Abstract
Rationale
Chronic obstructive pulmonary disease (COPD) is often associated with age-related systemic abnormalities that adversely affect the prognosis. Whether these manifestations are linked to the lung alterations or are independent complications of smoking remains unclear.
Objectives
To look for aging-related systemic manifestations and telomere shortening in COPD patients and smokers with minor lung destruction responsible for a decline in the diffusing capacity for carbon monoxide (DLCO) corrected for alveolar volume (KCO).
Methods
Cross-sectional study in 301 individuals (100 with COPD, 100 smokers without COPD, and 101 nonsmokers without COPD).
Measurements and Main Results
Compared to control smokers, patients with COPD had higher aortic pulse-wave velocity (PWV), lower bone mineral density (BMD) and appendicular skeletal muscle mass index (ASMMI), and shorter telomere length (TL). Insulin resistance (HOMA-IR) and glomerular filtration rate (GFR) were similar between control smokers and COPD patients. Smokers did not differ from nonsmokers for any of these parameters. However, smokers with normal spirometry but low KCO had lower ASMMI values compared to those with normal KCO. Moreover, female smokers with low KCO, had lower BMD and shorter TL compared to those with normal KCO.
Conclusions
Aging-related abnormalities in patients with COPD are also found in smokers with minor lung dysfunction manifesting as a KCO decrease. Decreased KCO might be useful, particularly among women, for identifying smokers at high risk for aging-related systemic manifestations and telomere shortening.
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with systemic manifestations including atherosclerosis, weight loss, osteoporosis, muscle atrophy and weakness, kidney dysfunction, and diabetes.[1–7] The mechanisms linking this systemic component to the lung disease remain unclear. Most of the studies addressing this issue found no association between systemic manifestations and degree of airflow limitation.[2] Associations with the severity of lung emphysema or of systemic inflammation have been reported but remain poorly understood.[5, 7–10] Improved understanding of the pathogenesis of systemic alterations in COPD may result in better preventive and therapeutic strategies.
COPD is an age-related condition, and accumulating evidence suggests a relationship with a global process of accelerated aging. Patients with COPD exhibit telomere shortening in circulating leukocytes compared to smokers without COPD [11, 12]. New data support an association between COPD and exaggerated lung-cell senescence that may contribute to the pathogenesis of the disease.[13–16]. Thus, one current hypothesis in COPD ascribes the systemic manifestations to a global aging process. Another possibility is that smoking, which is the most common cause of COPD, is also responsible for the systemic manifestations of the disease, independently from the lung function alterations [17]. We reasoned that comparing patients with COPD to smokers and nonsmokers might shed light on the role for lung alterations in the systemic aging-related manifestations. Moreover, we hypothesized that, in smokers without COPD, systemic manifestations may occur more frequently in individuals with than without lung dysfunction. One way to assess lung function in smokers is DLCO measurement, as a decrease DLCO has been proven sensitive for detecting lung dysfunction, even in patients without emphysematous lesions by computed tomography (CT) [18]. Analyses for data from smokers with lung dysfunction are not confounded by COPD-related factors such as pharmacological treatments, physical inactivity, exercise limitation, and gas exchange alterations. Furthermore, there are differences in the aging process between men and women [12, 19]. We therefore investigated three groups of individuals, patients with COPD, smokers without COPD (control smokers), and nonsmokers, taking into account cigarette-smoke exposure and gender.
Materials and Methods
In this cross-sectional study, we enrolled 301 participants including 100 with COPD, 100 smokers without COPD, and 101 nonsmokers, recruited at the Henri-Mondor Teaching Hospital, Créteil, France, between January 2009 and September 2012. The study was approved by the institutional review board of the Henri-Mondor Teaching Hospital (CPP, # 09–027). All participants provided written informed consent before inclusion.
Study population
Patients with clinically stable COPD were recruited prospectively at the pulmonology outpatient clinic and control smokers at the smoking-cessation clinic and at the clinical investigations center of the Henri-Mondor Teaching Hospital. Nonsmokers were healthy volunteers recruited from the general population by the clinical investigations center of the same hospital and evaluated clinically before study inclusion. Exclusion criteria were known chronic heart failure, malignancy, and inflammatory or metabolic conditions.
Each participant underwent spirometry, plethysmography, and DLCO measurement according to ATS/ERS consensus guidelines.[20] DLCO was measured using the single breath method. KCO, which is DLCO corrected for alveolar volume, was used for the analysis. DLCO and KCO were corrected for hemoglobin.
Control smokers and nonsmokers were required to have FEV1/FVC greater than 70%. Smokers without COPD were classified as having reduced KCO (<80%) or normal KCO (≥80%).
