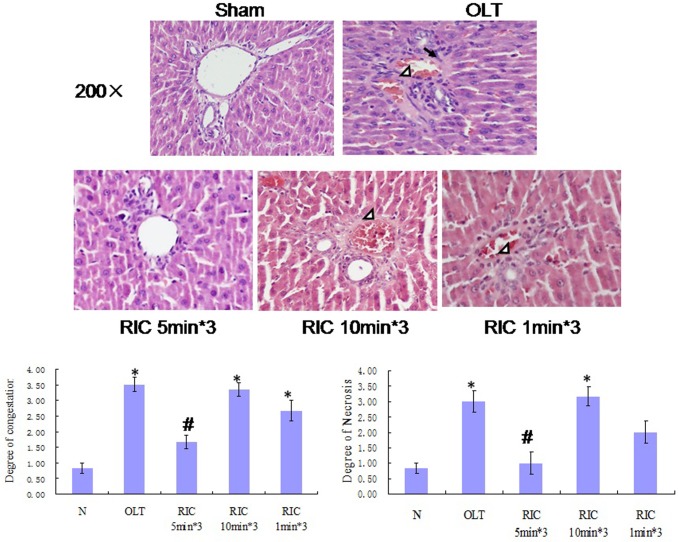
Comparing with N group, sinusoidal congestion of all other groups is elevated. Comparing with OLT, RIC 5min*3 diminished the sinusoidal congestion (P = 0.001) while in RIC 10min*3 and RIC 1min*3 are not. Among RIC groups, RIC 5min*3 has lower sinusoidal congestion than RIC 10min*3 (P = 0.002) while almost the same as RIC 1min. RIC 10min*3 shows severe congestion compared with RIC 1min*3. (Fig. 3)
Fig 3. Liver histopathological presentations of all groups (Haematoxylin and Eosin stain, original magnification ×200).
Hepatocyte necrosis (arrow) and congestion of the sinusoids and central vein (white arrow head). (Values are mean (s.e.m.) * p<0.05 compared with sham, # p<0.05 compared with OLT group).
Comparing with N group, multifocal areas of coagulated hepatic cellular necrosis level of all other groups are elevated. Comparing with OLT group, multifocal areas of coagulated hepatic cellular necrosis in RIC 5min*3 diminished (P = 0.026) while in RIC 10min*3 and RIC 1min*3 are not. Among RIC groups, RIC 5min*3 has lower hepatic cellular necrosis than RIC 10min*3 (P = 0,01) while almost the same as RIC 1min. RIC 10min*3 has more multifocal hepatic cellular necrosis compared with RIC 1min*3. (Fig. 3)
Optimal RIC algorithm improves the liver graft function
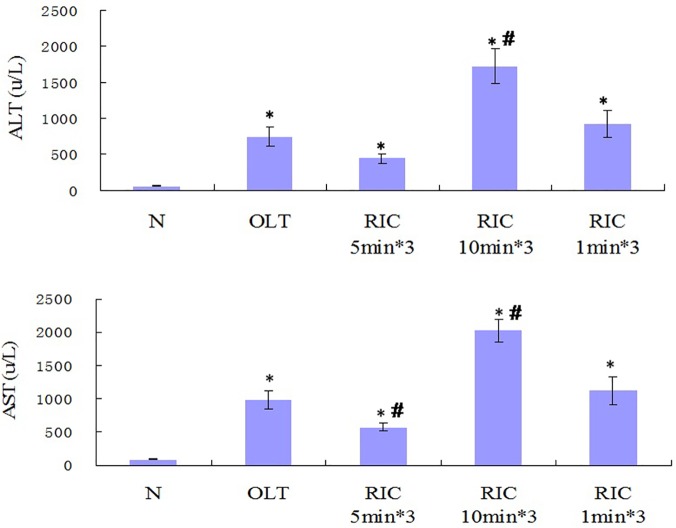
Comparing with N group, Serum ALT levels of all other groups are elevated. Comparing with OLT group, elevation of ALT level is seen in RIC 10min*3 (P <0.001) but not in RIC 5min*3 and RIC 1min*3. Among RIC groups, RIC 5min*3 has the lowest ALT level (VS RIC 10min*3, P <0.001; VS RIC 1min*3, P = 0.034). RIC 10min*3 shows higher ALT compared to RIC 1min*3. (Fig. 4)
Fig 4. ALT and AST levels of all groups.
