Abstract
Background
Pyrus pashia Buch.-Ham. ex D. Don. has been used conventionally by many communities in the Himalayan region for the management of gastrointestinal, respiratory, and vascular complications. Set against this background, this study was carried out to justify the scientific basis to validate folkloric uses of fruits of Pyrus pashia Buch.-Ham. ex D. Don. (Pp.Cr) in traditional systems of medicine.
Methods
The crude ethanol extract of fruits of Pyrus pashia Buch.-Ham. ex D. Don. (Pp.Cr) was tested in vitro on isolated rabbit jejunum, tracheal, and aorta preparations. The responses of tissues were recorded using isotonic transducers coupled with a PowerLab data acquisition system.
Results
The Pp.Cr on application (0.01–5.0 mg/ml) to isolated rabbit jejunum preparation exhibited relaxation through decrease in magnitude and frequency of spontaneous contractions. The Pp.Cr also exerted a relaxant (0.01–5.0 mg/ml) effect on K+(80 mM) induced contractions in isolated rabbit jejunum preparations and caused shifting of the Ca2+ curves (1.0–3.0 mg/ml) toward right in a manner similar to that of verapamil (3μM), possibly suggesting presence of Ca2+ channel blocking activity. Subsequently, Pp.Cr in a concentration-dependent fashion (0.01–10.0 mg/ml) caused relaxation of CCh (1μM) and K+ (80 mM) induced contractions in isolated rabbit tracheal preparations in a manner comparable to that of dicyclomine, suggesting that the observed relaxant effect is likely to be mediated through antimuscarinic and/or Ca2+ channel blocking activities. Moreover, when evaluated against isolated rabbit aortic preparations, the Pp.Cr in concentrations up to 10 mg/ml exhibited a contractile response that was found to be abolished subsequent to pretreatment of isolated tissue preparation with cyproheptadine (1μM), phentolamine (1μM), and losartan (1μM), suggesting that Pp.Cr may have some α-adrenergic, muscarinic, serotonergic, and angiotensin II activities.
Conclusions
The aqueous ethanolic extract of Pyrus pashia (Pp.Cr) exhibited spasmolytic, bronchodilator, and vaso-constrictive activities possibly through different mechanisms. The spasmolytic and bronchodilator activities are likely to be mediated through blockade of Ca2+ channels, while vasoconstrictive activity may be due to presence of a α-adrenergic, muscarinic, serotonergic, and angiotensin II agonistic component.
Introduction
Pyrus pashia Buch.-Ham. ex D. Don. (Rosaceae), is a medium size fruiting tree, known locally as Indian pear, Himalayan pear, Batangi, Tangai and Mehal [1,2]. In Pakistan, it is distributed widely in Chitral, Swat, Kaghan, Hazara, Muree, Poonch, Mirpur districts at elevations between 750 and 2500 m. The plant is well-known for its nutritional and therapeutic importance.
The leaves are bitter in taste, served as fodder for goats and sheep [3] as well as butter tea beverages by the Monpa community of Tawang, Arunachal Pradesh,India [4]. The fresh leaves are known to possess astringent, febrifuge, laxative and sedative properties and crushed leaves are used to improve cosmetic appearance by staining palms, feet and nails [5].The fruits are tasty and eaten by local people as diet and are known to be useful in constipation [6] while fruit is used to minimize thirst. Fruit juice is astringent and diuretic [7], and is used to manage dysentery [8], leishmaniasis [9], eye problems [10], digestive disorder, sore throat, irritability, abdominal pain, anemia [11].Lab investigations have proved antimicrobial, antioxidant, stomachic and hypoglycemic activities of fruit [12,13]. Decoctions containing dried fruits with other plant parts are used for improvement in spleen and stomach functions [14]. The fruit is added to cattle fodder to enhance milk production [15]. The barks of tree possess astringent, laxative, anthelmintics and febrifuge properties [16] and is used traditionally to manage digestive disorders [17–19]. The barks possess astringent and tonic properties and are used to manage sore throat, fever, peptic ulcer, gastric ulcer [20–25] and typhoid fever [18].
Phytochemical and pharmacological investigations of fruits revealed the presence of secondary metabolites like alkaloids, flavonoids, steroids, and tannins [26], lupeol, β-sitosterol, β-sitosterol—β-D-glucoside [9] and possesses antimicrobial activity against Klebsiella pneumonia, Shigella flexneri and Eschericia coli [26].Leaves has been known to contain arbutin, tannins, phlorhizin, pectin and amygdalin [27], chlorogenic acids, flavan-3-ols and arbutin, reported to possess antioxidant, anti-mutagenic and anti-carcinogenic activities [28–30]. Although used as a remedy for multiple ailments like airway and gastric and cardiovascular ailment, Pyrus pashia Buch.-Ham. ex D. Don is a very less-researched plant, as no detailed pharmacological studies exists on this plant. As part of series of experiments in our laboratory on validating tagged biological and physiological activities of medicinal plants [31–40] the current study was designed to investigate and validate the therapeutic potential of Pyrus pashia Buch.-Ham. ex D. Don in cardiovascular, respiratory and gastrointestinal ailments.