Aging-related systemic manifestations
Arterial stiffness (aortic pulse-wave velocity, PWV) was measured as the carotid-femoral pulse-wave velocity using the Complior Analyse device (Alam Medical, Vincennes, France) and was available in 294/301 subjects. Bone mineral density (BMD) at the hip (femoral neck) and lumbar spine was determined using dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare, UK). Complete dataset was obtained in 295/301 subjects. BMD is reported as the absolute value (g/cm2). T-scores were computed to classify participants as having normal BMD or osteoporosis (defined as T-score <-2.5 at either site). Appendicular skeletal muscle mass (ASMM) was measured as the fat-free soft-tissue masses of the arms and legs divided by height squared [21] and ASMM index (ASMMI) was then computed as ASMM divided by height squared. The cutoff for defining sarcopenia was two standard deviations below the mean sex-specific ASMMI values in the Rosetta Study of young adults (5.45 for females and 7.26 for males), as proposed by Baumgartner et al. [21]. Pinch and grip strengths were assessed using a standard handgrip dynamometer and pinch gauge (Baseline Evaluation Instruments, NY, USA), insulin resistance by calculating HOMA-IR (insulin·glucose)/22.5), and renal function by estimating the glomerular filtration rate (eGFR) using the Cockcroft-Gault formula.
Blood tests
Levels of 27 serum chemokines and growth factors were quantified using a bead-based cytometric immunoassay (Bio-Rad Laboratories, Hercules, CA). Among the 27 chemokines and growth factors, we focused on 12 for subsequent analysis in patients with COPD, control smokers, and nonsmokers (IL-1β, IL-1Ra, IL-6, IL-8, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, TNF-α, eotaxin). Measurement of telomere length in circulating leucocytes was assessed using the real-time quantitative polymerase chain reaction (RT-qPCR).[12] Telomere length measurement was obtained in 89/100 COPD patients, 90/100 control smokers and 94/101 non smokers.
Statistical analysis
Qualitative variables are reported as numbers and percentages, and quantitative variables as median, interquartile range, IQR.
Pulmonary function parameters, aging-related systemic manifestations, leukocyte counts, and plasma cytokine levels were compared across the three groups (COPD patients, control smokers, and nonsmokers) using the Chi2, Fisher exact, or Kruskal-Wallis test, as appropriate. When differences were significant, COPD patients were compared with control smokers, and control smokers with nonsmokers. After looking for a first-order interaction, to take into account potential confounding the analyses were also adjusted for gender and age (and pack-year value for the comparison of COPD patients to control smokers) by fitting logistic models for qualitative variables and nonparametric quantile regression models for quantitative variables. These models provide estimates of the median values of the dependent variable in each group conditional on the value of confounders [22]. Bootstrap resampling with 100 replications was performed to correct for potential heteroskedastic errors.
To assess whether minor lung destruction was associated with extrapulmonary manifestations of aging, we compared aging-related systemic manifestations, leukocytes, and cytokines between control smokers with normal vs. low KCO, using similar analyses. BMD and telomere length were performed separately in men and women because gender modified the relationships between these factors and KCO (significant interaction).
All tests were two-tailed. P values ≤0.05 were considered significant. Data were analyzed using Stata statistical software (StataCorp 12.1, College Station, TX).
Results
Clinical characteristics of the study population
Age, body mass index, and mean systemic arterial pressure did not differ across the three groups but COPD patients had a higher pack-year value compared to control smokers (Table 1). All the COPD patients were smokers, the percentage of current smokers was 46% among patients with COPD and 71% among control smokers (Table 1). Both DLCO and KCO were lower in COPD patients than in control smokers and in control smokers than in nonsmokers. The groups did not differ regarding treated diabetes or medical history. Inhaled steroids were used by 45 (45%) patients with COPD (S1 Table). Patients with COPD exhibited different levels of airflow limitation: GOLD stage was I in 8% of patients, II in 49%, III in 32%, and IV in 11%.
Table 1. Characteristics of the study patients.