(n = 6 in each group. Values are mean (s.e.m.) * p<0.05 versus with sham, # p<0.05 versus with OLT group).
Comparing with N group, Serum AST levels of all other groups are elevated. Compared with OLT group, increase in the AST level is found in RIC 10min*3 further (P <0.001) while obvious decrease in AST level in RIC 5min*3 (P = 0.047) but remains almost same in RIC 1min*3. Among RIC groups, RIC 5min*3 reported the lowest AST level (VS RIC 10min*3, P <0.001; VS RIC 1min*3, P = 0.011). RIC 10min*3 shows higher AST compared to RIC 1min*3. (Fig. 4)
Optimal RIC algorithm decreases MDA and MPO
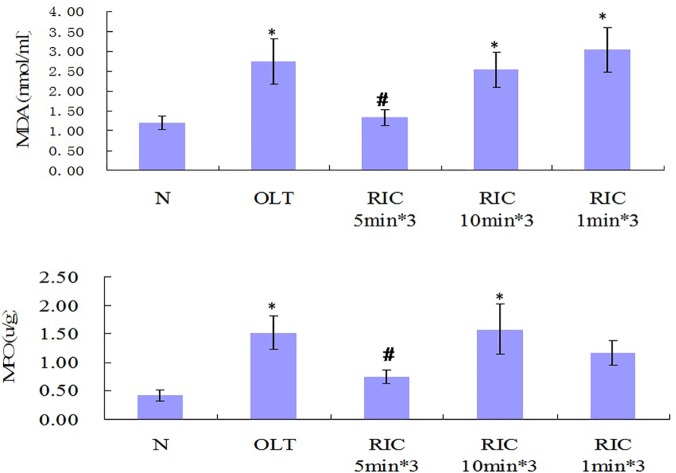
Comparing with N group, MDA levels of all other groups are elevated. Compared with OLT group, RIC 5min*3 diminished the MDA level (P = 0.006) while not in RIC 10min*3 and RIC 1min*3. Among RIC groups, RIC 5min*3 shows the lowest MDA level (VS RIC 10min*3, P = 0.014; VS RIC 1min*3, P = 0.002). RIC 10min*3 had lower MDA compared to RIC 1min*3. (Fig. 5)
Fig 5. MDA and MPO levels of all groups.
(n = 6 in each group. Values are mean (s.e.m.) * p<0.05 versus with sham, # p<0.05 versus with OLT group).
Comparing with N group, MPO levels of all other groups are elevated. Comparing with OLT group, markedly decrease in MPO level can be noticed in RIC 5min*3 (P = 0.002) but not in RIC 10min*3 and RIC 1min*3. Among RIC groups, RIC 5min*3 shows the lowest MPO level (VS RIC 10min*3, P = 0.002; VS RIC 1min*3, P = 0.022). RIC 10min*3 shows higher MPO compared to RIC 1min*3. (Fig. 5)
Optimal RIC algorithm does not increase CK
Comparing with N group, Serum CK levels of all other groups are elevated. Compared to OLT group, CK level is increased in RIC 10min*3 (P <0.0001) while not in RIC 5min*3 and RIC 1min*3. Among RIC groups, RIC 5min*3 shows lower CK level than RIC 10min*3 (P = 0.002) while the same as RIC 1min. RIC 10min*3 shows higher CK compared to RIC 1min*3. (Fig. 6)
Fig 6. CK levels of all groups.
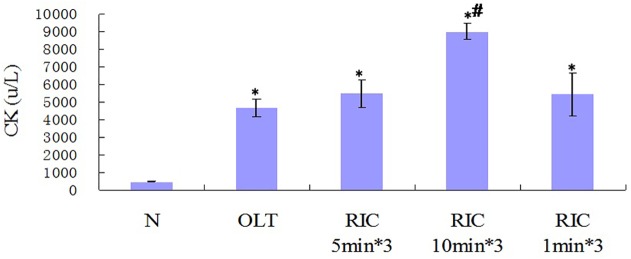
(n = 6 in each group. Values are mean (s.e.m.) * p<0.05 versus with sham, # p<0.05 versus with OLT group).