Materials and Methods
Collection of plant material and extraction
The fresh ripened fruits of Pyrus pashia Buch.-Ham. ex D. Don were collected from Abbottabad, Pakistan. No specific permissions were required for these locations/activities and we did not sample any endangered species. The plant material was got authenticated by kind cooperation of an expert taxonomist Professor Dr. Altaf Ahmad Dasti, at the Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan vide voucher numbers P. Fl. 364–7. The plant material was made free from contaminating materials adulterants and grinded to a coarse powder with the help of a special herbal grinder. The coarsely grinded herbal material was extracted by triple maceration procedure and about 1 Kg material was macerated with 70% aqueous-ethanol at room temperature for 1 week in amber colored container with occasional shaking. The soaked material was passed through muslin cloth to remove the organic debris and the fluid obtained was filtered through Whatmann-1 filter paper. The filtrates obtained following successive macerations were pooled and was evaporated under reduced pressure in a rotary evaporator (Buchi R-200 Switzerland) attached with re-circulating chiller (B-740) and vacuum pump (Buchivac V-500) at 37°C to a thick paste of semi solid consistency. The obtained crude extract was stored at -4°C in air tight jars. The approximate percentage yield of the crude extract was calculated to be 16.5%. The crude ethanolic extract of Pyrus pashia (Pp.Cr) was insoluble in distilled water and was rendered soluble by means of Tween 80 to prepare stock solution of 300 mg/ml, which was subjected to series of dilutions in normal saline to make 3mg/ml concentrations on the day of experiment. The final diluted vehicle was found without any effect on tissue response in control experiments.
Drugs and Chemicals
Acetylcholine chloride, carbachol, potassium chloride, verapamil hydrochloride and phenylephrine, magnesium chloride, ethylene tetra-acetic acid (EDTA) were purchased from Sigma Chemicals Co. St Louis, MO, USA. Calcium chloride, glucose, magnesium sulphate, potassium dihydrogen phosphate, sodium bicarbonate, sodium dihydrogen phosphate, and ethanol were obtained from Merck, Darmstadt, Germany. Ammonium hydroxide, sodium chloride, and sodium hydroxide were purchased from BDH Laboratory supplies, Poole, England.
The chemicals used in these experiments were of highest purity and reagent analytical research grade. Stock solutions and subsequent dilutions were made fresh in distilled water on the day of experiment. The drugs were made soluble in vehicles which were without any effect on tissues in control experiments.
Phytochemical screening
The crude ethanol extract of Pyrus pashia (Pp.Cr) was subjected to for qualitative phytochemical analysis for the possible presence of alkaloids, saponins, anthraquinones, coumarins, sterols, terpenes, flavonoids and phenols [41].
Experimental animals and their housing condition
Animals (♂/♀) used in this study were local strain rabbits (1.0–1.8 kg) purchased from the local market with age limit between 6–7 months. These were housed under controlled environmental condition (23–25°C) at the animal house of Faculty of Pharmacy, Bahauddin Zakariya University, Multan. The animals were provided with standard food and tap water ad libitum. The animals were deprived of food 24 hr prior to the experiments but were given free access to water. Rabbits were sacrificed following a blow on back of head to be used for in vitro studies. All the experiments performed complied with the rulings of Institute of Laboratory Animal Resources, Commission on Life Sciences [42] and approved by the Ethical Committee of Bahauddin Zakariya University, Multan (EC/12/2011 dated 16.02.2011).
In vitro experiments
In vitro experiments were performed following minor modification of the reported methods [31–34]. Briefly, tissue segments of jejunum, trachea and aorta from the rabbit were prepared and maintained adequately in the respective buffer solutions. The detailed explanation of each tissue extraction procedure is described below under the heading of tissue of interest.
Isolated rabbit jejunum preparations
The crude ethanolic extract of Pyrus pashia (Pp.Cr) was tested for the possible presence of either spasmolytic or spasmogenic activity by using isolated rabbit jejunum preparations. Isolated rabbit jejunum segments of approximately 2 cm in length were suspended in isolated tissue baths containing Tyrode’s solution, at 37°C, aerated with carbogen (95% O2 and 5% CO2). The composition of the Tyrode’s solution (mM) was: KCl (2.68), NaCl (136.9), MgCl2 (1.05), NaHCO3 (11.90), NaH2PO4 (0.42), CaCl2 (1.8) and glucose (5.55). A preload of 1 gm was applied and intestinal responses were recorded through isotonic transducer by Power Lab Data Acquisition System (AD Instruments, Sydney, Australia) attached to a computer installed with Lab Chart Software (Version 6). The tissues were allowed to equilibrate for at least 30 min. prior to the addition of any drug. Isolated rabbit jejunum preparations exhibit spontaneous rhythmic contractions and allow testing of the antispasmodic (relaxant) effect without application of an agonist [32,43]. The observed response of the test material was quantified by the application of doses in a cumulative fashion. The relaxant effects on the part of test substances were taken as the percent change in spontaneous contractions of the preparation recorded immediately before the addition of test substances.
The possible mechanism of the relaxant activity of the test materials were investigated through the relaxation of the observed sustained spasmodic contractions following exposure to high concentration of K+(80 mM) [44]. The test materials were applied in a cumulative manner to the sustained contractions to achieve concentration-dependent inhibitory responses [45]. The observed relaxant effect of the test materials on K+ (80 mM)-induced contraction was expressed as percent of the control contractile response.
Calcium channel blocking effect of the test substances were confirmed by the method described previously by Gilani et al [46]. The isolated rabbit jejunum preparations were allowed to stabilize in normal Tyrode’s solution, which were subsequently replaced for 30 min with Ca2+-free Tyrode’s solution to which EDTA (0.1 mM) was added in order to remove calcium from the tissues. This bath solution was further replaced with K+-rich and Ca2+-free Tyrode’s solution, having the following composition (mM): KCl (50), NaCl (91.04), MgCl2 (1.05), NaHCO3 (11.90), NaH2PO4 (0.42), glucose (5.55) and EDTA (0.1). Subsequent to an incubation period of 30 min., cumulative Ca2+ concentrations were applied to the tissue bath to obtain control calcium dose-response curves (DRCs). On achievement of the super-imposable control calcium dose-response curves (usually after two cycles), the tissues were then washed and allowed to equilibrated with the plant extract for 1 hr and then the concentration response curves of Ca2+ were recorded and compared to the control curves. The DRCs of Ca2+ were recorded in the presence of different concentrations of the plant extracts in tissue bath.