| Nonsmokers (n = 101) | Smokers without COPD (n = 100) | Patients with COPD (n = 100) | P value † | |
|---|---|---|---|---|
| Age, years | 59.5 [53.3–63.6] | 59.6 [53.6–64.1] | 60.6 [56.7–65.9] | 0.11 |
| Females/males, n | 36/65 | 41/59 | 28/72 | 0.15 |
| Pack-years | 30 [24–41] | 41.5 [32–62] | <0.001 | |
| Current smokers, n (%) | 71 (71) | 46 (46) | <0.001 | |
| BMI, Kg/m2 | 25.6 [23.2–28.0] | 25.5 [22.8–27.8] | 25.7 [22.4–29.0] | 0.91 |
| MAP, mmHg | 92 [87–98] | 93.3 [87–98] | 93.8 [87–100] | 0.58 |
| Pulmonary function parameters | ||||
| FEV1, L | 3.2 [2.5–3.8] | 2.9 [2.4–3.5] | 1.4 [1.1–2.0] | 0.000 |
| FEV1, % predicted | 109 [97–117] | 101 [90–112] | 55 [38–69] | 0.000 |
| FVC, L | 3.9 [3.1–4.6] | 3.7 [3.0–4.5] | 3.0 [2.3–3.6] | 0.000 |
| FVC, % predicted | 101 [90–116] | 100 [88–112] | 78 [65–93] | 0.000 |
| FEV1/FVC | 81 [78–85] | 79 [76–83] | 51 [42–64] | 0.000 |
| DLCO, % predicted | 87 [77–97] | 80 [69–88.5] | 54 [44–73] | 0.000 |
| KCO, % predicted | 92 [82–105] | 83 [72–95] | 67 [52–82] | 0.000 |
| SpO2, % | 97.0 [96.4–97.7] | 97.0 [96.4–97.5] | 96.3 [95.2–97.0] | 0.000 |
| 6-min walking distance, m | 600 [540–636] | 579 [510–627] | 510 [450–570] | 0.000 |
Definition of abbreviations: COPD, chronic obstructive pulmonary disease; % predicted, percentage of the predicted value; BMI, body mass index; MAP, mean arterial pressure; KCO, transfer factor coefficient of the lung for carbon monoxide; SpO2, oxygen saturation by pulse oximetry.
Data are median [interquartile range] unless stated otherwise.
†P value by Chi-square test, Fisher exact test, or nonparametric Kruskal-Wallis test, as appropriate, comparing the three populations (COPD patients, control smokers, and nonsmokers).
Aging-related systemic manifestations, telomere length, and cytokine plasma levels in patients with COPD compared to control smokers and nonsmokers
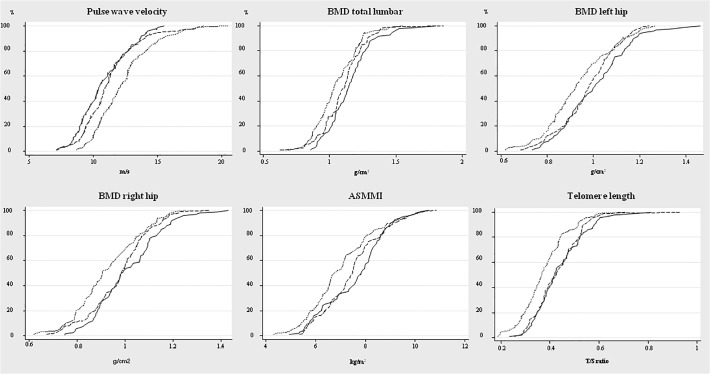
The three groups differed significantly regarding PWV, lumbar-spine and hip BMD, ASMMI, and TL when they are compared globally; but not HOMA-IR, eGFR, or muscle function tests (Table 2, Fig. 1). Compared to control smokers, patients with COPD had higher PWV and lower lumbar-spine and hip BMD, ASMMI, and TL; even after adjustment for age, gender, and pack-year history. Median values were not affected by adjustments. Sarcopenia was found in 21% of patients with COPD compared to 2% of control smokers (P<0.001) and osteoporosis in 21% of patients with COPD compared to 12% of control smokers (P = 0.16). No significant differences in these variables were observed between control smokers and nonsmokers, before or after adjustment for age and gender (Table 2).
Table 2. Comparison of aging-related systemic manifestations and telomere length in patients with chronic obstructive pulmonary disease, smokers without COPD, and nonsmokers.