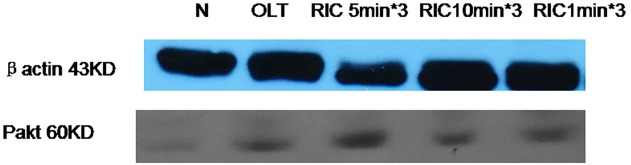
Optimal RIC algorithm up-regulates the PI3K-Akt protein expressions
The protein expression levels of PI3K Akt were measured by western blot analysis. The results revealed that the expression levels of PI3K Akt in the liver tissues were significantly increased in the RIC 5min*3 compared to OLT. (Fig. 7)
Fig 7. Expression of Pakt of all groups.
Discussion
According to our knowledge, it was first reported model of RIC in LT. No study in R-IPER comparing different times and protocols of limb occlusion was searched in Medline. This model was easily developed by a standard tourniquet applied immediately at the onset of recipient anhepatic phase with 1KG weight both sides (Fig. 2B), which did not increase CK level, in another word no further local mechanism trauma is induced. Besides, R-IPER especially 5min*3 markedly attenuated the IRI, improved liver function and reduced inflammation reaction, verifying the better outcomes in the liver graft of rats. This showed this model is easily developed, effective and particle.
Compared to the local ischemic conditionings (pre and post ischemic conditioning), RIC overcomes the main limitation of increasing total ischemic time which may bring sequence problems. Similarly to local ischemic conditionings, also there are three main components of the RIC algorithm: the duration of the initial reperfusion phase, the duration of each subsequent ischemia and reperfusion cycle and the number of cycles applied[27]. Some studies have proved that RIC could reduce IRI in small animal liver ischemia models [14, 13], however, according to our knowledge there is limited research so far to prove the effects of R-IPER on the liver graft[28]. 10min protocol[13] and 5min protocol [28] were showed protective role against hepatic IRI and enhanced the early antioxidative activity. In this study, we compared different R-IPER algorithm by selecting alternating cycles of brief reperfusion interrupted by brief ischemia starting at the beginning of anhepatic phase. It showed that only R-IPER 5min*3 induced the protective effects for the liver grafts. In all the groups, the R-IPER 10min*3 protocol showed the less protective effect, even worse than OLT for the indexes of ALT, AST and CK, Which is not the same as previous result[13].The possible reasons are related to the different animal model and the different weight used for the hind limb. On the other side, R-IPER 1min*3 was found almost the same with OLT, this may due to the stimulus of this protocol was not strong enough to induce the protective effect. So based on the observation, we can conclude that selection of the duration of ischemia and optimizing the protective protocol algorithm maybe associated to the degree of protection and can impact on the outcome of remote ischemic conditioning. The R-IPER 5min*3 protocol leads to more perceptible liver protection. This keeps in line with previous reports which 5min*4 were applied [17–19]. This finding provides the further evidence for the application of optimized RIC protocol in liver graft model.
Results from previous studies demonstrated the IRI suppression effects of RIC are through the multiple mechanisms [15, 29], though they are still not clarified yet. One of the major mechanism now known is to intervene the cascade of oxidative-stress-related events [30]. In present experiments, the protective effects have also been introduced in the OLT model of rat, which provides the further evidence that RIC protocol was feasible and practicable to apply in the OLT of rat model.
Our results showed the R-IPER 5min*3 exerted the protective effect on the morphological levels by attenuating the congestion and the hepatocyte necrosis (Fig. 3).On the functional level, it is demonstrated that all the groups except N group showed a significant increase of the ALT and AST. Conversely, the R-IPER 5min*3 dramatically improved in the protection of the grafts by inhibiting the increase in ALT and AST levels, which are the crucial biochemical parameter reflecting the function of liver. These results indicated there may be a component of morphological and biochemical adaptation of the graft to ischemia after RIC. In present study, R-IPER especially 5min*3 resulted in a marked reduction of lipid peroxidation product, MDA formation, and suppression of the infiltration of neutrophils causing to decrease the MPO level, which suggested that these protective effects may be associated with its enhanced capacities of antioxidant and anti-inflammatory activities with subsequently improvement in sinusoidal endothelial function following hepatic IRI of OLT.