Isolated rabbit tracheal preparations
The rabbit tracheas were dissected out and kept in Krebs solution of the following composition (mM): NaCl (118.2), NaHCO3 (25.0), CaCl2 (2.5), KCl (4.7), KH2PO4 (1.3), MgSO4 (1.2) and glucose (11.7). The trachea was cleaned free from the surrounding fatty tissues and rings of 2–3 mm width containing 2–3 cartilages were prepared. Each ring was opened by a longitudinal incision on the ventral side opposite to the smooth muscles layer to form a strip with smooth muscles layer in middle and cartilages on both sides. These tracheal preparations were mounted in 20 ml organ bath containing Krebs solution being maintained at 37°C and aerated with carbogen. A preload tension of 1g was applied and tissue preparations were allowed to be equilibrated for 1 hour prior to any challenge by the drug. Tissue preparations were stabilized by repeated applications of carbachol (1μM) until constant responses were recorded. The carbachol (1μM)- and high K+(80 mM)-induced sustained contractions were subsequently used for testing of different doses of the test material in a cumulative fashions. The isometric responses were recorded through Power Lab Data Acquisition System (AD Instruments, Sydney, Australia) attached to a computer installed with Lab Chart Software (Version 6). The standard drug with Ca+2 channel blocking effect (verapamil) was tested on high K+(80 mM)- and carbachol- induced spastic contractions in order to confirm the possible mechanism of action.
Isolated rabbit aorta preparation
The effect of Pp.Cr on systemic vascular resistance was assessed on isolated rabbit aorta preparations. Rabbits of either sex were sacrificed by a blow on the back of head and descending thoracic aorta was dissected out and kept in the normal Krebs solution having composition as described earlier. It was then cut vertically in 2–3mm width segments. Each isolated tissue segment was then hung in tissue organ bath (Radnoti) containing Kreb’s solution aerated with carbogen (95% oxygen and 5% carbon dioxide) at temperature 37 C. A pre-load of 2 g was applied to each preparation and allowed to equilibrate for a period of 1 hr. After equilibration, tissue was stabilized by repeated exposure to K+ (80mM) or phenylephrine (1μM) depending upon the protocol of the experiment. The vasorelaxant /vasoconstrictive effects of the test substances were studied by addition in tissue organ baths containing pre-stabilized tissue in a cumulative manner. Changes in isometric tension of aortic rings were obtained via force-displacement transducer (Model FORT100, WPI, USA) coupled to Power Lab data acquisition system (AD Instruments, Sydney, Australia) and computer running Lab Chart software (version 6).
Statistical analysis
The data is expressed as mean ± S.E.M. (n = 5) and median effective concentration (EC50) are given with 95% confidence intervals (CI) and the logarithmic dose response curves of different treatments were then plotted using Computer software “Graphpad Prism” (Graph Pad Software, San Diego, CA, USA).
Results
Preliminary phytochemical analysis
The Pp.Cr was subjected to preliminary phytochemical analysis to detect presence of the members of different phytochemical groups (i.e., alkaloids, tannins, saponins, coumarins, anthraquinones, sterols, flavonoids and terpenes) and results obtained indicated the presence of alkaloid, saponins, tannins, terpinoids, anthraquinones and flavonoids as ethanol soluble extractable constituents.
Effect on isolated rabbit jejunum preparations
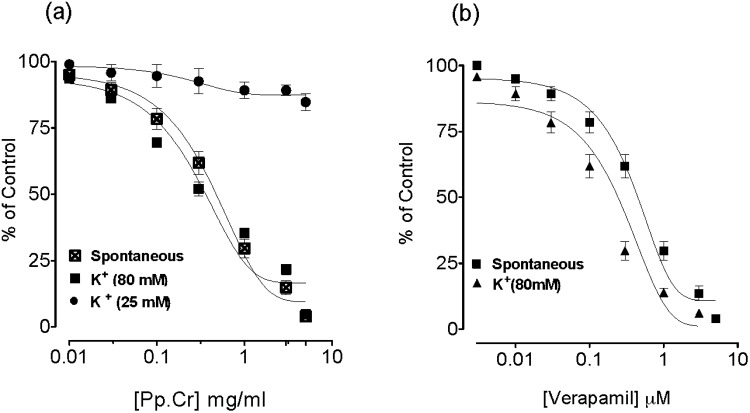
The Pp.Cr exerted relaxant effect on spontaneous contractions in isolated rabbit jejunum preparations in a manner proportional to concentration at isolated tissue bath, in concentration range of 0.01–5.0 mg/ml with EC50 value of 0.5131 mg/ml (95% CI: 0.3815–0.6902 mg/ml; n = 5) (Figs. 1b and 2a).
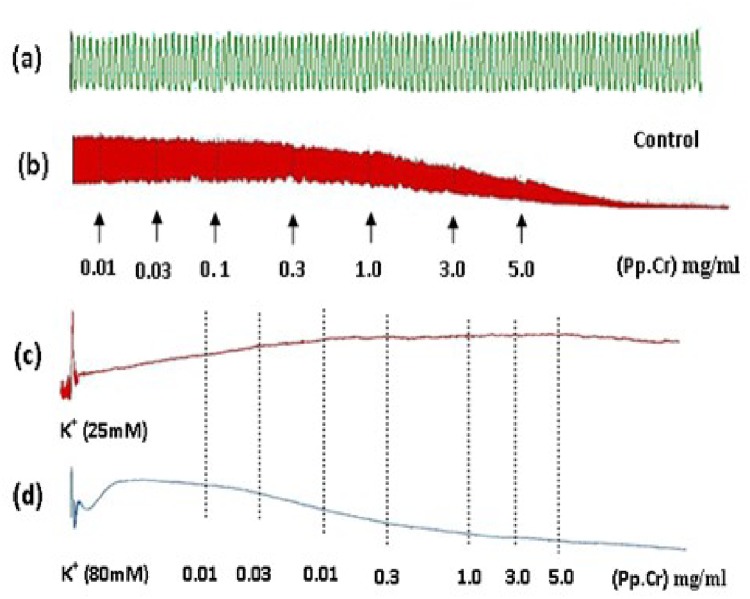
Fig 1. Original tracings presenting (a) spontaneous contraction of isolated rabbit jejunum in controlled biological environment and spasmolytic effect mediated by crude ethanolic extract of Pyrus pashia (Pp.Cr) on (b) spontaneous (c) low K+ (25 mM) and (d) high K+ (80 mM) induced tissue contraction.