| Adjustment | Nonsmokers | Smokers without COPD | COPD patients | P value † | P value * | ||
|---|---|---|---|---|---|---|---|
| S vs NS | COPD vs S | ||||||
| Pulse wave velocity, m/s | none | 10.4 [9.1–12,2] | 10.9 [9.7–12.2] | 12,1 [10.6–13.5] | 0.000 | 0.14 | 0.000 |
| gender and age | 10.6 | 11.1 | 12.0 | 0.000 | 0.23 | 0.003 | |
| gender, age, and pack years | - | 11.2 | 11.9 | - | - | 0.03 | |
| BMD, total lumbar, g/cm2 | none | 1.14 [1.04–1.24] | 1.11 [0.99–1.19] | 1.03 [0.93–1.18] | 0.000 | 0.08 | 0.02 |
| gender and age | 1.14 | 1.10 | 1.03 | 0.009 | 0.08 | 0.05 | |
| gender, age, and pack years | - | 1.09 | 1.04 | - | - | 0.09 | |
| BMD, left hip, g/cm2 | none | 1.00 [0.89–1.09] | 0.98 [0.89–1.06] | 0.92 [0.83–1.03] | 0.005 | 0.27 | 0.02 |
| gender and age | 1.00 | 0.99 | 0.91 | 0.001 | 0.55 | 0.00 | |
| gender, age, and pack years | - | 0.98 | 0.91 | - | - | 0.00 | |
| BMD, right hip, g/cm2 | none | 0.99 [0.90–1.10] | 0.99 [0.89–1.06] | 0.91 [0.83–1.04] | 0.000 | 0.19 | 0.01 |
| gender and age | 1,00 | 0.98 | 0.92 | 0.02 | 0.37 | 0.03 | |
| gender, age, and pack years | - | 0.98 | 0.93 | - | 0.03 | ||
| ASMMI, Kg/m2 | none | 7.8 [6.4–8.5] | 7.5 [6.6–8.1] | 6.8 [6.10–7.9] | 0.004 | 0.28 | 0.01 |
| gender and age | 7.5 | 7.5 | 6.9 | 0.03 | 0.99 | 0.03 | |
| gender, age, and pack years | - | 7.4 | 6.9 | - | - | 0.02 | |
| Sarcopenia, n (%) § | none | 3 (3) | 2 (2) | 26 (26) | 0.000 | 0.70 | 0.000 |
| gender and age | - | 0.000 | |||||
| gender, age, and pack years | - | 0.000 | |||||
| Pinch test, Kg | none | 7 [5–9] | 6 [5–8] | 6 [5–8] | 0.08 | - | - |
| Grip test, Kg | none | 38 [26–48] | 38 [26–45] | 36 [27–42] | 0.39 | - | - |
| HOMA-IR | none | 1.5 [1.1–2.4] | 2.0 [1.2–3.1] | 2.2 [1.2–3.1] | 0.10 | - | - |
| Glomerular flow rate, mL/min | none | 78.4 [68.9–89.6] | 79.6 [69.7–91,8] | 80.9 [69.8–94.6] | 0.18 | - | - |
| Telomere length (T/S) ratio | none | 0.42 [0.36–0.51] | 0.43 [0.36–0.50] | 0.37 [0.31–0.4] | 0.000 | 0.75 | 0.000 |
| gender and age | 0.42 | 0.41 | 0.37 | 0.006 | 0.75 | 0.05 | |
| gender, age, and pack years | - | 0.43 | 0.37 | - | - | 0.008 | |
Definition of abbreviations: COPD, chronic obstructive pulmonary disease; NS, nonsmokers; S, smokers; BMD, bone mineral density; ASMMI, appendicular skeletal muscle mass index; HOMA-IR, homeostatic model assessment of insulin resistance; T/S, ratio of telomere-repeat copy number over single-gene copy number
Glomerular flow rate was estimated using the Cockcroft-Gault formula.
Data are median [interquartile range] unless stated otherwise.
†P value by Fisher exact test, or nonparametric Kruskal-Wallis test, as appropriate, comparing the three populations (COPD patients, control smokers, and nonsmokers).
*P value of quantile regression models for adjusted analyses.
§ P value of logistic regression models for adjusted analyses.
Fig 1. Plots of cumulative percentages of aging-related systemic manifestations and telomere length in nonsmokers (—), control smokers (without COPD, ---) and COPD patients (….).
Among the 12 chemokines analyzed, IL-6, IL-8, and MCP-1 differed significantly across the three groups (Table 3). However, after adjustment for age, gender, and pack-year of exposure, no statistically significant differences were found between COPD patients and control smokers or between control smokers and nonsmokers. Control smokers had higher peripheral leukocyte counts compared to nonsmokers, even after adjustment for age and gender (Table 3). No significant differences were observed between patients with COPD and control smokers.
Table 3. Comparison of leukocytes and plasma inflammatory markers in patients with chronic obstructive pulmonary disease, smokers without COPD, and nonsmokers.