Evidence showed PI3-kinase and its downstream effector protein kinase B (Akt) as well as eNOS are known to play an important role against the reperfusion injury by regulating cellular activation, inflammatory responses and apoptosis[31]. As the redox-sensitive kinase, PI3-kinase/Akt may be activated by site-specific phosphorylation, when intracellular ROS levels changed, further result in eNOS activation and increased reperfusion NO generation, and subsequently exert the cytoprotective effect[32]. In present experiment, compared to control group, applying the R-IPER 5min*3 protocol significantly enhanced Akt phosphorylation at 3 hours after reperfusion in the liver graft. This study has demonstrated that RIC protected the liver graft of rat against IRI by increasing the activities of PI3K Akt, which is crucial for the protective effects against IRI in the graft liver of rat. However the further study for the detail role of P-Akt for this protective effect is still needed.
In conclusion, we successfully established a novel model of RIC in rat LT, the optimal R-IPER 5min*3 algorithm seemed to be more efficient to alleviate the detrimental effects of IRI of the liver graft in both functional and morphological categories, which depended on its antioxidative, anti-inflammation activities and activating PI3K Akt pathway. The strategy of R-IPER 5min*3 is relatively simple to perform, particularly during LT, and may be considered as a potentially applicable in the clinical field.
Supporting Information
(PDF)
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported, in part, by N National S&T Major Project (NO:2012ZX10002-017); National Natural Science Foundation of China (Nr 81000137); the Qianjiang Talent Program of Zhejiang Province, China (Nr2012R10045); and the Scientific Research program for the Returned Overseas Chinese Scholars, Ministry of Health, Zhejiang Province, China (J20112008). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Van Gelder F, de Roey J, Desschans B, Van Hees D, Aerts R, Monbaliu D, et al. Donor categories: heart-beating, non-heart-beating and living donors; evolution within the last 10 years in UZ Leuven and Collaborative Donor Hospitals. Acta Chir Belg. 2008;108:35–38. [PubMed] [Google Scholar]
- 2. Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. 10.1038/nrgastro.2012.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hicks M, Hing A, Gao L, Ryan J, Macdonald PS. Organ preservation. Methods Mol Biol. 2006;333:331–374. [DOI] [PubMed] [Google Scholar]
- 4. Jin LM, Liu YX, Zhou L, Xie HY, Feng XW, Li H, et al. Ischemic preconditioning attenuates morphological and biochemical changes in hepatic ischemia/reperfusion in rats. Pathobiology. 2010;77:136–146. 10.1159/000292647 [DOI] [PubMed] [Google Scholar]
- 5. Yan S, Jin LM, Liu YX, Zhou L, Xie HY, Zheng SS. Outcomes and mechanisms of ischemic preconditioning in liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:346–354. [PubMed] [Google Scholar]
- 6. Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762–768. [DOI] [PubMed] [Google Scholar]
- 7. Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. 10.1093/cvr/cvn114 [DOI] [PubMed] [Google Scholar]
- 8. Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151:1099–1103. 10.1016/j.neuroscience.2007.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury—a review. J Surg Res. 2008;150:304–330. 10.1016/j.jss.2007.12.747 [DOI] [PubMed] [Google Scholar]
- 10. Eberlin KR, McCormack MC, Nguyen JT, Tatlidede HS, Randolph MA, Austen WG Jr. Ischemic preconditioning of skeletal muscle mitigates remote injury and mortality. J Surg Res 2008;148:24–30. 10.1016/j.jss.2008.01.040 [DOI] [PubMed] [Google Scholar]
- 11. Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, Inubushi T, et al. Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of "remote preconditioning". J Am Coll Cardiol. 1999;33:556–564. [DOI] [PubMed] [Google Scholar]