Pp.Cr was added in increasing concentrations and values listed were the final tissue bath concentrations. Each tracing is a presentation of single jejunum used.
Fig 2. Spasmolytic effect observed in concentration-dependent manner by (a) a ethanolic extract of Pyrus pashia (Pp.Cr) and (b) verapamil on spontaneous- and high K+ (80 mM)-induced contractions in isolated rabbit jejunum preparations.
Values are the mean ± SEM, n = 5.
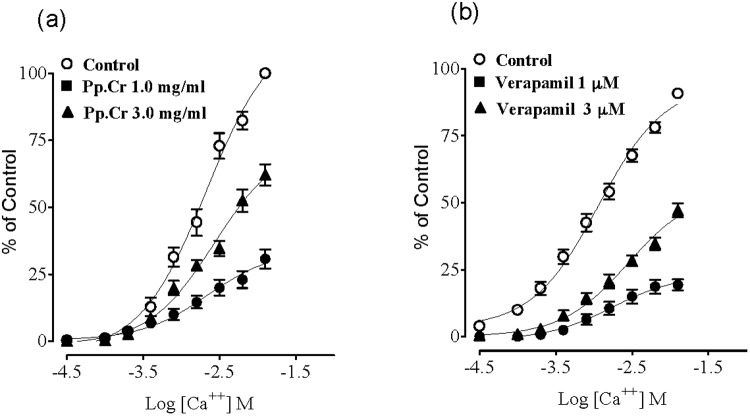
Pp.Cr was unable to relax K+ (25 mM)-induced spastic contractions (Fig. 1c and Fig. 2a), but exhibited complete relaxation of K+(80mM)-induced contractions in isolated rabbit preparations with EC50value of 0.3066 mg/ml (95% CI: 0.2226–0.4221 mg/ml; n = 5) (Figs. 1d and 2a). Verapamil relaxed the spontaneous and high K+ (80 mM) induced contractions with EC50 values of 0.1623 μM (95% CI: 0.1262–0.2086 μM; n = 5) and 0.1984 μM (95% CI: 0.1435–0.2744μM; n = 5) respectively (Fig. 2b). Moreover, pretreatment of the isolated rabbit jejunum preparations with Pp.Cr caused a rightward shift of concentration response curves for Ca2+ in a manner similar to that of verapamil (Fig. 3).
Fig 3. Calcium antagonizing effect of (a) ethanolic extract of Pyrus pashia (Pp.Cr) and (b) verapamil on concentration response curves of Ca2+ in isolated rabbit jejunum preparations.
Values are the mean ± SEM, n = 5. Control CaCl2.
Effect on isolated rabbit tracheal preparations
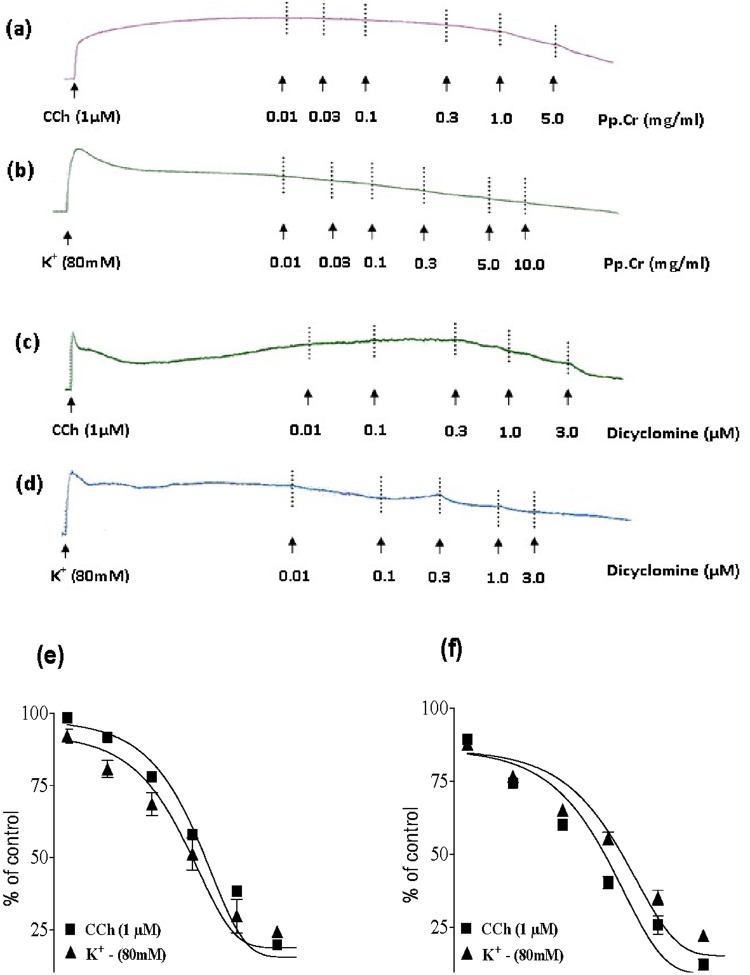
The Pp.Cr exerted relaxant effect on carbachol (CCh; 1 μM) and K+ (80 mM) induced contractions in isolated rabbit tracheal preparations with respective EC50 value of 0.5096 mg/ml (95% CI: 0.4046–0.635 mg/ml; n = 5) (Fig. 4) and 0.3839 mg/ml (95% CI: 0.2884–0.5111 mg/ml; n = 5) (Fig. 4).