| Adjustment | Nonsmokers | Smokers without COPD | COPD patients | P value † | P value * | ||
|---|---|---|---|---|---|---|---|
| S vs NS | COPD vs S | ||||||
| Leukocyte, Giga/L | none | 5.4 [4.6–6.4] | 6.5 [5.3–8.3] | 7.3 [5.8–8.65] | 0.000 | 0.000 | 0.13 |
| gender and age | 5.5 | 6.6 | 7.1 | 0.000 | 0.004 | 0.19 | |
| gender, age, and pack years | - | 6.6 | 7.0 | - | - | 0.50 | |
| IL-6, pg/mL | none | 14.7 [12.9–17.3] | 15.7 [12.9–18.5] | 16.5 [14.3–19.2] | 0.01 | 0.16 | 0.15 |
| gender and age | 14.7 | 15.5 | 16.4 | 0.02 | 0.19 | 0.17 | |
| gender, age, and pack years | - | 15.5 | 16.4 | - | - | 0.22 | |
| IL-8, pg/mL | none | 43.5 [38.9–50.4] | 47.0 [40.0–51.6] | 48.8 [42.7–53.4] | 0.008 | 0.17 | 0.11 |
| gender and age | 43.2 | 46.2 | 48.4 | 0.005 | 0.07 | 0.16 | |
| gender, age, and pack years | - | 47.5 | 48.5 | - | - | 0.59 | |
| MCP-1, pg/mL | none | 33.4 [27.6–42.2] | 37.3 [27.8–50.2] | 40.4 [28.1–56.8] | 0.02 | 0.12 | 0.19 |
| gender and age | 34.8 | 37.7 | 39.5 | 0.32 | - | - | |
| gender, age, and pack years | - | 36.5 | 39.1 | - | - | 0.56 | |
| TNF-α, pg/mL | none | 68.6 [58.3–86.0] | 67.8 [56.6–82.1] | 68.8 [56.6–84.0] | 0.83 | - | - |
| Eotaxin, pg/mL | none | 90.3 | 123.6 | 102.9 | 0.06 | - | - |
Definition of abbreviations: COPD, chronic obstructive pulmonary disease; IL, interleukin; MCP-1, monocyte chemotactic protein-1; TNF-α, tumor necrosis factor alpha
Data are median [interquartile range].
†P value by nonparametric Kruskal-Wallis test comparing the three populations (patients with COPD, control smokers, and nonsmokers).
*P value of quantile regression models for adjusted analyses.
Aging-related systemic manifestations, telomere length, and plasma cytokine levels in control smokers with normal spirometry and low KCO compared to control smokers with normal spirometry and normal KCO
Clinical characteristics were similar in these two subgroups but the percentage of women was significantly higher among control smokers with low KCO (59% vs. 32% for normal KCO, P = 0.01). Pack-year exposure was similar in control smokers with low and normal KCO (31 [25–43] vs. 30 [24–44], respectively, P = 0.77).
Because of a significant interaction (P<0.05) of gender with both BMD and TL, we analyzed these parameters separately in males and females. Low KCO was associated with low hip and lumbar-spine BMD in females but not in males. TL was shorter in females with low KCO compared to those with normal KCO (Table 4). Low KCO was associated with decreased ASSMI and muscle function (grip test) even after adjustment for age and gender but was not associated with PWV, HOMA-IR, or eGFR.
Table 4. Comparison of aging-related systemic manifestations and telomere length between smokers with low KCO and those with normal KCO .
| Univariate analysis | Adjusted for age and gender | |||||
|---|---|---|---|---|---|---|
| Normal KCO (n = 50) | KCO<80% (n = 39) | P* | Normal KCO (n = 50) | KCO<80% (n = 39) | P value † | |
| Pulse wave velocity, m/s | 11.2 [10–12] | 10.8 [9.7–12.2] | 0.77 | |||
| BMD, total lumbar, g/cm2 ** | ||||||
| In females | 1.19 [1.07–1.24] | 0.98 [0.91–1.12] | 0.001 | 1.18 | 1.02 | 0.003 |
| In males | 1.09 [1.05–1.29] | 1.14 [1.06–1.16] | 0.87 | - | - | - |
| BMD, left hip, g/cm2 ** | - | - | ||||
| In females | 0.98 [0.93–1.12] | 0.84 [0.76–0.94] | 0.002 | 0.99 | 0.85 | 0.007 |
| In males | 0.99 [0.95–1.08] | 1.02 [0.95–1.06] | 0.92 | |||
| BMD, right hip, g/cm2 ** | ||||||
| In females | 0.99 [0.95–1.08] | 0.88 [0.74–0.92] | 0.01 | 1.01 | 0.87 | 0.000 |
| In males | 1.01 [0.95–1.10] | 1.01 [0.96–1.05] | 0.71 | |||
| ASMMI, Kg/m2 | 7.7 [7.1–8.7] | 6.9 [5.9–7.6] | 0.000 | 7.6 | 7.00 | 0.03 |
| Pinch test, Kg | 6.5 [5–8.5] | 6 [4–8] | 0.13 | |||
| Grip test, Kg | 39 [29–46] | 32 [22–43] | 0.02 | 40.3 | 32.0 | 0.01 |
| HOMA-IR | 2.18 [1.15–3.26] | 1.94 [1.09–2.21] | 0.29 | |||
| Glomerular flow rate, mL/min | 84.9 [74.8–91.4] | 74.5 [64.4–93.5] | 0.12 | |||
| Telomere length (T/S) ratio** | ||||||
| In females | 0.52 [0.41–0.57] | 0.40 [0.36–0.46] | 0.023 | 0.50 | 0.40 | 0.037 |
| In males | 0.43 [0.4–0.5] | 0.46 [0.4–0.5] | 0.16 | |||
Definition of abbreviations: KCO, transfer coefficient of the lung for carbon monoxide; BMD, bone mineral density; ASMMI, appendicular skeletal muscle mass index; HOMA-IR, homeostatic model assessment of insulin resistance; T/S, ratio of telomere-repeat copy number over single-gene copy number
Data are median [interquartile range].