- 12. Pavione MA, Carmona F, de Castro M, Carlotti AP. Late remote ischemic preconditioning in children undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg.2012;144:178–183. 10.1016/j.jtcvs.2011.12.029 [DOI] [PubMed] [Google Scholar]
- 13. Wang F, Birch SE, He R, Tawadros P, Szaszi K, Kapus A, et al. Remote ischemic preconditioning by hindlimb occlusion prevents liver ischemic/reperfusion injury: the role of High Mobility Group-Box 1. Ann Surg.2009;251:292–299. [DOI] [PubMed] [Google Scholar]
- 14. Czigany Z, Turoczi Z, Bulhardt O, Hegedus V, Lotz G, Rakonczay Z, et al. [Remote ischemic conditioning: short-term effects on rat liver ischemic-reperfusion injury]. Orv Hetil. 2012; 153: 1579–1587. [DOI] [PubMed] [Google Scholar]
- 15. Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–1317. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–1890. [DOI] [PubMed] [Google Scholar]
- 17. Costa FL, Yamaki VN, Goncalves TB, Coelho JV, Percario S, Brito MV. Combined remote ischemic perconditioning and local postconditioning on liver ischemia-reperfusion injury. J Surg Res. 2014;192:98–102. 10.1016/j.jss.2014.05.046 [DOI] [PubMed] [Google Scholar]
- 18. Czigany Z, Turoczi Z, Kleiner D, Lotz G, Homeyer A, Harsanyi L, et al. Neural elements behind the hepatoprotection of remote perconditioning. J Surg Res. 2014;185:605–613. [DOI] [PubMed] [Google Scholar]
- 19. Czigany Z, Turoczi Z, Onody P, Harsanyi L, Lotz G, Hegedus V, et al. Remote ischemic perconditioning protects the liver from ischemia-reperfusion injury. J Surg Res. 2013;185:605–613. 10.1016/j.jss.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 20. Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64–69. [PubMed] [Google Scholar]
- 21. Shen K, Zheng SS, Park O, Wang H, Sun Z, Gao B. Activation of innate immunity (NK/IFN-gamma) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1070–1077. 10.1152/ajpgi.00554.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. [DOI] [PubMed] [Google Scholar]
- 23. Taki-Eldin A, Zhou L, Xie HY, Chen KJ, Yu D, He Y, et al. Triiodothyronine attenuates hepatic ischemia/reperfusion injury in a partial hepatectomy model through inhibition of proinflammatory cytokines, transcription factors, and adhesion molecules. J Surg Res. 2012;178:646–656. 10.1016/j.jss.2012.05.069 [DOI] [PubMed] [Google Scholar]
- 24. Nardo B, Caraceni P, Pasini P, Domenicali M, Catena F, Cavallari G, et al. Increased generation of reactive oxygen species in isolated rat fatty liver during postischemic reoxygenation. Transplantation. 2001;71:1816–1820. [DOI] [PubMed] [Google Scholar]
- 25. Peralta C, Fernandez L, Panes J, Prats N, Sans M, Pique JM, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100–113. [DOI] [PubMed] [Google Scholar]
- 26. Park KM, Cho HJ, Bonventre JV. Orchiectomy reduces susceptibility to renal ischemic injury: a role for heat shock proteins. Biochem Biophys Res Commun. 2005;328:312–317. [DOI] [PubMed] [Google Scholar]
- 27. Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G. Ischemic postconditioning: experimental models and protocol algorithms. Basic Res Cardiol. 2009;104:469–483. 10.1007/s00395-009-0040-4 [DOI] [PubMed] [Google Scholar]
- 28. Wang M, Shen J, Feng B, Gui L, Chen Q, Zhang B, et al. Remote ischemic preconditioning promotes early liver cell proliferation in a rat model of small-for-size liver transplantation. J Surg Res.2009;179:e245–253. 10.1016/j.jss.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 29. Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, et al. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2011;26:87–93. [DOI] [PubMed] [Google Scholar]
- 30. Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–345. 10.2119/2007-00134.Vardanian [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia–reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovascular Research. 2004;61:448–460. [DOI] [PubMed] [Google Scholar]
- 32. Ndiaye M, Chataigneau M, Lobysheva I, Chataigneau T, Schini-Kerth VB. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. 2005;19:455–457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.