Fig 4. Concentration dependent broncho-relaxant effect of a ethanolic extract of Pyrus pashia (Pp.Cr) (a, b and e) and dicyclomine (c,d and f) on carbachol (CCh: 1μM)- and high K+ (80 mM)- induced contractions in isolated rabbit tracheal preparations.
Values are the mean ± SEM, n = 5.
Comparison of the EC50 values of Pp.Cr for CCh (1μM) and K+(80 mM)-induced contractions pointed out lower numerical value of EC50 of Pp.Cr for K+ (80 mM) induced contractions than CCh -induced contractions, suggesting that Pp.Cr was more effective on K+ (80 mM)-induced contractions than on CCh-induced contractions. Similarly dicyclomine caused relaxation of CCh (1 μM) and K+(80 mM)-induced contractions with EC50 values of 0.1673 mg/ml (95% CI: 0.1280–0.2186 mg/ml; n = 5) and 0.3937 mg/ml (95% CI: 0.2728–0.5682 mg/ml, n = 5) (Fig. 4) respectively.
Effect on isolated rabbit aorta preparations
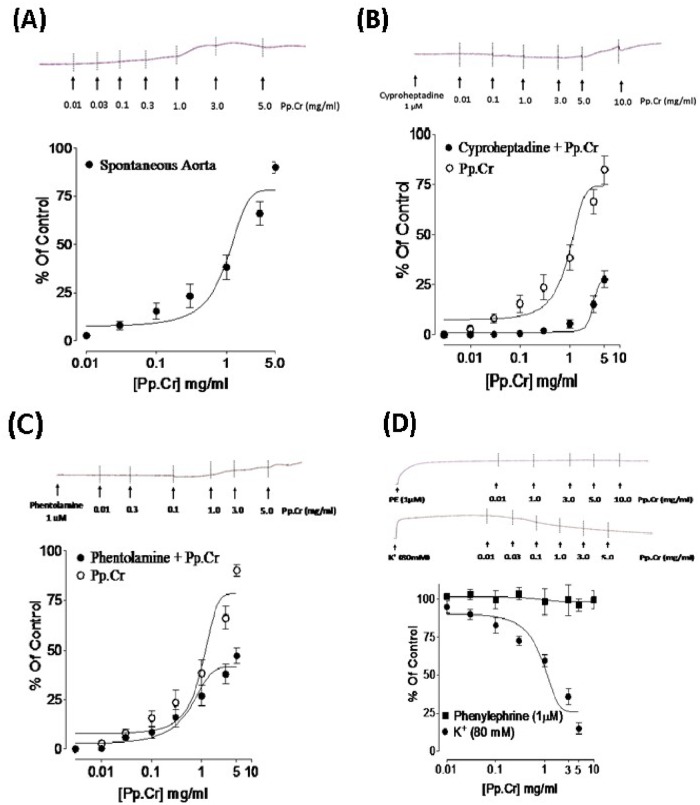
The Pp.Cr on application to the isolated rabbit aortic preparations exerted tissue bath concentration (0.3–5.0 mg/ml) dependent contractile response (Fig. 5A).
Fig 5. Tracings showing (A) Spasmogenic effect of Pyrus pashia (Pp.Cr) in concentration dependent manner on spontaneously contracting isolated rabbit aortic tissue.
Further, pretreatment of aortic tissue with each 1μM of cyproheptadine (B) and phentolamine (C) significantly inhibited the spasmogenic effect of Pp.Cr. When isolated aortic tissue was pre-contracted with phenylephrine (PE: 1μM)- and high K+ (80 mM), Pyrus pashia (Pp.Cr) exhibited concentration dependent vasodilator effect against high K+ while ineffective against phenylephrine (D). Values are the mean ± SEM, n = 5.
The percentage contractile responses corresponding to respective tissue bath concentrations of 0.01, 0.03, 0.1, 0.3, 1.0, 3.0 and 5.0 mg/ml were recorded to be 2.786 ± 0.16, 8.093 ± 0.73, 15.524 ± 0.56, 23.0498 ± 0.97, 38.236 ± 1.08, 66.105 + 1.631 and 90.00 ± 1.429. The Pp.Cr-induced contractile response was found to be decreased on pretreatment of tissues with cyproheptadine (1 μM) (Fig. 5B), phentolamine (1 μM) (Fig. 5C) and losartan (figure not shown). Furthermore, Pp.Cr did not relax phenylephrine (1 μM)-induced contractions in isolated rabbit aortic preparation (Fig. 5D) but relaxed the K+ (80mM)-induced contraction at tissue bath concentration range of 0.1–5 mg/ml with EC50 value of 0.5515 mg/ml (95% CI: 0.2602–1.169 mg/ml; n = 5) (Fig. 5D).