* P value by nonparametric Kruskal-Wallis test
†P value by quantile regression models adjusted for age and gender unless stated otherwise
** Females and males were analyzed separately because of a significant interaction between gender and aging-related parameters.
Peripheral leukocyte counts and plasma cytokine levels did not differ between control smokers with low vs. normal KCO (Table 5). Similar results were obtained when control smokers were classified based on DLCO lower or greater than 80% (data not shown).
Table 5. Comparison of inflammatory mediators between smokers with low KCO and those with normal KCO.
| Smokers without COPD | |||
|---|---|---|---|
| Normal KCO (n = 50) | KCO<80% (n = 39) | P value† | |
| Leukocytes, Giga/L | 6.3 [5.1–7.8] | 6.9 [6,0–8.4] | 0.19 |
| IL-6, pg/mL | 15.1 [12.9–18,5] | 16.9 [13,3–18.5] | 0.46 |
| IL-8, pg/mL | 44.7 [38,9–51.6] | 47.5 [41,8–51.6] | 0.30 |
| MCP-1, pg/mL | 35,8 [26.6–50,0] | 38.7 [27.3–50,2] | 0.62 |
| TNF-α, pg/mL | 66.5 [55.5–74.8] | 70.0 [59.5–85.9] | 0.35 |
| Eotaxin, pg/mL | 127 [92–185] | 125 [61–171] | 0.39 |
KCO, transfer factor coefficient of the lung for carbon monoxide; IL, interleukin; MCP-1, monocyte chemotactic protein-1; TNF-α, tumor necrosis factor alpha
Data are median [interquartile range].
† P value by nonparametric Kruskal-Wallis test
Discussion
Our data show that patients with COPD exhibit aging-related systemic manifestations and telomere shortening. These two characteristics were not significantly different between control smokers and nonsmokers, indicating that both were chiefly ascribable to COPD. Among control smokers, systemic manifestations and telomere shortening were more common in the subgroup with normal spirometry but low KCO than in those with normal KCO, particularly among women, supporting a link between lung dysfunction and aging-related systemic manifestations. Smokers susceptible to premature aging might therefore be identified based on a low KCO.
One major goal of this study was to evaluate whether age-related physiological and biological abnormalities occurred in patients with COPD compared to smokers without COPD and nonsmokers and whether these abnormalities were detectable in smokers with minor lung dysfunction defined as decreased CO diffusing capacity. Previous studies have been performed in patients with COPD or in smokers without COPD but did not assess lung function in the control smokers, leaving unclear whether the systemic manifestations were ascribable to smoking or to lung dysfunction [23]. Our patients with COPD had specific systemic manifestations that were not observed in control smokers, including increased arterial stiffness, bone and skeletal muscle loss, and TL shortening. Interestingly, patients with COPD did not differ from control smokers regarding insulin resistance or renal dysfunction, two other potential systemic manifestations of COPD. These data are consistent with recent studies showing that several systemic manifestations seen in COPD occur in combination [7]. A major finding from our study was that control smokers and nonsmokers did not differ regarding physiological and biological parameters. Thus, in the three groups, the physiological parameters and leukocyte TL changed in lockstep, with differences between COPD patients compared to control smokers and nonsmokers but no differences between control smokers and nonsmokers. These results establish that the systemic manifestations are chiefly ascribable to COPD and not to smoking.
Our results may appear to contradict previous studies documenting TL shortening in smokers.[23] Most of these studies did not involve lung function assessments and were therefore unable to evaluate TL independently from lung dysfunction. Here, we investigated smokers with normal spirometry and individualized a subgroup with minor lung dysfunction not detected by spirometry but responsible for a decrease in KCO. KCO reflects functional alveolar-capillary bed integrity.[24]. Low KCO was common among our control smokers without airflow obstruction, particularly among the women (57% compared to 25% in male smokers). The most striking results were obtained in female smokers with normal spirometry but low KCO, who exhibited decreased BMD and TL shortening. KCO values lower than 80% were closely associated with systemic manifestations in female smokers and, to a lesser degree, in male smokers. KCO measurement might therefore be valuable as a screening tool among smokers with normal spirometry, to identify individuals with early age-related systemic abnormalities. Low KCO may reflect early lung destruction before COPD onset, as lower baseline KCO is independently associated with worse symptoms and more rapid progression of emphysema and airflow limitation in heavy smokers [18, 25, 26]. However, further longitudinal studies are needed to determine whether smokers without airflow obstruction but with low KCO are at high risk for developing accelerated aging, COPD, and systemic manifestations.