Discussion
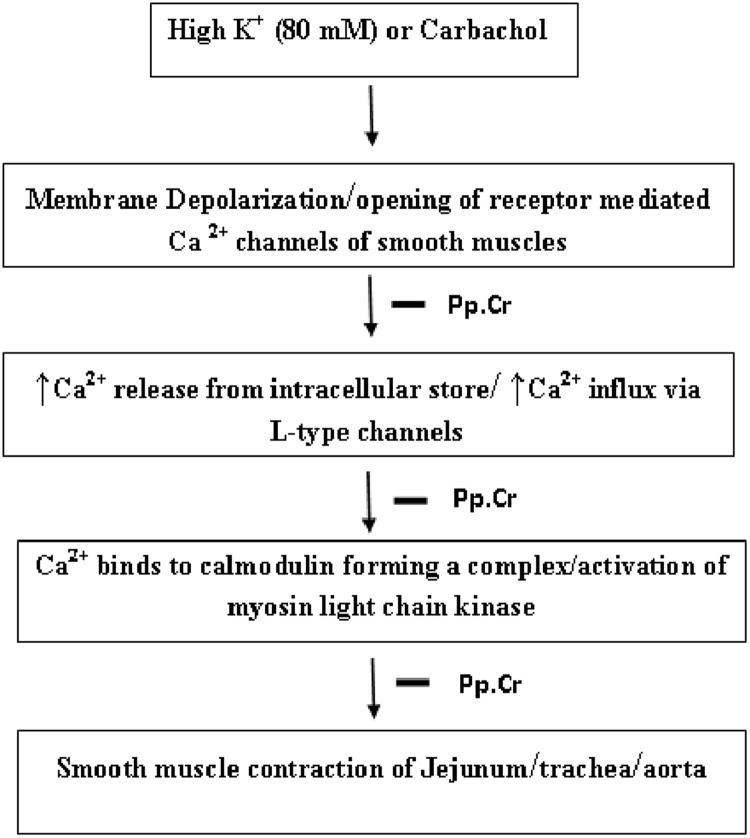
Pyrus pashia has folkloric repute for use in the management of gastrointestinal disorders in traditional systems of medicine, hence, Pp.Cr was subjected to a battery of tests to validate its use in native systems of medicine. The Pp.Cr on application to the spontaneous contractions in isolated rabbit jejunum preparations, suppressed the contractions in terms of magnitude and frequency. The spontaneous contractions in jejunum are function of periodic depolarization and repolarization, while action potential is generated through rapid influx of Ca+2 via the voltage dependent L channels (VDLCs) at the time of maximal depolarization [47]. The suppression of spontaneous movements in jejunum by Pp.Cr is presumed to be mediated through blockade of Ca+2 channels or alternatively via opening of K+ channels [32–33], which was concluded further by testing Pp.Cr on K+(25 mM)- and K+ (80 mM)-induced contractions in isolated rabbit jejunum preparations. The Pp.Cr did not cause much relaxation of K+(25 mM)-induced contractions, indicated that relaxant activity on the part of Pp.Cr was not mediated through opening of K+ channels. Alternatively, addition of Pp.Cr to tissue baths in cumulative manner completely relaxed the K+(80 mM)-induced contractions, suggested that the observed relaxant activity was likely to be mediated through blockade of Ca2+ channels. The contractile elements in smooth muscle preparations, i.e., rabbit jejunum, trachea and aorta are activated through increase in cytoplasmic free Ca2+ concentration via opening of the voltage dependent L-type Ca2+ channels (VDLCs) [48–49] or release of Ca2+ from sarcoplasmic stores [50]. The Pp.Cr exerted relaxant effect through blockade of Ca+2 channels and further inhibition of sequence of events, i.e., decrease in cystolic Ca2+ concentration, decrease in Ca2+ binding to calmodulin, decrease in Ca2+ calmodulin complex formation, decrease in activation of myosin light chain kinase (MLCK), decrease in phosphorylation of the myosine light chains, decrease in interaction between actin and myosin and inhibition of contractile phenomenon (Fig. 6). These speculations were confirmed further as pre-treatment of isolated rabbit jejunum preparations with Pp.Cr caused rightward shift of Ca2+ response curve in a manner similar to verapamil as a standard Ca2+ channel blocker [51]. The Ca2+ channel blockers are a class of therapeutic agents being effective in the management of hyperactive gut diseases. The bronchodilator potential of Pp.Cr was explored following application to carbachol (1 μM) and K+ (80 mM)-induced contractions in isolated rabbit tracheal preparations. The Pp.Cr addition to isolated tissue bath in cumulative manner, caused a concentration dependent relaxation of both CCh (1μM)- and K+(80 mM)-induced contractions. However, relaxant effect of Pp.Cr on K+(80 mM)-induced contractions was achieved at much lower tissue bath concentrations as compared to carbachol (1 μM)-induced contractions. The carbachol is one of the cholinergic drugs, causing increase in cytosolic Ca+2 concentrations. On the basis of above-mentioned arguments, presence of both activities, i.e., anti-muscarinic and blockade of Ca2+ channels was speculated in a manner similar to dicyclomine (Fig. 6).
Fig 6. Proposed mechanism of action of smooth muscle relaxation mediated by ethanol extract of Pyrus pashia (Pp.Cr) against high K+ (80 mM) and carbachol (1μM) induced contraction.
Sign—indicates site of inhibition by Pp.Cr.
Moreover, Pp.Cr exhibited contractile activity on isolated rabbit aortic preparation in tissue bath concentration range of 0.1–5 mg/ml, which was found to be minimized on pre-treatment of isolated rabbit aortic preparations with phentolamine, cyproheptadine and losartin and was concluded to be mediated through activation of multiple receptors like α-adrenergic, muscarininic, histaminergic, serotonergic and angiotensin II receptors [52]. The Pp.Cr also caused relaxation on K+ (80 mM)-induced contractions in isolated rabbit aortic preparations in a manner similar to isolated rabbit jejunum and rabbit tracheal preparations, hence Ca2+ channel blocking activity of Pp.Cr was confirmed in all types of smooth muscles preparations.
The Pp.Cr exhibited Ca2+ channel blocking activity in isolated rabbit tissue preparations (i.e., jejunum, trachea and aorta) which can be attributed to the presence of alkaloids and flavonoids among the constituents of Pyrus pashia detected in preliminary phytochemical screening. The phenylephrine-induced contractions in isolated rabbit aortic preparations were not relaxed on application of Pp.Cr because it involves activation of α-adrenergic receptors, activation of G-proteins, increase in Ca2+ influx, via opening of receptor-operated calcium channels (ROCs). The proposed mechanism of inhibition mediated by Pp.Cr in term of its relaxant effect is summarized in Fig. 6.
Conclusions
The aqueous ethanolic extract of Pyrus pashia (Pp.Cr) exhibited spasmolytic, bronchodilator and vaso-constrictive activities through different mechanisms. The spasmolytic and bronchodilator activities are likely to be mediated through blockade of Ca2+ channels, while vasoconstrictive activity may be due to presence of α-adrenergic, muscarinic, serotonergic and angiotensin II agonistic component. The Ca2+ channel blocking activitiy can be attributed to the phytochemical constituents of Pyrus pashia fruits (i.e., alkaloids, flavonoids, glycosides and anthraquinones).
Data Availability
All relevant data are within the paper.
Funding Statement
These authors have no support or funding to report.
References
- 1. Matin A, Khan MA, Ashraf M, Qureshi RA (2001) Traditional uses of herbs, shrubs and tree of Shogran valley, Mansehra, Pakistan. Pak J Bio Sci 4: 1101–1107. [Google Scholar]
- 2.Ahmad Z (2007) Country report on plant genetic resources for food and agriculture. Pakistan Agricultural Research Council, Islamabad, Pakistan.
- 3. Thapa B, Walker DH, Sinclair FL (1997) Indigenous knowledge of the feeding value of tree fodder. Animal Feed Sci Tech 67: 97–l14. [Google Scholar]
- 4. Tsering J, Gogoi BJ, Tag H (2012) Ethnobotany and phytochemical analysis of Pyrus pashia leaves. Int J Pharm Sci Res 3: 2721–2725. [Google Scholar]
- 5. Shinwari MI, Khan MA (1998) Multiple dimensions of ethnobotany and its present status in Pakistan. Hamdard Med 42: 5–10. [Google Scholar]
- 6. Abbasi AM, Khan MA, Khan N, Shah MH (2013) Ethnobotanical survey of medicinally important wildedible fruits species used by tribal communities of Lesser Himalayas-Pakistan. J Ethnopharm 148: 528–536. [DOI] [PubMed] [Google Scholar]
- 7. Meyers KJ, Watkins CB, Pritts MP, Liu RH (2003) Antioxidant and anti proliferative activities of strawberries. J Agri Food Chem 51: 6887–6892. [DOI] [PubMed] [Google Scholar]
- 8. Chettri N, Sharma E, Lama SD (2005) Non-timber forest produces utilization, distribution and status in a trekking corridor of Sikkim, India. Lyonia 8:89–101. [Google Scholar]
- 9. Khandelwal R, Paliwal S, Chauhan R, Siddiqui A (2008) Phytochemical screening of hexane soluble fraction of Pyrus pashia fruits. Oriental J Chem 24:773–774. [Google Scholar]
- 10.Lama YC, Ghimire SK, Aumeeruddy-Thomas A (2001) Medicinal Plants of Dolpo: Amchis’ Knowledge and Conservation. People and Plants Initiative and WWF Nepal Program, Nepal.
- 11. Saklani S, Chandra S (2012) Evolution of Garhwal Himalaya wild edible fruit Pyrus pashia pulp. J Pharm Res 5: 3030–3032. [Google Scholar]
- 12. Maga JA (1978) Simple phenol and phenolic compounds in food flavour. CRC Crit Rev Food Sci Nutr 10: 323–372. [DOI] [PubMed] [Google Scholar]
- 13. Shui G, Leong LP (2002) Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by HPLC. J Chromatograp A 977: 89–96. [DOI] [PubMed] [Google Scholar]
- 14. Jiangsu New Medical College (1986) Dictionary of Chinese Traditional Medicine. Shanghai People’s Press, Shanghai, China. [Google Scholar]
- 15. Kunwar RM, Mahat L, Sharma LN, Shrestha KP, Rivier L, et al. (1979) Editorial. J Ethnopharm 1:1. [Google Scholar]
- 16.Kala CP (2002) Medicinal plants of Indian trans-Himalaya. Bishen Singh Mahendra Pal Singh Dehradun, India.
- 17.Prajapati ND, Purohit SS, Sharma AK, Kumar T (2003) A handbook of medicinal plants. Agrobios, Jodhpur, India.