Differences between control smokers with low vs. normal KCO are not confounded by COPD-related factors such as exercise limitation, steroid use, hypoxemia, decreased BMI, smoking history, and current versus former smoking. Control smokers with low vs. normal KCO did not differ for age, BMI, smoking history, 6-minute walk test results, or O2 saturation; although they differed regarding systemic abnormalities. Moreover, most of our control smokers were current smokers. Thus, the differences found in our study between control smokers with low vs. normal KCO are probably ascribable to the lung disease or the process causing it, and not to the above-listed COPD-related factors. KCO is closely linked to the degree of lung emphysema in COPD patients. Low KCO in smokers is believed to reflect incipient smoking-induced destruction of both the lung parenchyma and the lung vessels.[24] KCO measurement can detect subtle lung dysfunction in patients with normal chest CT findings.[18] KCO is associated with increased plasma levels of circulating endothelial microparticles released from activated or apoptotic endothelial cells, which reflect vascular damage that may constitute an early step antedating emphysema development.[24] A role for emphysema in COPD-related systemic manifestations is supported by studies showing correlations of emphysema with low BMD and increased arterial stiffness in smokers with and without COPD.[9, 10] Taken together, these findings support a link between early lung parenchyma destruction and age-related systemic manifestations of COPD.
Smokers without COPD are generally chosen as controls for comparisons with COPD patients, as they are believed to be free from lung disease [6, 17]. This approach does not consider the heterogeneity of smokers without COPD, some of whom have early minor lung alterations that may be associated with alterations in extrapulmonary organs. In a study of 60 ex-smokers with COPD detected by spirometry, 60 control ex-smokers without COPD, and 60 nonsmokers, the COPD patients and control smokers were not significantly different regarding the prevalence of risk factors and comorbidities, whereas these prevalences differed between control smokers and nonsmokers, challenging the view that comorbidities are ascribable to lung alterations alone and not to smoking [17]. However, KCO was lower in the control smokers than in the nonsmokers (88% versus 98%). The comorbidity difference between control ex-smokers and nonsmokers may reflect the presence of minor lung dysfunction in many smokers without COPD.
A major issue is whether the pulmonary and systemic components of COPD result from a common pathogenic mechanism or whether the lung alterations drive the development of systemic senescence-related manifestations. Regarding the first hypothesis, accelerated aging, and not smoking, could be the main risk factor of developing both COPD and its systemic manifestations. Our observation in smokers with low KCO could suggest that accelerated aging could arrive early in the disease history. However, inclusion of COPD patients without a history of smoking or longitudinal studies evaluating aging manifestations in smokers are needed to answer this question. In support for the second hypothesis, we and others have shown that lung emphysema and COPD are associated with an accumulation of senescent cells in lung tissues.[13–16] These cells are still metabolically active and release many mediators that not only contribute to inflammation in COPD, but also can propagate the senescence process to neighboring cells in the lung and potentially to systemic organs in a paracrine manner.[27] Consistent with this mechanism, recent evidence indicates a role for circulating factors in aging-related manifestations.[28, 29] Candidate circulating factors consist of cytokines (e.g., IL-6 and IL-8), prostaglandins (e.g., PGE2), and chemokines (e.g., eotaxin) including serum interferon-gamma-inducible chemokines (e.g., IP-10).[15, 16, 28, 30] In the present study, some of these circulating factors were altered in patients with COPD and control smokers. However, IL-6, IL-8, IP-10, and eotaxin were not significantly different between control smokers with low vs. normal KCO. Of note is the previous finding of significant variations in endothelial microparticles in smokers with low KCO, indicating associated endothelial damage in the pulmonary vessels.[24] Further studies are therefore needed to identify circulating factors potentially involved in the aging process in patients with COPD or in smokers with early lung destruction manifesting only as low KCO.
Supporting Information
(DOC)
Acknowledgments
We gratefully acknowledge Matthieu Surenaud at the Luminex platform; Akara Y and Dominique Rideau for helping with telomere length measurements; Mourad Sarni and Noémie Nakache for recruiting patients and control subjects; Ahmed Hchikhat and Stephan Ribeil for performing aortic pulse-wave velocity measurements, dual-energy X-ray absorptiometry, and the pinch and grip tests; Isabelle Guilloteau, Marie-Andrée Dagnol, and Nathalie Guyot for performing the pulmonary function tests; Dylan Aissi for helping with data management; and Françoise Zerah for constructive criticism of the manuscript.
Data Availability
The data cannot be made publicly available due to legal and ethical restrictions from the French Commission nationale de l'informatique et des libertés. Data are from the Inserm translationnal C09-11 study. Requests for data use can now be made to the Scientific Manager of the study, Sonia Gueguen, (sonia.gueguen@inserm.fr) or to the corresponding author Dr. Laurent Boyer (laurent.boyer@hmn.aphp.fr).
Funding Statement
This research was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) grant C09-11. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33: 1165–1185. 10.1183/09031936.00128008 [DOI] [PubMed] [Google Scholar]
- 2. Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003; 107: 1514–1519. [DOI] [PubMed] [Google Scholar]
- 3. Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits L, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the Towards a Revolution in COPD Health study. Chest. 2009; 136: 1456–1465. 10.1378/chest.08-3016 [DOI] [PubMed] [Google Scholar]
- 4. Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier J, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002; 166: 809–813. [DOI] [PubMed] [Google Scholar]
- 5. Chandra D, Stamm JA, Palevsky PM, Leader JK, Fuhrman CR, Zhang Y, et al. The relationship between pulmonary emphysema and kidney function in smokers. Chest. 2002; 142: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007; 175: 1259–1265. [DOI] [PubMed] [Google Scholar]
- 7. Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013; 187: 728–735. 10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 8. Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186: 982–988. 10.1164/rccm.201206-1113OC [DOI] [PubMed] [Google Scholar]
- 9. Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011; 183: 885–890. 10.1164/rccm.201004-0666OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007; 176: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006; 27: 525–528. [DOI] [PubMed] [Google Scholar]
- 12. Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009; 179: 566–571. 10.1164/rccm.200809-1398OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006; 174: 886–893. [DOI] [PubMed] [Google Scholar]
- 14. Noureddine H, Gary-Bobo G, Alifano M, Marcos E, Saker M, Vienney N, et al. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ res. 2011; 109: 543–553. 10.1161/CIRCRESAHA.111.241299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amsellem V, Gary-Bobo G, Marcos E, Maitre B, Chaar V, Validire P, et al. Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011; 184: 1358–1366. 10.1164/rccm.201105-0802OC [DOI] [PubMed] [Google Scholar]
- 16. Dagouassat M, Gagliolo JM, Chrusciel S, Bourin MC, Duprez C, Caramelle P, et al. The cyclooxygenase-2-prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am J Respir Crit Care Med. 2013; 187: 703–714. 10.1164/rccm.201208-1361OC [DOI] [PubMed] [Google Scholar]
- 17. Van Remoortel H, Hornikx M, Langer D, Burtin C, Everaerts S, Verhamme P, et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013; 189: 30–38. [DOI] [PubMed] [Google Scholar]
- 18. Kirby M, Owrangi A, Svenningsen S, Wheatley A, Coxson HO, Paterson NA, et al. On the role of abnormal DLCO in ex-smokers without airflow limitation: symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax. 2013; 68: 752–759. 10.1136/thoraxjnl-2012-203108 [DOI] [PubMed] [Google Scholar]
- 19. Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, et al. Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001; 37: 381–385. [DOI] [PubMed] [Google Scholar]
- 20. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. The European respiratory journal. 1993; 16: 5–40. [PubMed] [Google Scholar]
- 21. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. American journal of epidemiology. 1998;147: 755–763. [DOI] [PubMed] [Google Scholar]
- 22. Koenker R HK. Quantile regression. Journal of Economic Perspectives, 2001; 15: 143–156. [Google Scholar]
- 23. Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005; 366: 662–664. [DOI] [PubMed] [Google Scholar]
- 24. Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, et al. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011; 184: 224–232. 10.1164/rccm.201012-2061OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohamed Hoesein FA, Zanen P, van Ginneken B, van Klaveren RJ, Lammers JW. Association of the transfer coefficient of the lung for carbon monoxide with emphysema progression in male smokers. Eur Respir J. 2011; 38: 1012–1018. 10.1183/09031936.00050711 [DOI] [PubMed] [Google Scholar]
- 26. Cauberghs M, Clement J, Van de Woestijne KP. Functional alterations accompanying a rapid decline in ventilatory function. The American review of respiratory disease. 1993; 147: 379–384. [DOI] [PubMed] [Google Scholar]
- 27. Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005; 120: 513–522. [DOI] [PubMed] [Google Scholar]
- 28. Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011; 477: 90–94. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764. [DOI] [PubMed] [Google Scholar]
- 30. Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008; 133: 1019–1031. 10.1016/j.cell.2008.03.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
The data cannot be made publicly available due to legal and ethical restrictions from the French Commission nationale de l'informatique et des libertés. Data are from the Inserm translationnal C09-11 study. Requests for data use can now be made to the Scientific Manager of the study, Sonia Gueguen, (sonia.gueguen@inserm.fr) or to the corresponding author Dr. Laurent Boyer (laurent.boyer@hmn.aphp.fr).