- 18. Shrestha PM, Dhillion SS (2003) Medicinal plant diversity and use in the highlands of Dolakha district, Nepal. J Ethnopharm 86: 81–96. [DOI] [PubMed] [Google Scholar]
- 19. Farooquee NA, Majila BS, Kala CP (2004) Indigenous knowledge systems and sustainable management of natural resources in a high altitude society in Kumaun Himalaya, India. J Hum Ecol 16:33–42. [Google Scholar]
- 20. Singh MP, Malla SB, Rajbhandari SB, Manandhar A (1979) Medicinal plants of Nepal: retrospects and prospects. Economic Bot 33:185–193. [Google Scholar]
- 21. Joshi AR, Edington JM (1990) The use of medicinal plants by two village communities in the Central Development Region of Nepal. Economic Bot 44:71–83. [Google Scholar]
- 22. Manandhar NP (1994) An ethnobotanical survey of herbal drugs of Kaski District, Nepal. Fitoterapia 65: 7–13. [Google Scholar]
- 23. Manandhar NP (1995) A survey of medicinal plants of Jajarkot District, Nepal. J Ethnopharm 48: 1–6. [DOI] [PubMed] [Google Scholar]
- 24. Manandhar NP (1990) Medico botany of Gorkha District, Nepal: an elucidation of medicinal plants. Int J Crude Drug Res 28: 17–25. [Google Scholar]
- 25. Joshi AR, Edington JM (1990) The use of medicinal plants by two village communities in the Central Development Region of Nepal. Economic Bot 44:71–83. [Google Scholar]
- 26. Saklani S, Chandra S (2012) In-vitro antimicrobial activity nutritional profile of medicinal plant of Garhwal, Himalaya. Int J Pharm Sci Res 3:268–272. [Google Scholar]
- 27. Hussein FTK (1983) Medicinal plants in Libya. Arab Encylopedia House, Beirut, Lebanon, pp. 7–822. [Google Scholar]
- 28. Challice JS, Williams AH (1968) Phenolic compound from genus Pyrus-II: A chemotaxonomic survey. Phytochem 7: 1781–1801. [Google Scholar]
- 29. Guven K, Yucel E, Cetintas F (2006) Antimicrobial activities of fruits of Crataegus and Pyrus species, Pharm Bio 44: 79–83. [Google Scholar]
- 30. Nassar MI, Mohamed TK, El-Toumy SA, Garra AH; El-Kashak WA, et al. (2011) Phenolic metabolites from Pyrus calleryana and evaluation of its free radical scavenging activity. Carbohydrate Res 346: 64–67. 10.1016/j.carres.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 31. Chaudhary MA, Imran I, Bashir S, Mehmood MH, Rehman NU, et al. (2012) Evaluation of gut modulatory and bronchodilator activities of Amaranthus spinosus Linn. BMC Complement Altern Med 12: 166–166. 10.1186/1472-6882-12-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janbaz KH, Haider S, Imran I, Zia-Ul-Haq M, De Martino L, et al. (2012) Pharmacological Evaluation of Prosopis cineraria (L.) Druce in gastrointestinal, respiratory, and vascular disorders. Evid Based Complement Altern Med 2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janbaz KH, Nisa M, Saqib F, Imran I, Zia-Ul-Haq M, De Feo V (2013) Bronchodilator, vasodilator and spasmolytic activities of methanolic extract of Myrtus Communis L. J Physiol Pharmacol 64: 479–484. [PubMed] [Google Scholar]
- 34. Janbaz KH, Arif J, Saqib F, Imran I, Ashraf M, et al. (2014) In-vitro and in-vivo validation of ethnopharmacological uses of methanol extract of Isodon rugosus Wall. ex Benth (Lamiaceae). BMC Complement Alternat Med 14:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zia-Ul-Haq M, Amarowicz R, Ahmad S, Qayum M, Ercişli S (2013) Antioxidant potential of mungbean cultivars commonly consumed in Pakistan. Oxid Communication 36:15–25. [Google Scholar]
- 36. Zia-Ul-Haq M, Ahmad S, Bukhari SA, Amarowicz R, Ercisli S, et al. (2014) Compositional studies and biological activities of some mash bean (Vigna mungo (L.) Hepper) cultivars commonly consumed in Pakistan. Bio Res 47:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zia-Ul-Haq M, Ahmad S, Amarowicz R, DeFeo V (2013) Antioxidant activity of the extracts of some cowpea (Vigna unguiculata (L) Walp.) cultivars commonly consumed in Pakistan. Molecules 18: 2005–2017. 10.3390/molecules18022005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zia-Ul-Haq M, Cavar S, Qayum M, Khan I, Ahmad S (2013) Compositional studies and antioxidant potential of Acacia leucophloea Roxb. Acta Bot Croat 72:27–31. [Google Scholar]
- 39. Zia-Ul-Haq M, Ahmad S, Qayum M, Ercişli S (2013) Compositional studies and antioxidant potential of Albizia Lebbeck (L.) Benth. Turk J Bio 37:25–32. [Google Scholar]
- 40. Zia-Ul-Haq M, Landa P, Kutil Z, Qayum M, Ahmad S (2013) Evaluation of anti-inflammatory activity of selected legumes from Pakistan: In vitro inhibition of Cyclooxygenase-2. Pak J Pharm Sci 26: 185–187. [PubMed] [Google Scholar]
- 41. Tona L, Kambu K, Ngimbi N, Cimanga K, Vlietinck AJ (1998) Antiamoebic and phytochemical screening of some Congolese medicinal plants. J Ethnopharmacol 61:57–65. [DOI] [PubMed] [Google Scholar]
- 42. National Research Council (1996) Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press. [Google Scholar]
- 43. Gilani AH, Shah AJ, Ghayur MN, Majeed K (2005) Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci 76: 3089–105. [DOI] [PubMed] [Google Scholar]
- 44. Farre AJ, Colombo M, Fort M, Gutierrez B (1991) Differential effects of various Ca2+ antagonists. Gen Pharmacol 22: 177–181. [DOI] [PubMed] [Google Scholar]
- 45. Van Rossum JM (1963) Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Thér 143: 299–330. [PubMed] [Google Scholar]
- 46. Gilani SN, Khan AU, Gilani AH (2010) Pharmacological basis for the medicinal use of Zanthoxylum armatum in gut, airways and cardiovascular disorders. Phytother Res 24:553–558. 10.1002/ptr.2979 [DOI] [PubMed] [Google Scholar]
- 47. Brading AF, Sneddon P (1980) Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol 70: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, et al. (1997) Calcium movements, distribution, and functions in smooth muscle. Pharm Rev 49:157–230. [PubMed] [Google Scholar]
- 49. Karaki H, Weiss B (1983) Mini-review: calcium release in smooth muscle. Life Sci 42:111–122. [DOI] [PubMed] [Google Scholar]
- 50. Godfraind T, Miller R, Wibo M (1986) Calcium antagonism and calcium entry blockade. Pharmacol Rev 38:321–416. [PubMed] [Google Scholar]
- 51. Fleckenstein A (1977) Specific pharmacology of Ca++ in myocardium, cardiac pacemakers and vascular smooth muscles. Rev Pharm Toxic 17:149–166. [DOI] [PubMed] [Google Scholar]
- 52. Bolton TB (1979) Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev 59: 606–718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.